Abstract
The need to understand the influence of high-level factors that shape species richness and population size is becoming more important as global biodiversity losses threaten biotic communities. Here we investigate the influence of ecological drift on periphytic diatom community composition in arctic lakes. If community composition is strongly influenced by drift, we hypothesize that (i) alpha diversity will increase with increasing lake size, due to the decreasing influence of drift-mediated local extinction, (ii) beta diversity will be greatest among small lakes and lowest in large lakes, and (iii) relationships between community size and environmental variables will be strongest in large lakes and weakest in small lakes. Analysis was conducted on periphytic diatoms accrued on artificial substrates in 44 shallow, thermokarst lakes over the 2009 growing season. Limnological and hydrological conditions at each site were described and their influence was removed to minimize the confounding effect of environmental heterogeneity. Alpha diversity was not significantly different among the lake sizes. Total beta diversity was significantly higher in the small lakes than the medium lakes. Beta diversity was primarily attributed to turnover, and small lakes had significantly higher turnover than medium and large lakes, while nestedness was not significantly different among lake-size classes. Periphytic diatom community composition and environmental variables were not significantly concordant in any lake-size class. Our results suggest that drift, as related to habitat size, is not a primary driver of periphytic diatom community composition in shallow, thermokarst arctic lakes.
Introduction
As predictions about the loss of global biodiversity grow increasingly pessimistic, there is a need to identify high-level factors that determine diversity to better understand community dynamics (Willis and Whittaker Citation2002). Vellend (Citation2010) emphasized four processes that have the potential to drive community composition; selection, drift, speciation and dispersal. Of these, drift is arguably the most overlooked and challenging to study directly (Vellend Citation2010). Drift is the inherently stochastic component of community change, resulting from random changes in species relative abundance due to birth and death rate or extinction. The influence of drift on community composition is predicted to increase as the size of a community decreases such that small habitats with fewer individuals are expected to be more strongly influenced by drift-mediated local extinction. It follows then, that these smaller habitats would have lower alpha diversity and greater beta diversity than larger habitats with larger populations (Hubbell Citation2001; Chase Citation2010; MacArthur and Wilson Citation1967).
A variety of approaches have been used to test for ecological drift in communities. Ricklefs and Lovette (Citation1999) compared species richness of birds, bats, butterflies, reptiles and amphibians to habitat area and habitat diversity on 19 islands and determined the relative contribution of area and habitat diversity by multiple regression. They detected a direct influence of area on species richness, possibly due to drift. In a study of small mammal diversity in forest fragments, Pardini et al. (Citation2005) found that fragment size influenced both small mammal abundance and diversity, and concluded that higher spatial variability in community composition among smaller fragments is likely due to stochastic extinctions. Gilbert and Levine (Citation2017) manipulated community size in annual plant communities and found support for ecological drift, which resulted in lower alpha diversity and higher beta diversity in small communities. Sydenham et al. (Citation2017) used linear mixed models to test the influence of dispersal limitation, ecological filtering and ecological drift in bee communities using elevation as a proxy for habitat size and found support for all three processes. A drawback of all these examples is that, with the exception of islands, habitat sizes were chosen somewhat arbitrability, and focus was on habitat patches or fragments, rather than habitats that naturally varied in size. Freshwater lakes and ponds are well suited to studies of alpha and beta diversity because they are distinct ecosystems with well-defined boundaries and therefore avoid this issue of fragment size selection (Chase and Myers, Citation2011). Another challenge in studying the influence of drift on ecological communities is extracting the influence of community size, often represented by habitat size (or ‘area per se’) while still accounting for environmental heterogeneity and the associated influence of spatially variable selection (Vellend Citation2016). In their study of small forest mammals, Pardini et al. (Citation2005) addressed this difficulty by analyzing the residuals of regressions of alpha and beta diversity against forest structure variables. The necessity of addressing these gaps is especially interesting in unique and at-risk ecosystems whose community dynamics are largely unexplained. However, this approach has never been applied to aquatic arctic habitats.
Periphytic diatoms are an important primary producer in high latitude lakes due to shallow water depth, short growing seasons and abundant benthic and macrophytic habitat (Squires and Lesack Citation2002; Sokal et al. Citation2008; Wiklund et al. Citation2010). Periphytic diatoms provide an opportunity to test the influence of drift on a biotic community because of their rapid growth cycles, small size and high abundance relative to larger organisms, and well-established taxonomy. Previous work in the Old Crow Flats (Yukon, Canada), a northern lake-rich landscape, found that water chemistry best explained the diatom community assembly deposited in lake sediment when compared to sediment and catchment properties, but only accounted for 17.18% of the variance in community composition (Balasubramaniam et al. Citation2017). Unexplained variance in the diatom community could potentially be attributed to stochastic processes, namely ecological drift.
To investigate the influence of ecological drift, we used the periphytic diatom community composition across 44 lakes in the Old Crow Flats during the open-water season of 2009 to determine the influence of habitat size on alpha diversity, beta diversity and the strength of relations between community composition and environmental variables. Environmental variables included water isotopes to capture hydrological conditions, water chemistry to capture limnological conditions and lake surface area as a measure of habitat size. If drift strongly influences community composition, we hypothesize that (i) alpha diversity will increase with increasing lake size, due to decreasing influence of drift-mediated local extinction, (ii) beta diversity will be greatest in small lakes and lowest in large lakes, due to higher drift-mediated local extinction in smaller lakes, resulting in more heterogeneity of community composition among lakes, and (iii) relationships between community composition and environmental variables will be strongest in large lakes and weakest in small lakes, because large lakes would be more influenced by environmental filtering whereas in smaller lakes this influence would be diminished by drift-mediated local extinctions. To address the concern that lake size and habitat heterogeneity could be confounded, limnological and hydrological conditions at each site were described and their influence was removed before evaluating the influence of lake area on periphyton diversity and the community composition–environment relationship.
Materials and methods
Study area
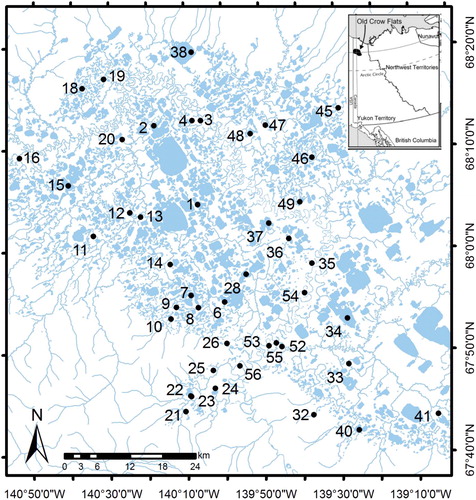
The Old Crow Flats (OCF) in northern Yukon is a 5600 km2 lake- and wetland-rich landscape where local communities have observed hydrologic change in the many shallow lakes of the area (Turner et al. Citation2014; ). Terrestrial vegetation broadly transitions from boreal–taiga to tundra (Yukon Ecoregions Working Group Citation2004). The landscape is underlain by relatively unconsolidated glaciolacustrine sediment and is within a region of continuous permafrost (Yukon Ecoregions Working Group Citation2004). Lakes were chosen for sampling in 2007 based on their cultural significance and to capture the range of environmental characteristics representative of the lakes in the region (Turner et al. Citation2010). For this study we selected data from 44 lakes where the diatom community was sampled (). We then classified lakes as either small, medium or large according to whether they fell within the lower, middle, and upper quantiles of lake surface area, respectively. Small lakes (n = 15) ranged from 0.002 to 0.127 km2, medium lakes (n = 14) ranged from 0.133 to 0.845 km2 and large lakes (n = 15) ranged from 0.963 to 12.60 km2.
Figure 1. Location of study lakes (black circles) in the Old Crow Flats (Yukon, Canada). Numbers indicate lake name for consistency with prior work in the study region (Turner et al. Citation2010).
Study sites and data collection
Water isotopes were collected in July of 2009 from the center of each lake to capture hydrologic conditions, as described in Turner et al. (Citation2010; ). Water quality data was also collected at the same time in July, as described by Balasubramaniam et al. (Citation2015). Briefly, specific conductivity was recorded at ∼30 cm below the water surface using a YSI 600QS multi-meter. Total phosphorus (TP), total nitrogen (TN), nitrite + nitrate (NO2+NO3), ammonia (NH3), silica (SiO2), major ions (Ca2+, Cl−, Mg2+, Na+, K+, SO42−), and pH and alkalinity (Alk), total dissolved phosphorus (TDP), dissolved inorganic carbon (DIC) and dissolved organic carbon (DOC) were analyzed from water samples at Environment Canada’s National Laboratory for Environmental Testing (NLET) in Burlington, Ontario, following standard protocols (Environment Canada Citation1996). Chl-a concentrations were determined using standard fluorescence techniques (Stainton et al. Citation1977).
Periphytic algae were collected using artificial substrates deployed at the onset of the ice-free season (June) and collected in the fall (September) before ice-cover. Artificial substrates have proven effective for periphytic algae collection in shallow, northern lakes (Wiklund et al. Citation2010; MacDonald et al. Citation2012), and provide a standardized substrate for colonization which leads to greater comparability among lakes. Accrual time exerts a significant influence on the composition of diatom communities in the OCF (MacDonald et al. Citation2012) and another benefit of artificial samplers is that they allow for consistent accrual time among sites. Samplers followed a design developed by Wiklund et al. (Citation2010) and consisted of a wooden float (62 cm long × 6.3 cm wide × 1.6 cm thick) holding two sampling units each containing 5 polypropylene sheets 4 cm wide by 14 cm long (Poly Disposables Ltd.) that serve as the artificial substratum. One sampler was deployed per lake, and each sampler was suspended vertically in the water column at 25 cm below the water surface. Upon retrieval, polypropylene sheets were placed individually in opaque canisters and stored frozen. Diatom analysis was performed on one randomly selected sheet. Microscope slides for diatom analysis were prepared by strong acid digestion to remove organic material (Ruhland et al. Citation1999). Diatom slurries were dried onto 18 mm circular coverslips and mounted onto microscope slides using Napthrax. Diatom slides were analyzed using a compound light microscope at 1000× magnification under oil immersion. A count of 300 diatom valves were identified to the lowest taxonomic level possible using the keys from Krammer and Lange-Bertalot (Citation1986–1991).
Data analysis
The number of counted individuals of each diatom taxon was used as an index of relative abundance. To minimize the influence of environmental heterogeneity between lakes, principal components analysis (PCA; Goodall, 1954) was performed on the environmental variables in a correlation matrix using PC-ORD V.7 (MjM Software Design, Gleneden Beach, Oregon, 2017) to identify the main environmental gradients. The correlation matrix was centered and standardized by variable to allow comparison of variables with different units of measurement. All variables except for pH were log-transformed to meet assumptions of multivariate normality. Alpha diversity, calculated as the number of diatom taxa per lake, was then regressed against the eigenvalue-weighted sum of the significant PCA axes, effectively factoring out the influence of hydrological and limnological differences among sites. To test for a difference in mean alpha diversity between the lake sizes after accounting for environmental factors, residuals of this regression from small (n = 15), medium (n = 14) and large (n = 15) lakes were compared with a one-way ANOVA.
Beta diversity was calculated for all pair-wise combinations of sites within each of the three lake size classes. Beta diversity can reflect two different phenomena (e.g., Harrison Citation1999; Baselga Citation2010): nestedness and turnover. Nestedness occurs when sites with a smaller number of species contain subsets of the species occurring in sites with greater diversity (Wright and Reeves Citation1992; Ulrich and Gotelli Citation2007). Turnover occurs when different sites contain distinct sets of species (Qian et al. Citation2004). To explore the influence of these phenomena, beta diversity at each site was partitioned into the two components using the betapart package in R (Baselga and Orme Citation2012; Baselga Citation2013). To minimize the influence of environmental heterogeneity, pairwise Bray-Curtis dissimilarity matrices for overall, nested and turnover beta diversity from small, medium and large lakes were regressed against a pairwise Bray-Curtis dissimilarity matrix of hydrologic and water quality variables, which were log-transformed and relativized by variable maximum. The residuals of this regression were used to test for differences in overall, nested and turnover beta diversity un-related to hydrological and limnological differences among sites. A one-way ANOVA was used to test for differences in each component of residual beta diversity among the three lake-size categories. A Tukey’s pairwise comparison was used when differences among groups were determined to be significant (p < 0.05). Mantel tests between the diatom taxa and the log-transformed environmental variables in each of the small, medium and large lake classes were performed to assess concordance between periphytic diatom community composition and environmental variables, and resulting p-values were compared to assess the strength of the relationship between community composition and the environment within each size class.
Results
In total, 60 diatom taxa were identified among the 44 lakes. The most abundant diatom taxon was Achnanthidium minutissimum (Kützing).
Alpha diversity
To control for environmental factors, alpha diversity was first regressed against significant axes of a PCA of hydrological and limnological variables. The first two axes of the PCA were significant (p < 0.01) and explained 63.1% of the variance in hydrological and limnological variables in the 44 lakes (Appendix 1). To capture the environmental variance, the eigenvalue-weighted sum of both significant axes for each lake was used in subsequent analyses. Alpha diversity was significantly related to the hydrological and limnological conditions captured in the two significant PCA axes (R2 = 0.23, p = 0.001; ). After removing the effects of hydrological and limnological conditions, residual alpha diversity was not significantly related to lake surface area (R2 = 0.02, p = 0.370; ). Mean residual alpha diversity was not significantly different among the three lake size classes (, ). We did not find support for our hypothesis that alpha diversity attributed to area per se increases with increasing lake size due to the decreasing influence of drift-mediated local extinction.
Figure 2. Scatterplots showing the relationships among the 44 lakes of the Old Crow Flats between periphytic diatom community alpha diversity and environmental variables represented by the eigenvalue-weighted sum of PCA axis 1 and 2 scores, summarized by the sum of eigenvalue weighted site scores of the first and second axis of a Principal Component Analysis (PCA), on water isotopes and water chemistry (top panel). Relationship of residual alpha diversity and lake surface area (bottom panel).
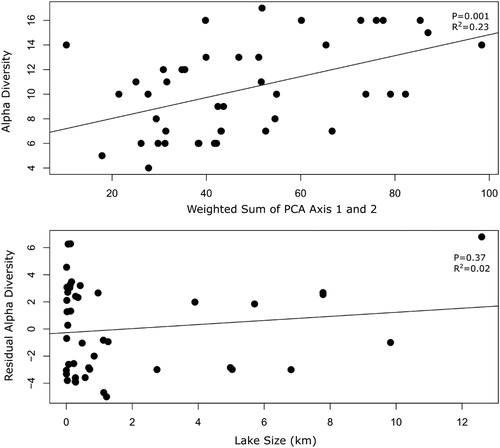
Figure 3. Alpha diversity of diatoms, after statistically controlling for environmental heterogeneity, in small (n = 15), medium (n = 14) and large (n = 15) lake-size categories in the Old Crow Flats (Yukon, Canada). Periphyton samples accrued during the ice-free season in 2009. Error bars represent standard error.
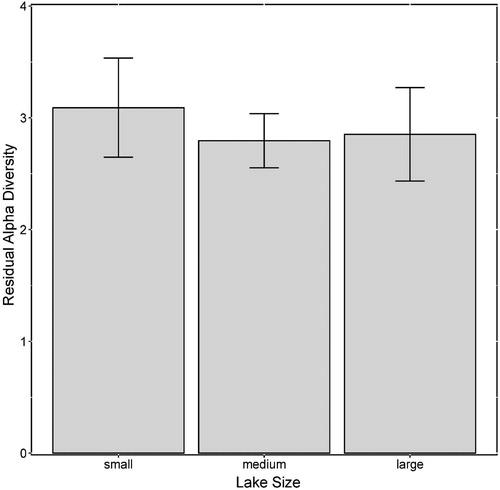
Table 1. ANOVA and Mantel test results comparing alpha diversity, beta diversity and community composition-environment congruence in differently sized lake categories in the Old Crow Flats.
Beta diversity
Within each of the three lake size classes, neither total pairwise beta diversity nor its components were significantly related to pairwise environmental dissimilarity (), in other words lakes with more similar hydrology and water chemistry did not have more similar diatom community composition. Beta diversity and its components were significantly different among the three lake size classes (). Average overall beta diversity was greatest in the small-lakes and decreased with increasing lake size (small = 0.68 ± 0.018 SE, medium = 0.58 ± 0.025 SE, and large = 0.47 ± 0.01 SE; ). Turnover accounted for ∼99.9% of the total beta diversity in small, medium and large lakes ().
Figure 4. Pairwise relationships between beta diversity of diatoms and environmental dissimilarity in small, medium and large lake-categories in the Old Crow Flats (Yukon, Canada). Periphyton samples accrued during the ice-free season in 2009. Beta diversity here is partitioned into its turnover and nested components.
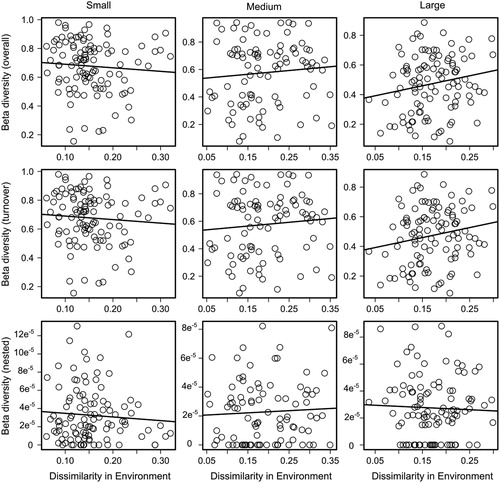
Figure 5. Mean and standard error of total and residual beta diversity of diatoms in small (n = 15), medium (n = 14) and large (n = 15) lake size-categories in the Old Crow Flats (Yukon, Canada). Beta diversity is partitioned into total (dark grey), turnover (light grey) and nestedness (white) components. Error bars represent standard error.
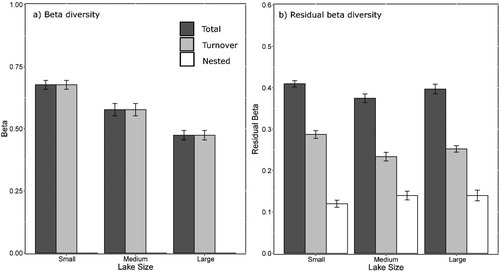
After statistically controlling for the effect of hydrologic and limnologic variation on beta diversity and its components, much of the observed relationship between lake size and diatom beta diversity disappeared (). Residual overall beta diversity was marginally significantly different between the small and medium lakes () with small lakes containing the greatest pairwise residual beta diversity (0.41 ± 0.0076 SE) followed by large lakes (0.40 ± 0.012 SE), and then medium lakes (0.38 ± 0.010 SE). Turnover accounted for 70, 62 and 64% of the residual overall beta diversity in the small, medium and large lakes, respectively, and was significantly different among lake-size classes (, ). Nestedness comprised 30, 38 and 36% of residual overall beta diversity in the small, medium and large lakes, respectively, contributing relatively more to residual overall beta diversity than it did to overall beta diversity. Yet, residual nestedness did not differ significantly among the lake-size classes. The data yields limited support for the hypothesis that beta diversity will be greatest in small lakes and lowest in large lakes after accounting for environmental heterogeneity. We found significantly greater residual total and turnover beta diversity in small lakes, but residual beta diversity and its components did not decrease between the medium and large lake classes and were not consistently lowest in the large lake class.
Environment-diatom community composition concordance
Periphytic diatom community composition and environmental variables were not concordant in any of the lake size classes (). In other words, we found that the between-lake similarity in periphytic diatom community composition was unrelated to between-lake similarity in environmental variables for all three lake-size classes (Mantel r values not significantly different from 0). Thus, we must reject our hypothesis that the relationships between community composition and the environmental variables would be strongest in large lakes and weakest in small lakes.
Discussion
A central question in ecology is how biotic communities assemble in nature. Shallow lakes in the arctic and subarctic provide an opportunity to assess the influence of ecological drift (as opposed to environmental filtering) on periphytic diatom communities. In the Old Crow Flats, previous aquatic community studies determined that spatially variable selection exerts only weak control on diatom communities (Balasubramaniam et al. Citation2017). Our analyses support this conclusion, as we found weak and non-significant concordance between diatom community composition and a comprehensive matrix of hydrologic and water quality parameters, regardless of lake size. This would imply that some other high-level process (sensu Vellend Citation2016) must be driving diatom community assembly in these shallow lakes. Yet, we found little evidence to support our hypothesis that ecological drift was a key determinant of diatom community composition.
Alpha diversity
We predicted that residual alpha diversity would be proportional to lake size, as drift-mediated local extinction would be more prominent in smaller lakes. Contrary to our prediction, residual alpha diversity attributed to area per se was not significantly different among the lake size classes. This differs from previous studies in plants (Gilbert and Levine Citation2017) and small mammals (Pardini et al. Citation2005) that found evidence for ecological drift in smaller communities. A possible explanation is that stabilizing niche differences could have reduced the effect of ecological drift (Chesson Citation2000). Increasing growth rate when at low abundance and decreasing growth at high abundance would reduce the risk of diatom taxon extinction in the smaller lakes. Another possibility is that high dispersal in this landscape offsets drift-mediated extinctions, resulting in constant alpha diversity regardless of lake size (Chase and Myers Citation2011; Vellend Citation2016).
Beta diversity
We predicted that beta diversity would be inversely related to lake size, as greater drift-meditated local extinction in smaller lakes should result in less homogeneity in community composition. In each lake-size class, nestedness contributed a negligible fraction to total beta diversity, with the majority of beta diversity attributed to turnover. Further, there were no trends evident in nestedness, which was low across all lake size classes. Trends in total beta diversity were driven by trends in turnover. Before factoring out the influence of environmental conditions, there was a strong and significant trend of diminishing beta diversity and turnover with increasing lake size. However, once environmental conditions were statistically controlled for (i.e., residual beta diversity) to isolate area effects per se, overall beta diversity and turnover values were reduced and the relative importance of nestedness increased somewhat. Though statistically significant differences remained, with residual overall beta diversity and residual turnover in small lakes being the greatest, the trend of decreasing beta diversity with increasing lake size was weakened to the extent that we find only partial support for the prediction that drift-mediated local extinction in smaller lakes would lead to greater beta diversity in smaller lake even after accounting for any lake-size related differences in environmental heterogeneity. Given that total and residual beta diversity were primarily attributed to turnover (), environmental filtering likely plays a limited role in shaping diatom communities (Chase and Myers Citation2011).
Community composition and environment concordance
Our last prediction was that community composition and environmental variables would be most strongly concordant in large lakes, where the influence of environmental filtering would be less eroded by drift-mediated local extinctions. We found little support for a trend of increasing concordance with increasing lake size, as although concordance values did increase with lake size, they were not significantly different from zero for our three lake-size classes.
Other potential drivers of community composition
The high degree of beta diversity attributed to turnover, even after accounting for environmental conditions, may be the result of processes such as dispersal and priority effects (Fukami et al. Citation2005; Chase and Myers Citation2011). Dispersal between sites may reduce the effect of drift-mediated species loss in small lakes, thereby serving as a buffer that reduces the difference in beta diversity between the small and large communities (Leibold et al. Citation2004; Vellend Citation2016). Diatoms in lotic environments disperse mainly by hydrologic connections, wind or animal vectors such as water birds (Kristiansen Citation1996). Whether diatoms are ubiquitous or dispersal is an important component of their community compositions is still debated (Soininen Citation2007). In a review of studies on diatom communities by Soininen (Citation2007), spatial factors accounted for 20–30% of variation in communities and a greater number of studies found a significant influence of spatial configuration than those that found strict local environmental control. In the Old Crow Flats, spring snowmelt produces a period of high hydrologic connectivity which can be prolonged in lakes with tree and shrub dominated catchments, relative to lakes with sparsely vegetated catchments, due to increased snowmelt resulting from trapped winter snowfall (Turner et al. Citation2010). This may create a gradient of connectivity that could account for the pattern of diatom community composition we observed. While the use of standardized sampling approaches across habitat sizes is consistent with previous work (i.e. Pardini et al. Citation2005), incomplete sampling of all periphytic diatom taxa present in lakes may also influence the observed patterns. However, a recent study by Roden et al. (Citation2018) found incomplete data sets were capable of accurately depicting beta diversity. Unmeasured environmental variables, including length of the photoperiod, lake depth/bathymetry, vegetation community characteristics, or shading might also contribute to differences in diatom community composition observed among our 44 lakes.
Conclusions
Testing for evidence of high-level processes and their relative contributions to community composition provides insight that can inform further research on the specific mechanisms behind observed patterns in species distribution (Vellend Citation2016; Letten et al. Citation2017). This approach also allows for a more synthetic understanding of ecological communities and tests the predictive capabilities of ecological theories. Here we test the hypothesis that ecological drift is an important driver of periphytic diatom community composition in arctic lakes. Despite its predicted importance, we did not detect strong evidence of ecological drift in structuring periphytic diatom communities in the Old Crow Flats.
Environmental filtering must play a role in diatom community assembly, as statistically controlling for variation in hydrology and water chemistry left little variance in alpha and beta diversity among lake-size classes. However, these environmental conditions must not be the main driver of variance in diatom community composition, based on the weak and insignificant concordance between environmental conditions and diatom community composition. There are several other community interactions that likely also influence diatom communities, including dispersal, stabilizing niche differences and priority effects (Leibold et al. Citation2004; Fukami et al. Citation2005; Chase and Myers Citation2011). These other processes merit further study and might account for the observed patterns in alpha diversity, beta diversity and environment-diatom congruence among lake size classes.
Widespread reductions in lake surface area have been documented in discontinuous permafrost zones of Siberia and Alaska (Yoshikawa & Hinzman, Citation2003; Smith et al. Citation2005) as well as in the Canadian Arctic (e.g. Smol and Douglas Citation2007; Carroll et al. Citation2011). Changes in the overall hydrology of northern landscapes may have unexpected consequences for the biotic communities that rely on shallow freshwater ecosystems. Additionally, the use of diatoms as ecological indicators in these landscapes requires a robust understanding of underlying ecological processes affecting community composition. Future studies can further our understanding of the high-level processes that drive diatom community assembly in these changing landscapes, and could help project their trajectories.
Notes on contributors
Casey R. Remmer is a PhD candidate at the University of Waterloo, Canada. She studies the relationship between hydrology and diatoms in northern shallow lakes.
Courtney D. Robichaud is a PhD candidate at the University of Waterloo, Canada. She studies wetland and community ecology, with a focus on the effects of invasive species on community structure.
Heather C. Polowyk is an MSc student at the University of Waterloo, Canada. She studies community ecology, with a focus on species at risk and their interaction with invasive species.
Dr. Rebecca C. Rooney is an Associate Professor at the University of Waterloo, Canada. She is a wetland ecologist who studies the drivers of community assembly, the fate and effects of contaminants, and the influence of disturbance on freshwater ecosystems.
Acknowledgements
We are grateful to Wathiq Jassim Mohammed for providing the diatom count data, and Kevin Turner and Ann-Marie Balasubramaniam for providing the environmental and lake area data. We thank those involved in the field and laboratory during data collection.
Disclosure statement
The authors declare that no personal or institutional conflict of interest has influenced this research.
Additional information
Funding
References
- Balasubramaniam AM, Hall RI, Wolfe BB, Sweetman JN, Wang X. 2015. Source water inputs and catchment characteristics regulate limnological conditions of shallow subarctic lakes (Old Crow Flats, Yukon, Canada). Can J Fish Aquat Sci. 72(7):1058–1072.
- Balasubramaniam AM, Medeiros AS, Turner KW, Hall RI, Wolfe BB. 2017. Biotic responses to multiple aquatic and terrestrial gradients in shallow subarctic lakes (Old Crow Flats, Yukon, Canada). Arctic Sci. 3(2):277–300.
- Baselga A. 2010. Partitioning the turnover and nestedness components of beta diversity. Global Ecol Biogeogr. 19(1):134–143.
- Baselga A, Orme DL. 2012. Betapart: an R package for the study of beta diversity. Methods Ecol Evol. 3(5):808–812.
- Baselga A. 2013. Separating the two components of abundance-based dissimilarity: balanced changes in abundance vs. abundance gradients. Methods Ecol Evol. 4(6):552–557.
- Carroll ML, Townshend JRG, DiMiceli CM, Loboda T, Sohlberg RA. 2011. Shrinking lakes of the Arctic: Spatial relationships and trajectory of change. Geophys Res Lett. 38:L20406.
- Chase JM. 2010. Stochastic community assembly causes higher biodiversity in more productive environments. Science. 328(5984):1388–1391.
- Chase JM, Myers JA. 2011. Disentangling the importance of ecological niches from stochastic processes across scales. Philos Trans R Soc Lond B Biol Sci. 366(1576):2351–2363.
- Chesson P. 2000. Mechanisms of maintenance of species diversity. Annu Rev Ecol Syst. 31(1):343–366.
- Environment Canada. 1996. Manual of analytical methods. The National Laboratory of Environmental Testing. Burlington, ON: Canada Centre for Inland Waters.
- Fukami T, Martijn Bezemer T, Mortimer SR, Putten WH. 2005. Species divergence and trait convergence in experimental plant community assembly. Ecol Lett. 8(12):1283–1290.
- Gilbert B, Levine JM. 2017. Ecological drift and the distribution of species diversity. Proc R Soc B. 284(1855):20170507.
- Harrison S. 1999. Local and regional diversity in a patchy landscape: native, alien, and endemic herbs on Serpentine. Ecology. 80(1):70–80.
- Hubbell SP. 2001. The unified neutral theory of biodiversity and biogeography. Princeton, NJ: Princeton University Press.
- Krammer K, Lange-Bertalot H. 1986–1991. Susswasserflora von Mitteleuropa, Band 2. Stuttgart: Gustav Fischer Verlag.
- Kristiansen J. 1996. Dispersal by freshwater algae a review. Hydrobiologia. 336:151–157.
- Leibold MA, Holyoak M, Mouquet N, Amarasekare P, Chase JM, Hoopes MF, Holt RD, Shurin JB, Law R, Tilman D, et al. 2004. The metacommunity concept: a framework for multi-scale community ecology. Ecol Lett. 7(7):601–613.,
- Letten AD, Ke PJ, Fukami T. 2017. Linking modern coexistence theory and contemporary niche theory. Ecol Monogr. 87(2):161–177.
- MacArthur RA, Wilson EO. 1967. The theory of island biogeography. Princeton, NJ: Princeton University Press.
- MacDonald LA, Balasubramaniam AM, Hall RI, Wolfe BB, Sweetman JN. 2012. Developing biomonitoring protocols for shallow Arctic lakes using diatoms and artificial substrate samplers. Hydrobiologia. 683(1):231–248.
- Pardini R, de Souza SM, Braga-Neto R, Metzger JP. 2005. The role of forest structure, fragment size and corridors in maintaining small mammal abundance and diversity in an Atlantic forest landscape. Biol Conserv. 124(2):253–266.
- Qian H, Ricklefs RE, White PS. 2004. Beta diversity of angiosperms in temperate floras of eastern Asia and eastern North America. Ecol Lett. 8(1):15–22.
- Ricklefs RE, Lovette IJ. 1999. The roles of island area per se and habitat diversity in the species area relationships of four Lesser Antillean faunal groups. J Anim Ecol. 68(6):1142–1160.
- Roden JV, Kocsis AT, Zuschin M, Kiessling W. 2018. Reliable estimates of beta diversity with incomplete sampling. Ecology. 99(5):1051–1062.
- Ruhland K, Karst T, Paterson A, Gregory-Eaves R, Smol JP, Cumming BF. 1999. Standard Sediment Sample Preparation Methods for Siliceous Microfossils (Diatoms and Chrysophyte Scales and Cysts). PEARL: Paleoecological Environmental Assessment and Research Laboratory, Department of Biology Queens University.
- Smith L, Sheng Y, MacDonald G, Hinzman L. 2005. Disappearing arctic lakes. Science. 308(5727):1429.
- Smol JP, Douglas MSV. 2007. Crossing the final ecological threshold in high Arctic ponds. Proc Natl Acad Sci USA. 104(30):12395–12397.
- Soininen J. 2007. Environmental and spatial control of freshwater diatoms—a review. Diatom Res. 22(2):473–490.
- Sokal MA, Hall RI, Wolfe BB. 2008. Relationships between hydrological and limnological conditions in lakes of the Slave River Delta (NWT, Canada) and quantification of their roles on sedimentary diatom assemblages. J Paleolimnol. 39(4):533–550.
- Squires MM, Lesack LFW. 2002. Water transparency and nutrients as controls on phytoplankton along a flood-frequency gradient among lakes of the Mackenzie Delta, western Canadian Arctic. Can J Fish Aquat Sci. 1349:1339–1349.
- Stainton MP, Capel MJ, Armstrong FAJ. 1977. The chemical analysis of freshwater. 2nd edition. Canadian Fisheries and Marine Service Miscellaneous Special Publication 25.
- Sydenham MAK, Moe SR, Kuhlmann M, Potts SG, Roberts SPM, Totland Ø, Eldegard K. 2017. Disentangling the contributions of dispersal limitation, ecological drift, and ecological filtering to wild bee community assembly. Ecosphere. 8:01650.
- Turner KW, Wolfe BB, Edwards TWD. 2010. Characterizing the role of hydrological processes on lake water balances in the Old Crow Flats, Yukon Territory, Canada, using water isotope tracers. J Hydrol. 386(1-4):103–117.
- Turner KW, Wolfe BB, Edwards TWD, Lantz TC, Hall RI, Larocque G. 2014. Controls on water balance of shallow thermokarst lakes and their relations with catchment characteristics: a multi-year, landscape-scale assessment based on water isotope tracers and remote sensing in Old Crow Flats, Yukon (Canada). Glob Change Biol. 20(5):1585–1603.
- Ulrich W, Gotelli NJ. 2007. Null model analysis of species nestedness patterns. Ecology. 88(7):1824–1831.
- Vellend M. 2010. Conceptual synthesis in community ecology. Q Rev Biol. 85(2):183–206.
- Vellend M. 2016. The theory of ecological communities. Princeton, UK: Princeton University Press.
- Wiklund JA, Bozinovski N, Hall RI, Wolfe BB. 2010. Epiphytic diatoms as flood indicators. J Paleolimnol. 44(1):25–42.
- Willis KJ, Whittaker RJ. 2002. Species diversity-scale matters. Science. 295(5558):1245–1248.
- Wright DH, Reeves JH. 1992. On the meaning and measurement of nestedness of species assemblages. Oecologia. 92(3):416–428.
- Yoshikawa K, Hinzman L. 2003. Shrinking thermokarst ponds and groundwater dynamics in discontinuous permafrost near Council, Alaska. Permafrost Periglac Process. 14(2):151–160.
- Yukon Ecoregions Working Group 2004. Old Crow basin. In C.A.S. Smith, J.C. Meikle, and C.F. Roots, editors. Ecoregions of the Yukon Territory: biophysical proper- ties of Yukon landscapes. PARC Technical Bullentin No. 04-01. Summerland, B.C: Agriculture and Agri-Food Canada; pp. 115–122.
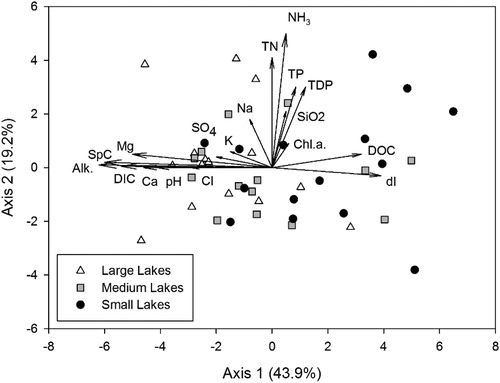
Appendix 1: Principal Components Analysis
Principal Components Analysis (PCA) on hydrologic and limnologic data resulted in two significant axes (p < 0.01) that cumulatively explained 63.1% of the variance in hydrology and water chemistry among the 44 lakes. Axis significance was determined running 999 randomizations and the p-value was calculated as the number of randomizations with an eigenvalue for that axis that was equal to or larger than the observed eigenvalue for that axis plus one divided by the total number of randomizations plus one. The proportion of variance represented in PCA ordination axis one was 43.9%, while axis two independently represented 19.2% of the variance.
Axis one is negatively correlated with pH (eigenvector = −0.78), Alkalinity (eigenvector = −0.95), DIC (eigenvector = −0.93), Specific conductivity (eigenvector = −0.95), Sulphate (eigenvector = −0.71), Cl (eigenvector = −0.64), Ca (eigenvector = −0.88), Mg (eigenvector = −0.91), K (eigenvector = −0.44), Na (eigenvector = −0.52) and positively correlated with DOC (eigenvector = 0.71) and isotopic composition of input water (eigenvector = 0.78). Axis two is positively correlated with dissolved silica (eigenvector = 0.58), NH3 (eigenvector = 0.82), TN (eigenvector = 0.76), TDP (eigenvector = 0.71), TP (eigenvector = 0.82) and Chl.a (eigenvector = 0.40).
Based on the dispersion of lake-size classes, there is no greater hydrologic and chemical heterogeneity within the large-lake class than within the small-lake class. There is substantial overlap among lake size classes in ordination space, particularly along axis 2. However, there is a slight difference in the range of axis 1 scores of small- and large-lake classes, whereby small lakes may have higher DOC and isotopic composition of input water (dI) and large lakes may have higher DIC, alkalinity, specific conductance, Mg, Ca, SO4, and Cl.