Abstract
Understanding the ecological mechanisms driving the dynamics of field rotifer populations remains a challenge in ecology. Brachionus angularis (Rotifera) is preferred in such studies because of their planktonic behavior, high fecundity, and absence of cryptic species. In this study, one-year zooplankton samples were collected twice a month from a subtropical lake and the roles of abiotic and biotic factors regulating the population dynamics of B. angularis were analyzed. The sampled B. angularis were composed of two discontinuous populations: ‘summer and autumn’ and ‘spring and winter’, segmented by high mixis rates. The ‘summer and autumn’ population had high egg ratios, but low egg volumes. Water temperature was the main factor driving temporal dynamics in density, thereby counteracting the negative effect of top-down force. Based on the synchronous dynamics of two fractions of chlorophyll-a, the densities of B. angularis, its potential competitors and predators, together with the reverse correlations between the egg ratio and population density, and Secchi-disk depth, we found that bottom-up and top-down forces were plausible factors regulating variations in population density in spring (March and April). However, the density peak of B. angularis in winter might be primarily ascribed to recruitment from the sediment. We further addressed the necessity for diverse evidence from the speciation of cryptic species, abundance, reproduction, and morphology, in understanding the population dynamics of a field rotifer population.
Introduction
Population dynamics have long attracted the interest of ecologists and are recognized as the central subject in population ecology, with a focus on how and why population numbers change in both time and space (Turchin Citation2003). As a common constituent of zooplankton in aquatic ecosystems, Rotifera is considered a model organism for understanding population dynamics because of its short generation time, high population growth rate, and strong trophic interactions (Yoshida Citation2005).
The population dynamics of rotifers are generally controlled by abiotic and biotic factors, among which food supply (bottom-up effect) and predation (top-down force) are crucial for structuring the community and regulating the annual population variability (Hunter and Price Citation1992; Ooms-Wilms et al. Citation1999; Yoshida et al. Citation2003; Waervågen and Andersen, Citation2018). Sommer et al. (Citation1986) proposed a verbal Plankton Ecology Group (PEG) model demonstrating the standard seasonal succession pattern of zooplankton, including rotifers, from various lakes in a temperate zone. Nevertheless, whether or not abiotic (e.g. water temperature) or biotic factors (e.g. food resources and predation pressure) have more critical influences on the dynamics of rotifers is still under debate. Some published studies have reported that the importance of resource limitation differs among rotifer species (Merriman and Kirk Citation2000; Cordova et al. Citation2001) and have shown that Synchaeta spp. population dynamics are more closely controlled by resources than Keratella spp. (Merriman and Kirk Citation2000), while Polyarthra vulgaris may be more strongly affected by other factors, despite the common occurrence of food limitation (Cordova et al. Citation2001). Yoshida et al. (Citation2003) suggested that rotifer abundance among lakes is mainly determined by bottom-up forces, but their temporal changes within individual lakes are highly associated with top-down forces from copepod predation (Yoshida et al. Citation2000).
Apart from the aforementioned environmental variables, sexual reproduction, cyclomorphosis, and the presence of cryptic rotifer species may be associated with the temporal dynamics of the rotifer population. Monogonont rotifers often produce diapausing eggs to overcome unfavorable conditions and possess a specific morphology that discourages predation by carnivorous copepods and rotifer Asplanchna spp., resulting in a subsequent decline in the population growth and density of these prey rotifers (Schröder Citation2005; Sarma et al. Citation2011; García-Roger et al. Citation2017; Tarazona et al. Citation2017). The co-occurrence of cryptic rotifer species with a high degree of morphological similarity but a distinct response of ecological traits to environmental change is widespread (Gilbert and Walsh Citation2005; Fontaneto et al. Citation2009; Obertegger et al. Citation2012; Leasi et al. Citation2013; Wen et al. Citation2016). Rotifer population dynamics in natural bodies of water can be disrupted by the putative coexistence of cryptic species. Accordingly, whether or not sexual reproduction, morphological variation, and the differentiation of cryptic species over time attribute to understand the relationship between environmental conditions and rotifer population dynamics merits further investigation.
Brachionus angularis is one of the most frequent rotifer species in plankton dominated by Microcystis spp. (Green Citation2011). It is preferred in aquaculture and ecotoxicology research focusing on the effects of environmental factors on population growth rate (Gama-Flores et al. Citation2004; Hu and Xi Citation2008; Ogata et al. Citation2011; Miracle et al. Citation2014; Matus et al. Citation2017). Theoretically, B. angularis is also an ideal test animal to reveal the ecological mechanisms regulating its natural population dynamics, due to its planktonic behavior, high fecundity (Walz Citation1995), and more importantly, lack of cryptic species speciation. Our previous study in Lake Jinghu found that in the absence of copepods, population dynamics of ‘summer and autumn’ B. angularis are mostly affected by water temperature (Wen et al. Citation2011b). Furthermore, the rotifer Keratella cochlearis showed a lower reproductive ability and population growth rate than B. angularis (Walz Citation1987) and is more vulnerable to water temperature than predation pressure from copepods (Meyer et al. Citation2017). Hence, we hypothesized that the temporal dynamics of B. angularis populations might be principally driven by water temperature, even if prospective predators, including copepods, were abundant.
In the present study, the annual dynamics of B. angularis in a subtropical lake was studied with the following aims: (1) to explore the relative importance of abiotic and biotic factors affecting B. angularis population dynamics; (2) to test the hypothesis that water temperature is more important than predation risk in regulating seasonal dynamics of this rotifer; and (3) to identify whether the sexual reproduction and morphological variation of B. angularis during growing seasons can help in understanding the forces driving its population dynamics.
Materials and methods
Study sites and zooplankton sampling
Lake Tingtang is a shallow and slightly eutrophic lake located in Wuhu City on the south bank of the Yangtze River, with an average depth of 1.5 m and a surface area of 13.47 ha (Wen et al. Citation2011a).
The zooplankton samples, including rotifers, cladocerans, and copepods, were collected at two fixed stations twice a month from February 2009 through January 2010. Those collected at the end of October 2009 after a period of continuous bad weather were not harvested successfully. On each sampling date, a qualitative sample was obtained by hauling a 25 μm net along a wide range of surface water to determine the presence of rotifer species. A quantitative sample was collected from each station by filtering 15 L of integrated lake water (5 L of water from the surface to the bottom at 0.5 m intervals) through another 25 μm net. The retained rotifer samples were fixed in situ with 4% sucrose formaldehyde (Haney and Hall Citation1973). Rotifers were counted in at least three Sedgewick-Rafter subsamples using an Olympus BH-2 microscope at 100× magnification. Based on the fixed samples and living materials, rotifers were identified to the species level according to Koste (Citation1978), while cladocerans and copepods were identified to the genus and order levels, respectively.
Environmental variables
Physicochemical conditions, including water temperature, pH, Secchi-disk depth (SD), dissolved oxygen (DO), and NH4+-N concentrations were measured in each station during rotifer sampling. To quantify the chlorophyll-a content representative of edible algae or other associated food resources (Auer et al. Citation2004), 1.0 L of water from another 15 L of integrated lake water was sampled in two fractions: one was filtered through a 25 μm plankton net (Chl-a < 25 μm) and one was unfiltered (Chl-a), indicating the biomass of the algae with a smaller volume and all phytoplankton, respectively. Annual dynamics and measurement methods of the contents of these environmental variables have been described in detail by Wen et al. (Citation2017).
Cladocerans and rotifers have an overlapping food niche, consuming particles 1–20 μm in size (de Bernardi et al. Citation1987). The genera Brachionus (excluding B. angularis), Keratella, and Filinia have been recognized as feeding guild rotifers (i.e. microphagous species based on their feeding strategy) (Obertegger et al. Citation2011). Consequently, cladocerans and feeding guild rotifers were considered potential competitors of B. angularis in the present study.
The effects of environmental variables on the density and egg ratio of the B. angularis population
Under field conditions, the egg ratio (ER) is a useful indicator for prediction about the pattern of population growth in planktonic rotifers (Edmondson Citation1960; Sarma et al. Citation2005). Egg ratio is calculated using the formula: ER = total amictic eggs/total amictic females (Edmondson Citation1960). The mixis rate of the B. angularis population (MR) was used to depict the degree of sexual reproduction that does not affect the population growth of monogonont rotifers, which is calculated as MR = total mictic females/total females. Morphological measurements of B. angularis were performed under 100× magnification, and body size and egg volume were analyzed according to Sarma and Rao (Citation1987).
All data were tested for normality using the one-sample Kolmogrov-Smirnov procedure. Thus, the data that were inconsistent with the normal distribution were ln(X + 1) transformed. A paired-sample t-test was used to detect differences in environmental variables between two sampling stations. To represent the potential environmental conditions associated with the population dynamics of B. angularis during a growing season with a low degree of sexual reproduction, principal component analyses (PCA) were performed using the Paleontological Statistics Software Package (PAST) (Hammer et al. Citation2001).
Product-moment correlations were conducted between each environmental variable measured in this study and the population density, egg ratio, body size, and egg volume of B. angularis. The environmental conditions included the abiotic (selected by PCA) and biotic factors. Factors that were significantly correlated with density and egg ratio, as well as morphological parameters, were used as independent variables in a multiple-regression model using a stepwise forward selection procedure (F to enter =1) (López et al. Citation2007). Beta-coefficients were calculated in the model to assess relative importance of each independent variable to predict the density, egg ratio, body size, and egg volume (López et al. Citation2007). All analyses were performed using SPSS version 16.0 (SPSS Inc., Chicago, IL).
Results
Annual dynamics of abiotic variables and crustacean zooplankton
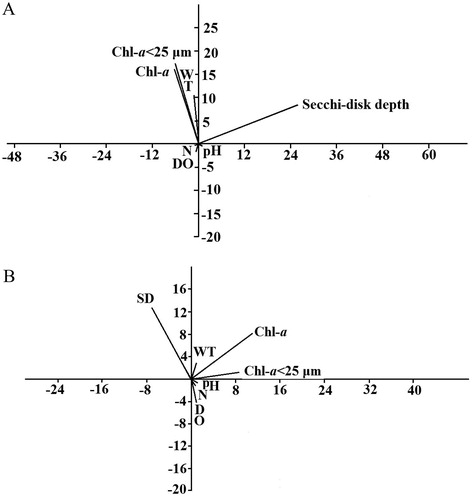
The dynamics of abiotic factors over one year in Lake Tingtang were described by Wen et al. (Citation2017). A paired-sample t-test indicated that there were no significant differences in physicochemical variables between the two sampling stations in Lake Tingtang (p > 0.05). The water temperature and two fractions of Chl-a peaked in summer (June through August 2009) with low values recorded in winter (February 2009, December 2009, and January 2010) (). Notably, two fractions of Chl-a increased from early to late March 2009, but decreased at the beginning of April 2009 (). The pH values varied within a relatively narrow range of 7.5 to 8.5, while NH4+-N fluctuated considerably on a temporal scale with a concentration of less than 2.0 mg/L. The PCA revealed that water temperature, Chl-a, SD, and these three factors coupled with DO were the most important variables related to the changes in abiotic factors during winter, spring, and summer through autumn in Lake Tingtang ().
Figure 1. Annual dynamics of two fractions of Chl-a (Chl-a and Chl-a < 25 μm) in Lake Tingtang (Wen et al.,Citation2017). The dotted and solid arrows indicate the variation trends of two fractions of Chl-a in March and April 2009, respectively.
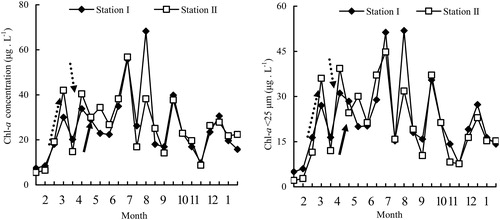
Figure 2. Principal component analyses of the environmental factors measured in this study. a: winter and spring; b: summer and autumn.
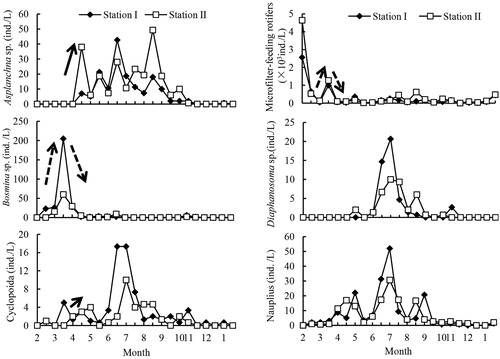
Cyclopoida and its nauplii prevailed in the copepod assemblages, whose densities were high in June and September 2009 (). The cladoceran community was dominated by small species belonging to Bosmina and Diaphanosoma, which were detected from February to April 2009 (winter and spring), and between June and October 2009 (summer and autumn), respectively (). The rotifer Asplanchna spp. was recorded between early April and November 2009 ().
Variation in the population density of B. angularis
The annual average population density of B. angularis at station I and station II was 40.3 ind./L and 42.6 ind./L, with peaks of 298.0 ind./L and 223.3 ind./L, respectively. No significant distinctions in the densities were observed between stations except for those occurring in late July (paired-sample t-test, p > 0.05). The B. angularis population clearly declined in May and November 2009 when the mixis rate exhibited a sudden increase () and the average densities in winter and spring were higher than those in summer and autumn. Population density of B. angularis in spring (March through April 2009) suddenly increased from early March 2009 to late March 2009, followed by a rapid decline until late April (). Similarly, two fractions of Chl-a and the density of Bosmina spp. were also observed from early March through late April 2009.
Figure 4. Dynamics of population density, egg ratio, and mixis rate of the B. angularis population in Lake Tingtang. The dotted and solid arrows indicate the density dynamics of the B. angularis population in March and April 2009, respectively.
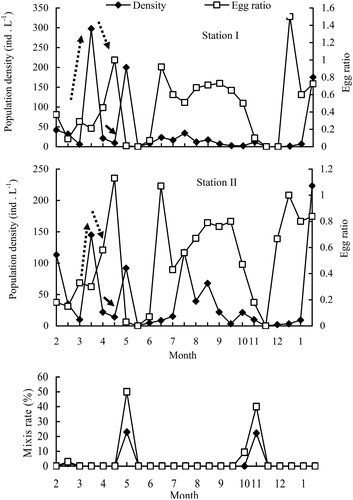
Regardless of sexual reproduction of B. angularis in this study, Pearson correlation indicated that the annual population density of this rotifer was positively correlated with the feeding guild rotifers (). However, B. angularis in Lake Tingtang could be divided into two discontinuous populations by the boundaries of the high mixis rate occurring in May and November 2009 (), referring to the ‘winter and spring’ population (before May 2009 and after November 2009) and the ‘summer and autumn’ population (between May and November 2009). The ‘winter and spring’ population density of B. angularis was positively correlated with the unique factor of feeding guild rotifers and that of the ‘summer and autumn’ population density was also positively associated with water temperature (, ), potential competitors (Diaphanosoma spp. and feeding guild rotifers), and potential predators (Cyclopoida, copepods, and Asplanchna spp.) ().
Figure 5. Regression relationships between density and egg ratio of the B. angularis population and water temperature during summer and autumn. The population density was ln (X + 1) transformed.
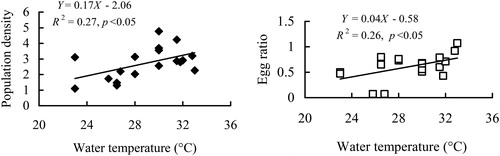
Table 1. The Pearson correlation coefficients between population density and egg ratio of B. angularis and environmental factors in Lake Tingtang. (The densities of the zooplankton listed in the table were ln (X + 1) transformed; *p < 0.05; **p < 0.01).
Variations in the egg ratio and morphological parameters of B. angularis
The egg ratios of the B. angularis population rapidly decreased in May and November 2009 when mixis rates were high, and then increased in both summer and December 2009 through January 2010 (). It is noteworthy that the egg ratio in the B. angularis population showed an increasing trend between February 2009 and April 2009, remaining at a high level from June through September 2009 ().
The egg ratio of B. angularis from year-round data was negatively associated with SD, but was positively associated with two fractions of Chl-a (). The ‘winter and spring’ population displayed reverse relationships with SD and the densities of B. angularis and feeding guild rotifers (, ), but showed positive correlations with two fractions of Chl-a (, ). Multiple regressions suggested that SD was the most important factor affecting egg ratios of B. angularis of the ‘winter and spring’ population (). However, the egg ratio of the ‘summer and autumn’ population was positively associated with the unique factor of water temperature (, ).
Figure 6. Regression relationships between egg ratio of the B. angularis population and Secchi-disk depth (a), Chl-a < 25 μm (b) and population density of B. angularis (c and d) during winter and spring. The population density in C and D was ln (X + 1) transformed and the values obtained in January 2010 when a density peak occurred were ignored in D.
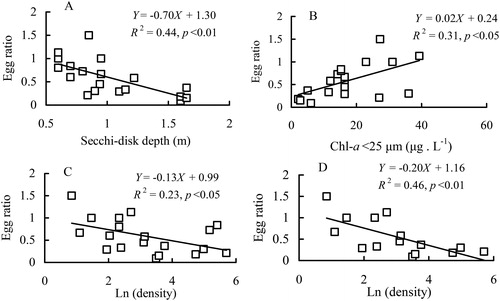
Table 2. Forward stepwise regression analyses between dependent variables of egg ratio (Y1: year-round data; Y2: winter and spring population), body size (Y3), and egg volume (Y4) and independent variables of environmental factors.
Over one year, excluding May and November 2009, body size and egg volume of B. angularis were both negatively correlated with water temperature, two fractions of Chl-a, and potential competitors and predators (), but were both positively correlated with SD, DO, and feeding guild rotifers (). Multiple regressions suggested that only water temperature was a factor influencing body size and egg volume of B. angularis over time (, ).
Figure 7. Regression relationships between body size and egg volume of B. angularis and water temperature in Lake Tingtang.
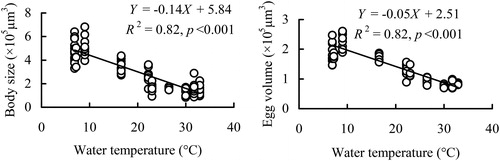
Table 3. The Pearson correlation coefficients between body size and egg volume of B. angularis and environmental factors in Lake Tingtang. (The densities of the zooplankton listed in the table were ln (X + 1) transformed; *p < 0.05; **p < 0.01).
Discussion
The ecological mechanisms driving B. angularis dynamics of ‘winter and spring’ population in Lake Tingtang
Rotifer population dynamics are governed by abiotic and biotic factors, but which of these contributes more to shaping the seasonal patterns of abundance is contested. The classic PEG model states that rotifer abundance is expected to increase in early spring when algal food is abundant, and then decline with increased abundance of large zooplankton (their predators and competitors) (Sommer et al. Citation1986). During winter and spring in the present study, the population density of B. angularis was related to the unique factor of feeding guild rotifers based on correlation and regression analyses. However, during early March through early April 2009, the population density of B. angularis underwent synchronous dynamics with two fractions of Chl-a and the densities of potential competitors, including Bosmina spp. and feeding guild rotifers, indicating that the B. angularis population was likely controlled by food resources, which is consistent with the PEG model. Additionally, the population density of B. angularis continued to decline until the end of April, coupled with an increase in both fractions of Chl-a and a decrease in the densities of Bosmina spp. and feeding guild rotifers. Moreover, potential predators of B. angularis, including Asplanchna spp. and Cyclopoida copepods, maintained increasing trends in density. These findings suggest that the B. angularis population that occurred in April might be determined by top-down forces instead of a bottom-up effect.
With respect to the population in winter, a sudden density peak and higher abundance of B. angularis were observed in January 2010 and early February 2009 when water temperature and Chl-a concentration were at a lower level, together with the absence of potential competitors in the water column of Lake Tingtang, including Bosmina spp., Cyclopoida copepods, and carnivorous Asplanchna spp. This interesting phenomenon could not be explained by water temperature, food resources, competition effects, or predation pressure. It has, however, been largely attributed to recruitment from the sediment, which is both crucial for shaping rotifer communities and conducive to understanding temporal changes in rotifer abundance (Zhang et al. Citation2015). The resting eggs of a cold-water strain of B. angularis are inclined to hatch from the sediment at a lower temperature (Schröder 2005). Hence, the density peak in B. angularis that occurred in January 2010 in Lake Tingtang can be considered to correspond to recruitment from the sediment.
Inverse relationships between the egg ratio of planktonic rotifers and their density, as well as transparency, generally indicate that food resources or short periods of low food availability are limiting for rotifers (Sarma et al. Citation2005). During winter and spring, the egg ratio of B. angularis in Lake Tingtang was negatively associated with SD and population density, while it was positively and linearly correlated with two fractions of Chl-a, suggesting that a food shortage probably occurred in winter and early spring when there was a lower Chl-a concentration and a higher density of this rotifer and feeding guild rotifers.
Is water temperature or predation risk more important in regulating the population dynamics of B. angularis during summer and autumn when its potential predators are abundant?
Yoshida (Citation2005) suggests that negative correlations of abundance between competing species have been used to imply strong interspecific competition for food. During summer and autumn, in the present study, the population density of B. angularis showed positive correlations with potential competitors and predators. Moreover, the density of B. angularis increased during June and July 2009 when Diaphanosoma spp., feeding guild rotifers, Asplanchna spp., and Cyclopoida copepods were abundant. Competition and predation effects played minor roles in regulating abundance of the B. angularis population. During summer and autumn, higher concentrations of both fractions of Chl-a were detected in Lake Tingtang. Concurrently, population density of B. angularis was positively correlated with zooplankton, such as Diaphanosoma spp. and feeding guild rotifers, and was not significantly related to its own egg ratio. Taken together, these findings suggest food limitation and competition pressure by herbivorous crustaceans and feeding guild rotifers were likely to be less important in regulating dynamics of B. angularis dwelling in the warm water column. The cladoceran community in this study was dominated by smaller species belonging to Bosmina and Diaphanosoma, rather than the larger Daphnia spp., which has also been found in most of 29 shallow lakes located along the middle and lower Chinese Yangtze River (Wang et al. Citation2007). The dominance of small crustacean species might be the consequence of intensive predation from fish on Daphnia spp. (Brooks and Dodson Citation1965), which thereby reduced interference and exploitative competition pressure to rotifers from cladocerans.
However, Cyclops vicinus (a species of Cyclopoid copepod) prefers to prey on the smaller B. angularis of two otherwise similar morphs with very different sizes (Brandl 2005). In the presence of Asplanchna brightwellii, B. angularis develops inducible defense patterns such as an increasing lorica thickness and hardness, leading to decreased reproduction as a fitness cost (Yin et al. Citation2017). Moreover, B. angularis is the first prey eliminated in experimental groups consisting of a two-prey (Brachionus calyciflorus and B. angularis) – two-predator (Asplanchna intermedia and Mesocyclops thermocyclopoides) system, even at a low density of M. thermocyclopoides (another species of Cyclopoid copepod) (Kumar and Rao Citation2001). These findings concerning predator-prey interactions indicated that the population dynamics of B. angularis should be affected by top-down forces from predators like Asplanchna spp. and copepods. However, other invertebrate predators of rotifers, including Chaoborus spp., were not recorded in this study because zooplankton was sampled near midday when Chaoborus spp. had likely migrated into sediment (Havens et al. Citation2007).
During summer and autumn in Lake Tingtang, the population density and egg ratio of B. angularis were positively correlated with water temperature, with egg ratio maintaining higher values in this phase, indicating that the reproduction and growth of this rotifer were promoted by a high temperature. Regarding morphological features, both body size and egg volume of B. angularis in Lake Tingtang decreased with water temperature. Planktonic rotifers usually produce larger and fewer eggs or smaller and more eggs to maximize population growth (Sarma and Rao Citation1987). We conclude, therefore, that the B. angularis population adopted the ecological strategy of producing relatively more and smaller eggs induced by a higher water temperature during summer and autumn, which may be the main ecological mechanism controlling the temporal dynamics and somewhat counteracting the adverse effects of top-down forces. Notably, the density of potential predators such as Asplanchna spp. was negatively correlated with the body size of B. angularis in Lake Tingtang. Yin et al. (Citation2017) confirmed that B. angularis will maintain its body size when exposed to kairomones produced by Asplanchna spp. Hence, this inverse relationship was likely to be an erroneous perception from temperature effect on the body size of this rotifer. Overall, water temperature is more important in regulating population dynamics of B. angularis than predation pressure during summer and autumn when potential predators are abundant.
The importance of multi-dimensional analysis in understanding the dynamics of natural rotifer population
Determining whether cryptic species exist in the water column is the first step in discussing the ecological mechanisms driving seasonal dynamics of natural rotifer populations. B. angularis is an excellent target in such a study, because no reports on the speciation of cryptic species have been made. Furthermore, Sun (Citation2012) performed a study examining seasonal variation in the genetic structure of a B. angularis population in Lake Tingtang and did not find the differentiation of cryptic species based on internal transcribed spacer (ITS) sequence analyses.
With respect to the correlations from year-round data presented in this study, it is very difficult to understand the ecological mechanisms that drive the seasonal dynamics of B. angularis because no persuasive relationships have been examined between the population density and the measured environmental variables. The division of seasonal population of B. angularis by the degree of sexual reproduction, together with the synchronous variations in the abundance of B. angularis and its potential food resources, competitors, and predators, helped reveal the underlying forces controlling population dynamics across a growing season. Egg ratio has been confirmed to be an important life history parameter in predicting patterns of population growth of planktonic rotifers under field conditions and is more valuable in evaluating rotifer population responses to environmental conditions than population density alone (Korstad et al. Citation1995; Sarma et al. Citation2005). Additionally, the combined features of egg ratio and morphological parameters, such as body size and egg volume, have contributed to the detection of the ecological strategy of planktonic rotifers under controlled conditions (Sarma and Rao Citation1987), which is of crucial importance for understanding population dynamics of field rotifers in response to the external environment. Hence, sexual reproduction and various ecological parameters, including abundance, egg ratio, and morphology, need further study to determine how rotifers respond to changing environmental conditions.
In summary, two discontinuous populations of B. angularis in Lake Tingtang were divided by high mixis rates in May and November 2009. The ‘summer and autumn’ population dynamics of B. angularis were controlled primarily by water temperature, which somewhat counteracted the negative effect of predation on reducing the density of B. angularis. The ‘winter and spring’ population dynamics of B. angularis might be controlled by different ecological factors. Between early March and early April, the population dynamics of B. angularis were likely controlled by food resources (bottom-up effect), then likely regulated by predation pressure (top-down effect) in April. However, the density peak in winter might be ascribed to recruitment from the sediment. Furthermore, we addressed the necessity for diverse evidence, including the seasonal population divided by sexual reproduction degree and the application of various ecological parameters of monogonont rotifer populations, in investigating how rotifer populations respond to varying environmental conditions.
Notes on contributors
Xin-Li Wen is an associate professor at College of Life Sciences, Anhui Normal University, where he worked for many years as a teacher and ecologist, particularly in zooplankton ecology.
Ying-Hao Xue has got his master degree from College of Life Sciences, Anhui Normal University, and worked for many years at Rural Energy and Environment Agency, Ministry of Agriculture, Beijing, China.
Gen Zhang has got his master degree from College of Life Sciences, Anhui Normal University, and got his Doctor degree from the Hong Kong University of Science and Technology.
Xian-Ling Xiang is a professor at College of Life Sciences, Anhui Normal University, where he worked for many years as a teacher and ecologist, particularly in zooplankton ecology.
Yi-Long Xi is a professor at College of Life Sciences, Anhui Normal University, where he worked for many years as a teacher and ecologist, particularly in zooplankton ecology.
Acknowledgments
We are grateful to Shenzhen Nobel Science and Technology Service Co., Ltd. for the valuable comments on the manuscript and corrections of the English.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was funded by National Natural Science Foundation of China [grant number 41877417], the Anhui Provincial Natural Science Foundation [grant number 1808085MC79], Natural Science Foundation of Educational Committee of Anhui Province [grant number KJ2017A320], and the Foundation of Provincial Key Laboratories for Conservation and Utilization of Important Biological Resources in Anhui.
References
- Auer B, Elzer U, Arndt H. 2004. Comparison of pelagic food webs in lakes along a trophic gradient and with seasonal aspects: influence of resource and predation. J Plankton Res. 26(6):697–709.
- Brandl Z. 2005. Freshwater copepods and rotifers: predators and their prey. Hydobiologia. 546(1):475–489.
- Brooks JL, Dodson SI. 1965. Predation, body size, and composition of plankton. Science. 150(3692):28–35.
- Cordova SE, Giffin J, Kirk KL. 2001. Food limitation of planktonic rotifers: field experiments in two mountain ponds. Freshwater Biol. 46(11):1519–1527.
- de Bernardi R, Giussani G, Manca M. 1987. Cladocera: predators and prey. Hydrobiologia. 145(1):225–243.
- Edmondson WT. 1960. Reproductive rates of rotifers in natural populations. Mem Ist Ital Idrobiol. 12:21–77.
- Fontaneto D, Kaya M, Herniou EA, Barraclough TG. 2009. Extreme levels of hidden diversity in microscopic animals (Rotifera) revealed by DNA taxonomy. Mol Phylogenet Evol. 53(1):182–189.
- Gama-Flores JL, Sarma SSS, Nandini S. 2004. Acute and chronic toxicity of the pesticide methyl parathion to the rotifer Brachionus angularis (Rotifera) at different algal (Chlorella vulgaris) food densities. Aqua Ecol. 38(1):27–36.
- García-Roger EM, Carmona MJ, Serra M. 2017. Modes, mechanisms and evidence of bet hedging in rotifer diapause traits. Hydrobiologia. 796(1):223–233.
- Gilbert JJ, Walsh EJ. 2005. Brachionus calyciflorus is a species complex: mating behavior and genetic differentiation among four geographically isolated strains. Hydrobiologia. 546(1):257–265.
- Green J. 2011. Geographical variation in with Microcystis blooms. Hydrobiologia. 662(1):197–204.
- Hammer Ø, Harper DAT, Ryan PD. 2001. PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron. 4(1):1–9.
- Haney J, Hall D. 1973. Sugar-coated Daphnia: a preservation technique for Cladocera. Limnol Oceanogr. 18(2):331–333.
- Havens KE, East TL, John RB. 2007. Zooplankton response to extreme drought in a large subtropical lake. Hydrobiologia. 589(1):187–198.
- Hu HY, Xi YL. 2008. Demographic parameters and mixis of three Brachionus angularis Goss (Rotatoria) strains fed on different algae. Limnologica. 38(1):56–62.
- Hunter MD, Price PW. 1992. Playing chutes and ladders: heterogeneity and the relative roles of bottom-up and top-down forces in natural communities. Ecology. 73:724–732.
- Korstad J, Neyts A, Danielsen T, Overrein I, Olsen Y. 1995. Use of swimming speed and egg ratio as predictors of the status of rotifer cultures in aquaculture. Hydrobiologia. 313-314(1):395–398.
- Koste W. 1978. Rotatoria: Die Rädertiere Mitteleuropas. Berlin (Germany): Gebrüder Borntraeger.
- Kumar R, Rao TR. 2001. Effect of the cyclopoid copepod Mesocyclops thermocyclopoides on the interactions between the predatory rotifer Asplanchna intermedia and its prey Brachionus calyciflorus and B. angularis. Hydrobiologia. 453/454:261–268.
- Leasi F, Tang CQ, De Smet WH, Fontaneto D. 2013. Cryptic diversity with wide salinity tolerance in the putative euryhaline Testudinella clypeata (Rotifera, Monogononta). Zool J Linn Soc. 168(1):17–28.
- López C, Soto LM, Dávalos-Lind L, Lind O. 2007. Summer dynamics of egg-ratio of the rotifer Keratella cochlearis (Gosse, 1851) in a eutrophic reservoir: a field study on affecting factors. Hydrobiologia. 589:175–185.
- Matus LN, Rodríguez RV, Jiménez LA, Castro MJ, Ocampo CJA, Castro MG, Dávila F, Castro CAE. 2017. Population density comparison of Brachionus angularis (Gosse, 1851) cultured in laboratory at 19°, 21° and 25 °C, fed with bacteria produced in Biofloc system. Fish Aqua Stud. 5:13–17.
- Merriman JL, Kirk KL. 2000. Temporal patterns of resource limitation in natural populations of rotifers. Ecology. 81:141–149.
- Meyer MF, Hampton SE, Ozersky T, Rusanovskaya OO, Woo KH. 2017. Vulnerability of rotifers and copepod nauplii to predation by Cyclops kolensis (Crustacea, Copepoda) under varying temperatures in Lake Baikal, Siberia. Hydrobiologia. 796(1):309–318.
- Miracle MR, Vicente E, Sarma SSS, Nandini S. 2014. Planktonic rotifer feeding in hypertrophic conditions. Int Rev Hydrobiol. 99(1-2):141–150.
- Obertegger U, Fontaneto D, Flaim G. 2012. Using DNA taxonomy to investigate the ecological determinants of plankton diversity: explaining the occurrence of Synchaeta spp. (Rotifera, Monogononta) in mountain lakes. Freshwater Biol. 57(8):1545–1553.
- Obertegger U, Smith HA, Flaim G, Wallace RL. 2011. Using the guild ratio to characterize pelagic rotifer communities. Hydrobiologia. 662(1):157–162.
- Ogata Y, Tokue Y, Yoshikawa T, Hagiwara A, Kurokura H. 2011. A Laotian strain of the rotifer Brachionus angularis hold promise as a food source for small-mouthed larvae of freshwater fish in aquaculture. Aquaculture. 312(1-4):72–76.
- Ooms-Wilms AL, Postema G, Gulati RD. 1999. Population dynamics of planktonic rotifers in Lake Loosdrecht, the Netherlands, in relation to their potential food and predators. Freshwater Biol. 42(1):77–97.
- Sarma SSS, Gulati R, Nandini S. 2005. Factors affecting egg-ratio in planktonic rotifers. Hydrobiologia. 546(1):361–373.
- Sarma SSS, Rao TR. 1987. Effect of food level on body size and egg size in a growing population of the rotifer Brachionus patulus Muller. Arch Hydrobiol. 111245–253.
- Sarma SSS, Resendiz RAL, Nandini S. 2011. Morphometric and demographic responses of brachionid prey (Brachionus calyciflorus Pallas and Plationus macracanthus (Daday)) in the presence of different densities of the predator Asplanchna brightwellii (Rotifera: Asplanchnidae). Hydrobiologia. 662(1):179–187.
- Schröder T. 2005. Diapause in monogonont rotifers. Hydrobiologia. 546(1):291–306.
- Sommer U, Gliwicz ZM, Lampert W, Duncan A. 1986. The PEG model of seasonal succession of planktonic events in fresh waters. Arch Hydrobiol. 106:433–471.
- Sun Q. 2012. Annual variation in population genetic structure and ecological characteristics of Brachionus angularis in Tingtang Lake [Thesis for Master degree]. Wuhu: Anhui Normal University.
- Tarazona E, García-Roger EM, Carmona MJ. 2017. Experimental evolution of bet hedging in rotifer diapause traits as a response to environmental unpredictability. Oikos. 126(8):1162–1172.
- Turchin P. 2003. Complex population dynamics: a theoretical/empirical synthesis. Princeton (NJ): Princeton University Press.
- Walz N. 1987. Comparative population dynamics of the rotifers Brachionus angularis and Keratella cochlearis. Hydrobiologia. 147(1):209–213.
- Walz N. 1995. Rotifer populations in plankton communities: energetics and life history strategies. Experientia. 51(5):437–453.
- Wang SB, Xie P, Wu SK, Wu AP. 2007. Crustacean zooplankton distribution patterns and their biomass as related to trophic indicators of 29 shallow subtropical lakes. Limnologica. 37(3):242–249.
- Wen XL, Feng RN, Zhang G, Xue YH, Xi YL. 2017. Temporal variations of chlorophyll-a and their relationships with abiotic and biotic factors in two small shallow lakes. J Lake Sci. 29:1421–1432.
- Wen XL, Xi YL, Qian FP, Zhang G, Xiang XL. 2011a. Comparative analysis of rotifer community structure in five subtropical shallow lakes in East China: role of physical and chemical conditions. Hydrobiologia. 661303–316.
- Wen XL, Xi YL, Yang YF, Zhang XA, Zhang G. 2011b. Temperature is the key factor controlling population dynamics of Brachionus angularis in Lake Jinghu during summer and autumn. J Freshwater Ecol. 26(2):277–286.
- Wen XL, Xi YL, Zhang G, Xue YH, Xiang XL. 2016. Coexistence of cryptic Brachionus calyciflorus (Rotifera) species: roles of environmental variables. J Plankton Res. 38(3):478–489.
- Waervågen SB, Andersen T. 2018. Seasonal quantitative dynamics and ecology of pelagic rotifers in an acidified boreal lake. J Limnol. 77(1):147–163.
- Yin XW, Jin W, Zhou YC, Wang PP, Zhao W. 2017. Hidden defensive morphology in rotifers: benefits, costs, and fitness consequences. Sci Rep. 7:4488.
- Yoshida T. 2005. Toward the understanding of complex population dynamics: planktonic community as a model system. Ecol Res. 20(5):511–518.
- Yoshida T, Ban S, Takenouchi T, Aono T, Ishikawa Y, Mikami H, Takano K, Imada K, Yasutomi R, Takeuchi K. 2000. Top down control of population dynamics of the dominant rotifers in two mesotrophic lakes in Hokkaido, Japan. Fundam Appl Limnol. 148(4):481–498.
- Yoshida T, Urabe J, Elser JJ. 2003. Assessment of “top-down” and “bottom-up” forces as determinants of rotifer distribution among lakes in Ontario, Canada. Ecol Res. 18(6):639–650.
- Zhang H, Ekvall MK, Xu J, Hansson LA. 2015. Counteracting effects of recruitment and predation shape establishment of rotifer communities under climate change. Limnol Oceanogr. 60(5):1577–1587.