 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The food habits of three non-native cichlid fishes, Mayan cichlid (Mayaheros urophthalmus), Mozambique tilapia (Oreochromis mossambicus) and Nile tilapia (O. niloticus), in the lowermost Chao Phraya River basin, Thailand, was examined by stomach contents analysis. The index of preponderance, an index of the importance of prey items, was calculated from two relative metrics of prey quantity: percent frequency and percent volume. The index of niche breadth and the overlap coefficient were calculated to compare the breadth of food habits among the size classes and species groups. The M. urophthalmus mainly preyed on fish scales, detritus and aquatic invertebrates (molluscs and crustaceans). The O. mossambicus and O. niloticus fed mostly on detritus. The diets of the latter two species overlapped almost completely; however, green filamentous algae mixed with detritus was observed in the diet of the O. niloticus only. The observation that fish scales were a predominant food source in the stomach of M. urophthalmus (high importance value 45.48%) was specific to this study area.
Introduction
Nile tilapia, Oreochromis niloticus, and Mozambique tilapia, O. mossambicus, are common African cichlid fishes that have been introduced widely all over the world for aquaculture (Canonico et al. Citation2005; De Silva Citation2006). They now can be found throughout most of Southeast Asia (Arthington et al. Citation1984; Ang et al. Citation1989; Chou and Lam Citation1989; Eidman Citation1989; Juliano et al. Citation1989; Piyakarnchana Citation1989; Welcomme and Vidthayanon Citation2003; Canonico et al. Citation2005; Peterson et al. Citation2005; Weyl Citation2008; Grammer et al. Citation2012; Lowe et al. Citation2012; Russell et al. Citation2012; Welcomme et al. Citation2016). In addition, the Mayan cichlid, Mayaheros urophthalmus, (and hybrids of this species), which is endemic to the Atlantic slope of Central America, has been traded as an aquarium fish worldwide (Nico et al. Citation2007). It has been documented in some parts of Southeast Asia, including Singapore, Sulawesi in Indonesia, around Manila Bay in the Philippines and the lowermost Chao Phraya River basin in Thailand (Nico et al. Citation2007; Herder et al. Citation2012; Kwik et al. Citation2013; Ordoñez et al. Citation2015).
In Thailand, all three of these fish species were introduced into the wild in the middle of the last century due to escaped aquarium trade (Welcomme and Vidthayanon Citation2003). The lowermost Chao Phraya River basin is a sympatric region where all three fishes can be found in Thailand. Recently, the Thai government banned the rearing, release and transportation of four alien cichlid species in Thailand, including O. mossambicus and M. urophthalmus (The Nation, Citation2018). While reporting the establishment of non-native fishes in new region is a critical first step for reducing ecological impacts of invasion (Corsini-Foka and Economidis Citation2007; Galil Citation2008), it also is important to determine the role the non-native organisms play in their new habitat. This provides a clear picture of the issues necessary to mitigate the ecological problems associated with the invasion. One of these important aspects is their diets (Hughes Citation1986; Getabu Citation1994; Kadye and Booth Citation2012; Kalogirou et al. Citation2012).
Some preliminary studies of diet in these three non-native cichlid fishes showed that exotic O. niloticus and indigenous O. mossambicus mostly consumed plant detritus in the Limpopo River system, South Africa (Zengeya et al. Citation2011). Gut contents analysis suggested that the main food source of O. niloticus was plant material in the lower Nile River basin (Abdelghany Citation1993). However, Njiru et al. (Citation2004) showed that this species consumed various prey items, such as insects, plants, fishes and small aquatic invertebrates. Oreochromis mossambicus has been known as an opportunistic omnivore that consumes terrestrial vegetation, aquatic plant materials, zooplankton, aquatic and terrestrial macro-vertebrates, tadpoles and fish (Wager and Rowe–Rowe Citation1972; Bruton and Boltt Citation1975; Mathavan et al. Citation1976; De Silva et al. Citation1984; DeMoor et al. Citation1986; Jameson Citation1991; Arthington and Bluhdorn Citation1994; Fuselier Citation2001; Komarkova and Tavera Citation2003). One study in Asia found that O. mossambicus acted as a detrivore, herbivore and carnivore in different lakes in Sri Lanka (De Silva et al. Citation1984). The diet of M. urophthalmus ranged from herbivore, to omnivore, and carnivore depending on sites in their native range in Mexico and Belize (Caso Chávez et al. Citation1986; Martinez-Palacios and Ross Citation1988; Chávez-Lóvez et al. Citation2005; Vaslet et al. Citation2012). Bergmann and Motta (Citation2005) reported that M. urophthalmus was an omnivore in south Florida, where this species had been established as an exotic species. From these previous studies, it is clear that the food habits of these three cichlid fishes can vary widely depending on the location where they were introduced.
To date, comprehensive investigation of the food habits of the three non-native cichlid fishes is lacking in the lowermost Chao Phraya River basin. Nico et al. (Citation2007) conducted a limited survey of stomach contents of M. urophthalmus and suggested that this species was an omnivore, which was consistent with most previous studies. In the current study, we performed quantitative stomach contents analysis of these three non-native cichlid fishes to determine diet composition in the lowermost Chao Phraya River basin.
Materials and methods
Collections
The Chao Phraya System is one of the largest river systems in Southeast Asia, (approximately 372 km in length and 160,000 km2 in area) and serves as the origin of four rivers (the Pin, Wan, Yom and Nan Rivers) that start in the highlands of northern Thailand (Van BeeK Citation1995). This system is divided into an upper and lower basin by a narrow section at the confluence of these four rivers in Nakhon Sawan Province (Van Beek Citation1995; Komori et al. Citation2012). The Chao Phraya System joins with the Sakae Krang River and the Pa Sak River, and the Tha Chin River branches off at Chai Nat Province in the lower basin of this river system. The so-called ‘Chao Phraya River’ normally is the mainstream part of the lower basin of the river system (Van Beek Citation1995; Komori et al. Citation2012). The present study was conducted at the lowermost Chao Phraya River basin which is defined here as a delta area expanding along the main stream from the point where this river and the Pa Sak River meet to the mouth of the river. The lower Chao Phraya River basin previously formed a vast delta area, an amphibious environment that was not suitable for human life. The delta was developed when European colonials arrived and a number of canals were constructed in the area (Takaya Citation1987). These canals, which provide native aquatic animals with artificial refuges and habitats, were the main locations of investigation in this study ().
Figure 1. Map showing collection localities (black dots) in the lowermost Chao Phraya River basin in the present study. Broken lines indicate provincial boundaries. Provincial names are in italic.
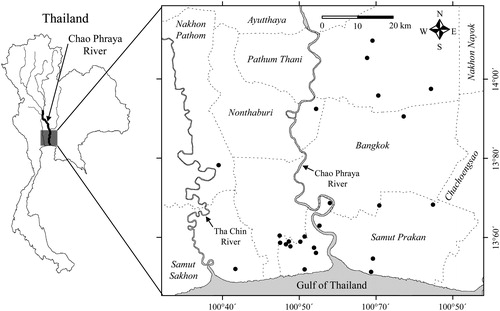
The study sites were selected from sampling locations where M. urophthalmus, O. mossambicus or O. niloticus had been collected in previous work from 2007 to 2010 by the Research Laboratory of Ichthyology, Faculty of Fisheries, Kasetsart University (RLIKU) (Prachya Musikasinthorn, unpublished data). When adequate sampling sites were encountered or found from observations during fieldwork or interviews with local fishermen, additional sampling was conducted at these sites.
All fish were collected using cast nets and scoop nets in small canals (number of sites = 24; ), 50 to 130 cm in depth, in the lower Chao Phraya River basin from September 2014 to October 2015. The collected fish were anaesthetised in ice water and immediately fixed in 10% formalin. For large specimens, formalin stock liquid was injected into the stomach directly to prevent digestion. After 10 days in formalin, the specimens were washed in fresh water and then stored in 75% ethanol. The number of specimens varied from 1 to 33 per collection, depending on study location.
Stomach contents analysis
The stomachs were extracted from the specimens after measurement of standard length (SL) and wet weight (WW) using calipers and a gravimeter. SL was measured from the tip of the upper jaw to the posterior end of the midlateral point of the hypural plate. The stomach contents were removed and sorted into 12 major functional categories (detritus, mixture of detritus and filamentous algae, filamentous algae, plant matter, benthic crustaceans, molluscs, adult fishes, fish bones, fish larvae, fish eggs, fish scales and artificial matter). For comparison among growth stages, specimens were divided into three size classes in each species group when all analyses were performed ().
Table 1. Distribution of standard length (SL) in each size class of three non-native cichlid fishes.
The contribution of each prey functional category to the overall stomach contents was assessed using two indices: percent frequency of occurrence (% F) and percent composition by volume (%V) (Hyslop Citation1980; Bowen Citation1996) (). %V was estimated using a modified method of Platter and Potter (Citation2001) by evenly spreading the contents of the stomach on waterproof paper ruled into 1 mm squares in approximately 1 mm thickness and examining them under a microscope. The number of squares occupied by each food category on the waterproof paper was later converted to the percent volume for each food category.
Table 2. Percent frequency of occurrence (%F) and percent composition by volume (%V) of functional prey categories of three non-native cichlid fishes in three size classes.
For the assessment of the importance of each prey category, the index of preponderance (IOPa) (Natrajan and Jhingran Citation1962; Sreeraj et al. Citation2006) was calculated:
where S is the number of prey types, Fa is the frequency of occurrence of species a and Va is the percent composition by volume of species a. To facilitate comparisons among species, IOPa was converted into percent IOPa (%IOPa).
To estimate prey item diversity of the three non-native cichlids, the index of niche breadth (INB) (Smith and Zaret Citation1982) was calculated for each individual stomach as
where n is the number of prey items and pi is the proportion of the total samples belonging to the ith prey item group (Smith and Zaret Citation1982).
To express dietary overlap between cichlids of different species and sizes, the overlap coefficient (Okj) was calculated using volume (%V) following Schoener (Citation1970):
where pik and pij are the proportions of the total samples belonging to the ith prey item group of species k and species j, respectively. The value of Okj may range from zero (indicating a specialised diet or no overlap) to 1.0 (indicating an equal use of food resources or complete overlap), with values >0.6 considered to indicate a biologically significant overlap (Wallace Citation1981; Langton Citation1982).
To test for significant differences in diet between cichlids of different species and sizes, analysis of similarity (ANOSIM) was performed on the Bray-Curtis similarity matrix using the vegan package in R (Oksanen et al. Citation2018) on statistic software R version 3. 4. 4 (R Core Team Citation2017). ANOSIM is a non-parametric test that uses a permutation procedure applied to a ranked similarity matrix to test whether there is a significant difference between two or more groups (Doi and Okamura Citation2011). Though this statistic was developed for testing differences of community composition among study sites, it also has been used to test differences of food items among taxonomic group and size classes (i.e. Platter and Potter Citation2001; Maddern et al. Citation2007; Zengeya and Marshall Citation2007; Muñoz et al. Citation2011).
Results
A total of 289 specimens (92 M. urophthalmus, 131 O. mossambicus and 66 O. niloticus) were collected between September 2014 and October 2015 at 18 sites around the lowermost Chao Phraya River basin. No new cichlid species were observed in the study area.
This study area was located in a tropical monsoon climate, and the mean monthly temperature during the study period was 29.1 °C and ranged from 26.5 to 31.1 °C (Japan Meteorological Agency Citation2008). There likely were differences in rainfall and water depth between dry and rain seasons. However, most of this study area was located in coastal areas, where the environment was affected by more tides rather than rainfall (Takaya Citation1987).
In M. urophthalmus, the SL ranged from 53.6 to 134.7 mm, with a mean (± SE) of 79.5 ± 18.7 mm; and WW ranged from 6.4 to 98.1 g, with a mean of 24.6 ± 20.1 g. In O. mossambicus, the SL ranged from 66.2 to 164.2 mm, with a mean of 115.5 ± 23.2 mm; and WW ranged from 8.6 to 138.3 g, with a mean of 57.4 ± 32.9 g. Finally, in O. niloticus. SL ranged from 76.5 to 183.5 mm, with a mean of 119.6 ± 27.9 mm, while WW ranged from 15.7 to 232.5 g, with a mean of 74.5 ± 52.4 g. On the whole, specimens of M. urophthalmus were smaller and no individuals of this species were placed in the third size class (151–200 mm) ().
The IOP value revealed that overall size classes, the most important dietary item was detritus (). In O. niloticus there were no significant differences in diet among size classes (ANOSIM, R = –0.09, P > 0.1), and the overlap coefficients ranged from 0.99 to 1.00, indicating an almost complete overlap in diet (). There was a tendency for the INB to decrease through the size classes ().
Table 3. Index of preponderance (IOPa) of functional prey categories and index of niche breadth (INB) in each size classes of three non-native cichlid fishes.
Table 4. Overlap coefficient (Okj) of stomach contents among size classes of three non-native cichlid fishes.
For O. niloticus, there there were no significant differences in diet among the size classes (ANOSIM, R = –0.07, P > 0.1), and the overlap coefficients ranged from 0.96 to 1.00 (). The most important food source was detritus over all the size classes (). The INB values of O. mossambicus were nearly the same for all the size classes (). There were no significant differences in the diets between O. mossambicus and O. niloticus (ANOSIM, R = –0.02, P > 0.1). However, the overlap coefficient in diets between the two species was high overall size classes, ranging from 0.96 to 1.00 (). The secondary prey items of O. mossambicus were crustaceans, fish eggs and fish larvae. In contrast, the diet of O. niloticus also contained filamentous algae and no aquatic invertebrates overall size classes (). According to additional observations under the binocular stereomicroscope, the larvae in the diet of O. mossambicus were larvae of cichlid fishes.
No significant differences in important prey items among size classes were observed in M. urophthalmus (ANOSIM, R = 0.03, P > 0.1). The IOP results showed differences in important prey items among size classes in M. urophthalmus. In addition to detritus as a main prey item, other important food resources for smaller-sized fish (MU1) were fish scales, whereas crustaceans and molluscs were the main food resources for the larger-size class (MU2) (). The scales from stomachs of MU1 probably were eaten from live fish, because other fish body fractions, such as eyes, skins and muscle, were not found in the stomach. The INB value of M. urophthalmus in the smaller-sized fish (MU1) was smaller than in the larger-sized fish (MU2) (). Additional comparisons of scale morphology among the one removed from stomachs and the one from fish specimens under a microscope showed that the scales in the diet of M. urophthalmus came from all species of three non-native cichlid fishes. All adult fish found in the diet of M. urophthalmus were identified as non-native mosquito fish (Gambusia affinis).
There were significant differences among the three cichlid fishes in their prey items (ANOSIM, R = 0.25, P < 0.01). The INB value of M. urophthalmus was the highest of the three cichlid fishes, indicating that M. urophthalmus utilised more widely varied prey resources, particularly fish in the smaller-size class, compared with the other two cichlid fishes.
The overlap coefficient was higher than 0.6 in all the size classes of O. niloticus and O. mossambicus as well as among larger-sized M. urophthalmus (), indicating that prey items of these groups overlapped significantly. The overlap coefficient was significantly higher in smaller (MU1) than in larger M. urophthalmus. No significant overlap was observed with the other size groups of the other two tilapiine fishes ().
Discussion
Previous work has shown that the food habits of the non-native cichlid fishes can be hihgly variable depending on where they are sampled. For example, M. urophthalmus acted as an herbivore, omnivore or carnivore in three different lagoons in Mexico (Caso Chávez et al. Citation1986; Martinez-Palacios and Ross Citation1988; Chávez-Lóvez et al. Citation2005). Thus, in order to determine the food habits of these three species in the lowermost Chao Phraya River basin, it must be established in their new environment.
The present study found that the main food resource of O. niloticus and O. mossambicus was detritus, with filamentous algae and small aquatic animals as secondary prey items of these species, respectively. By contrast, the diet of M. urophthalmus was more variable and changed with size classes. The main food item of small fish (MU1) was fish scale, but larger fish (MU2) tended to consume various aquatic animals, such as crustaceans and molluscs. Prey items of the three cichlid fishes mostly overlapped, except that only small M. urophthalmus ate fish scale.
The main food resources of O. niloticus in the lowermost Chao Phraya River basin were consistent with previous studies, that examined the proportion (%) by volume of food items (Zengeya et al. Citation2011, Citation2013). However, other studies conducted in its native range found that O. niloticus was omnivorous and consumed diverse prey items, including various insects, algae and crustaceans, depending on the surroundings (Njiru et al. Citation2004; Canonico et al. Citation2005; Oso et al. Citation2006). Oreochromis mossambicus are normally recognised as opportunistic omnivores in their native and introduced ranges (Wager and Rowe–Rowe Citation1972; Bruton and Boltt Citation1975; Mathavan et al. Citation1976; De Silva et al. Citation1984; DeMoor et al. Citation1986; Jameson Citation1991; Arthington and Bluhdorn Citation1994; Fuselier Citation2001; Komarkova and Tavera Citation2003). For example, by examining the percentage of dietary contribution by volume and occurrence, Maddern et al. (Citation2007) documented that this species utilised various food items, like algae, vegetable matter, terrestrial insects, diptera, ephemeroptera and silt/biofilm…etc. in Western Australia.
The findings from the current study supported the work of Zengeya et al. (Citation2011), which was conducted in a non-native habitat that also included O. niloticus and O. mossambicus. Zengeya et al. (Citation2011) suggested that O. mossambicus and O. niloticus in the Limpopo River in South Africa switched their primary food resource in favour of more detritus due to the recession of the river in the dry season. Thus, it also may be possible that the preferred prey of these two species may be lacking in the lowermost Chao Phraya River basin, a hypothesis that will need to be investigated further.
There is some evidence that O. mossambicus uses fish eggs and larvae as food resources (Berrios-Hernandez and Snow Citation1983; Macintosh and de Silva Citation1984; Panastico et al. Citation1988). However, in our study, only mature females consumed fish eggs and larvae (n = 12, SL = 115.5 ± 13.3 mm), suggesting that the eggs and the larvae found in their stomach was that probably were swallowed by mouth-brooding individuals accidentally when they were captured in the cast net.
The prey items of M. urophthalmus differed among size classes. The main prey items were detritus with fish scales consumed by the smaller-size class (MU1), and detritus with crustaceans and molluscs in fish of the larger-size class (MU2). The life history of M. urophthalmus is diverse, depending on the environment, and its food habits can vary widely (Loftus Citation1987; Martinez-Palacios et al. Citation1990; Martinez-Palacios and Ross Citation1992; Faunce and Lorenz Citation2000; Bergmann and Motta Citation2005) (). For example, there are clear differences in the diet of M. urophthalmus in their native and introduced range. In their native range in Belize, their main food items were crustaceans, detritus and plant material. However, in non-native Florica, they primarily consumed fish, ostracods and filamentous algae (Bergmann and Motta Citation2005; Vaslet et al. Citation2012) (). In the current study, M. urophthalmus fed as an omnivore. Some previous studies also found fish scales in the M. urophthalmus diet, but they made up a small contribution to overall prey (e.g. Martinez-Palacios and Ross Citation1988; Chávez-Lóvez et al. Citation2005). In the study of Martinez-Palacios and Ross (Citation1988), fish scales were found (%V = 0.45 – 5.24) in the larger fish (>150 mm), whereas they were found in the diet of smaller fish in this study. In our study, fish scales were an important secondary prey item, whereas in Martinez-Palacios and Ross’s study, fish scales were one of many options.
Table 5. Review of analyses of stomach contents performed on Mayaheros urophthalmus specimens from native and non-native ranges.
To the best of our knowledge, this study is the first study to investigate the food habits of non-native fishes in the lowermost Chao Phraya River basin. The capital, Bangkok, is located in the centre of this area and the physical environment and the biota of aquatic ecosystems in this area are continually changing. Cichlid fishes change their food habits depending on environmental changes in the region (e.g. Canonico et al. Citation2005). The continuous investigation of the food habits of cichlid fishes in this area is necessary to evaluate the ecological impacts on native ecosystems. Because there are prey items of animal origin in the diet of M. urophthalmus and this species has become established relatively recently, M. urophthalmus should be monitored carefully. Additionally, in the future, an extension of the methodology is needed to evaluate the broader impact of non-native cichlid fishes on native ecosystems because it is difficult to determine the actual prey items that are absorbed and assimilated in the body and their effects on food web dynamics (Okuda Citation2012). It will be important to undertake more detailed descriptions of the microhabitats, to determine the stomach contents of native fishes and other aquatic animals and to utilise more powerful ecological tools that can investigate trophic levels and food webs.
This study has shown that the ecology of non-native fishes is not always the same in an introduced region, even for the same species. Thus, research on non-native fish ecology should be performed site-specifically and multidimensionally, taking into account ecological, geological and human cultural factors. Site-specific research will be essential in the future, considering the possible impacts of non-native fishes on the environment, particularly in areas where non-native fishes are introduced frequently and their actual status is not clear.
Notes on contributors
Daiki Tomojiri is a postgraduate in invasion ecology and area studies in Graduate School of Asian and African Area Studies, Kyoto University.
Prachya Musikasinthorn is an assistant professor of Department of Fishery Biology, Faculty of Fisheries, Kasetsart University, Thailand, where he conducts research on taxonomy, systematics and biogeography of freshwater fishes of southern Asia. He also conducts surveys on alien fish species in the region.
Akihisa Iwata is a professor of Kyoto University, Japan, where he conducts research on fish taxonomy, ecology and conservation biology including alien species problems in East and Southeast Asia.
Acknowledgements
We appreciate the financial support provided by a scholarship for Japanese students through the Heiwa Nakajima Foundation. We thank Dr. Anong Chirapart, Dr. Charumas Meksumpun and Dr. Shettapong Meksumpun for their help and advice in fieldwork and research techniques. Pakorn Tongboonkua, Suchati Taothong and Mr. Saiyon Nakkamin assisted with field sampling and interviews with local people, and their help is greatly appreciated. Finally, we thank the local people and the people concerned who helped to complete the field survey.
Disclosure statement
The authors declare that there is no conflict of interest regarding the publication of this article.
References
- Abdelghany AE. 1993. Food and feeding habits of Nile tilapia from the Nile river at Cairo, Egypt. In Reinertsen H, Dahle LA, Jorgensen L, editors. Fish farming technology. Rotterdam: AA Balkema; p. 447–454.
- Ang KJ, Gopinath R, Chua TE. 1989. The status of introduced fish species in Malaysia. In De Silva SS, editor. Exotic Aquatic Organisms in Asia. Proceedings of the Workshop on Introduction of Exotic Aquatic Organisms in Asia. Asian Fisheries Society, Manila; p. 71–82.
- Arthington AH, McKay RJ, Russell DJ, Milton DA. 1984. Occurrence of the introduced Cichlid Oreochromis mossambicus (Peters) in Queensland. Mar Freshw Res. 35(2):267–272.
- Arthington AH, Bluhdorn DR. 1994. In: Dudgeon D, Lam PKS, editors. Distribution, genetics, ecology and status of the introduced cichlid, Oreochromis mossambicus, in Australia. Stuttgart (FRG): Schweizerbart’sche Verlagsbuchhandl; p. 53–62
- Bergmann GT, Motta PJ. 2005. Diet and morphology through ontogeny of the nonindigenous Mayan cichlid ‘Cichlasoma (Nandopsis)’ urophthalmus (Günther 1862) in southern Florida. Environ Biol Fish. 72(2):205–221.
- Berrios-Hernandez JM, Snow JR. 1983. Comparison of methods for reducing fry losses to cannibalism in tilapia production. Progr Fish-Cult. 45(2):116–118.
- Bowen SH. 1996. Quantitative description of the diet. In: Murphy BR, Willis DW, editors. Fisheries techniques. Bethesda: American Fisheries Society; p. 513–522.
- Bruton MN, Boltt RE. 1975. Aspects of the biology of Tilapia mossambica Peters (Pisces: Cichlidae) in a natural fresh-water lake (Lake Sibaya, South Africa). J Fish Biol. 7(4):423–445.
- Canonico GC, Arthington A, Mccrary JK, Thieme ML. 2005. The effects of introduced tilapias on native biodiversity. Aquat Conserv: Mar Freshw Ecosyst. 483:463–483.
- Caso Chávez M, Yáñez-Arancibia, Lara-Domínguez AL. 1986. Biologia, ecologia y dinamica de poblaciones de Cichlasoma urophthalmus (Gunter) (Pisces: Cichlidae) en habitat de Thalassia testudinum y Rhizophora mangle, Laguna de Terminos, sur del Golfo de Mexico. Biotica. 11:79–111.
- Chávez-Lóvez R, Peterson MS, Brown-Peterson NJ, Morales-Gómez, Franco-López 2005. Ecology of the Mayan Cichlid, Cichlasoma urophthalmus Günther, in the Alvarado Lagoonal System, Veracruz, Mexico. Gulf Caribb Res. 16:1–10.
- Chou LM, Lam TJ. 1989. Introduction of exotic aquatic species in Singapore. In De Silva SS, editor. Exotic Aquatic Organisms in Asia. Proceedings of the Workshop on Introduction of Exotic Aquatic Organisms in Asia, Asian Fisheries Society, Manila, p. 91–97.
- Corsini-Foka M, Economidis PS. 2007. Alien and vagrant ichthyofauna in Hellenic waters. In Papaconstantinou K, Zenetos A, Vassilopoulou V, Tserpes G, editors. State of hellenic fisheries. Athens: Hellenic Centre for Marine Research; p. 43–53.
- De Silva SS, Perera MK, Maitipe P. 1984. The composition, nutritional status and digestibility of the diets of Sarotherodon mossambicus from nine man-made lakes in Sri Lanka. Environ Biol Fish. 11(3):205–219.
- De Silva SS, Nguyen TTT, Abery NW, Amarasinghe US. 2006. An evaluation of the role and impacts of alien finfish in Asian inland aquaculture. Aquacult. Res. 37(1):1–17.
- DeMoor FC, Wilkinson RC, Herbst HM. 1986. Food and feeding habits of Oreochromis mossambicus (Peters) in hypertrophic Hartbeesport Dam, South Africa. S Afr J Zool. 21:170–176.
- Doi H, Okamura H. 2011. Similarity indices, ordination, and community analysis tests using the software R. Jpn. J. Ecol. 61:3–20.
- Eidman HM. 1989. Exotic aquatic species introduction into Indonesia. In De Silva SS, editors. Exotic Aquatic Organisms in Asia. Proceedings of the Workshop on Introduction of Exotic Aquatic Organisms in Asia, Asian Fisheries Society, Manila; p 57–62.
- Faunce C, Lorenz J. 2000. Reproductive biology of the introduced Mayan cichlid, Cichlasoma urophthalmus, within an estuarine mangrove habitat of southern Florida. Environ Biol Fishes. 58(2):215–225.
- Froese R, Pauly D, Editors. 2018. FishBase. World Wide Web electronic publication. www.fishbase.org (06/2018).
- Fuselier L. 2001. Impacts of Oreochromis mossambicus (Perciformes: Cichlidae) upon habitat segregation among cyprinodontids (Cyprinodontiformes) of a species flock in Mexico. Rev Biol Trop. 49:647–656.
- Galil BS. 2008. Alien species in the Mediterranean Sea – Which, when, why? Hydrobiologia. 606(1):105–116.
- Getabu A. 1994. A comparative study on the feeding habits of Oreochromis niloticus (Linnaeus) in Nyanza Gulf Lake Victoria and sewage fish ponds. Okemwa E, Wakwabi EO, Getabu A, editors. Proceedings of the Second EEC Regional Seminar on Recent Trends of Research on Lake Victoria Fisheries, ICIPE SCIENCE Nairobi; p. 93–103.
- Grammer GL, Slack WT, Peterson MS, Dugo MA. 2012. Nile tilapia Oreochromis niloticus (Linnaeus, 1758) establishment in temperate Mississippi, USA: multi-year survival confirmed by otolith ages. Aquat Invasions. 7(3):367–376.
- Herder F, Schliewen UK, Geiger MF, Hadiaty RK, Gray SM, McKinnon JS, Walter RP, Pfaender J. 2012. Alien invasion in Wallace’s Dreamponds: records of the hybridogenic “flowerhorn” cichlid in Lake Matano, with an annotated checklist of fish species introduced to the Malili Lakes system in Sulawesi. Aquat Invasions. 7(4):521–535.
- Hughes NF. 1986. Changes in the feeding biology of the Nile perch, Lates niloticus (L.) (Psces: Centropomidae), in Lake Victoria, East African since its introduction in 1960, and its impact on the native fish community of the Nyanza Gulf. J Fish Biol. 29(5):541–548.
- Hyslop EJ. 1980. Stomach contents analysis – a review of methods and their application. J Fish Biol. 17(4):411–429.
- Jameson JD. 1991. Plankton feeding habit of Oreochromis mossambicus (Cichlidae) held in cages, in Sathiar Reservoir, Tamil Nadu. Comp Physiol Ecol. 16:72–75.
- Juliano RO, Guerrero R, III, Ronquillo I. 1989. The introduction of exotic aquatic species in the Philippines. In De Silva SS, editors. Exotic Aquatic Organisms in Asia. Proceedings of the Workshop on Introduction of Exotic Aquatic Organisms in Asia, Asian Fisheries Society, Manila; p. 83–90.
- Kadye TW, Booth AJ. 2012. Integrating stomach content and stable isotope analyses to elucidate the feeding habits of non-native sharptooth catfish Clarias gariepinus. Biol Invasions. 14(4):779–795.
- Kalogirou S, Mittermayer F, Pihl L, Wennhage H. 2012. Feeding ecology of indigenous and non-indigenous fish species within the family Sphyraenidae. J Fish Biol. 80(7):2528–2548.
- Komarkova J, Tavera R. 2003. Steady state of phytoplankton assemblage in the tropical Lake Catemaco (Mexico). Hydrobiologia. 502:187–196.
- Komori D, Nakamura S, Kiguchi M, Nishijima A, Yamazaki D, Suzuki S, Kawasaki A, Oki K, Oki T. 2012. Characteristics of the 2011 Chao Phraya River flood in Central Bangkok. Hydrol Res Lett. 6(0):41–46.
- Kwik JTB, Kho ZY, Quek BS, Tan HH, Yeo DCJ. 2013. Urban stormwater ponds in Singapore: potential pathways for spread of alien freshwater fishes. BioInvasions Records. 2(3):239–245.
- Langton RW. 1982. Diet overlap between Atlantic cod, Gadus morhua, Silver hake, Merluccius bilinearis, and fifteen other northwest Atlantic finfish. Fish Bull. 80:745–759.
- Loftus WF. 1987. Possible establishment of the Mayan cichlid (Cichlasoma urophthalmus (Guenther) (Pisces: Cichlidae), in Everglades National Park, Florida. Florida Sci. 50:1–6.
- Lowe MR, Wu W, Peterson MS, Brown-Peterson NJ, Slack WT, Schofield PJ. 2012. Survival, growth and reproduction of non-native Nile tilapia II: fundamental niche projections and invasion potential in the northern Gulf of Mexico. PLoS One. 7(7):e41580.
- Macintosh DJ, de Silva SS. 1984. The influence of stocking density and food ration on fry survival in Oreochromis mossambicus and O. niloticus female 9 O. aureus male hybrids reared in a closed circulated system. Aquaculture. 41(4):345–335.
- Maddern MG, Morgan DL, Gill HS. 2007. Distribution, diet and potential ecological impacts of the introduced Mozambique mouthbrooder Oreochromis mossambicus Peter (Pisces: Cichlidae) in Western Australia. J R Soc West Aust. 90:203–214.
- Martinez-Palacios CA, Ross LG. 1988. The feeding ecology of the Central American cichlid Cichlasoma urophthalmus (Gunther). J Fish Biol. 33(5):665–670.
- Martinez-Palacios CA, Ross LG, Rosado-Vallado M. 1990. The effects of salinity on the survival and growth of juvenile Cichlasoma urophthalmus. Aquac Res. 91(1-2):65–75.
- Martinez-Palacios CA, Ross LG. 1992. The reproductive biology and growth of the Central American cichlid Cichlasoma urophthalmus (Günther). J Appl Ichthyol. 8(1–4):99–109.
- Mathavan S, Vivekanandan E, Pandian TJ. 1976. Food utilization in the fish Tilapia mossambica fed on plant and animal foods. Helgolander Wiss Meeresunters. 28:66–70.
- Muñoz RC, Currin CA, Whitfield PE. 2011. Diet of invasive lionfish on hard bottom reefs of the Southeast USA: insights from stomach contents and stable isotopes. Mar Ecol Prog Ser. 432:181–193.
- Natrajan AV, Jhingran AG. 1962. Index of Preponderance—a method of grading the food elements in the stomach analysis of fishes. Indian J Fish. 8:54–59.
- Nico LG, Beamish WH, Musikasinthorn P. 2007. Discovery of the invasive Mayan cichlid fish “Cichlasoma” urophthalmus (Günther 1862) in Thailand, with comments on other introductions and potential impacts. Aquatic Invasions. 2(3):197–214.
- Njiru M, Okeyo-Owuor JB, Muchiri M, Cowx IG. 2004. Shifts in the food of Nile tilapia, Oreochromis niloticus (L.) in Lake Victoria, Kenya. Afr J Ecol. 42(3):163–170.
- Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, Solymos P, Henry M, Stevens H, Szoecs E, Wagner H. 2018. vegan: Community Ecology Package. R package version 2.5-2. (https://CRAN.R-project.org/package=vegan).
- Japan Meteorological Agency 2008. World climate data tools. http://www.data.jma.go.jp/gmd/cpd/monitor/climatview/graph_mkhtml.php?&n=48455&p=24&s=1&r=0&y=2015&m=10&e=0&k=0
- Okuda N. 2012. How to access ecosystem structure and function of paddy fields using stable isotopes. Jpn J Ecol. 62:207–215.
- Ordoñez JFF, Asis AMJM, Catacutan BJ, Dela Pena J, Santos MD. 2015. First report on the occurrence of invasive black-chin tilapia Sarotherodon melanotheron (Ruppell, 1852) in Manila Bay and Mayan cichlid Cichlasoma urophthalmus (Gunther, 1892) in the Philippines. BioInvasions Records. 4(2):115–124.
- Oso JA, Ayodele IA, Fagbuaro O. 2006. Food and feeding habits of Oreochromis niloticus (L.) and Sarotherodon galilaeus (L.) in a tropical reservoir. World J Zool. 1:118–121.
- Panastico JB, Dangilan MMA, Eguia RV. 1988. Cannibalism among different sizes of tilapia (Oreochromis niloticus) fry/fingerlings and the effect of natural food. In: Pullin RSV, Tonguthai K, Maclean JL, editors. The second international symposium on Tilapia in aquaculture. ICLARM Conference, Manila, p. 465–468 Pearce
- Platter ME, Potter CI. 2001. Partitioning of food resources amongst 18 abundant benthic carnivorous fish species in marine waters on the lower west coast of Australia. J Mar Biol Ecol. 261:31–54.
- Peterson MS, Slack WT, Woodley CM. 2005. The occurrence of non-indigenous Nile tilapia, Oreochromis niloticus (Linnaeus) in coastal Mississippi, USA: ties to aquaculture and thermal effluent. Wetlands. 25(1):112–121.
- Piyakarnchana T. 1989. Exotic aquatic species in Thailand. In De Silva SS, editor. Exotic Aquatic Organisms in Asia. Proceedings of the Workshop on Introduction of Exotic Aquatic Organisms in Asia, Asian Fisheries Society, Manila, p. 119–124.
- R Core Team. 2017. R: A language and environment for statistical computing. (https://www.r-project.org/)
- Russell DJ, Thuesen PA, Thomson FE. 2012. A review of the biology, ecology, distribution and control of Mozambique tilapia, Oreochromis mossambicus (Peters 1852) (Pisces: Cichlidae) with particular emphasis on invasive Australian populations. Rev Fish Biol Fish. 22(3):533–554.
- Schoener T. 1970. Nonsynchronous spatial overlap of lizards in patchy habitats. Ecology. 51(3):408–418.
- Smith EP, Zaret TM. 1982. Bias in estimating niche overlap. Ecology. 63(5):1248–1253.
- Sreeraj N, Raghavan R, Prasad G. 2006. The diet of Horabagrus brachysoma (Günther), an endangered bagrid catfish from Lake Vembanad (South India). J Fish Biol. 69(2):637–642.
- Takaya Y. 1987. Agricultural development of a tropical delta: a study of the Chao Phraya Delta. translated by Peter Hawkes. Monoghraphs of the Center for Southeast Asian Studies, Kyoto University. Honolulu: University of Hawaii Press; p. 269.
- The Nation. 2018. Thai Fisheries Dept cracks down on cichlid. traffickers https://forum.thaivisa.com/topic/1030219-thai-fisheries-dept-cracks-down-on-cichlid-traffickers/ (accessed on 23 January 2019).
- van Beek S. 1995. The Chao Phya: river in transition. Kuala Lumpur: Oxford University Press.
- Vaslet A, France C, Baldwin CC, Feller IC. 2012. Dietary habits of juveniles of the Mayan cichlid, Cichlasoma urophthalmus, in mangrove ponds of an offshore islet in Belize, Central America. Neotrop Ichthyol. 10(3):667–674.
- Wager VA, Rowe–Rowe DT. 1972. The effects of Tilapia rendalli and T. mossambica on aquatic macrophytes and fauna in five ponds. S Afr J Sci. 68:257–260.
- Weyl OLF. 2008. Rapid invasion of a subtropical lake fishery in central Mozambique by Nile tilapia, Oreochromis niloticus (Pisces: Cichlidae). Aquatic Conserv: Mar Freshw Ecosyst. 18(6):839–851.
- Wallace RK. 1981. An assessment of diet-overlap indexes. Trans Am Fish Soc. 110(1):72–76.
- Welcomme RL, Baird IG, Dudgeon D, Halls A, Lamberts D, Mustafa MG. 2016. Fisheries of the rivers of Southeast Asia. In Craig JE, editor. Freshwater fisheries ecology. 1st ed. Hoboken: Wiley.
- Welcomme RL, Vidthayanon C. 2003. The impacts of introductions and stocking of exotic species in the Mekong Basin and policies for their control. Mekong River Commission (MTC) Technical Paper No. 9. Phnom Penh. Cambodia, 38.
- Welcomme RL. 1992. A history of international introductions of inland aquatic species. ICES Mar. Sci Symp. 194:3–14.
- Zengeya TA, Marshall BE. 2007. Trophic interrelationships amongst cichlid fishes in a tropical African reservoir (Lake Chivero, Zimbabwe). Hydrobiologia. 592(1):175–182.
- Zengeya TA, Booth AJ, Bastos ADS, Chimimba DT. 2011. Trophic interrelationships between the exotic Nile tilapia, Oreochromis niloticus and indigenous tilapiine cichlids in a subtropical African river system (Limpopo River, South Africa). Environ Biol Fish. 92(4):479–489.
- Zengeya TA, Robertson MP, Booth AJ, Chimimba CT. 2013. Ecological niche modeling of the invasive potential of Nile tilapia Oreochromis niloticus in African river systems: concerns and implications for the conservation of indigenous congenerics. Biol Invasions 15(7):1507–1521.
