Abstract
Floods affect fish populations in the short term (e.g. entrainment, mortality) and long term (e.g. recruitment, habitat availability), but oftentimes long-term effects are overlooked. In 2011, a catastrophic flood with record peak flows (4,200 m3/s) occurred in Lake Sharpe, a mainstem Missouri River reservoir. The flood’s immediate impacts on the Lake Sharpe walleye (Sander vitreus) population have been documented, but long-term effects on natal recruitment and habitat use have not been assessed. We used otolith chemistry to evaluate spatial patterns in walleye natal recruitment and late-summer (i.e. July − September) habitat use in years before, during, and after the flood to gain insight into long-term flood impacts on the Lake Sharpe walleye population. From 2004 to 2013, all walleye hatched in embayments and main channel habitats as opposed to stilling basins or tributaries, and the majority of age ≥ 1 fish (i.e. age-1 or older) used the former habitats in late summer. During the flood year, natal recruitment remained stable relative to pre-flood levels in embayments (26% pre-flood, 20% during flood) and main channels (74% pre-flood, 80% during flood). Habitat use of age ≥ 1 walleye was generally unaltered by the flood as it remained consistent in embayments and main channel habitats before, during, and after the disturbance. Hence, the Lake Sharpe walleye population was largely unaffected by the flood in terms of natal recruitment and habitat use. Our results highlight the resilience of the Lake Sharpe walleye population to a catastrophic flood, providing fisheries professionals with an otolith chemistry approach for quantifying flood effects on natal recruitment and habitat use and developing spatially informed management approaches (e.g. habitat protection/rehabilitation, harvest regulations).
Introduction
In large river systems, longitudinal and lateral connectivity are maintained by floods and drive primary production and fisheries productivity (Ward and Weins Citation2001). Upstream and downstream environments are linked by longitudinal connectivity such that nutrient concentrations and fish assemblages at particular locations are affected by upstream biotic and abiotic factors. In addition, migratory fishes rely on longitudinal connectivity to move among habitats (Park et al. Citation2003). As with longitudinal connectivity, floods maintain lateral connectivity with floodplain habitats (e.g. embayments, tributaries) in large rivers (Ward et al. Citation1999), causing biotic and abiotic characteristics of the main channel to be influenced by, and influence, floodplain environments. For instance, floods increase primary production in floodplains by depositing nutrients that are eventually recycled to the main channel as floodwaters recede (Bayley Citation1995).
Although floods play a critical role in providing lateral river–floodplain connectivity in natural rivers, this function can be diminished in impounded systems (i.e. reservoirs). Impoundment reduces habitat connectivity and heterogeneity by disrupting the natural flow regime of rivers (Ward and Weins Citation2001). As a result, impoundments are susceptible to sedimentation and reservoir aging, which affect – and often reduce – connectivity between the main channel and floodplain habitats (Juracek Citation2015). For example, sedimentation decreases river–floodplain connectivity by washing substrates into areas that formerly connected main channel and floodplain habitats, leading to habitat homogenization (Juracek Citation2015). Reductions in, or loss of, river–floodplain connectivity can influence main channel fisheries because floodplains provide reproductive, nursery, refuge, and overwintering habitats for fish throughout their life stages (Rosenfeld et al. Citation2008). Larval fish densities are often greater in floodplain habitats than main channels due to warmer water temperatures, higher nutrient (e.g. phosphorus, nitrogen) concentrations, and greater zooplankton abundance (Hall et al. Citation2003; Klumb et al. Citation2003; Schemel et al. Citation2004). In addition, floodplains serve as reproductive areas for forage fishes such as gizzard shad (Dorosoma cepedianum) that support socioeconomically important sport fisheries (Fincel et al. Citation2014; Radigan et al. Citation2018a).
In 2011, late-melting snow and high spring rainfall in the Rocky Mountains and Great Plains caused the largest flood in recorded history in the Missouri River (USACE Citation2012, ). Runoff totaled an unprecedented 75.2 billion m3 from May through early July, causing record water levels () and discharges through all dams on Missouri River reservoirs and exceeding system capacity by >20% upstream of Sioux City, Iowa, USA (USACE Citation2012). Despite more than US$395 million in disaster relief funding from the Federal Emergency Management Agency (Freeman and Jones Citation2012), the flood caused multi-billion dollar damages to residential, industrial, agricultural, and transportation infrastructure, including $1 billion in damage expenses incurred by the U.S. Army Corp of Engineers (Grigg et al. Citation2011). Although floodwaters initially connected the main channel of Lake Sharpe (a Missouri River reservoir) with floodplain habitats (e.g. embayments, tributaries) during the disturbance, extreme sedimentation resulting from the flood ultimately caused a significant reduction in river-floodplain connectivity (R. Hanten, South Dakota Game, Fish and Parks, July 2017, pers. comm.). By reducing river–floodplain connectivity in Lake Sharpe, the flood may have altered habitat-specific patterns in fish natal recruitment (i.e. the percentage of fish that hatch in particular habitats and survive to a given age [e.g. fall of the first year, age-1]) by, for example, changing movement patterns into and out of floodplain areas or altering floodplain nursery habitats (Junk et al. Citation1989).
Figure 1. The 2011 Missouri river flood as depicted by (A) peak gauge height (m) from 1988 to 2017 and (B) monthly gauge height in 2011 for the United States Geological Survey gauge in Pierre, South Dakota, in Lake Sharpe. Data were obtained from Alexander et al. (Citation2001).
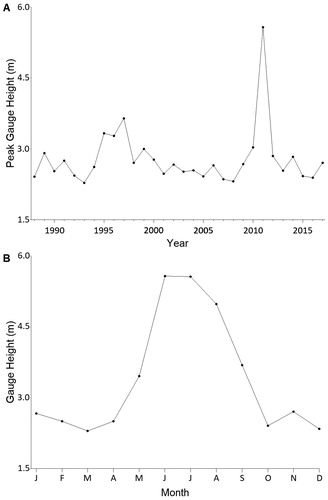
Image 1. Collection of age-0 walleye in Lake Sharpe, South Dakota (photographed here) was an important step in otolith chemistry research to assess walleye natal recruitment and habitat use. Image courtesy of William J. Radigan.

However, effects of the 2011 flood on socioeconomically important sport fish populations such as walleye (Sander vitreus) have primarily been investigated in Lake Oahe (upstream of Lake Sharpe; Carlson et al. Citation2016a) or at a multi-reservoir scale in the Missouri River (Carlson et al. Citation2016b, Citation2017), rather than in Lake Sharpe itself. For instance, downstream movements of Lake Oahe walleye were considerably longer and more frequent than upstream movements during the 2011 flood (Carlson et al. Citation2016a). At a multi-reservoir scale, some walleye moved to downstream impoundments (including Lake Sharpe) due to flood-induced entrainment through dams (Carlson et al. Citation2017). In Lake Sharpe, similar percentages of walleye annually moved downstream (41%) or upstream (47%) before the 2011 flood, with few fish (12%) remaining in the same locations year to year (Carlson et al. Citation2017). Most walleye remained in the same location (42%) or moved downstream (40%) during the flood year, but the percentages of downstream/upstream/no movement were approximately the same (30–35%) after the flood. Despite this movement research in Lake Sharpe, there is a pressing need to examine other aspects of walleye environmental history (e.g. natal recruitment, habitat use) before and after the flood. Lake Sharpe supports a socioeconomically important, multimillion-dollar walleye fishery (Hanten et al. Citation2014), so understanding effects of the flood on walleye environmental history in this reservoir is informative for maintaining a successful walleye management program. Moreover, the flood provided an uncommon opportunity to quantify associations among discharge, walleye natal recruitment, and walleye habitat use and use this information to develop strategies for fisheries management within Lake Sharpe and across Missouri River reservoirs. Hence, objectives of this study were to use otolith chemistry to (1) quantify natal recruitment (as opposed to spawning) in various habitat types (i.e. embayment, main channel, stilling basin, tributary) before, during, and after the flood year (2011), and (2) quantify late-summer (i.e. July–September) habitat use in the habitat types listed above before, during, and after the flood year. Emphasis was placed on late-summer habitat use due to an otolith ablation protocol (see Methods) that focused on annuli, which form in late summer for Missouri River walleye (Graff et al. Citation2014). It was hypothesized that floodplain habitats (e.g. embayments, tributaries) would be particularly important for walleye natal recruitment and that occupancy of relatively deep, cool main channels would dominate late-summer habitat use (Riis Citation1985; Carlson et al. Citation2016a, Citation2016b, Citation2017). In addition, it was expected that the flood would reduce natal recruitment and habitat use in flood-affected areas such as embayments by decreasing river-floodplain connectivity and the benefits that it provides for young fish (e.g. nursery and refuge habitats, abundant food; Junk et al. Citation1989).
Methods
Study site
This study was conducted in Lake Sharpe, a Missouri River reservoir in central South Dakota. Spanning 23,020 ha from Oahe Dam to Big Bend Dam, Lake Sharpe had a mean depth of 9.5 m and a maximum depth of 23.7 m. The northern end of Lake Sharpe has a stilling basin, two principal embayments (i.e. Hipple Lake, La Framboise), one major tributary (i.e. Bad River), a canal (i.e. Marion’s Garden Complex), and several smaller tributaries. The southern end of the reservoir is characterized by main channel habitats and few floodplain environments (e.g. embayments, tributaries).
Trace element sampling
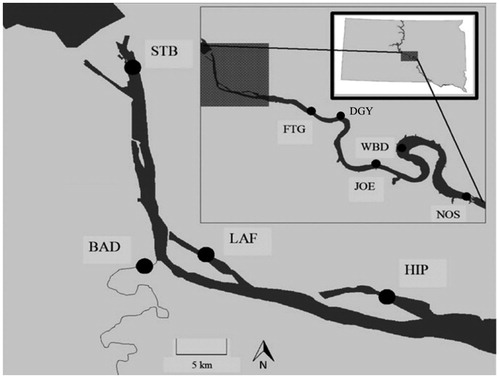
Water samples were collected to determine if water chemistry met requirements for otolith chemistry research (i.e. spatial variability, temporal stability). Water was sampled from nine locations in Lake Sharpe in late summer (i.e. July-September) 2012 and late summer 2014 in main channel habitats (N = 5), embayments (N = 2), a stilling basin (N = 1), and the Bad River (). Samples were collected using a syringe filtration method (Shiller Citation2003) employed in previous Missouri River otolith chemistry studies (Carlson et al. Citation2016a; Radigan et al. Citation2018a, Citation2018b, Citation2018c). Samples were stored in sealed coolers to prevent evaporation and associated chemical changes. Trace element (i.e. strontium [Sr]; barium [Ba]) and calcium (Ca) concentrations were measured using high-resolution inductively coupled plasma mass spectrometry (ICPMS) at the University of Southern Mississippi. All concentrations were converted to micromolar ratios against Ca (μmol/mol) as this element is a pseudointernal standard (Bickford and Hannigan Citation2005; Ludsin et al. Citation2006; Whitledge et al. Citation2007). Surficial and bedrock geology are temporally stable in Missouri River impoundments and cause temporally consistent water signatures (Bickford and Hannigan Citation2005), a notion that has been verified in Lake Sharpe and other Missouri River reservoirs (Carlson et al. Citation2016a, Citation2016b, Citation2017; Radigan et al. Citation2018a, Citation2018b, Citation2018c). Based on these previous studies, it was predicted that water Sr:Ca and Ba:Ca would be temporally consistent in Lake Sharpe. To test this prediction, a United States Geological Survey database (i.e. National Stream Water-Quality Monitoring Network for Region 10, including South Dakota; Alexander et al. Citation2001) was used to obtain historical (1978–1986, 1997–2000) late-summer Sr:Ca and Ba:Ca signatures for a mainstem Lake Sharpe site (i.e. Pierre, the only Lake Sharpe location in the database). Historical Sr:Ca and Ba:Ca ratios for this site were then compared with current (2012, 2014) ratios collected at the same time of year (i.e. late summer) to evaluate temporal patterns in water chemistry (see Statistical Analysis section for full details).
Figure 2. Sampling locations for water chemistry (all sites) and walleye Sander vitreus, including age-0 fish (all sites but DGY) and age ≥ 1 fish (all sites), in Lake Sharpe, South Dakota, between Fall 2012 and Fall 2014. Site abbreviations (upstream to downstream) are as follows: STB = Stilling Basin, BAD = Bad River, LAF = La Framboise Embayment, HIP = Hipple Lake Embayment, FTG = Fort George (mainstem), DGY = De Grey (mainstem), JOE = Joe Creek (mainstem), WBD = West Bend (mainstem), NOS = North Shore (mainstem). Modified from Radigan et al. (Citation2018b).
Fish sampling
Age-0 walleye were collected in late summer 2012 and late summer 2014 in combination with water sampling at all sites except De Grey (, , ). Walleye that were age ≥ 1 (i.e. age-1 or older) were also sampled in late summer 2012 and late summer 2014 at all sites where water sampling occurred (, ). Fish sampling involved shoreline seining (30.5-m-long x 2.4-m-deep net, 6.4-mm mesh), 24-h gillnetting (46-m-long net, 15–51-mm bar mesh), and boat electrofishing (pulsed DC, 6-8 A, 60 pulses/s). Shoreline seining was performed using a boat, which navigated an arc around a researcher holding the net on the shoreline. Electrofishing was used to collect fish when shoreline seining was not possible due to submerged rocks/trees. Total fish sampling effort (i.e. seine and gill net soak time, electrofishing time) in the main channel and floodplain habitats was proportional to the availability of these habitats throughout Lake Sharpe. Fish sampling throughout the entire reservoir ensured that environmental history evaluation was representative of the Lake Sharpe walleye population.
Table 1. Sample sizes of age–0 and age ≥ 1 walleye Sander vitreus collected in Lake Sharpe, South Dakota, in late summer (i.e. July–September) 2012 and late summer 2014.
Otolith chemistry
Walleye were sacrificed immediately after collection and placed on ice to preclude stress-induced fluctuations in otolith trace element signatures (Milton and Chenery Citation1998). Both sagittal otoliths were removed from every fish (age-0 and age ≥ 1) in a laboratory using plastic, triple acid-washed forceps (Campana et al. Citation2000; Brazner et al. Citation2004). Two independent readers aged otoliths before chemical analysis (correspondence between readers >90%). Otoliths were triple-rinsed in distilled, ultrapure water; air-dried for >24 h, and stored in acid-washed 2-mL polypropylene microcentrifuge tubes (Zeigler and Whitledge Citation2010). After initial cleaning, otoliths from age ≥ 1 fish were embedded in Epo-Fix epoxy and sectioned in the transverse plane using a low-speed Isomet diamond saw (Buehler, Lake Bluff, IL). The saw blade was cleaned after each section with aluminum oxide lapping film (3-µm grit) to prevent otolith contamination. Due to the small size and fragility of age-0 otoliths, they were placed in thermoplastic cement and sanded in the sagittal plane without sectioning. All otoliths were sanded and polished with 3 M Wetordry sandpaper (400 grit) and aluminum oxide lapping film, mounted on petrographic slides (Donohoe and Zimmerman Citation2010), and sonicated in ultrapure water.
Otolith concentrations of 86Sr, 88Sr, 137Ba, and 138Ba were measured via laser ablation ICPMS using an Agilent Technologies 7500a quadrupole inductively coupled plasma mass spectrometer coupled to a New Wave Research UP-213-nm laser with helium as the carrier gas at the University of California–Davis Interdisciplinary Center for ICPMS. The following laser settings were used: 70% energy, 10 Hz pulse rate, 40 μm spot size, 50 s acquisition, and 25 s background. A calibration standard (U.S. Geological Survey synthetic glass standard GSE-1G) and two additional reference standards (GSD-G1 and MACS-3) were used as quality controls for verification of instrument accuracy and precision. Standards were ablated in three to five locations after every 10-15 samples to adjust for possible instrument drift. Specialized computer programs (Glitter 4.4, GEMOC CSIRO, Macquarie Research Ltd., Macquarie University, Sydney, Australia; ICP-MS MassHunter, Agilent Technologies, Santa Clara, California) were used to correct for gas blank, matrix, and drift effects and convert isotopic counts to elemental concentrations (μg/g). Mean limits of detection for 88Sr and 137Ba were 0.62 ppm and 0.03 ppm, respectively; concentrations of these elements were well above detection limits in all otoliths. Otolith signatures were reported as an element:Ca ratios (μmol/mol) because Ca is a pseudointernal standard (Whitledge et al. Citation2007).
Otolith trace elemental concentrations were measured using spot analyses with a 15-s laser warm-up time, a 20-s ablation, and a 95-s washout time after each ablation. Integration times for 43Ca (0.01s), 88Sr (0.05 s), and 137Ba (0.05 s) were repeated for each ablation. Age-0 and age ≥ 1 otoliths were spot-ablated along transects encompassing the core (representing natal origins), the outer edge of each annulus (representing late-summer habitat use for Missouri River walleye; Graff et al. Citation2014), and the terminal edge. Temporal matching of water and age-0 otolith signatures was a requirement for evaluating natal recruitment and habitat use of age ≥ 1 walleye. In most cases, water samples and age-0 walleye from particular sites were collected on the same day in late summer, so water and otolith signatures were temporally matched by ablating otoliths at terminal edges, which corresponded with same-day water sampling and reflected recently occupied environments ( Zeigler and Whitledge Citation2010, Citation2011). In a few cases, logistical sampling constraints and low fish catches caused delays between water sampling and age-0 walleye collection (i.e. fish collected after water). When this happened, water and otolith Sr:Ca and Ba:Ca signatures were synchronized by enumerating the days separating water and age-0 fish sampling and ablating otoliths an equivalent number of daily rings from otolith edges. Otolith signatures of age ≥ 1 walleye were also temporally matched with late-summer water sampling (both historical and current) because otoliths were ablated on the outer edge of each annulus (i.e. late-summer habitat use; Graff et al. Citation2014). Moreover, because floodwaters declined from peak levels in mid-May through June, late-summer otolith signatures of age ≥ 1 walleye reflected habitat use after the flood, as opposed to during the disturbance, when water chemistry could potentially have been inconsistent with Sr:Ca and Ba:Ca ratios before and after the flood (Voss et al. Citation2014). Temporal separation between the flood and walleye annulus formation prevented the need for water and fish collection during dangerous flood conditions and allowed assessment of flood-induced patterns in natal recruitment and habitat use.
Statistical analysis
Spatial and temporal patterns in Lake Sharpe Sr:Ca and Ba:Ca ratios were evaluated using analysis of variance (ANOVA) with Tukey’s Honestly Significant Difference tests for multiple comparisons. Water data were log10 transformed if they did not meet assumptions for normality and homoscedasticity as inferred from Shapiro-Wilk tests and Levene’s tests, respectively. Temporal patterns in water chemistry were evaluated by comparing mean Sr:Ca and Ba:Ca signatures and 95% confidence intervals (CIs) between the historical and current periods for the Pierre site (Alexander et al. Citation2001). Relationships between water and age-0 mean terminal otolith signatures were assessed at corresponding collection sites using least-squares linear regression (Munro et al. Citation2005; Zeigler and Whitledge Citation2010).
It was presumed that age-0 walleye (as opposed to age ≥ 1 fish) did not move from natal locations between hatching and capture in late summer, a common assumption in walleye otolith chemistry studies ( Carlson et al. Citation2016a, Citation2016b, Citation2017 ). This notion implies that otolith terminal edge signatures – ablated to ensure temporal matching with historical and current water sampling (see above) – reflected the chemistry of natal locations. This assumption was verified by ablating otolith cores and edges, which established the chemical similarity of these regions (i.e. age-0 fish did not move between hatch and capture). Hence, known-location, late-summer age-0 otolith signatures were reliable ‘blueprints’ for evaluating natal recruitment and habitat use of age ≥ 1 walleye via a bivariate approach (i.e. Sr:Ca used as the primary element, Ba:Ca used secondarily), as in previous otolith chemistry research (Carlson et al. Citation2016a; Radigan et al. Citation2018a). In particular, K-sample nearest neighbor discriminant analysis was used to measure the accuracy with which age-0 natal otolith signatures could be used to classify age-0 fish to known natal habitats and sites and ultimately identify natal origins of age ≥ 1 fish. This nonparametric method permits reliable classification of age-0 fish to their natal locations when otolith data do not meet assumptions of normality or homoscedasticity (Bickford and Hannigan Citation2005). A leave-one-out jackknife procedure was performed for all models (k = 2–8) and allowed identification of the model with the lowest error rate (k = 2), which was used to assess natal recruitment and late-summer habitat use of age ≥ 1 fish using the known-location age-0 data set. Natal recruitment was defined as the percentage of age ≥ 1 walleye from particular year classes that possessed otolith Sr:Ca and Ba:Ca signatures indicative of hatching in particular habitats (i.e. embayments, main channels, stilling basins, tributaries). Similarly, habitat use was defined as the percentage of outer-edge (i.e. late-summer) annuli signatures corresponding to use of particular habitats. All analyses involved a standard level of statistical significance (α < 0.05) and were performed in program R version 3.1.3 (R Development Core Team Citation2015).
Results
Fish sampling
A total of 43 age-0 walleye were collected in main channels (N = 25 individuals), embayments (N = 10), a stilling basin (N = 5), and a tributary (N = 3; ). A total of 163 age ≥ 1 walleye were sampled in main channels (N = 87 individuals), embayments (N = 30), a stilling basin (N = 26), a tributary (N = 10), and a canal (N = 10; ).
Water and otolith chemistry
Water Sr:Ca ratios were spatially heterogeneous in Lake Sharpe (F5,36 = 7.09, P < 0.01) and temporally stable at the Pierre site, with 95% CIs overlapping between historical (4.767 ± 0.090 mmol/mol) and current (4.906 ± 0.063 mmol/mol) time periods. Similarly, water Ba:Ca ratios varied spatially in Lake Sharpe (F5,36 = 4.54, P < 0.01) and were consistent over time at the Pierre site, with overlapping historical (0.230 ± 0.015 mmol/mol) and current (0.211 ± 0.007 mmol/mol) water signatures. Linear regressions between water and age-0 otolith chemistry were positive and proportional for Sr:Ca (otolith Sr:Ca = 0.08 + 0.23*water Sr:Ca, R2 = 0.99, P < 0.01) and Ba:Ca (otolith Ba:Ca = 0.00050 + 0.02*water Ba:Ca, R2 = 0.99, P < 0.01). Age-0 walleye were classified with high accuracy to habitat types (83% mean accuracy; ) but not individual sites (45%), reflecting the geological similarity of the mainstem sites sampled.
Table 2. Results of k-sample nearest neighbor discriminant analysis with leave-one-out jackknife cross-validation using Ba:Ca and Sr:Ca ratios of 43 age-0 walleye Sander vitreus collected in late summer 2012 and 2014 in Lake Sharpe, South Dakota.
Table 4. Habitat-specific patterns of walleye Sander vitreus natal recruitment (percentage of fish, with number of individuals in parentheses) and late-summer habitat use (percentage of otolith annuli, with number of annuli in parentheses) in embayments, the main channel, a stilling basin, and a tributary before, during, and after the 2011 flood in Lake Sharpe, South Dakota.
Table 5. Late-summer habitat use of age ≥ 1 walleye Sander vitreus, expressed as the percentage of otolith annuli (number of annuli in parentheses) with chemical signatures indicative of embayments, the main channel, a stilling basin, and a tributary from 2004 to 2013 in Lake Sharpe, South Dakota.
Natal recruitment
Accurate classification of age-0 walleye to their natal habitats using otolith Sr:Ca and Ba:Ca ratios made these signatures reliable for assessing spatial patterns in natal recruitment of age ≥ 1 walleye. Between 2004 and 2013, the majority of age ≥ 1 walleye hatched in main channel habitats (mean 79%, range 61–100%; ) and embayments (mean 21%, range 0–39%; ). No walleye collected in this study hatched in the stilling basin or tributary habitats. Individual sites with classification accuracies sufficient for site-specific natal recruitment evaluation (i.e. ≥ 80% accuracy) included the Bad River, Hipple Lake, and the Lake Sharpe stilling basin. Of these sites, only Hipple Lake contributed to walleye hatching, with mean annual recruitment of four percent (0–18% range; ) between 2004 and 2013.
Table 3. Percent natal contribution (# individuals in parentheses) of all habitat types and specific sites (i.e. those with ≥ 80% classification accuracy) to the walleye Sander vitreus population in Lake Sharpe, South Dakota, organized by year class from 2004 to 2013.
Overall, natal recruitment in embayments remained stable before (26%), during (20%), and after (21%) the flood year (). Similarly, natal recruitment in main channel habitats remained stable before (74%), during (80%), and after (79%) the flood year.
Habitat use
Accurate classification of age-0 walleye to natal habitats using Sr:Ca and Ba:Ca ratios also made these signatures reliable for evaluating habitat use of age ≥ 1 walleye. Walleye commonly occupied main channel habitats (mean 80% of annuli, range 73–95%) and embayments (mean 20% of annuli, range 5–26%) in late summer (). Only one walleye occupied the tributary in late summer, and no walleye used the stilling basin. The 2011 flood did not affect main channel habitat use, as occupancy was consistent before (mean 80% of annuli), during (mean 77%), and after (mean 80%) the flood year (). Similarly, embayment habitat use was stable before (mean 20% of annuli), during (mean 23%), and after (mean 20%) the flood year.
Discussion
Results from this study demonstrate that embayments were important habitats, relative to their area, for walleye natal recruitment in Lake Sharpe before, during, and after the flood year. For instance, Hipple Lake comprises only 0.8% (178 ha) of Lake Sharpe by surface area, but its annual natal recruitment averaged four percent and ranged up to 18% in 2012, contributions that are 5–23 times greater than expected under a linear contribution–surface area relationship. These findings build on previous research by illustrating a contrast between Lake Sharpe – where main channels are numerically the most important natal recruitment habitats and embayments have a relatively high natal contribution relative to their size – and nearby Lake Oahe, where walleye hatch in tributaries disproportionately more than other habitats (Carlson et al. Citation2016a, Citation2016b). This important distinction between the two reservoirs likely reflects the relative scarcity of tributaries in Lake Sharpe, compared to the numerical and areal abundance of tributaries in Lake Oahe (Galat et al. Citation2005). Main channels are the predominant habitat type in Lake Sharpe and tend to have environmental conditions that are suitable for natal recruitment (e.g. rocky substrates, relatively cool water temperatures, adequate food availability; Bozek et al. Citation2011; Fincel, Citation2011). Lake Sharpe embayments are even more favorable for walleye natal recruitment (e.g. high nutrient availability, plentiful food, abundant macrophytes and woody debris that protect against predators and swift currents; Turner et al. Citation1994; Brown et al. Citation2001; Madsen et al. Citation2001; Hall et al. Citation2003; Pease et al. Citation2006). However, embayment abundance is low and the collective surface area of these habitats is small compared to main channel environments in Lake Sharpe, explaining their lower numerical importance for natal recruitment.
In addition to demonstrating the resilience of the walleye population to the 2011 flood in terms of natal recruitment and habitat use, which were largely unaffected by the disturbance, this study provides insight into the mechanisms through which resilience occurred. Hypothetically, extensive sedimentation during the flood (Dixon et al. Citation2015; Carlson et al. Citation2016c) could have decreased walleye natal recruitment in embayments by degrading walleye spawning and nursery habitats, reducing river–floodplain connectivity (and thus walleye movement to and from spawning/nursery areas), or a combination of the two (Busch et al. Citation1975; Cheng et al. Citation2006). By illustrating that natal recruitment and habitat use of Lake Sharpe walleye were largely similar before, during, and after the flood year, results from this study indicate that spawning and nursery habitats were not degraded nor was river–floodplain connectivity reduced to the extent of affecting walleye. Ongoing research confirms findings presented herein that walleye natal recruitment and habitat use in Lake Sharpe embayments were not significantly altered by the 2011 flood (M. J. Fincel, South Dakota Game, Fish and Parks, July 2018, unpubl. data).
Results from this study have important implications and applications for walleye management in Lake Sharpe and other Missouri River reservoirs. For instance, in Lake Sharpe, fisheries professionals should focus on protecting and rehabilitating walleye spawning conditions in main channel habitats (e.g. rocky substrates, cool water temperatures; Bozek et al. Citation2011; Fincel Citation2011) shortly after large floods (i.e. within 1–2 years) because these locations contribute greatest to natal recruitment. However, over longer temporal scales, fisheries professionals should prioritize preserving and supplementing embayment habitats (e.g. spawning, nursery, refuge) in Lake Sharpe given their disproportionate importance for walleye natal recruitment relative to their surface area (collectively < 2% of Lake Sharpe). In addition, fisheries professionals should consider habitat addition (e.g. riprap shelves) as a way to supplement extant spawning habitats and provide a buffer against potential habitat destruction during future floods. Although it did not impact the Lake Sharpe walleye population in terms of natal recruitment and habitat use, river–floodplain connectivity still decreased considerably throughout all Missouri River reservoirs as a result of the flood (Dixon et al. Citation2015; Carlson et al. Citation2016c). Fisheries professionals should promote efforts to maintain and increase river–floodplain connectivity at a multi-reservoir scale and continue monitoring walleye natal recruitment and habitat use in embayments and main channels to ensure that contributions of these habitats to walleye populations are sustained and enhanced in all reservoirs.
In the present study, otolith chemistry was used to evaluate the relative importance of different habitat types to walleye natal recruitment and habitat use in response to a historic flood, an analysis that is informative for fisheries and water management in Lake Sharpe and other Missouri River reservoirs. Results presented herein suggest that the 2011 flood – a record disturbance – did not significantly reduce walleye natal recruitment or habitat use in Lake Sharpe. Indeed, the resilience of the Lake Sharpe walleye population to the flood is an important consideration for managing reservoir water levels in ways that promote multiple fisheries and water management objectives (e.g. angling, hydropower, irrigation). However, there is a need for further research evaluating how walleye respond to hydrological changes resulting from reservoir management actions (e.g. controlled water releases), as opposed to flooding. Findings from the present study also help fill knowledge gaps regarding the relative importance of different habitat types (i.e. embayment, main channel, stilling basin, tributary) for walleye natal recruitment and habitat use, providing a foundation for fisheries management in Lake Sharpe. For instance, the most important habitats for walleye natal recruitment in Lake Sharpe (i.e. main channels, embayments) should be prioritized over less productive habitats (e.g. stilling basin, tributaries) for broodstock collection, habitat protection/rehabilitation, and harvest regulations.
We encourage future researchers to explore additional applications of otolith chemistry (e.g. entrainment, invasive species management; Carlson et al. Citation2016d) in relation to – if not in combination with – traditional environmental history methods such as mark-recapture and radiotelemetry (Pollock et al. Citation2004; Landsman et al. Citation2011). Moreover, although Sr:Ca and Ba:Ca are effective otolith signatures for habitat-specific research in Lake Sharpe, future researchers should explore the efficacy of other signatures (e.g. 87Sr/86Sr, δ18O, δ13C) for investigating environmental history of walleye and other fishes within and beyond Lake Sharpe, perhaps at finer spatial scales (e.g. specific sites). Future researchers should also seek to maximize fish sample sizes (particularly during floods), monitor long-term patterns in Lake Sharpe water chemistry at multiple sites, and thereby overcome limitations of the present study. Overall, otolith chemistry was an effective tool for evaluating effects of the 2011 flood on walleye natal recruitment and habitat use in Lake Sharpe. As the predominant habitat type in Lake Sharpe, main channels had numerically greater natal recruitment and habitat use than embayments and other habitats. Even so, embayments were disproportionately important for natal recruitment and habitat use relative to their surface area, suggesting that walleye management should balance habitat protection/rehabilitation in main channels and embayments. Demonstrating the resilience of Lake Sharpe walleye to a historic flood, this study will help fisheries professionals quantify flood effects on fish populations and develop spatially informed approaches for habitat conservation, harvest regulations, and related management activities.
Notes on contributors
William J. Radigan, MS, conducts fisheries research in collaboration with the Department of Natural Resource Management at South Dakota State University.
Andrew K. Carlson, PhD, is a Distinguished Fellow in the Center for Systems Integration and Sustainability at Michigan State University.
Mark J. Fincel, PhD, is a senior fisheries biologist with the South Dakota Department of Game, Fish and Parks
Brian D.S. Graeb, PhD, is an associate professor in the Department of Natural Resource Management at South Dakota State University.
Acknowledgments
The authors thank J. Mecham, R. Johnston, J. Breeggeman, C. Hayer, T. Rapp, R. Novak, A. De Geoi, and C. Shake for field and laboratory assistance; C. Longhenry, B. Hanten, H. Meyer, K. Potter, G. Knecht, J. Sorenson, R. Trible, B. Larson, and B. Beel of South Dakota Game, Fish and Parks for field assistance and technical advice; the Mobridge, SD and Yankton, SD field crews for field assistance; A. Shiller and lab members at the University of Southern Mississippi for providing water sampling kits and measuring water chemistries in samples; and G. Barford, J. Commisso, J. Glessner, and A. Cole at the University of California-Davis for assistance with otolith chemistry analyses. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the Federal Aid in Sport Fish Restoration program [grant number 3M5526], [grant number 1531], [grant number 3M3328]; the South Dakota Department of Game, Fish and Parks [grant number 1526]; the United States Fish and Wildlife Service, South Dakota Agricultural Experiment Station [grant number SD00H439-12]; and the South Dakota State University.
References
- Alexander RB, Slack JR, Ludtke AS, Fitzgerald KK, Schertz TL. 2001. Data from selected U.S. Geological Survey national stream water-quality monitoring networks (WQN). USGS Digital Data Series DDS-37 [accessed 19 January 2019]. https://pubs.usgs.gov/dds/wqn96cd/
- Bayley PB. 1995. Understanding large river-floodplain ecosystems. BioScience. 45(3):153–158. doi:10.2307/1312554
- Bickford N, Hannigan R. 2005. Stock identification of walleye via otolith chemistry in the Eleven Point River, Arkansas. North Am J Fish Manag. 25(4):1542–1549. doi:10.1577/M04-189.1
- Bozek MA, Haxton TJ, Raabe JK. 2011. Walleye and sauger habitat. In: Barton BA, editor. Biology, management, and culture of walleye and sauger. Bethesda (MD): American Fisheries Society; p. 133–198.
- Brazner JC, Campana SE, Tanner DK. 2004. Habitat fingerprints for Lake Superior coastal wetlands derived from elemental analysis of yellow perch otoliths. Trans Am Fish Soc. 133(3):692–704. doi:10.1577/T03-091.1
- Brown RS, Power G, Beltaoa S. 2001. Winter movements and habitat use of riverine brown trout, white sucker and common carp in relation to flooding and ice break-up. J Fish Biol. 59(5):1126–1141. doi:10.1111/j.1095-8649.2001.tb00180.x
- Busch WN, Scholl RL, Hartman WL. 1975. Environmental factors affecting the strength of walleye (Stizostedion vitreum vitreum) year-classes in western Lake Erie, 1960–1970. J Fish Res Bd Can. 32(10):1733–1743. doi:10.1139/f75-207
- Campana SE, Chouinard GA, Hanson JM, Frechet A, Brattey J. 2000. Otolith elemental fingerprints as biological tracers of fish stocks. Fish Res. 46(1–3):343–357. doi:10.1016/S0165-7836(00)00158-2
- Carlson AK, Bailey PE, Fincel MJ, Graeb BDS. 2016a. Otoliths as elemental tracers of walleye environmental history: insights for interjurisdictional fisheries management. Lake Reserv Manag. 32:1–12. doi:10.1080/10402381.2016.1203845
- Carlson AK, Fincel MJ, Graeb BDS. 2016. Otolith microchemistry reveals natal origins of walleyes in Missouri River reservoirs. North Am J Fish Manag. 36(2):341–350. doi:10.1080/02755947.2015.1135214
- Carlson AK, Fincel MJ, Graeb BDS. 2017. Otolith chemistry indicates walleye movement and entrainment in a large serial reservoir system. Fish Manag Ecol. 24(3):217–229. doi:10.1111/fme.12224
- Carlson AK, Fincel MJ, Longhenry CM, Graeb BDS. 2016. Effects of historic flooding on fishes and aquatic habitats in a Missouri River delta. J Freshw Ecol. 31(2):271–288. doi:10.1080/02705060.2015.1128989
- Carlson AK, Phelps QE, Graeb BDS. 2017. Chemistry to conservation: using otoliths to advance recreational and commercial fisheries management. J Fish Biol. 90(2):505–527. doi:10.1111/jfb.13155
- Cheng F, Zika U, Banachowski K, Gillenwater D, Granata T. 2006. Modeling the effects of dam removal on migratory walleye (Sander vitreus) early life stages. River Res Applic. 22(8):837–851. doi:10.1002/rra.939
- Dixon MD, Boever CJ, Danzeisen VL, Merkord CL, Munes EC, Scott ML, Johnson WC, Cowman TC. 2015. Effects of a ‘natural’ flood event on the riparian ecosystem of a regulated large‐river system: the 2011 flood on the Missouri River, USA. Ecohydrol . 8(5):812–824. doi:10.1002/eco.1613
- Donohoe CJ, Zimmerman CE. 2010. A method of mounting multiple otoliths for beam-based microchemical analyses. Environ Biol Fish. 89(3-4):473–477. doi:10.1007/s10641-010-9680-3
- Fincel MJ. 2011. Productivity and trophic interactions in the Missouri River impoundments. Ph.D. Dissertation. Brookings, SD: South Dakota State University. 248 pp.
- Fincel MJ, Dembkowski DJ, Chipps SR. 2014. Influence of variable rainbow smelt and gizzard shad abundance on walleye diets and growth. Lake Reserv Manag. 30(3):258–267. doi:10.1080/10402381.2014.914989
- Freeman B, Jones R. 2012. Status of flood recovery. Missouri River Flood Task Force [accessed 19 January 2019]. http://www.nwd-mr.usace.army.mil/rcc/MRFTF/docs/StatusofFloodRecovery.pdf
- Galat DL, Berry CR, Gardner WM, Hendrickson JC, Mestl GE, Power GJ, Stone C, Winston MR. 2005. Spatiotemporal patterns and changes in Missouri River fishes. In: Rinne JN, Hughes RM, Calamusso B, editors. Historical changes in large river fish assemblages of the Americas. Bethesda (MD): American Fisheries Society; p. 249–291.
- Graff BJ, Dembkowski DJ, Wuellner MR. 2014. Phenology of annulus formation in walleye and smallmouth bass otoliths. TOFISHSJ. 7(1):1–7.
- Grigg N, McCarthy C, Lawrence B, Ockerman D. 2011. Review of the regulation of the Missouri River mainstem reservoir system during the flood of 2011. Fort Collins (CO): Colorado State University [accessed 19 January 2019]. http://www.nwd-mr.usace.army.mil/rcc/MRFTF/docs/MRIndependentReviewPanel.pdf
- Hall SR, Pauliukonis NK, Mills EL, Rudstam LG, Schneider CP, Lary SJ, Arrhenius F. 2003. A comparison of total phosphorus, chlorophyll a, and zooplankton in embayment, nearshore, and offshore habitats of Lake Ontario. J Great Lakes Research. 29(1):54–69. doi:10.1016/S0380-1330(03)70415-8
- Hanten R, Fincel MJ, Meyer H, Potter K, Smith M. 2014. Annual fish population and angler use, harvest and preference surveys on Lake Sharpe, South Dakota, 2014 Pierre (SD): South Dakota Department of Game, Fish and Parks. Fisheries Division Report No.: 15-06.
- Junk W, Bayley PB, Sparks RE. 1989. The flood pulse concept in river-floodplain systems. Can Spec Publ Fish Aquat Sci. 106:110–127.
- Juracek KE. 2015. The aging of America’s reservoirs: in-reservoir and downstream physical changes and habitat implications. J Am Water Resour Assoc. 51(1):168–184. doi:10.1111/jawr.12238
- Klumb RA, Rudstam LG, Mills EL, Schneider CP, Sawyko PM. 2003. Importance of Lake Ontario embayments and nearshore habitats as nurseries for larval fishes with emphasis on alewife (Alosa pseudoharengus). J Great Lakes Res. 29(1):181–198. doi:10.1016/S0380-1330(03)70426-2
- Landsman SJ, Nguyen VM, Gutowsky LFG, Gobin J, Cook KV, Binder TR, Lower N, McLaughlin RL, Cooke SJ. 2011. Fish movement and migration studies in the Laurentian Great Lakes: research trends and knowledge gaps. J Great Lakes Res. 37(2):365–379. doi:10.1016/j.jglr.2011.03.003
- Ludsin SA, Fryer BJ, Gagnon JE. 2006. Comparison of solution-based versus laser ablation inductively coupled plasma mass spectrometry for analysis of larval fish otolith microelemental composition. Trans Am Fish Soc. 135(1):218–231. doi:10.1577/T04-165.1
- Madsen JD, Chambers PA, James WF, Koch EW, Westlake DF. 2001. The interaction between water movement, sediment dynamics and submersed macrophytes. Hydrobiologia. 444(1/3):71–84. doi:10.1023/A:1017520800568
- Milton DA, Chenery SR. 1998. The effect of otolith storage methods on the concentrations of elements detected by laser-ablation ICPMS. J Fish Biol. 53(4):785–794. doi:10.1111/j.1095-8649.1998.tb01832.x
- Munro AR, McMahon TE, Ruzycki JR. 2005. Natural chemical markers identify source and date of introduction of an exotic species: lake trout (Salvelinus namaycush) in Yellowstone Lake. Can J Fish Aquat Sci. 62(1):79–87. doi:10.1139/f04-174
- Park Y, Chang J, Lek S, Cao W, Brosse S. 2003. Conservation strategies for endemic fish species threatened by the Three Gorges Dam. Conserv Biol. 17(6):1748–1758. doi:10.1111/j.1523-1739.2003.00430.x
- Pease AA, Davis JJ, Edwards MS, Turner TF. 2006. Habitat and resource use by larval and juvenile fishes in an arid-land river (Rio Grande, New Mexico). Freshwater Biol. 51(3):475–486. doi:10.1111/j.1365-2427.2005.01506.x
- Pollock KH, Jiang HH, Hightower JE. 2004. Combining telemetry and fisheries tagging models to estimate fishing and natural mortality rates. Trans Am Fish Soc. 133(3):639–648. doi:10.1577/T03-029.1
- R Development Core Team 2015. R: a language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing.
- Radigan WJ, Carlson AK, Fincel MJ, Graeb BDS. 2018. Otolith chemistry as a fisheries management tool after flooding: the case of Missouri River gizzard shad. River Res Applic. 34(3):270–278.doi:10.1002/rra.3247
- Radigan WJ, Carlson AK, Fincel MJ, Graeb BDS. 2018. Assessing the utility of otolith chemistry for management of six freshwater fishes from a river-reservoir system. North Am J Fish Manage. 38(2):316–326. doi:10.1002/nafm.10024
- Radigan WJ, Carlson AK, Kientz JL, Chipps SR, Fincel MJ, Graeb BDS. 2018. Species- and habitat-specific otolith chemistry patterns inform riverine fisheries management. River Res Applic. 34(3):279–287. doi:10.1002/rra.3248
- Riis JC. 1985. Walleye movement, harvest and angler use on Lake Oahe, South Dakota, 1981–1984. Pierre: South Dakota Game, Fish and Parks. Completion Report 84–4.
- Rosenfeld JS, Raeburn E, Carrier PC, Johnson R. 2008. Effects of side channel structure on productivity of floodplain habitats for juvenile Coho Salmon. North Am J Fish Manag. 28(4):1108–1119. doi:10.1577/M07-027.1
- Schemel LE, Sommer TR, Müller-Solger AB, Harrell WC. 2004. Hydrologic variability, water chemistry and phytoplankton biomass in a large floodplain of the Sacramento River, CA, U.S.A. Hydrobiologia. 513(1):129–139. doi:10.1023/B:hydr.0000018178.85404.1c
- Shiller AM. 2003. Syringe filtration methods for examining dissolved and colloidal trace element distributions in remote field locations. Environ Sci Technol. 37(17):3953–3957. doi:10.1021/es0341182
- Turner TF, Trexler JC, Miller GL, Toyer KE. 1994. Temporal and spatial dynamics of larval and juvenile fish abundance in a temperate floodplain river. Copeia. 1:174–183. doi:10.2307/1446683
- USACE (United States Army Corps of Engineers) 2012. Post 2011 flood event analysis of Missouri River mainstem flood control storage. Omaha (NE): USACE Omaha District [accessed 19 January 2019]. http://www.nwd-mr.usace.army.mil/rcc/reports/pdfs/Post2011FloodEventAnalysisofMainstemFloodControlStorage.pdf
- Voss BM, Peucker-Ehrenbrink B, Eglinton TI, Fiske G, Wang ZA, Hoering KA, Montluçon DB, LeCroy C, Pal S, Marsh S, et al. 2014. Tracing river chemistry in space and time: Dissolved inorganic constituents of the Fraser River, Canada. Geochim Cosmochim Acta. 124:283–308. doi:10.1016/j.gca.2013.09.006
- Ward JV, Tockner K, Schiemer F. 1999. Biodiversity of floodplain river ecosystems: ecotones and connectivity. Regul Rivers: Res Mgmt. 15(1–3):125–139. doi:10.1002/(SICI)1099-1646(199901/06)15:1/3≤125::AID-RRR523≥3.0.CO;2-E
- Ward JV, Weins JA. 2001. Ecotones of riverine ecosystems: role and typology, spatio-temporal dynamics, and river regulation. Intern J Ecohydro Hydrobiol. 1:25–36.
- Whitledge GW, Johnson BM, Martinez PJ, Martinez AM. 2007. Sources of nonnative centrarchids in the upper Colorado River related to stable isotope and microchemical analyses of otoliths. Trans Am Fish Soc. 136(5):1263–1275. doi:10.1577/T06-045.1
- Zeigler JM, Whitledge GW. 2010. Assessment of otolith chemistry for identifying source environment of fishes in the lower Illinois River, Illinois. Hydrobiologia. 638(1):109–119. doi:10.1007/s10750-009-0033-1
- Zeigler JM, Whitledge GW. 2011. Otolith trace element and stable isotopic compositions differentiate fishes from the Middle Mississippi River, its tributaries, and floodplain lakes. Hydrobiologia. 661(1):289–302. doi:10.1007/s10750-010-0538-7
