?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Understanding consumption patterns and preferences of particular life stages for fish species can clarify potential dietary overlap and identify energy flow within aquatic communities. Age-0 white bass (Morone chrysops) have been documented to consume a variety of prey items and zooplankton are a common occurrence. The importance of particular zooplankton taxa and available sizes will likely impact prey selectivity, which is not understood for age-0 white bass. The objectives of this study were to evaluate food habits, prey electivity, and size selectivity of age-0 white bass in an irrigation reservoir from July to September 2015 and 2016. By number, age-0 white bass consumed mostly zooplankton from July to September. Consumption of fish become an important component of the diet for 22% of fish starting at 80 mm TL. Calanoida were the primary zooplankton taxa consumed and selected for by age-0 white bass. In relation to other zooplankton taxa, Calanoida were one of the most abundant taxa groups available and age-0 white bass displayed size selective feeding by consuming the larger Calanoida available in the environment. While Calanoida are not the largest taxa available, their high density appears to result in higher consumption by age-0 white bass compared to other available taxa.
Introduction
During their first year of life, age-0 fishes consume a variety of taxa as a result of changes in growth and/or gape limitations. Commonly, exogenous feeding begins with zooplankton and then shifts to macroinvertebrates before sometimes culminating in a shift to piscivory (Post Citation2003; Uphoff et al. Citation2019). While age-0 fish are initially zooplanktivorous, zooplankton can continue to be consumed throughout the first year of life and the zooplankton taxa selected for may vary depending on the size of fish and the zooplankton size and availability. For example, larval freshwater drum (Aplodinotus grunniens) selection for zooplankton taxa and sizes changed with increasing fish size (Sullivan et al. Citation2012). Improving the understanding of prey resource utilization can assist managers in understanding the potential for interspecies competition and energy flow within the system and potentially predict problems given changing zooplankton community structure or abundance.
White bass (Morone chrysops) are a popular reservoir sport fish (Quist et al. Citation2002) found to consume a variety of diet items including zooplankton, macroinvertebrates, and fish (Van Den Avyle et al. Citation1983; Saul et al. Citation1985; Pothoven and Höök Citation2015). Previous studies have provided insight on age-0 white bass prey electivity (Martin et al. Citation1981; Beck et al. Citation1998; Lemke et al. Citation2003) but data on size selectivity are limited. Age-0 white bass selected for the largest zooplankton in the zooplankton community in a South Dakota reservoir (Michaletz et al. Citation1987) and selected for larger Daphnia lumholtzi during a ten-day study in an Illinois lake (Lemke et al. Citation2003).
Understanding the importance of zooplankton taxa to age-0 white bass can identify community energy flow and allow predictive assessments of potential environmental changes (i.e., aquatic invasive species, climate change, and drought) on zooplankton densities. Fish can either consume prey in proportion to their availability in the environment (opportunistic feeding) or they can selectively prey on particular taxa in greater proportion to their availability (selective feeding; Newkirk and Schoenebeck Citation2018) which helps describe the taxa that are integral to growth and development of age-0 white bass. The optimal foraging theory suggests that individuals consume prey with the greatest energetic benefit (Breck Citation1993). Therefore, feeding behaviors of age-0 white bass should focus on prey that are most calorically beneficial. Thus, the objectives of this study are to (1) describe food habits, (2) evaluate prey electivity, and (3) evaluate potential size selectivity of age-0 white bass during July to September.
Study site
Harlan County Reservoir is located in south-central Nebraska along the Republican River (). Harlan County Reservoir covers more than 5362 ha with 121 km of shoreline at conservation pool (1946 msl). Intensive drawdowns occur during the agriculture irrigation season to provide water for Nebraska and Kansas (USBR 1996). Harlan County Reservoir oscillates between eutrophic and hypereutrophic states and has limited stratification due to wind exposure and water withdrawal activities (Olds et al. Citation2011). The result is that the reservoir has limited macrophytic development.
Figure 1. A map of Harlan County Reservoir, Nebraska (Peterson et al. Citation2005).
Methods
Sampling protocol
Age-0 white bass sampling occurred in July, August, and September 2015 and 2016 following procedures outlined in Miller et al. (Citation2018a). Age-0 white bass were collected with bag seines (15.25 m long; 0.63-cm mesh, bar measure), boat electrofishing (10–12 A of pulsed-DC current at 100–200 V), and overnight small-mesh gill nets (1.83 × 60.96 m with equal compliments of 1.27 and 1.90 cm bar mesh) until 50 individuals were collected each month. Age-0 white bass retrieved from sampling gear were measured for total length (mm) and immediately placed on ice and transported to the University of Nebraska at Kearney, where they were frozen for later diet and aging assessment.
Environmental zooplankton community assessments from the reservoir occurred in July, August, and September in 2015 and 2016 to establish environmental availability from 15 standardized sites (Peterson et al. Citation2005). The numerical proportions of zooplankton in the environment were calculated from standardized zooplankton sampling that occurred within 5 days of the white bass collections each month. Zooplankton samples were collected using a Wisconsin plankton net (0.5 m diameter with 80 µm mesh) towed vertically from the substrate to the surface. Samples were preserved in a sucrose-buffered 4% formalin solution to prevent osmotic distortion (Haney and Hall Citation1973), then identified and quantified following the procedures of Peterson et al. (Citation2005). Densities were calculated for the following zooplankton taxa: Calanoida, Cyclopoida, copepod nauplii, Daphnia pulicaria, Daphnia retrocurva, Daphnia lumholtzi, and Diaphanosoma. Reservoir means derived from the sampling sites were utilized to assign the monthly relative reservoir community composition.
Diet analysis
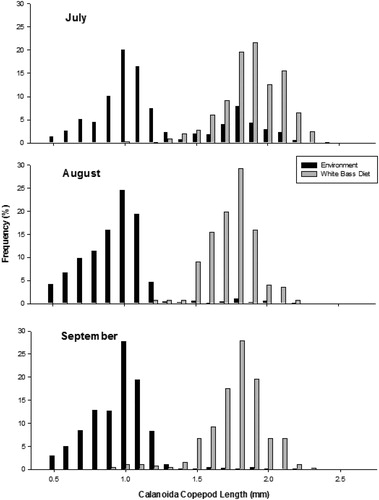
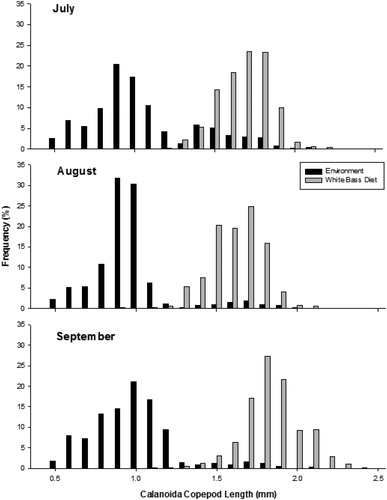
Diet contents were categorized as fish (species level), macroinvertebrates (family level), copepods (order level), or cladocerans (genus level). Individual wet weights were recorded for macroinvertebrates and fish (nearest 0.1 g). If possible, total length (nearest mm) was recorded for fish found in stomachs. Up to 25 individuals of each copepod order and cladoceran genus from each diet were measured for body length (nearest 0.1 mm) and biomass was calculated using length-dry weight conversions (O’Brian and deNoyelles 1974; McCauley Citation1984; Culver et al. Citation1985; Eisenbacher Citation1998). Zooplankton dry weights were converted to wet weight with a 1:10 (dry:wet) ratio (Balvay Citation1987). Food habits were calculated by percent number and percent weight for each fish and then averaged by month. Zooplankton length distributions were compared between those collected from the environment and those from white bass stomachs for July, August, and September in 2015 and 2016 using a Kolmogorov-Smirnov test with a significance level of α ≤ 0.05.
Feeding electivity
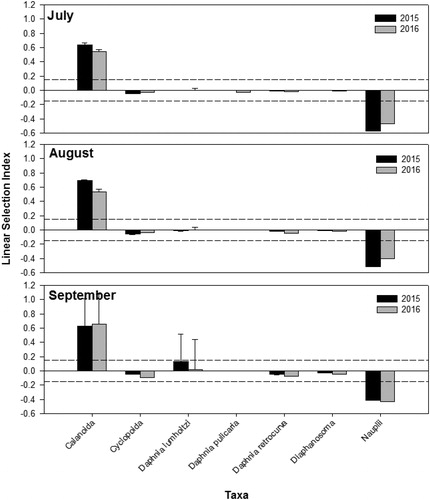
Zooplankton selection by age-0 white bass were quantified using the linear index of food selection (Strauss Citation1979). Strauss’s index value () can range from total avoidance (–1) to absolute selectivity (1) for a given prey item. Similar to previous studies (Newkirk and Schoenebeck Citation2018; Thiessen et al. Citation2018), a value of ±0.15 was chosen as the cutoff to determine selectivity or avoidance. Therefore, we defined opportunistic prey selection as electivity values between 0.15 and –0.15, prey selection as values >0.15, and prey avoidance as <–0.15 (Sullivan et al. Citation2011, Citation2012). Standard errors for electivity indices were calculated for each taxa consumed by age-0 white bass (Graham and Sprules Citation1992).
Results
A total of 300 fish were collected, 11 of those had empty stomachs, and therefore 289 were utilized for diet assessment (). A total of 240 out of 289 age-0 white bass had consumed zooplankton. Calanoida prey represented between 27.8 and 99.0% by number of diet items during all sampling months (). Additional prey items consumed by age-0 white bass in 2015 and 2016 included gizzard shad, Diptera, Hemiptera, and zooplankton taxa including the exotic zooplankton Daphnia lumholtzi. Gizzard shad were consumed in all months but July 2016 but were only present in the diets of 48 out of 289 individual age-0 white bass (). The inclusion of gizzard shad as a prey item for age-0 white bass occured around 80 mm and was a large diet component for those fish who consumed shad.
Table 1. Average length (mm) and sample size (n) for age-0 white bass from July–September 2015–2016 in Harlan County Reservoir, Nebraska.
Table 2. Prey by percent number and weight (g) of age-0 white bass diets in July, August, and September from 2015 to 2016 in Harlan County Reservoir, Nebraska.
Age-0 white bass demonstrated both prey taxa and size selectivity. During both years, age-0 white bass positively selected for Calanoida, consumed other zooplankton taxa in proportion to their availability, and avoided abundant copepod nauplii (). Additionally, age-0 white bass selected for larger zooplankton during all months of this study (). In 2015, age-0 white bass selected for some of the largest Calanoida that were available in the environment in July (D = 0.523, P < 0.001), August (D = 0.636, P < 0.001), and September (D = 0.545, P < 0.001) (). Similarly, in 2016, age-0 White Bass selected for some of the largest Calanoida that were available in the environment in July (D = 0.500, P < 0.001), August (D = 0.575, P < 0.001), and September (D = 0.475, P < 0.001) ().
Figure 2. Electivity index of age-0 white bass collected in Harlan County Reservoir, Nebraska in July, August, and September 2015–2016. Age-0 white bass diets containing zooplankton were utilized for electivity calculation. The dashed lines represent the selectivity threshold.
Discussion
Age-0 white bass diets by number consisted primarily of zooplankton, but occasionally contained macroinvertebrates and gizzard shad. Similarly, other studies documented zooplankton as a primary diet item for age-0 white bass throughout their first year of life (Beck et al. Citation1998; Willis et al. Citation2002; Pothoven and Höök Citation2015). However, as this study confirmed, there is potential for prey items such as macroinvertebrates and fish to be present in age-0 white bass food habits and when present these prey items may constitute a majority of the prey by weight. Fish prey has a higher energetic value than zooplankton (Cummins and Wuycheck Citation1971; Miranda and Muncy Citation1989) and the ability of some age-0 white bass to use this prey item is calorically important and may increase individual growth thereby broadening intracohort variability in length and potentially provide greater overwinter survival for the larger individuals within a cohort (Post Citation2003; Uphoff et al. Citation2019). Shifts to alternative prey resources, especially those that have higher caloric potential, can be regulated by prey availability or fish size (Wu and Culver Citation1992; Post Citation2003; Uphoff et al. Citation2019) and further investigations would be recommended to determine the role of these factors in feeding behavior of age-0 white bass.
Age-0 white bass size appears to influence food habits as the occurrence of higher trophic level prey items (especially age-0 gizzard shad) were observed at ≥80 mm TL. Age-0 walleye within the study reservoir have been observed to display a similar ontogenetic diet shift from consuming Calanoida to age-0 gizzard shad at 47 and 77 mm (Uphoff et al. Citation2019). However, in contrast to age-0 white bass, Uphoff et al. (Citation2019) found the entire cohort of age-0 walleye eventually shifted to piscivory and thereafter largely consumed gizzard shad. Age-0 gizzard shad availability is fairly consistent and not suspected to be limiting in this reservoir (Miller et al. Citation2018b), suggesting gizzard shad would be available for age-0 white bass to consume. Yet in this study only 22% of age-0 white bass >80 mm consumed gizzard shad, however, all age-0 white bass >150 mm consumed 100% gizzard shad. Furthermore, for the 42 white bass between 80 and 150 mm who did consume gizzard shad, 40 of those had 100% shad based diets. Considering macroinvertebrates and gizzard shad have a greater caloric value than zooplankton, there is likely a different mechanism that explains the inconsistent use of age-0 gizzard shad as prey items for age-0 white bass <150 mm. We would hypothesize that these simultaneously occuring different feeding strategies (zooplanktivourous and piscivory) within the cohort would result in greater growth for piscivorous white bass and would increase intracohort variability.
Age-0 white bass in this study demonstrated selective feeding behavior consuming a high proportion of Calanoida relative to their environmental availabilty. High selectivity values for Calanoida are especially telling given the high density of available Calanoida in the environment as high prey densities would generally result in lower electivity values. In contrast, other zooplankton taxa were largely consumed in proportion to their availability (except nauplii). Calanoida densities averaged 60.2 ± 3.0 SE L-1 throughout this study which was almost ten times those of cladocerans such as Daphnia retrocurva 6.6 ± 0.5 SE L-1, Daphnia pulicaria 7.0 ± 2.0 SE L-1, and Daphnia lumholtzi 1.6 ± 0.2 SE L-1. In addition to density, we hypothesize zooplankton size also played a factor in prey taxa selection. For example, copepod nauplii were abundant, but the smallest zooplankton taxa averaging 0.2 ± 0.0 SE mm in the environment and were not selected for by age-0 white bass in this study or in a mainstem river reservoir (Martin et al. Citation1981). Age-0 white bass consumed Calanoida with an average length of 1.0 ± 0.0 SE mm throughout the study which was a similar mean length to Daphnia pulicaria 1.0 ± 0.0 SE mm, Daphnia retrocurva 1.1 ± 0.0 SE mm, and Daphnia lumholtzi 1.3 ± 0.0 SE mm. Given the similar lengths between Calanoida and cladocerans, we hypothesize age-0 white bass selected for Calanoida due to their greater density and caloric value compared to cladocearns (Cummins and Wuycheck Citation1971), which would conform to the optimal foraging theory (Breck Citation1993).
Size selectivity of prey was evident in the selection of larger Calanoida. Furthermore, the abundance of the larger Calanoida decreased throughout both years from July to September, potentially due to age-0 white bass consumption. Calanoida species have multiple stages of development (Ferrari Citation1995) and one hypothesis is the binomial length distribution observed in the environmental samples represents both immature (copepodid) and adult calanoid copepods with age-0 white bass selecting for the larger, more advanced stages of development. Another hypothesis would be the presence of multiple species of Calanoida, each with different maximum sizes such that age-0 white bass are selecting for the larger species of Calanoida (e.g., Aglaodiaptomus). Future identification of copepods to genus or by life stage, while time consuming, may provide insight into these competing hypotheses.
Acknowledgements
We thank staff of the Kearney Field Office of the Nebraska Game and Parks Commission and the University of Nebraska at Kearney for help in the field and lab, especially J. Kreitman, B. Newcomb, B. Eifert, C. Hadan, J. Hasz, B. Roberg, T. Jackson, S. Eilers, B. Peterson, J. Thiessen, B. Schall, M. Perrion, B. Andersen, M. Wright, J. Laux, B. O’Connor, K. Miller, T. Kozeal, Z. Ondrak, and J. Cabela. We thank J. Hirsch for zooplankton expertise and J. Davis for graphical assistance.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Brett T. Miller is a former MS student and currently a district fisheries biologist for the Kansas Department of Wildlife, Parks and Tourism in Clay Center, Kansas.
Casey W. Schoenebeck is a former associate professor of biology at the University of Nebraska at Kearney. He is currently the Sentinel Lakes Program coordinator in the Fisheries Research Unit for the Minnesota Department of Natural Resources in Glenwood, Minnesota.
Keith D. Koupal is an irrigation reservoir specialist in the Fisheries Division for the Nebraska Game and Parks Commission in Kearney, Nebraska.
References
- Balvay PG. 1987. Equivalence entre quelques parametres estimatifs de 1'abondance du zooplancton total. Schweiz Z Hydrol. 49(1):75–84.
- Beck HD, Starostka AB, Willis DW. 1998. Diet overlap of age-0 walleye and white bass in Lake Poinsett, South Dakota. J Freshwater Ecol. 13(4):425–431.
- Breck JE. 1993. Foraging theory and piscivorous fish: are forage fish just big zooplankton? Trans Am Fish Soc. 122(5):902–911.
- Culver DA, Boucherle MM, Bean DJ, Fletcher JW. 1985. Biomass of freshwater crustacean zooplankton from length-weight regressions. Can J Fish Aquat Sci. 42(8):1380–1390.
- Cummins KW, Wuycheck JC. 1971. Caloric equivalents for investigations of ecological energies. Int Ver Theor Angew. 18:1–158.
- Eisenbacher ME. 1998. Effects of the exotic cladoceran Daphnia lumholtzi (Sars) on the growth rate and prey selection of bluegill sunfish (Lepomis macrochirus Rafinesque) [master’s thesis]). Springfield (MO): Missouri State Univerisity.
- Ferrari FD. 1995. Six copepodid stages of Ridgewayia klausruetzleri, a new species of copepod crustacean (Ridgewayiidae: Calanoida) from the barrier reef in Belize, with comments on appendage development. Proc Biol Soc Wash. 108:180–200.
- Graham DM, Sprules WG. 1992. Size and species selection of zooplankton by larval and juvenile walleye (Stizostedion vitreum vitreum) in Oneida Lake, New York. Can J Zool. 70(10):2059–2067.
- Haney JF, Hall DJ. 1973. Sugar-coated Daphnia: a preservation technique for Cladocera. Limnol Oceanogr. 18(2):331–333.
- Lemke AM, Stoeckel JA, Pegg MA. 2003. Utilization of the exotic cladoceran Daphnia lumholtzi by juvenile fishes in an Illinois River floodplain lake. J Fish Biol. 62(4):938–954.
- Martin DB, Mengel LJ, Novotny JF, Walburg CH. 1981. Spring and summer water levels in a Missouri River reservoir: effects on age-0 fish and zooplankton. Trans Am Fish Soc. 110(3):370–381.
- McCauley E. 1984. The estimation of the abundance and biomass of zooplankton in samples. In: Downing JA and Rigler FH, editors. A manual on methods for the assessment of secondary productivity in fresh waters. Oxford (UK): Blackwell Scientific; p. 228–265.
- Michaletz PH, Unkenholz DG, Stone CC. 1987. Prey size selectivity and food partitioning among zooplanktivorous age-0 fishes in Lake Francis Case, South Dakota. Am Midl Nat. 117(1):126–138.
- Miller BT, Peterson BC, Koupal KD, Schoenebeck CW. 2018. Long-term changes in biotic and abiotic factors influence larval gizzard shad (Dorosoma cepedianum) annual peak density. J Freshwater Ecol. 33(1):173–181.
- Miller BT, Schoenebeck CW, Koupal KD. 2018. Gear- and season -specific catch rates of age-0 walleye and white bass: standard sampling recommendations for Great Plains reservoirs. North Am J Fish Manage. 38(4):903–910.
- Miranda LE, Muncy RJ. 1989. Bioenergetic values of shads and sunfishes as prey for largemouth bass. Proc Annu Conf SEAFWA. 43:153–163.
- Newkirk JI, Schoenebeck CW. 2018. Prey electivity of the slimy sculpin within the Lake Superior-North Watershed. J Freshwater Ecol. 33(1):327–333.
- Olds BP, Peterson BC, Koupal KD, Farnsworth-Hoback KM, Schoenebeck CW, Hoback WW. 2011. Water quality parameters of a Nebraska reservoir differ between drought and normal conditions. Lake Reserv Manage. 27(3):229–234.
- Peterson BC, Fryda NJ, Koupal KD, Hoback WW. 2005. Daphnia lumholtzi, an exotic zooplankton, invading a Nebraska reservoir. Prairie Nat. 37:11–19.
- Post DM. 2003. Individual variation in the timing of ontogenetic niche shifts in largemouth bass. Ecology. 84(5):1298–1310.
- Pothoven SA, Höök TO. 2015. Feeding ecology of invasive age-0 white perch and native white bass after two decades of co-existence in Saginaw Bay, Lake Huron. AI. 10(3):347–357.
- Quist MC, Guy CS, Bernot RJ, Stephen JL. 2002. Ecology of larval white bass in a large Kansas reservoir. North Am J Fish Manage. 22(2):637–642.
- Saul BM, Wilson JL, Peterson DC, Richardson JM. 1985. Food habits and growth of young-of-the-year white bass in two east Tennessee reservoirs. Proc Annu Conf SEAFWA. 36:115–124.
- Strauss RE. 1979. Reliability estimates for Ivlev's electivity index, the forage ratio, and a proposed linear index of food selection. Trans Am Fish Soc. 108(4):344–353.
- Sullivan CL, Schoenebeck CW, Koupal KD, Hoback WW, Peterson BC. 2011. Patterns of age-0 gizzard shad abundance and food habits in a Nebraska irrigation reservoir. Prairie Nat. 43:110–116.
- Sullivan CL, Koupal KD, Hoback WW, Peterson BC, Schoenebeck CW. 2012. Food habits and abundance of larval freshwater drum in a south central Nebraska irrigation reservoir. J Freshwater Ecol. 27:111–121.
- Thiessen JD, Koupal KD, Schoenebeck CW, Shaffer JJ. 2018. Food habits of imperiled plains topminnow and diet overlap with invasive western mosquitofish in the central Great Plains. Trans Nebr Acad Affil Soc. 38:1–9.
- Uphoff CS, Schoenebeck CW, Koupal KD, Pope KL, Hoback WW. 2019. Age-0 walleye Sander vitreus display length-dependent diet shift to piscivory. J Freshwater Ecol. 34(1):27–36.
- US Bureau of Reclamation [USBR]. 1996. Resource management assessment Republican River Basin: Water service contract renewal. Washington (DC): US Bureau of Reclamation.
- Van Den Avyle MJ, Higginbotham BJ, James BT, Bulow FJ. 1983. Habitat preferences and food habits of young-of-the-year striped bass, white bass, and yellow bass in Watts Bar Reservoir, Tennessee. North Am J Fish Manage. 3(2):163–170.
- Willis DW, Paukert CP, Blackwell BG. 2002. Biology of white bass in eastern South Dakota glacial lakes. North Am J Fish Manage. 22(2):627–636.
- Wu L, Culver DA. 1992. Ontogenetic diet shift in Lake Erie: age-0 yellow perch (Perca flavescens): a size-related response to zooplankton density. Can J Fish Aquat Sci. 49(9):1932–1937.