Abstract
Fluoxetine, a selective serotonin reuptake inhibitor (SSRI), is frequently detected in surface waters globally, yet the effects of SSRIs on ecological processes at environmentally realistic concentrations are not currently known. We used a controlled, replicated artificial stream experiment to expose biofilm, algal and stream insect communities to two different concentrations of fluoxetine: 20 ng/L (typical concentration detected in surface waters) and 20 µg/L (concentration shown to influence insect emergence and algal productivity). We quantified a range of community and ecosystem response metrics over the course of the 21d experiment including; algal biomass (chl-a), net ecosystem production (NEP), gross primary production (GPP), ecosystem respiration (ER) and invertebrate emergence. At 20 ng/L, fluoxetine significantly suppressed algal colonization on rocks, and reduced GPP after 13 days, but by day 21 chl-a, NEP and GPP did not differ between treatments and control. Fluoxetine increased ER on leaves where invertebrates were excluded, but had no effect on leaves accessible to invertebrates. Streams receiving 20 ng/L of fluoxetine had adult insects from the order Diptera emerge sooner and at a greater rate than control streams. Our results suggest that ecosystem function, including primary production and respiration, and invertebrate population dynamics are sensitive to SSRIs and that fluoxetine may alter these key processes concentrations found in the environment.
Introduction
Pharmaceuticals are ubiquitous in aquatic ecosystems worldwide and are now identified as environmental contaminants of concern (Kolpin et al. Citation2002; Monteiro and Boxall Citation2010; Boxall et al. Citation2012; Richmond et al. Citation2017). Pharmaceuticals are dispersed into the aquatic environment largely via waste water sources and typically at low concentrations (ng/L to µg/L) (Daughton and Ternes Citation1999; Boxall et al. Citation2012; Rosi-Marshall and Royer Citation2012). Although many pharmaceuticals are broken down by a variety of processes including microbially induced decomposition and UV degradation, their constant resupply from sources such as wastewater treatment plants makes them ‘pseudo-persistent’ (Daughton Citation2003). Despite the ubiquity of pharmaceuticals, their effects at levels typically present in the environment on aquatic biota and ecosystem processes remains largely unknown (Rosi-Marshall and Royer Citation2012; Richmond et al. Citation2017).
Antidepressants are a globally prescribed suite of pharmaceuticals commonly detected in surface waters and include selective serotonin reuptake inhibitors (SSRIs), which act in humans by increasing levels of brain serotonin, a natural neurotransmitter responsible for behavior and mood (Brooks et al. Citation2005; Styrishave et al. Citation2011). Since its introduction in 1987, the SSRI fluoxetine, trade name Prozac, has been among the most prescribed antidepressants worldwide (Wong et al. Citation1995). Whilst many studies have focused on the toxicological effects of fluoxetine on model aquatic organisms (Brooks et al. Citation2003), and how fluoxetine alters organismal behavior (Ford and Fong Citation2015; Pelli and Connaughton Citation2015; Martin et al. Citation2017) just how fluoxetine influences stream communities and key ecosystem functions including primary production and respiration at these low concentrations remains largely unknown.
Benthic biofilms are critical to aquatic environments, contributing to the heterotrophic and autotrophic function of aquatic ecosystems, and are an important basal resource in many stream food webs (Minshall Citation1978). Gross primary production (GPP) within biofilms occurs with a range of concurrent heterotrophic processes including ecosystem respiration (ER), facilitating a variety of biogeochemical transformations (Cummins Citation1974). GPP has been shown to be inhibited by non-monotonic exposure to antidepressants (fluoxetine and citalopram at 20 µg/L, Richmond et al. Citation2016) and other pharmaceuticals including diphenhydramine, a common antihistamine (Rosi-Marshall et al. Citation2013) and amphetamines (Lee et al. Citation2016). Similarly, ER has been shown to be suppressed by caffeine, diphenhydramine, cimetidine and ciprofloxacin (Rosi-Marshall et al. Citation2013), amphetamines (Lee et al. Citation2016) and antidepressants (Richmond et al. Citation2016). Pharmaceuticals can also cause non-lethal adverse effects and changes in biofilm community composition (Drury et al. Citation2013; Lee et al. Citation2016). Pharmacology studies have provided evidence that fluoxetine and other SSRIs have antimicrobial abilities, including antibacterial activity against gram positive bacteria (Kalaycı et al. Citation2014).
In addition to autochthonous GPP, allochthonous inputs of organic matter from riparian landscapes are a key contributor to the energy pool in many streams (Fisher and Likens Citation1973; Tank et al. Citation2010) and the consumption and decomposition of organic matter by detritivores, bacteria and fungi are key ecosystem processes in freshwater ecosystems (Hieber and Gessner Citation2002). Some contaminants, specifically pesticides and heavy metals, can adversely affect the diversity of stream detritivores and alter decomposition rates (Gessner et al. Citation2010). However, decomposition studies involving pharmaceuticals are limited, and it is not clear whether there are any impacts at concentrations most commonly detected, for example leaf decomposition was not affected by addition of the antihistamine fexofenadine, but this compound did influence mediated carbon and nitrogen cycling (Jonsson et al. Citation2015). Fluoxetine is known to have a relatively high absorption capacity to soil and organic matter (Kwon and Armbrust Citation2008), making stream organic matter a potentially contaminated food source for stream insects and microbes.
Exposure of stream insects to pharmaceuticals may result in disruption to insect-mediated processes. As regulators of nutrient cycling, aquatic insects serve as an important food resource for higher trophic levels both in and out of the streams (e.g. fishes and riparian spiders or birds, respectively). Aquatic insects are the main mediator of energy flow through stream food webs and from streams into adjacent terrestrial environments via insect emergence (Baxter et al. Citation2005). Anthropogenic pollution can affect insect emergence. For example, heavy metal exposure (Schmidt et al. Citation2013), the herbicide atrazine (Dewey Citation1986) and the pesticide diazinon (Arthur et al. Citation1983) are all associated with reduced emergence. Conversely when exposed to pharmaceuticals, insect emergence has been observed to increase when exposed to amphetamines (1 µg/L, Lee et al. Citation2016), and antidepressants (fluoxetine and citalopram at 20 µg/L, Richmond et al. Citation2016).
In this study, we investigated the effects of environmentally realistic concentrations of the common antidepressant, fluoxetine, on a range of stream community and ecosystem processes: GPP, ER, net ecosystem production (NEP), microbial functional composition and organic matter decomposition, algal biomass, stream insect community composition and emergence. We conducted an experiment using two concentrations: 20 ng/L, the median background concentration of fluoxetine detected in surface waters of the US (Silva et al. Citation2015), and 20 µg/L which has been shown previously to have ecological effects (Richmond et al. Citation2016). There has been an increased number of studies reporting the sub lethal effects of fluoxetine on single aquatic taxa at low concentrations (e.g. Ford and Fong Citation2015; Martin et al. Citation2017), and a call for more research observing nontoxic endpoints (Richmond et al. Citation2017), therefore, we predicted that we would observe changes in ecosystem processes at both concentrations, and that these changes would follow traditional monotonic dose responses, with a greater effect observed at 20 µg/L than at 20 ng/L.
Materials and methods
Leaf pack construction and deployment
Experiments were carried out in the artificial stream facility at the Cary Institute of Ecosystem Studies, Millbrook NY. We used red maple (Acer rubrum) for leaf pack colonization as it has a moderate breakdown rate (k = 0.005–0.010 day−1) (Webster and Benfield Citation1986) and was found adjacent to East Branch Wappinger Creek, Dutchess County, NY (4th order woodland stream). We collected leaves in October 2014 following abscission and allowed them to air-dry naturally for 14 days. We packaged 10 g of leaf material into leaf packs in two ways. The first used 15 × 20 cm large 1 cm polypropylene mesh bags allowing colonization of stream invertebrates (hereafter referred to as ‘colonized’). The second group of leaf material was packaged into 10 × 12 cm organic muslin cotton bags, preventing colonization by stream invertebrates (referred to hereafter as ‘no-invertebrates’). We tethered leaf packs to steel rebar in East Branch Wappinger Creek in close proximity to the artificial stream facility, and incubated leaf packs in situ for 14 days to allow for microbial, fungal and invertebrate colonization. East Branch Wappinger Creek was chosen due to its proximity, good biotic metrics of instream health and because it has very limited sources of pharmaceutical contamination (Richmond et al. Citation2016).
Artificial stream and invertebrate colonization
We conducted our experiment over 21 days in summer 2015. We filled 15 composite fiberglass artificial streams (4 m × 15.5 cm × 15 cm) with 60 L of low nutrient ground water (NH4+, PO43−, TP below detection <0.02 mg/L, NO3− and TN <0.04 mg/L) without any pharmaceutical contaminants. The water in the streams was re-circulated by stainless steel paddle wheels and maintained at a constant velocity of 0.238 m s−1, powered by Dayton DC gear motors and a speed controller (Dayton Co, Niles, Illinois).
To colonize streams with macroinvertebrates, bacteria and fungi we used methods similar to those detailed in Richmond et al. (Citation2016). We transferred both types of leaf packs into the artificial streams. We transferred six ‘no invertebrate’ leaf packs, taking care to ensure no invertebrates were attached to the cotton packs, and six ‘colonized’ leaf packs (with invertebrates) to the streams. In order to ensure retention of all invertebrates in colonized leaf packs, we carefully collected those leaf packs in 20 L buckets in groups of six. The contents of each bucket were then passed through a 125 μm mesh sieve to retain invertebrates but exclude fine particles which may make streams turbid. The contents of the sieve, along with the six colonized leaf packs were then placed into artificial streams, ensuring each stream was treated equally. We distributed the leaf packs (6 of each type per artificial stream) evenly within the stream, tethering alternating no invertebrate and colonized leaf packs. We added 1 L of clean quartz rock substrate (size distribution 10–15 cm across largest axis) (Maryland River Rock, Ayres Supply, Inc., Pennsylvania) to each stream as habitat and substrate for algal growth. We used quartz rock because it does not react with organic chemicals (e.g. PPCPs). All rocks were placed in haphazard clusters, to mimic natural riffle – run benthic conditions. Each stream was covered with fine mesh netting (size 0.25 mm) to exclude colonizing invertebrates from outside the stream and to capture emerging adults. Bacteria, fungi and invertebrate communities were then left to acclimate for 24 h prior to dosing with fluoxetine.
Experimental design
We randomly divided the 15 artificial streams into 3 experimental treatments: control, 20 ng/L fluoxetine and 20 µg/L fluoxetine, with 5 replicates per treatment. We created a concentrated stock solution of fluoxetine by adding 0.1341 g of fluoxetine HCl (AK Scientific, Union City, California) to 1 L of ultra-pure water. From this concentrated stock solution, we performed a 1:1000 dilution to make a second, dilute stock solution. For each replicate, we added 10 mL of either the concentrated stock solution (20 µg/L treatment), the dilute stock solution (20 ng/L treatment) or ultra-pure water only (control treatment) to raise fluoxetine concentrations to the respective levels. We calculated how much fluoxetine HCl to add to the concentrated solution based upon our target concentrations and standard concentration-volume calculations. To mimic waste water treatment plant (WWTP) discharges, we dosed streams every second day with a goal to emulate the pseudo-persistence nature of pharmaceuticals in environmentally relevant conditions. To determine fluoxetine concentrations from streams we also collected whole water samples in 250 mL glass amber jars at day 21.
Ecosystem and community responses
We collected water samples weekly in 60 mL Nalgene™ bottles for analysis of ammonium (NH4+), nitrate (NO3−) and phosphate (PO43−). We also collected 20 mL water samples in glass septum vials for dissolved organic carbon (DOC) analysis. DOC samples were filtered through Whatman™ glass fiber filters (pore size: 1.5 µm) and acidified with 100 µL of 0.5 M sulfuric acid (H2SO4). Water chemistry samples were analyzed by the Cary Institute of Ecosystem Studies Analytical Laboratory (Millbrook, NY 12545, USA) using flow injection analysis (FIA). To determine fluoxetine concentrations from streams we also collected whole water samples in 250 mL glass amber jars at day 21. Samples were frozen and sent to the Nebraska Water Center at the University of Nebraska for extraction using liquid chromatography tandem mass spectrometry (LC-MS/MS); the method detection limit for fluoxetine was 0.5 ng/L. Samples were analyzed using a direct injection method, which eliminated any associated effects of low recovery, using the instrument detection limit of 0.100 pg/µL as a basis for determining the lowest levels of fluoxetine detection (for further information on methodology and method validation, see Lee et al. Citation2016).
We measured ER using one leaf pack of each type (colonized and no invertebrates leaf packs) on day 13 and at the end of the 21-day experiment using dark bottle incubations (adapted from Hill et al. Citation2002). We carefully opened each leaf pack and transferred a consistent amount of leaf matter into a gas-tight glass jar. We filled jars containing leaf material with water from the respective treatment and in addition to leaf material, we filled a blank jar with only stream water from each replicate. We recorded initial dissolved oxygen (DO) concentrations and temperature of each jar using an ODO probe (model 550, Yellow Springs Instruments, OH). We replaced any water lost during initial DO measurement, and recapped jars, taking care to ensure all oxygen bubbles were eliminated, finally placing jars back in their corresponding treatment and covering them with an opaque plastic bag to ensure darkness. We incubated jars in the dark for 3 h and recorded a final DO concentration. We also measured ER and GPP of the quartz rocks that we added to the streams for substrate complexity, as these rocks were colonized by biofilms throughout the experiment. We measured ER and GPP using the same light-dark incubation approach (GPP incubations were done in full sunlight). Due to sampling error, one replicate from the µg/L treatment was lost during GPP measurements at day 13, and we overestimated incubation time for ER resulting in very low changes of O2, thus have also reported NEP (the change in O2 in sunlight). To estimate whole-stream metabolism, we deployed 6 combination DO and temperature probes (miniDOT, Precision Measurement Engineering, CA), in two streams per treatment. In order to extrapolate DO measurements for other streams, spot measurements were taken over a 24-h period on 4 occasions during the experiment. We used the Bayesian Single-station Estimation (BASE) to estimate whole-stream GPP and ER (Grace et al. Citation2015).
After incubations, we collected leaf matter from each jar and quantified ash-free dry mass (AFDM) by combustion of leaf matter for 12 h at 550 °C. We scrubbed all biofilms from rocks, and filtered subsamples through separate 0.7 µm glass fiber filters (Whatman). One filter was used to measure chlorophyll-a (Chl-a) using methanol extraction (Wetzel and Likens Citation1991) and the other filter was used to measure AFDM by loss-on-combustion (Steinman et al. Citation1996). We then took pictures of each rock with a ruler for scale and quantified the surface area of each rock using Image J digital software (Rasband Citation1997). We scaled GPP and ER per rock surface area (mg O2 cm−2 h−1) and per AFDM (mg O2 g−1 AFDM−1 h−1). At the end of the artificial stream experiment we also calculated individual whole-stream Chl-a and AFDM by scrubbing all surfaces and substrates and processing a filtered subsample for Chl-a and AFDM as described above.
To examine the effect of fluoxetine treatments on microbial functional composition, we collected 6 leaf discs (3.8 cm2) per replicate stream (3 from each pack type) on day 13 and day 21, placing each leaf disc in an individual foil pouch with a small wad of wet tissue to provide moisture. Leaf discs were then transported in a cooler on ice to the University of Notre Dame where they were analyzed within 24 h of being sent for community-level functional profiling using a Biolog® EcoPlateTM (BIOLOG, Inc., CA, USA), a tool for rapid assessment of microbial community functional composition (i.e. metabolic potential or respiration). Leaf discs were shaken in a mild saline solution to suspend microbial communities and centrifuged. The supernatant was diluted and pipetted into well plates. Each well plate provides 3 replicates of 31 carbon sources that are respired by microbial communities, subsequently inducing color development via the redox dye tetrazolium violet (Garland and Mills Citation1991). Inoculated well plates were maintained at 20 °C and color development, measured by absorbance, was analyzed every 12 hours for 3 days at 590 nm (Spectra Max M2, Molecular Devices, CA, US). We calculated average well color development (AWCD) as the absorbance of a well with a designated carbon source minus the absorbance of a control well (i.e. no carbon source) divided by the number of wells with designated carbon sources; this approach corrects color development in each well to account for any differences in cell density (Garland Citation1997). AWCD provides a quantitative metric that has been used successfully to examine spatial and temporal patterns of microbial function (Garland Citation1997), as well as microbial response to contaminants like heavy metals (Ellis et al. Citation2001) and hydrocarbons (Bundy et al. Citation2004) and has previously been used to assess the effects of PPCPs in soil communities (Duan et al. Citation2018).
We collected emerged individuals from each stream replicate every 24 h using a modified vacuum (Craftsman Model No. 315.115710). Individuals were placed in 70% ethanol for identification and biomass measurements. We placed emerged adults from each stream on small petri-dishes in a desiccator for 24 h, we then weighed each sample to estimate biomass. We collected all invertebrates from each stream at the end of the 21d experiment. We carefully brushed all invertebrates from rocks, leaf packs and stream surfaces into the stream water. We then sieved the contents of each stream through a 250 µm mesh sieve. Macroinvertebrates were then preserved in 70% ethanol for enumeration and identification. All individuals were identified to family level, and to genus level where possible (Merritt and Cummins Citation1996; Wiggins Citation1996). As Hydropsychids were abundant in all streams, we also measured the length of Hydropyschids using Image J, to apply length-mass regressions specific to Hydropsychidae (a = 0.0046, b = 2.926) (Benke et al. Citation1999) to estimate biomass.
We collected all leaf packs remaining at the end of the experiment, and emptied the contents of the packs into paper bags. Leaf matter was then left to air dry in the greenhouse for 3 weeks before being weighed to determine percentage mass loss for each stream and treatment.
Statistical analysis
We conducted all statistical analysis in R (version 3.11; R Project for Statistical Computing, Vienna, Austria), with the exception of community analysis. To analyze the effects of fluoxetine on biofilm GPP, NEP, ER, AFDM and chlorophyll a, insect biomass and leaf mass we used a 2-way analysis of variance (ANOVA), with fluoxetine treatment and sample day as the two main factors. To meet the assumptions of ANOVA (normality and heteroscedasticity) we log(x) transformed all data, except for ER on rock biofilms and leaves colonized and with no-insects. If a 2-way ANOVA produced a significant result we used a Tukey’s post hoc test for multiple comparisons. To measure effects of fluoxetine treatments on whole-stream GPP through time (mg O2 m−2 h−1), whole stream ER (mg O2 m−2 h−1), number of emerged adult dipterans and dipteran mass we used a linear mixed effect (LME) model, using the restricted maximum likelihood method and a continuous autoregressive temporal structure to account for day as the repeated measure. This model was run using the nlme package in R with the lme() function (R Core Team Citation2014; Pinheiro et al. Citation2009). To test for differences in invertebrate community composition and microbial community activity (EcoPlatesTM) we used analysis of similarity (ANOSIM; PRIMER, version 6 (Clarke and Gorley Citation2006)). We also conducted multiple one way ANOVAs to examine abundance differences between treatments in the top 10 most abundant invertebrate taxon.
We acknowledge that the nature of artificial stream experiments restricts our statistical power due to low replication (n = 5). For example to obtain a statistical power of 80% with a moderate effect size where α = 0.05, would require a sample size in excess of n = 39 (Champely Citation2007). Therefore, low statistical power could not be avoided. To address this issue, we report statistical significance as p < 0.1, where statistical power is 64% (Lee et al. Citation2016), and we also provide exact p-values throughout the results to allow the reader to infer conclusions on effects of specific treatments. We set the critical value to α = 0.1 due to our limited replication and statistical power, and we were more willing to make a type I error than a type II error due to the novelty of our experiment.
Results
Water chemistry and fluoxetine concentrations
DOC measured at day 13 and day 21 did not differ among treatments (F2,12 = 0.085, p = 0.919), nor was there a difference in DOC concentrations between sampling times (day 13 and day 21) and treatment (treatment × time interaction: F2,12 = 0.657, p = 0.536). Concentrations of NO3− and PO43− did not differ statistically between treatments on day 1 (F2,12 = 0.302, p = 0.745 and F2,12 = 0.802, p = 0.471); however, concentrations in all streams were below detection limits (NO3− <0.02, PO43− <0.002) at day 14 and day 21. NH4+ concentrations at all sampling points were below detection limits (<0.02 mg/L). Fluoxetine concentrations measured at the conclusion of the experiment (one day after final amendment with fluoxetine) were below the method detection limit (0.5 ng/L) in 20 ng/L treated streams, and at 0.989 µg/L in 20 µg/L treated streams.
Biofilm response to fluoxetine
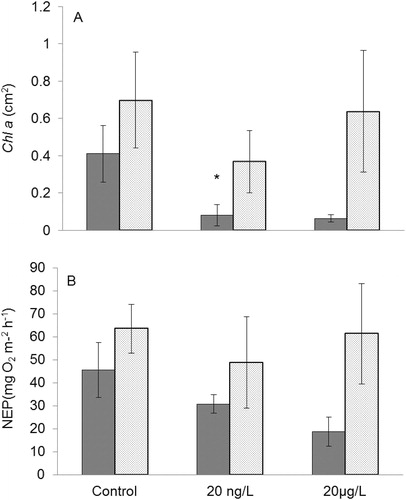
Fluoxetine had a significant effect on Chl-a (µg/cm2) measured from biofilms that had established on quartz stream rocks (F2,24 = 3.749, p = 0.038). Chl-a was lower in 20 ng/L treatments relative to control streams (Tukey post hoc, p = 0.0313, ), but there was no statistically significant difference in 20 µg/L treatments compared to control (p = 0.238). Chl-a also increased significantly between measurements (day 13 and day 21) (F1,24 = 8.419, p = 0.008), but no treatment × day interaction was observed (day 13 or day 21). When examining percentage change, Chl-a was suppressed by 84% in 20 ng/L treatments and non-significantly by 85% in 20 µg/L when comparing mean concentrations at day 13, this non statistically significant trend continued when comparing means at day 21; 20 ng/L suppressed Chl-a by 47%, but only by 9% in 20 µg/L treatments. Fluoxetine did not significantly alter NEP between day 13 and day 21 (mg O2 m2 h−1) measured on quartz rock biofilms (F2,23 = 1.25, p = 0.305, ), nor was there a treatment × day interaction (F2,23 = 0.789, p = 0.466). We observed a significant treatment effect on GPP (mg O2 m2 h−1) (F2,23 = 2.890, p = 0.076) and GPP in 20 ng/L treated streams was significantly lower than control streams (Tukey post hoc: p = 0.073); however, GPP was not significantly different between 20 µg/L and control treatments (Tukey post hoc: p = 0.827), and we observed no significant treatment × day interaction (F2,23 = 0.046, p = 0.955). When standardized by AFDM, GPP (mg O2 g−1 AFDM h−1) did not differ between treatment and control (F2,23 = 1.346, p = 0.280), although there was a significant increase in GPP from day 13 to day 21 across all treatments (F2,23 = 4.171, p = 0.053) and we observed no treatment × day interaction (F2,23 = 0.264, p = 0.771).
Figure 1. (A) Mean (±1 SE) biofilm chlorophyll a (chl a) at day 13 (dark gray bars) and day 21 (light gray bars), (B) mean (±1 SE) biofilm net ecosystem production (NEP) at day 13 (dark gray bars) and day 21 (light gray bars). Asterisks (*) indicate means that differ statistically between treatment and control (p < 0.1).
Fluoxetine did not significantly alter rates of ER on biofilm covered quartz rocks when standardized by area (mg O2 mg−1 m2 d−1) (F2,24 = 0.672, p = 0.52), nor was there a treatment × day interaction (F2,24 = 0.637, p = 0.538). We also observed no treatment effect when standardizing ER by AFDM (mg O2 mg−1 AFDM d−1) (F2,24 = 0.395, p = 0.678), nor was there are treatment × day interaction (F2,24 = 0.201, p = 0.819).
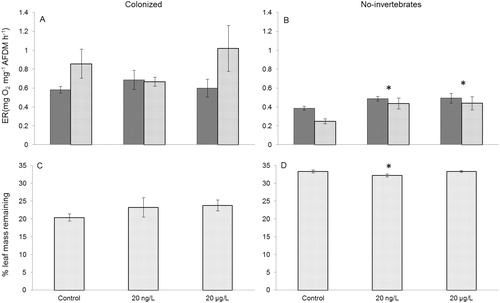
There was no fluoxetine effect on colonized leaf-pack ER (F2,24 = 1.394, p = 0.267, ); however, fluoxetine treatments significantly increased ER on no-invertebrate leaf biofilms (F2,24 = 6.964, p = 0.004, , Tukey post hoc comparisons p < 0.05 for both treatments). ER on leaves also significantly increased over time in all streams regardless of treatment (F2,24 = 4.583, p = 0.043) but there was no significant treatment × day interaction (F2,24 = 0.589, p = 0.562). Percentage of leaf mass remaining after day 21 differed between fluoxetine treated streams and controls for no-invertebrate leaf packs (F2,12 = 2.942, p = 0.0913, ). Leaf mass remaining was significantly lower in the 20 ng/L treatment compared to the control, (Tukey post hoc: p = 0.057), but not significantly different in 20 µg/L treatment compared to control (p = 0.1). No differences were observed for colonized leaf pack percentage mass remaining (F2,12 = 0.894, p = 0.435, ).
Figure 2. (A) Mean (±1 SE) ecosystem respiration (ER) on leaves at day 13 (dark gray bars) and day 21 (light gray bars) with invertebrates (B) and without invertebrates on day 13 (dark gray bars) and day 21 (light gray bars) (C), percentage leaf mass remaining (±1 SE) at day 21 with invertebrates (D) and without invertebrates (B). Asterisks (*) indicate means that differ statistically between treatment and control (p < 0.1).
At the end of the experiment, there was no statistical difference for whole-stream Chl-a concentrations (F2,12 = 1.152, p = 0.351). Total stream AFDM did not differ between fluoxetine treated streams and control streams (F2,12 = 2.699, p = 0.108). Continuous measurements of stream GPP did not differ between streams treated with fluoxetine and control streams (LME: F3,113 = 0.202, p = 0.827), and whole stream ER also was not different among streams (LME: F3,113 = 1.208, p = 0.412).
Response of microbial community functional composition
Color development occurred in each BIOLOG plate over time confirming carbon source utilization as an indication of microbial respiration; however, there were no differences among treatments. Additionally, the number of wells with color development (i.e. positive responses) remained consistent across all treatments and time points. At day 13, dissimilarity in microorganism community activity differed between each fluoxetine treatments and controls on both no-invertebrate leaves (ANOSIM, global R = 0.045, p = 0.08) and colonized leaves (ANOSIM, global R = 0.049, p = 0.07). However global R is small, suggesting that these differences may be small. At day 21, results suggest differences between 20 ng/L and control streams (pairwise: R = 0.231, p = 0.001) and 20 ng/L and 20 µg/L streams (pairwise: R = 0.0126, p = 0.007) in microbial functional composition on no-invertebrate leaves (ANOSIM, global R = 0.109, p = 0.002), however, again, global R is still near 0, therefore differences may be small, as is the case with observed differences in microorganism community activity detected on colonized leaves between fluoxetine treatments and control (ANOSIM, global R = 0.047, p = 0.055).
Invertebrate community response to fluoxetine
At the conclusion of the experiment, there was no difference in stream invertebrate community composition between treated streams (20 ng/L of fluoxetine, 20 µg/L of fluoxetine) or control streams (), this was further confirmed by conducting an analysis of similarities (ANOSIM, global R = −0.008, p = 0.475) nor was there a difference in diversity (Shannon–Wiener: F2,12 = 0.247, p = 0.785) or average taxon richness (F2,12 = 0.363, p = 0.703). However, the number of individuals of Leptocerridae mayflies differed between fluoxetine treated streams and control, streams (F2,10 = 3.04, p = 0.093). Leptoceriidae numbers were higher in both fluoxetine treatments (20 ng/L p = 0.042, 20 µg/L p = 0.065). There was no difference in total Hydropsychidae mass (mg) (F2,12 = 0.084, p = 0.92), nor was there a statistical difference in mean individual mass (F2,12 = 1.785, p = 0.21).
Table 1. Mean (±1SE) number of the 10 most abundant aquatic insects per treatment (in order of decreasing abundance of insects in control streams) and results of 1-way analysis of variance (ANOVA).
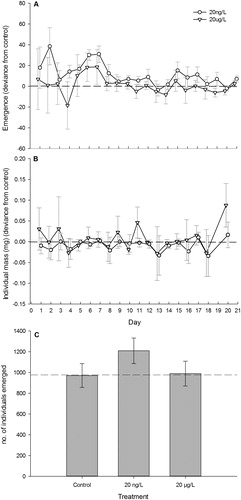
The average number of individuals emerged over the duration of the experiment was not statistically different among treatments (LME: F2,12 = 0.499, p = 0.619, ). Daily total stream biomass (g) of emerged dipterans in treated streams was not significantly different from the control streams (LME: F2,12 = 0.3796, p = 0.692, ). Average cumulative total emergence mass (g) was also not significantly different between treatment and control (F2,12 = 0.105, p = 0.901).The average mass of individuals (mg) emerged over the duration of the experiment was also not statistically different (LME: F2,12 = 0.0312, p = 0.969) Finally, there was no significant difference in cumulative insect emergence between treatments or control streams (F2,12 = 1.239, p = 0.324, ).
Figure 3. (A) Mean (±1 SE) dipteran individuals emerged in response to fluoxetine treatment (20 ng/L and 20 µg/L) over 21d duration. Treatments have been normalized against controls, represented by a dashed line at 0. (B) Mean (±1 SE) dipteran individual mass emerged in response to fluoxetine treatment (20 ng/L and 20 µg/L) over 21d duration. Treatments have been normalized against controls, represented by a dashed line at 0. (C) Mean (±1 SE) cumulative dipteran emergence in response to fluoxetine treatment (20 ng/L and 20 µg/L). Dashed line is representative of mean cumulative emergence in control streams.
Discussion
It is well established that PPCPs are present in surface waters globally (Daughton and Ternes Citation1999). The ubiquity of PPCPs and the fact that these compounds are designed to be biologically active at low concentrations, raises concerns about effects on non-target organisms (Monteiro and Boxall Citation2010). It is only recently that studies have begun to acknowledge the potential for sub-lethal effects of pharmaceuticals on aquatic biota and ecosystem functions (Proia et al. Citation2011; Hoppe et al. Citation2012; Rosi-Marshall et al. Citation2013; Lee et al. Citation2016; Richmond et al. Citation2016; Richmond et al. Citation2017). In this study, we explored the ecological effects of fluoxetine at 20 µg/L (shown to have previous effects on algae and aquatic insects (Richmond et al. Citation2016)) and 20 ng/L, which is the median concentration reported in freshwaters across the USA (Silva et al. Citation2015). Our study further establishes that fluoxetine has the capacity to alter ecosystem processes; biofilm colonization, GPP, NPP and ER all were affected by fluoxetine, showing that extremely low, but environmentally realistic concentrations pose an ecological risk.
Some contaminants including pesticides and heavy metals can reduce the diversity of microbial communities and invertebrate shredders, altering decomposition rates in streams (Gessner et al. Citation2010); however, the extent to which pharmaceuticals, specifically fluoxetine, impact organic matter decomposition and microbial diversity remains largely unknown. Throughout the experiment, we observed no change in ER by colonized leaf biofilms exposed to fluoxetine; however, ER was higher on leaves with no-invertebrates when exposed to fluoxetine, irrespective of concentration, suggesting that the effects of fluoxetine on ER is mediated by the presence or absence of aquatic insects. In the absence of invertebrates, fluoxetine appears to affect microbial heterotrophic efficiency. This effect may be masked when invertebrates are present, and further research is warranted to identify how and why fluoxetine affected microbially mediated decomposition but not invertebrate-mediated decomposition.
Surprisingly, in the presence of invertebrates and fluoxetine treatments, we observed an increase in leaf mass remaining, but this increase was not significant. In contrast, there was a significant decrease in leaf mass remaining when invertebrates were excluded. One possible explanation for this response could be that in the absence of invertebrates, fluoxetine stimulates microbial activity, increasing respiration, whereas in the presence of invertebrates, respiration is maintained via grazing. This may be supported by the significant increase in rates of ER observed in ng/L treatments which did not contain invertebrates. Fluoxetine and other SSRIs have been found to possess antimicrobial properties, mainly affecting gram positive bacteria (Munoz-Bellido et al. Citation2000). When leaves were exposed to fluoxetine, there may have been a suppression of gram positive bacteria, leading to an increase in potential fluoxetine resistant gram negative bacteria such as Flavobacterium, a genus often found within biofilms (Besemer et al. Citation2012), which may have increased respiration. Fluoxetine inhibits the neurotransmitter serotonin. Serotonin is known to occur in fungi and other organisms (El-Merahbi et al. Citation2015) and is also known to inhibit in vitro viability of fungal cells, particularly those from the Candidae family (Lass-Flörl et al. Citation2003). It is possible that fluoxetine, working as a selective serotonin inhibitor, modulates serotonin, reducing its antifungal properties and increasing fungal activity.
BIOLOG® plates have been used to examine microbial communities in freshwater systems, including streamside channels (Kreutzweiser and Capell Citation2003) and lakes (Christian and Lind Citation2006). However, we know of only one previous study that has used this tool in aquatic biofilms exposed to a mixture of pharmaceuticals (Lawrence et al. Citation2005), where, in general no pharmaceutical treatment altered carbon utilization. We observed no change in microbial activity using BIOLOG® redox plates, which is consistent with our findings that community respiration did not differ among fluoxetine treatments. This is contradictory to pharmacological studies that suggest SSRIs may exhibit antimicrobial properties (Kalaycı et al. Citation2014), which could alter microbial communities. BIOLOG® plates provide a convenient and time effective method of characterizing microbial community activity and diversity; however, this method may be biased towards easily extracted microbes (Stefanowicz Citation2006), and therefore BIOLOG® plates may only provide a conservative estimate. Using enumeration techniques to rigorously standardize inoculum densities and examining color development through time (Garland Citation1997) may have detected differences in the rates of color development that we were unable to determine. Previous studies examining microbial community response to contaminants (e.g. heavy metals) have successfully created community level physiological profiles using BIOLOG® plates (Barranguet et al. Citation2003). Additionally, utilizing DNA techniques may have detected subtle changes or shifts in composition, not just functional diversity measured via carbon source utilization (Lee et al. Citation2016).
Similar to results observed in our previous study (Richmond et al. Citation2016), we found that biofilm NEP and GPP was marginally suppressed in fluoxetine treated streams. Our previous study measured how fluoxetine and citalopram (another SSRI) influenced functionality on established biofilms. In the current study we started with bare rocks rather than pre-colonized algal covered rocks, allowing us to test algal colonization rates rather than alterations to an already established biofilm. In this study, the initial suppression of GPP may be due to delays in algal colonization in replicates exposed to fluoxetine, evident by the reduction of chlorophyll a concentrations. The suppression of algal functionality observed in this study raises concerns about biofilm vulnerability in systems where algal reestablishment rates are high and pharmaceutical contaminants are common, for example urban systems. Similar delayed colonization was observed when biofilms were exposed to the common PPCPs caffeine, ciprofloxacin and diphenhydramine (Rosi-Marshall et al. Citation2013). By the end of the 3-week experiment in the 20 µg/L fluoxetine treatments, average algal biomass and GPP had recovered to similar levels observed in control streams. In contrast, in the low (20 ng/L) fluoxetine treated streams, there was lower algal mass, but no differences in GPP or photosynthetic efficiency compared to the control. It is possible, however, that these streams would eventually reach the level of other treatments, but fluoxetine appears to have delayed rates of colonization.
The concentrations of fluoxetine used in this study are below those that have been found to be toxic to algae (39 µg/L; Brooks et al. Citation2003). However, it appears that the lower, environmentally-realistic concentrations did disrupt algal colonization and affected primary productivity on day 13. We did not observe a reduction in whole-stream production, and these findings are similar with those reported elsewhere. Subtle reductions in GPP using biofilm covered rocks, do not necessarily translate to detectable changes at the whole-stream scale (Richmond et al. Citation2016), potentially due to a shift in algal communities to the water column or other habitats. Algal communities form vital components of stream ecosystems and the environmentally relevant ng/L concentration reduced rates of algal colonization. This suggests that in frequently disturbed streams where drugs like fluoxetine are commonly detected (e.g. urban streams), the consequences for colonization dynamics after disturbance may be an important endpoint to consider when examining the effects of these compounds, particularly in light of the fact that even small rainfall events cause enough of a disturbance to reset GPP in urban streams (Reisinger et al. Citation2017).
The concentrations of fluoxetine in this study did not cause invertebrate larval mortality, alter community composition, or taxon richness in stream benthic fauna, which is similar to previous findings (Richmond et al. Citation2016). It is very interesting to note that these common metrics (e.g. those that investigate changing composition of macroinvertebrate communities) were not sensitive to fluoxetine exposure. Although not statistically significant, we did find that relative to the control streams, the numbers of emerging individuals were 20% higher, the biomass of dipterans emerged was 17% higher, the cumulative density and biomass of emerged insects were15% and 25% higher, respectively, in the 20 ng/L treatments. Moreover, the average biomass (i.e. size at emergence) of individuals was 10% less in 20 ng/L treatments compared to the control streams. Although the high amount of variation inherent with measuring emergence, coupled with modest statistical power, may have limited our ability to detect significant effects, the direction of these patterns is consistent with previous results (Richmond et al. Citation2016). These findings suggest that there may be effects of fluoxetine and other SSRI’s on aquatic insect emergence and highlight the need for more research on this topic. Furthermore, Krams et al. (Citation2018), recently discovered that the resting metabolic rate of rapidly developing crickets, significantly increased in the presence of a SSRI and they hypothesized that SSRIs may promote selection for increased development.
Evidence suggests that SSRIs, including fluoxetine, have endocrine disrupting capabilities in vertebrates and invertebrates by therapeutically altering serotonin levels which are known to modulate reproductive output, feeding and behavioral traits (Mennigen et al. Citation2011). Fluoxetine is known to increase spawning rates in bivalves (Fong Citation1998; Lazzara et al. Citation2012), and when exposed to 40 µg/L of fluoxetine and food limiting conditions, female Daphnia produced greater amounts of offspring, but at a reduced size (Campos et al. Citation2016). We found a non-significant decrease in individual biomass of emerged dipteran adults; this is contradictory to results observed in our previous study (Richmond et al Citation2016). Alterations to dipteran emergence can have cascading effects throughout the environment, as emerged insects serve as an important resource subsidy to terrestrial landscapes (Baxter et al. Citation2005), and can facilitate the movement of contaminants to riparian predators (Laws et al. Citation2016). Fluoxetine and 65 other pharmaceuticals were recently detected in riparian spiders which feed exclusively on emerged aquatic insects (Richmond et al. Citation2018), further explaining that fluoxetine may enable greater rates of stream-riparian contaminant flux. Although the mechanisms and mode of action are unknown, fluoxetine has been shown to induce reproductive processes in bivalves (Fong Citation1998) and enhance growth of crayfish (Tierney et al. Citation2016). However, individual size has been negatively correlated to contaminants including heavy metals and pesticides (Kiffney and Clements Citation1993; Bruner et al. Citation1994).
Fluoxetine has a high absorption capacity to organic material (Kwon and Armbrust Citation2006), thus, this may explain the low concentrations of fluoxetine detected in the water column at the conclusion of this study, despite constant addition. In a recent analysis of almost 70 pharmaceuticals in aquatic food webs, fluoxetine was found to bioaccumulate within aquatic insects downstream of a WWTP at an average concentration of 794 ng/g and concentrations within aquatic invertebrates were three orders of magnitude higher than concentrations typically measured in stream water (Richmond et al. Citation2018). Therefore food-borne exposure and adsorption of pharmaceuticals to organic material may be an underappreciated threat to wildlife. Accumulation of pharmaceuticals in aquatic biota, rather than direct effects from water column exposure may be an important take-home point from this study. Our study does not attempt to describe a dose–response curve; rather, given there is so little information on the effects of pharmaceuticals, at environmentally realistic concentrations on ecosystem structure and function. Coupled with the relatively new knowledge that fluoxetine can accumulate in invertebrate tissues (Richmond et al. Citation2018), the lack of significant effects on the community composition or emergence patterns of macroinvertebrates observed in this experiment suggest that although these taxa may not be highly sensitive to exposure to these compounds, they may serve as a means by which waterborne exposure to drugs may be transferred to higher trophic levels in river and riparian food webs (e.g. fishes, spiders, birds and bats).
The collective influence of pharmaceuticals on aquatic ecosystem function remains largely unknown; however, these results begin to suggest multiple ecological effects of extremely low, yet environmentally realistic, concentrations of pharmaceuticals on algae, bacteria and invertebrates. Although there was a lack of statistical significance, this study provides some evidence that fluoxetine may disrupt and disturb ecosystem processes at these low concentrations. There has been an increased number of studies reporting the non-monotonic effects of fluoxetine on aquatic organisms (e.g. Ford and Fong Citation2015; Martin et al. Citation2017), and a call for more research observing nontoxic endpoints (Richmond et al. Citation2017). In this study, fluoxetine altered algal colonization and changed respiration in the absence of invertebrates. These results may be important in natural streams where ecosystem processes must respond to the pseudo-persistent occurrence of fluoxetine in addition to other disturbances. Secondly, it is possible, though not statistically significant, that insect emergence increased and individual size decreased when exposed to fluoxetine. This finding, coupled with previous evidence that emergence is affected by serotonin-disrupting compounds (e.g. Lee et al. Citation2016; Richmond et al. Citation2016) suggests that these types of compounds may affect this important ecological process and emphasizes the need for more research. Studies that examine the effects of PPCPs and other emerging contaminants on ecological endpoints are necessary to fully understand the ecological threats that these novel pollutants pose.
Acknowledgments
We thank Abigail Bline and Heather Malcom for assistance with data collection and setup and running of the artificial stream experiment.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Erinn K. Richmond
Dr. Erinn K. Richmond is a freshwater ecologist with a passion for aquatic insects and the streams and rivers they live in. Erinn completed her Ph.D. in 2017 examining the ecological effects of pharmaceuticals and personal care products on aquatic ecosystems. During her Ph.D. Erinn spent time abroad conducting experiments and working with scientist at the Cary Institute of Ecosystem Studies in NY, USA. Erinn is currently a post-doctoral research fellow within the Water Studies Centre at Monash University, where she is continuing research on the ecological effects of pharmaceuticals and their uptake in food webs.
Emma J. Rosi
Senior Scientist Emma J. Rosi’s research focuses on how human activities affect aquatic life and water quality in diverse ecosystems around the world. She is particularly interested in novel and emerging contaminants such as pharmaceuticals, and also studies the effects of nutrient enrichment. In addition to water quality, her work examines aquatic food webs (invertebrates and fishes), and the role of the hippopotamus in nutrient cycling. Her research seeks to inform sound stewardship of freshwater resources, and she often speaks to the public and policymakers. She serves on the Ecological Processes and Effects Committee of the US Environmental Protection Agency’s Science Advisory Board.
Alexander J. Reisinger
Assistant Professor Alexander J. Reisinger is an assistant professor of urban soil and water quality, and specializes in the ecosystem ecology and biogeochemistry of urban environments. He is a member of the Sustainability Human and Ecological Development group. He focuses on the ecosystem functions of nutrient and energy cycling and the effect of traditional (e.g. nutrients) and novel (e.g. pharmaceuticals) contaminants on these functions. Dr. Reisinger received his Ph.D. from the University of Notre Dame.
Brittany R. Hanrahan
Dr. Brittany R. Hanrahan is a research biologist at the US Department of Agriculture (USDA). She is an aquatic ecologist with expertise in stream biogeochemistry and nutrient cycling in watersheds dominated by agricultural land use. Dr. Hanrahan received her Ph.D. in 2017 from the University of Notre Dame.
Ross M. Thompson
Professor Ross M. Thompson is Director and Chair of Water Science in the Institute for Applied Ecology at the University of Canberra. Ross is a freshwater ecologist with interests in the study of biodiversity and the restoration of landscapes. His fundamental research is in food web ecology; seeking the rules that determine how natural communities assemble and persist. His applied research addresses the ways in which food webs can be influenced by anthropogenic factors including urbanization, land clearance, pharmaceutical contamination, river flow diversion and restoration, and invasion. He has an active research program on aquatic biodiversity and ecosystem function in urban and rural landscapes. Ross has published more than 90 papers, 10 book chapters and more than 200 scientific reports. His work has strong links to government and industry, and Ross sits on a number of senior technical advisory panels for local, state and federal research programs.
Michael R. Grace
Associate Professor Michael R. Grace’s research interests include: aquatic chemistry of lakes, rivers and estuaries; biogeochemical cycling of nutrients (N, P, S and C); environmental analytical chemistry; using stable isotopes to investigate aquatic nutrient cycling and ecosystem functioning; aquatic ecosystem process measurements (e.g. whole stream metabolism, denitrification); investigating the effects of urbanization on aquatic ecosystems; integrating science into Natural Resource Management; and ecosystem ecology.
References
- Arthur JW, Zischke JA, Allen KN, Hermanutz RO. 1983. Effects of diazinon on macroinvertebrates and insect emergence in outdoor experimental channels. Aquat Toxicol. 4(4):283–302.
- Barranguet C, van den Ende FP, Rutgers M, Breure AM, Greijdanus M, Sinke JJ, Admiraal W. 2003. Copper-induced modifications of the trophic relations in riverine algal-bacterial biofilms. Environ Toxicol Chem. 22(6):1340–1349.
- Baxter CV, Fausch KD, Saunders CW. 2005. Tangled webs: reciprocal flows of invertebrate prey link streams and riparian zones. Freshw Biol. 50(2):201–220.
- Benke AC, Huryn AD, Smock LA, Wallace JB. 1999. Length-mass relationships for freshwater macroinvertebrates in north america with particular reference to the southeastern United States. J N Am Benthol Soc. 18(3):308–343.
- Besemer K, Peter H, Logue JB, Langenheder S, Lindström ES, Tranvik LJ, Battin TJ. 2012. Unraveling assembly of stream biofilm communities. ISME J. 6(8):1459–1468.
- Boxall ABA, Rudd MA, Brooks BW, Caldwell DJ, Choi K, Hickmann S, Innes E, Ostapyk K, Staveley JP, Verslycke T, et al. 2012. Pharmaceuticals and personal care products in the environment: what are the big questions? Environ Health Perspect. 120(9):1221–1229.
- Brooks BW, Kevin Chambliss C, Stanley JK, Ramirez A, Banks KE, Johnson RD, Lewis RJ. 2005. Determination of select antidepressants in fish from an effluant dominated stream. Environ Toxicol Chem. 24(2):464–469.
- Brooks BW, Foran CM, Richards SM, Weston J, Turner PK, Stanley JK, Solomon KR, Slattery M, La Point TW. 2003. Aquatic ecotoxicology of fluoxetine. Toxicol Lett. 142(3):169–183.
- Bruner KA, Fisher SW, Landrum PF. 1994. The role of the zebra mussel, Dreissena polymorpha, in contaminant cycling: I. The effect of body size and lipid content on the bioconcentration of PCBs and PAHs. J Great Lakes Res. 20(4):725–734.
- Bundy JG, Paton GI, Campbell CD. 2004. Combined microbial community level and single species biosensor responses to monitor recovery of oil polluted soil. Soil Biol Biochem. 36(7):1149–1159.
- Campos B, Rivetti C, Kress T, Barata C, Dircksen H. 2016. Depressing antidepressant: fluoxetine affects serotonin neurons causing adverse reproductive responses in Daphnia magna. Environ Sci Technol. 50(11):6000–6007.
- Champely S. 2007. PWR: Basic functions for power analysis. R Package Version 1.1. [Computer software]. Available from https://cran.r-project.org/web/packages/pwr/pwr.pdf.
- Christian BW, Lind OT. 2006. Key issues concerning biolog use for aerobic and anaerobic freshwater bacterial community-level physiological profiling. Int Rev Hydrobiol. 91(3):257–268.
- Clarke K, Gorley R. 2006. User manual/tutorial. Plymouth: PRIMER-E Ltd.
- Cummins KW. 1974. Structure and function of stream ecosystems. BioScience. 24(11):631–641.
- Daughton CG. 2003. Cradle-to-cradle stewardship of drugs for minimizing their environmental disposition while promoting human health. I. Rationale for and avenues toward a green pharmacy. Environ Health Perspect. 111(5):757–774.
- Daughton CG, Ternes TA. 1999. Pharmaceuticals and personal care products in the environment: agents of subtle change? Environ Health Perspect. 107(suppl 6):907–938.
- Dewey SL. 1986. Effects of the herbicide atrazine on aquatic insect community structure and emergence. Ecology. 67(1):148–162.
- Drury B, Scott J, Rosi-Marshall EJ, Kelly JJ. 2013. Triclosan exposure increases triclosan resistance and influences taxonomic composition of benthic bacterial communities. Environ Sci Technol. 47(15):8923–8930.
- Duan M, Gu J, Wang X, Li Y, Li P, Yin Y. 2018. Combined effects of compost containing sulfamethazine and zinc on pakchoi (Brassica chinensis L.) growth, soil sulfonamide resistance genes, and microbial communities. Arch Agron Soil Sci. 64(2):231–243.
- El-Merahbi R, Löffler M, Mayer A, Sumara G. 2015. The roles of peripheral serotonin in metabolic homeostasis. FEBS Lett. 589(15):1728–1734.
- Ellis RJ, Neish B, Trett MW, Best JG, Weightman AJ, Morgan P, Fry JC. 2001. Comparison of microbial and meiofaunal community analyses for determining impact of heavy metal contamination. J Microbiol Methods. 45(3):171–185.
- Fisher SG, Likens GE. 1973. Energy flow in Bear Brook, New Hampshire: an integrative approach to stream ecosystem metabolism. Ecol Monogr. 43(4):421–439.
- Fong PP. 1998. Zebra mussel spawning is induced in low concentrations of putative serotonin reuptake inhibitors. Biol Bull. 194(2):143.
- Ford AT, Fong PP. 2015. The effects of antidepressants appear to be rapid and at environmentally relevant concentrations. Environ Toxicol Chem. 35(4):794–798.
- Garland JL. 1997. Analysis and interpretation of community-level physiological profiles in microbial ecology. FEMS Microbiol Ecol. 24(4):289–300.
- Garland JL, Mills AL. 1991. Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level sole-carbon-source utilization. Appl Environ Microbiol. 57(8):2351–2359.
- Gessner MO, Swan CM, Dang CK, McKie BG, Bardgett RD, Wall DH, Hättenschwiler S. 2010. Diversity meets decomposition. Trends Ecol Evol. 25(6):372–380.
- Grace MR, Giling DP, Hladyz S, Caron V, Thompson RM, Mac Nally R. 2015. Fast processing of diel oxygen curves: estimating stream metabolism with BASE (BAyesian Single-station Estimation). Limnol Oceanogr: Methods. 13(3):103–114.
- Hieber M, Gessner MO. 2002. Contribuitions of stream detrivores, fungi and bacteria to leaf breakdown based on biomass estimates. Ecology. 83(4):1026.
- Hill BH, Herlihy AT, Kaufmann PR. 2002. Benthic microbial respiration in Appalachian Mountain, Piedmont, and Coastal Plains streams of the eastern U.S.A. Freshw Biol. 47(2):185–194.
- Hoppe PD, Rosi-Marshall EJ, Bechtold HA. 2012. The antihistamine cimetidine alters invertebrate growth and population dynamics in artificial streams. Freshw Sci. 31(2):379–388.
- Jonsson M, Ershammar E, Fick J, Brodin T, Klaminder J. 2015. Effects of an antihistamine on carbon and nutrient recycling in streams. Sci Total Environ. 538:240–245.
- Kalaycı S, Demirci S, Sahin F. 2014. Antimicrobial properties of various psychotropic drugs against broad range microorganisms. Curr Psychopharmacol. 3(3):195–202.
- Kiffney PM, Clements WH. 1993. Bioaccumulation of heavy metals by benthic invertebrates at the Arkansas River, Colorado. Environ Toxicol Chem. 12(8):1507–1517.
- Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT. 2002. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. Streams, 1999 − 2000: a national reconnaissance. Environ Sci Technol. 36(6):1202–1211.
- Krams I, Trakimas G, Kecko S, Elferts D, Krams R, Luoto S, Rantala MJ, Mänd M, Kuusik A, Kekäläinen J, et al. 2018. Linking organismal growth, coping styles, stress reactivity, and metabolism via responses against a selective serotonin reuptake inhibitor in an insect. Sci Rep. 8(1):8599.
- Kreutzweiser DP, Capell SS. 2003. Benthic microbial utilization of differential dissolved organic matter sources in a forest headwater stream. Can J For Res. 33(8):1444–1451.
- Kwon J-W, Armbrust KL. 2006. Laboratory persistance and fate of fluoxetine in aquatic environments. Environ Toxicol Chem. 25(10):2561–2568.
- Kwon J-W, Armbrust KL. 2008. Aqueous solubility, n-octanol-water partition coefficient, and sorption of five selective serotonin reuptake inhibitors to sediments and soils. Bull Environ Contam Toxicol. 81(2):128–135.
- Lass-Flörl C, Fuchs D, Ledochowski M, Speth C, Dierich MP, Würzner R. 2003. Antifungal properties of 5-hydroxytryptamine (serotonin) against Candida species in vitro. J Med Microbiol. 52(2):169–171.
- Lawrence JR, Swerhone GDW, Wassenaar LI, Neu TR. 2005. Effects of selected pharmaceuticals on riverine biofilm communities. Can J Microbiol. 51(8):655–669.
- Laws J, Heppell K, Sheahan D, Liu C-F, Grey J. 2016. No such thing as a free meal: organotin transfer across the freshwater–terrestrial interface. Freshw Biol. 61(12):2051–2062.
- Lazzara R, Blázquez M, Porte C, Barata C. 2012. Low environmental levels of fluoxetine induce spawning and changes in endogenous estradiol levels in the zebra mussel Dreissena polymorpha. Aquat Toxicol. 106–107:123–130.
- Lee SS, Paspalof AM, Snow D, Richmond EK, Rosi-Marshall EJ, Kelly JJ. 2016. Occurrence and potential biological effects of amphetamine on stream communities. Environ Sci Technol. 50(17):9727–9735.
- Martin JM, Saaristo M, Bertram MG, Lewis PJ, Coggan TL, Clarke BO, Wong BBM. 2017. The psychoactive pollutant fluoxetine compromises antipredator behaviour in fish. Environ Pollut. 222:592–599.
- Mennigen JA, Stroud P, Zamora JM, Moon TW, Trudeau VL. 2011. Pharmaceuticals as neuroendocrine disruptors: lessons learned from fish on Prozac. J Toxicol Environ Health B. 14(5–7):387–412.
- Merritt RW, Cummins KW. 1996. An introduction to the aquatic insects of North America. Dubuque, IA: Kendall Hunt.
- Minshall GW. 1978. Autotrophy in stream ecosystems. BioScience. 28(12):767–771.
- Monteiro S, Boxall AA. 2010. Occurrence and fate of human pharmaceuticals in the environment reviews of environmental contamination and toxicology. In: Reviews of environmental contamination and toxicology. Vol. 202. New York: Springer; p. 53–154.
- Munoz-Bellido JL, Munoz-Criado S, Garcı̀a-Rodrı̀guez JA. 2000. Antimicrobial activity of psychotropic drugs: selective serotonin reuptake inhibitors. Int J Antimicrob Agents. 14(3):177–180.
- Pelli M, Connaughton VP. 2015. Chronic exposure to environmentally-relevant concentrations of fluoxetine (Prozac) decreases survival, increases abnormal behaviors, and delays predator escape responses in guppies. Chemosphere. 139:202–209.
- Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D., R Core Team. 2009 nlme: linear and nonlinear mixed effects models. R package version 3.1 – 96. [Computer software]
- Proia L, Morin S, Peipoch M, Romaní AM, Sabater S. 2011. Resistance and recovery of river biofilms receiving short pulses of Triclosan and Diuron. Sci Total Environ. 409(17):3129–3137.
- R Core Team. 2014. R: a language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing.
- Rasband W. 1997. ImageJ 2012. Bethesda (MD): US National Institutes of Health.
- Reisinger AJ, Rosi EJ, Bechtold HA, Doody TR, Kaushal SS, Groffman PM. 2017. Recovery and resilience of urban stream metabolism following Superstorm Sandy and other floods. Ecosphere. 8(4):e01776.
- Richmond EK, Grace MR, Kelly JJ, Reisinger AJ, Rosi EJ, Walters DM. 2017. Pharmaceuticals and personal care products (PPCPs) are ecological disrupting compounds (EcoDC). Elem Sci Anth. 5:66.
- Richmond EK, Rosi EJ, Walters DM, Fick J, Hamilton SK, Brodin T, Sundelin A, Grace MR. 2018. A diverse suite of pharmaceuticals contaminates stream and riparian food webs. Nat Commun. 9(1):4491.
- Richmond EK, Rosi-Marshall EJ, Lee SS, Grace MR, Thompson RM. 2016. Antidepressants in stream ecosystems: influence of selective serotonin reuptake inhibitors (SSRIs) on algal production and insect emergence. Freshw Sci. 35(3):845–855.
- Rosi-Marshall EJ, Kincaid DW, Bechtold HA, Royer TV, Rojas M, Kelly JJ. 2013. Pharmaceuticals suppress algal growth and microbial respiration and alter bacterial communities in stream biofilms. Ecol Appl. 23(3):583–593.
- Rosi-Marshall EJ, Royer TV. 2012. Pharmaceutical compounds and ecosystem function: an emerging research challenge for aquatic ecologists. Ecosystems. 15(6):867–880.
- Schmidt TS, Kraus JM, Walters DM, Wanty RB. 2013. Emergence flux declines disproportionately to larval density along a stream metals gradient. Environ Sci Technol. 47(15):8784–8792.
- Silva LJG, Pereira AMPT, Meisel LM, Lino CM, Pena A. 2015. Reviewing the serotonin reuptake inhibitors (SSRIs) footprint in the aquatic biota: Uptake, bioaccumulation and ecotoxicology. Environ Pollut. 197:127–143.
- Stefanowicz A. 2006. The Biolog plates technique as a tool in ecological studies of microbial communities. Pol J Environ Stud. 15(5):669.
- Steinman AD, Lamberti GA, Leavitt PR. 1996. Biomass and pigments of benthic algae. In: FR. Hauer, GA. Lamberti (Eds.), Methods in stream ecology. Vol. 2, (pp. 357–379). Oxford, UK: Elsevier. doi:10.1016/B978-012332908-0.50024-3
- Styrishave B, Halling-Sørensen B, Ingerslev F. 2011. Environmental risk assessment of three selective serotonin reuptake inhibitors in the aquatic environment: a case study including a cocktail scenario. Environ Toxicol Chem. 30(1):254–261.
- Tank JL, Rosi-Marshall EJ, Griffiths NA, Entrekin SA, Stephen ML. 2010. A review of allochthonous organic matter dynamics and metabolism in streams. J N Am Benthol Soc. 29(1):118–146.
- Tierney AJ, Hanzlik KN, Hathaway RM, Powers C, Roy M. 2016. Effects of fluoxetine on growth and behavior in the crayfish Orconectes rusticus. Mar Freshw Behav Physiol. 49(2):133–145.
- Webster J, Benfield E. 1986. Vascular plant breakdown in freshwater ecosystems. Annu Rev Ecol Syst. 17(1):567–594.
- Wetzel RG, Likens GE. 1991. Limnological analyses. New York: Springer Verlag; p. 391.
- Wiggins GB. 1996. Larvae of the North American caddisfly genera (Trichoptera). Toronto: University of Toronto Press.
- Wong DT, Bymaster FP, Engleman EA. 1995. Prozac (fluoxetine, Lilly 110140), the first selective serotonin uptake inhibitor and an antidepressant drug: twenty years since its first publication. Life Sci. 57(5):411–441.
