Abstract
The Susquehanna River basin is one of the largest and most diverse watersheds in the northeastern United States, however, its historically renowned Micropterus dolomieu (smallmouth bass–SMB) fishery has been in decline since the mid-2000s. Agricultural herbicide runoff has been identified as a major risk for Susquehanna basin SMB populations given their effects as endocrine disrupting compounds (EDCs). During the summers of 2016 and 2017, we assessed potential threats to SMB populations in 11 tributaries to the Juniata River, the second largest tributary to the Susquehanna River. Passive water samplers were installed for 38–39 days in ecologically important tributaries to quantify six common herbicides, and SMB were collected from nine sites in 2016 and 2017 to assess their health and morphology. Our passive water samplers showed markedly higher EDC concentrations than has previously been documented in the Juniata basin, with atrazine occurring at all sites and in the highest concentrations (11.09–91.02 ng/L). SMB blood samples revealed complete prevalence (100%) of vitellogenin, an egg protein precursor, in male fishes further confirming previous rates male vitellogenisis. Additionally, SMB hepatosomatic index (HSI) was statistically higher in female SMB than in male SMB (P < 0.001), and higher than many previous regional SMB studies further highlighting a contaminant-based stressor. Finally, a geometric morphometric analysis of SMB body shape indicated morphologies to be significantly site-based. Morphological differences were in line with the ram-suction feeding continuum, further revealing potential vulnerability in SMB sub-populations where EDCs may alter food web dynamics and prey availability. Overall, our study of the Juniata River Basin highlights high EDC concentrations alongside high rates of male vitellogenisis and elevated HSI, and proposes novel theory for morphological vulnerability in SMB sub-populations.
Introduction
Anthropogenic pollutants, such as endocrine disrupting compounds (EDCs), threaten the sustainability of freshwater ecosystems across the world from organismal to population levels and across multiple taxa. EDCs in the form of herbicides are a major concern in the United States and beyond due to their high prevalence yet mixed consensus with regard to their environmental impacts (Rohr and McCoy Citation2010; Solecki et al. Citation2017; Matthiessen et al. Citation2018). Atrazine is arguably the most significant agricultural EDC in the United States at this time, as it is the most widely used herbicide on broadleaf crops including corn, soybeans, and sorghum (Farruggia et al. Citation2016). Most recently, the US EPA’s risk assessment concluded concentrations of atrazine at 5 ug/L are likely to have reproductive effects on fish, yet its application has been outright banned for agricultural use in the European Union since 2003 (Bethsass and Colangelo Citation2006; Farruggia et al. Citation2016).
Endocrine disrupting compounds, such as atrazine, possess the ability to mimic estrogen, thereby inducing vitellogenisis in male fishes and other taxa, causing cascading reproductive effects (Hayes et al. Citation2011; Matthiessen et al. Citation2018). In male fishes, concentrations of vitellogenin are naturally very low, however, when fish are exposed to EDCs they can express high vitellogenin concentrations, comparable to concentrations of female fishes (Hiramatsu et al. Citation2006; Lee Pow et al. Citation2017). Effects of EDCs such as intersex, reduced gonadal development and viability, and skewed sex ratios can therefore have detrimental effects on fish at organismal and population levels (Kolpin et al. Citation2013; Hillis et al. Citation2015; Lee Pow et al. Citation2017).
However, there’s a lack of studies investigating agricultural EDCs’ potential influence on smallmouth bass (Micropterus dolomieu; SMB) populations at the smaller tributary scale, and therefore we sought to fill this research gap. Our study details high EDC presence in ecologically important tributaries and high presence of male SMB expressing vitellogenin, which represent anthropogenic threats to SMB in the Juniata River Basin. Additionally, a geometric morphometric analysis revealed potential vulnerabilities in piscivorous SMB morphotypes in several Juniata basin subpopulations given EDCs more longstanding effect on vertebrate prey items than invertebrates.
Study site
The Susquehanna River basin is one of the largest and most diverse watersheds in the northeastern United States, draining nearly half of Pennsylvania, and support both cold-water and warm-water fisheries (Mcllnay Citation2002). The basin is renowned for its SMB fishing which has an annual economic impact of 630 million dollars (Allen et al. Citation2013). The Juniata River is the Susquehanna’s second largest tributary (3400 square miles) and is largely comprised of forested ridges, agriculture in the valleys, and a few urban centers (LeFevre Citation2005). Recently the entire Susquehanna watershed has received a lot of attention due to its degradation from a previously premier SMB fishery (Arway and Smith Citation2013). In December 2015, a collaborative assessment between the Pennsylvania Fish and Boat Commission, Pennsylvania Department of Environmental Protection, and others detailed potential perturbations to SMB populations (Shull and Pulket Citation2015). The conclusions of this report highlighted EDCs, as well as pathogens and parasites, as the two most likely stressors on SMB populations, with little known about either throughout the basin.
Methods
Site selection
Sites (n = 11) within the Juniata Basin were selected to survey major tributaries across varying land covers and broad spatial distribution (). Watersheds were delineated for all sites using 30 m DEM projections and hydrology tools in ArcGIS 10.5. Land cover statistics (% row crop, % forested, etc.) were calculated for each watershed using the 2011 National Land Cover Database (Homer et al. Citation2015) (). Other watershed characteristics (basin area, slope, and stream density) were calculated using the USGS StreamStats data tool (Ries et al. Citation2004) ().
Table 1. Site statistics for study sites in Juniata River Basin, Pennsylvania sampled in June and July of 2016 and 2017.
Passive water samplers
Polar Organic Chemical Integrative Samplers (POCIS) (n = 6), were deployed by anchoring samplers to the streambed with 1-m long rebar for 38–39 days between 31 May and 8 July 2016. This sampling period was chosen to match previous sampling periods in the region and when fish would be collected (Blazer et al. Citation2014; Iwanowicz et al. Citation2019). Passive water samplers were chosen because they are designed to accumulate contaminants, such as herbicides, throughout the period to replicate biological exposure. A field blank was used during installation and recollection to assess potential contamination while samplers were handled and prepared outside the stream environment. After the deployment period, passive water samplers were recollected, immediately stored on ice, frozen, and shipped to Environmental Sampling Technologies (St. Joseph, Montana, USA) for sample extraction. Samples were extracted with 25 mL methanol, blown down using ultra high purity nitrogen gas, and the extracts from each of the three sampling membranes were pooled. Pooled extracts were blown down, filtered through glass fiber filter paper with methanol, and transferred to yield 5 mL of concentrated pooled extract from each site. Extracts were sent to Anatek labs (Moscow, Idaho) for a screen of five herbicides and one metabolite (atrazine, metolachlor, alachlor, simazine, prometon, and desethylatrazine (DEA)), with a detection limit of 50 ng from pooled-extracts for each chemical. We used the quantified chemical masses to estimate in-stream herbicide concentrations according to Mazzella et al. (Citation2007).
Fish collection and processing
We used backpack electrofishing and angling to collect SMB (n = 65) from 10 sites from 25 May to 3 August of 2016 (n = 37) and 29 May to 20 July of 2017 (n = 29), and white suckers (n = 7) from one site, Clover, on 13 July 2017 as Clover did not have smallmouth bass but were abundant in white suckers. We felt it was worthwhile assessing white suckers at this site as multiple sucker species have been assessed alongside SMB in previous ecotoxicologal work in the region (Blazer et al. Citation2014). Collected fish were euthanized via immersion in Tricaine MS-222, following humane procedures and in compliance with approved Juniata College humane animal use protocol. Post-euthanasia, SMB were pinned to a foam board to splay each fin and photographed with a ruler in the frame for geometric morphometric analysis (Hall et al. Citation2018). In 2017, blood was collected from the caudal vein of SMB (n = 22) and white suckers (n = 7) using heparinized capillary tubes. Collected blood was chilled on ice for later in lab analysis. Fish were further dissected to determine sex, and livers were weighed to calculate hepatosomatic index (HSI).
Blood vitellogenin analysis
Within 12 hours of collection, blood samples were centrifuged, measured for hematocrit, and then blood plasma was isolated and transferred to microtubes where a 1:1 dilution was made using SDS sample buffer. Samples were then placed on a heat block at 100 °C for 4 minutes, and then ran through a 7.5% 15-well SDS-PAGE electrophoresis gel alongside a pair of molecular weight markers. Gels were stained with Brilliant Blue for 18–24 hours on a rocker table and viewed on a light box for presence of vitellogenin around 200 kDa (Orlando et al. Citation1999).
Geometric morphometric and data analysis
A total of 15 homologous landmarks were placed on each SMB photograph, and scales were set using the StereoMorph package in R-studio (R Core Team Citation2015). Landmark data of each sample was uploaded to MorphoJ for statistical analysis (Klingenberg Citation2011). A full Procrustes superimposition was used to remove the effects of size, rotation, and translation on point placement (Dryden and Mardia Citation1998). A covariate matrix was generated on Procrustes coordinates, and then analyzed using a canonical variate analysis (CVA) with stream site as a classifier group, and a permutation test of 100,000 pairwise distances was used on the CVA for site-based comparisons, similar to previous studies (Elmer et al. Citation2014; Stafford et al. Citation2014; Hall et al. Citation2018). Sites within one stream mile of each other were combined after initial analysis showed strong overlap between geographically close sites, and further literature supported one stream mile is within SMB’s home range (Gatz and Adams Citation1994; Humston et al. Citation2010). Only two sites were combined and are referred to singularly as ‘Standing Stone’ throughout the study. Wire frame graphs were generated for each canonical variate to visualize morphological differences. All variables were verified for normality, and herbicide concentrations, land cover (% agriculture, % forest, etc.), watershed characteristics (basin slope, area, etc.), and morphological data were analyzed using linear regressions. Sex-based variables were analyzed with t-tests, and all statistics were run in R-studio with significance considered at α < 0.05.
Results
Herbicides
Herbicide presence was confirmed at all POCIS (n = 6), with atrazine being the only herbicide detected at all sites, ranging from 11.09 to 91.02 ng/L. Metolachlor was also prevalent, being detected at five sites and ranging from 3.47 to 47.45 ng/L (). Only one site (Kishacoquillas) had all six herbicides present, which were at relatively low levels compared to other sites. While atrazine was observed at all sites, its respective metabolite, DEA, was not detected at two sites. The field blank did not show any potential contamination while handling the passive water samplers outside the stream. Statistically herbicides did not show a significant relationship to land cover or watershed characteristics.
Table 2. Estimated herbicide concentrations (ng/L) from passive water samplers in Juniata River tributaries, Pennsylvania, USA for 38–39 days between May 31st and July 8th, 2016.
Blood vitellogenin and hepatosomatic index
Presence of vitellogenin in blood plasma was confirmed in 100% of male (n = 11, n = 5) and female (n = 11, n = 2) SMB and white suckers, respectively, collected in 2017 from five total sites. Hematocrit was not significantly related to fish length, weight, sex, HSI, or site-based variables. HSI was however statistically higher in female SMB (n = 24, [mean ± CI], 1.61 ± 0.36) than in male SMB (n = 26, [mean ± CI], 1.01 ± 0.84) (t38.1=5.03, P < 0.001).
Geometric morphometrics
A geometric morphometric analysis was conducted on Juniata basin SMB sub-populations (n = 9) with sample sizes ranging from 4 to 18 SMB per site. Analysis indicated statistically significant variation in morphologies across sampled sub-populations ( and ). CV1 accounted for most of the variation (43.14%) where morphological differences largely occurred in the jaw, body depth, and caudal peduncle, with the positive end of the CV1 axis (CV1+) having smaller jaws, deeper body depth, and wider caudal peduncles ( and ). CV2 accounted for less variation (19.82%), with minor morphology differences occurring in the jaw and body depth areas ( and ). Site averaged Procrustes shapes were analyzed for correlations with all site-based variables, but none were significant. Additionally, sex influence on body morphology was assessed using a CVA with sex as a factor, as well as linear measurements of commonly sex-linked variables including body length standardized upper and lower jaw length, but none were significant; P = 0.6910, P = 0.9311, and P = 0.7692, respectively.
Figure 1. Morphological canonical variate plot (CV1 and CV2 with % variation) for SMB (n = 69) sampled from the Juniata River basin, PA, USA in the summers of 2016 and 2017. Ellipses are drawn to 95% confidence intervals. Illustrations summarize major morphological changes that occur in CV1 where the most variation is accounted for. Fish Illustration: Ted Walke.
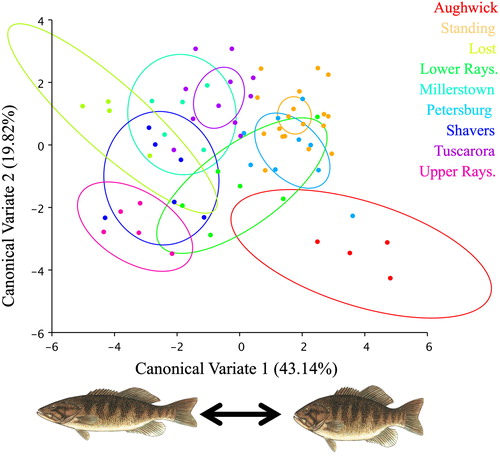
Figure 2. Wire frame graphs of CV1 and CV2 displaying morphological shifts in smallmouth bass from the Juniata River basin, PA, USA captured in summers of 2016 and 2017. Red lines show the positive-extreme shift (CV+) in morphology from the black lines (consensus-average shape) according to the canonical variate analysis (). For example, the most extreme CV1+ site, Aughwick, has a morphology most closely represented by the red wire frame in CV1.
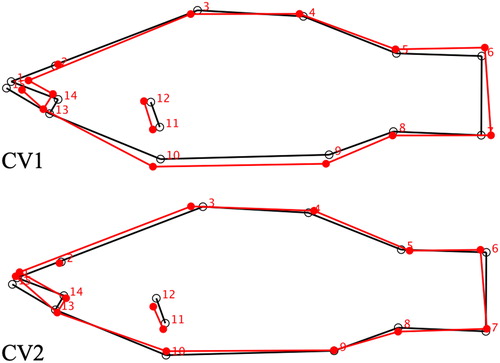
Table 3. P-values from permutation tests (100,000 permutation rounds) for Procrustes distances among site groups in canonical variate analysis (CVA).
Discussion
EDCs
EDC concentrations in this study were found to be markedly higher than previous studies both locally and across the region. Specifically, we found atrazine concentrations in Juniata River tributaries to be upwards of 3 times higher than previously sampled in the mainstem Juniata River, and relatively high compared to other studies across the Chesapeake Bay basin (Kolpin et al. Citation2013; Blazer et al. Citation2014; Walsh et al. Citation2018; Iwanowicz et al. Citation2019). We attribute the relatively high concentrations in this study to relative watershed size where it was previously shown in the Chesapeake Bay basin by Hall et al. (Citation1999) that both atrazine and metolachlor concentrations were at their respective maximums at an intermediate tributary scale and at their minimums in main-stem rivers and bays. The lack of statistical significance between EDCs and land cover and/or other variables is likely due to the low sample size in POCIS EDC sampling (n = 6), which was limited by the high cost of EDC analysis. Overall, our results highlight relatively high levels of multiple EDCs in Juniata River tributaries, likely related to the watershed scale we assessed, and warrants further EDC monitoring of smaller scale tributaries in the region.
Beyond high concentrations of EDCs, extensive EDC overlap especially atrazine and metolachlor is an additional concern due to synergistic effects of EDCs. In the six tributaries we assessed, all but one of them had multiple EDCs present at detectable levels (). While extensive EDC overlap is not uncommon, a chemical-by-chemical approach to EDCs has been largely criticized, and a more cumulative approach is advised for EDC risk assessment (Kortenkamp Citation2007; Alvarez et al. Citation2009; Van den Brink et al. Citation2019). Further, the lack of field research on the ‘potency’ of individual EDCs on individual fish species therefore makes it difficult to justly determine the biological risk of overlapping EDCs on SMB. Nevertheless, the overlap of EDCs shown in this study suggests an increasing risk to the Juniata River basin biota that should further be investigated with synergies in mind.
The EDC concentrations and potential synergies in this study pose a high reproductive and developmental risk for SMB as they occurred in ecologically important tributaries. Tributaries are essential spawning and refuge habitat for juvenile SMB in late spring and early summer, and it is suggested to be the most influential time for a year class’s success (Gerber and Haynes Citation1988; Humston et al. Citation2010). The timing and location of high EDC concentrations therefore poses a severe risk for juvenile life stages as it is SMB’s, as well as many other fishes, most vulnerable time period (Hiramatsu et al. Citation2006; Kolpin et al. Citation2013; Walsh et al. Citation2018). For the current issue of Susquehanna River basin SMB decline, EDC exposure during spawning is especially important given the SMB decline has been strongest in the young-of-year, juvenile life stages (Arway and Smith Citation2013). Ultimately, these results suggest high concentrations and potential synergies of EDCs in Juniata River tributaries are of biological importance to the regional SMB decline (Kolpin et al. Citation2013).
Blood vitellogenin and HSI
The high prevalence (100%) of blood vitellogenin presence in our study confirms previous rates of male vitellogenisis at the local and global scale. Although SDS-PAGE only gives presence/absence results, this study expands the well-documented rates of male vitellogenisis in fishes of the Northeastern US to smaller scale tributaries in the Juniata River basin. Widespread abnormal vitellogenisis has also coincided with intersex (testicular oocytes) in male fishes, being observed in several fish species within the Centrarchidae and Catastomidae families, and has largely been attributed to EDCs from point and nonpoint sources of EDCs such as waste-water effluent and herbicides, respectively (Blazer et al. Citation2007; Blazer et al. Citation2014; Iwanowicz et al. Citation2016; Lee Pow et al. Citation2017). Overall, alongside the growing body of EDC exposure studies in the Northeastern US, this study highlights extensive EDC pollution potentially contributing to the overall regional decline in SMB abundance. Further, these results expand our knowledge of Micropterus’s sensitivity to EDCs, and justifies their potential use as an indicator of EDC pollution (Iwanowicz et al. Citation2016; Lee Pow et al. Citation2017).
Female SMB HSI was significantly higher than male SMB HSI, and was also elevated compared to other studies in the region during similar study periods (seasonally) (Lee Pow et al. Citation2017; Pinkney et al. Citation2017). HSI is often used as a measure of fish health and when it is abnormally high it can be an indication of poor fish health (Orlando et al. Citation1999; Sepùlveda et al. Citation2003). Often studies compare HSI against site-based (% agriculture, EDC concentrations) or biological metrics (oocyte index, gonadosomatic index, vitellogenin levels) rather than sex-based lines (Lee Pow et al. Citation2017; Pinkney et al. Citation2017). However, our study’s site-based measures did not show significant relations to HSI, rather HSI was highly sex-based. Mention of sex-based HSI differences in SMB is rare as the aforementioned studies with Micropterus pooled sexes in statistical analyses. Given the aforementioned ecological indicators of this study (EDCs and vitellogenin), our HSI results represent yet another potential indicator of high EDC exposure in Juniata River basin SMB. These results also suggest future studies assessing HSI should extend analyses to sex-based variables rather than only site-based or biological measures.
Geometric morphometrics
Morphological differences were shown in Juniata River basin SMB, where the morphological differences were significantly site-based indicating unique morphologies at the tributary, sub-population level. Body morphology of fishes are often pressured by biotic (food web characteristics) and/or abiotic (flow, habitat, etc.) factors, and therefore are sensitive to respective site-based ecosystem alterations (Robinson and Wilson Citation1994; Wahlström Citation2001; Franssen Citation2011). Specific to the SMB morphologies in this study, the larger jaw and fusiform body depth on CV1- (sites Lost, Upper Rays., Shavers) is more associated with ram-style feeding, while the smaller jaw and deeper body depth on CV1+ (sites Aughwick, Standing Stone, Petersburg) is more associated with suction feeding (Norton Citation1991; Norton and Brainerd Citation1993; Sass and Motta Citation2002) (). Ecologically, the ram feeding morphology (CV1-) accentuates higher swimming/cruising speed and is more adept to chase and capture baitfish. In contrast, the suction feeding morphology (CV1+) has better maneuverability and the smaller jaw has higher suction potential making it more adept to prey on smaller prey items, like macroinvertebrates, around complex in-stream cover (Norton Citation1995; Wainwright and Richard Citation1995; Bloodworth and Marshall Citation2005).
Specific site-based morphologies reveal yet another vulnerability in Juniata Basin SMB given potential food web perturbations of EDCs. Since morphologies strongly relate to site based conditions, broader ecosystem effects of EDCs could be an additional stressor for SMB. For example, while EDCs effect both vertebrates and invertebrates, they are thought to have a more longstanding effect on vertebrate populations that experience fecundity related complications, whereas invertebrates are thought to rebound faster from EDC exposure (Solomon et al. Citation1996; Rohr and McCoy Citation2010; Farruggia et al. Citation2016). In the case of Juniata River SMB sub-populations, food webs more reliant on vertebrates could experience significant changes, such as ram-feeding SMB with morphologies suggestive of baitfish reliance (sites Aughwick, Standing Stone, Petersburg) (). If these food webs experience these alterations, ram-feeding, piscivorous SMB would have to transition their diets to macroinvertebrates; a prey item they are less suited for capturing, which would be bioenergetically costly (Svanbäck and Eklöv Citation2004). Although we did not assess direct food web dynamics in this study, such concentrations of EDCs known to be capable of changing food web dynamics and resource availability, and the site-specific morphologies shown here highlight a potential vulnerability yet to be suggested. Although there is a wealth of toxicological studies on EDCs, more study needs to be directed to assessing EDC’s potential to go beyond simply impairing reproductive dynamics of organisms.
Conclusions
Here we confirmed high prevalence of EDCs in ecologically important tributaries alongside high presence of male vitellogenisis. This study provides evidence of potentially higher risks for biota at smaller tributary scales where EDCs are more concentrated than levels in diluted in main-stem sites previously monitored. We further propose how populations adapted to their site-specific conditions could be vulnerable to EDC effects on food web dynamics, going beyond the well-studied reproductive effects. Future work would contribute significantly by quantifying vitellogenin levels in tributary SMB and baitfish to investigate such threats to food web dynamics and the ability for populations to morphologically adapt to changing conditions.
Acknowledgments
We would like to acknowledge Elijah Hall and JD Weyant for their determination in collecting fish samples. We would also like to thank Kelsey Pfau and Devin Beck for their help with sample collection. We’d also like to thank EST Labs, Anatek Labs, and Dr. Sharon Yohn for their help in sample analysis. Additionally, we want to thank the US Fish and Wildlife Service (Lora Zimmerman) for providing funding for this project, as well the Pennsylvania Fish and Boat Commission and Juniata College’s IACUC committee for approving this work.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Benjamin E. Martin
Ben Martin is currently a PhD student at University of Wisconsin-Madison studying the predator-prey dynamics of an invasive zooplankton (spiny water flea) and native fish. His project is focused on the potential to control spiny water flea populations with predation pressure from native fish, and understanding biological adaptations in light of invasions.
Christopher J. Grant
Ben Martin is currently a PhD student at University of Wisconsin-Madison studying the predator-prey dynamics of an invasive zooplankton (spiny water flea) and native fish. His project is focused on the potential to control spiny water flea populations with predation pressure from native fish, and understanding biological adaptations in light of invasions.
Christopher J. Grant is an assistant professor of Biology at Juniata College. His research lab focuses on involving undergraduate students in research that explores the ecological and toxicological processes that center around freshwater aquatic ecosystems. He is especially interested in trophic dynamics, contaminant bioaccumulation, and conservation of native fishes.
References
- Allen T, Southwick R, Howlett D. 2013. Sportfishing in America: an economic force for conservation. Alexandria (VA): American Sportfishing Association.
- Alvarez DA, Cranor WL, Perkins SD, Schroeder VL, Iwanowicz LR, Clark RC, Guy CP, Pinkney AE, Blazer VS, Mullican JE, et al. 2009. Reproductive health of bass in the Potomac, USA, drainage: Part 2. Seasonal occurrence of persistent and emerging organic contaminants. Environ Toxicol Chem. 28(5):1084–1095.
- Arway JA, Smith G. 2013. The Susquehanna River—a fishery in decline. Fisheries. 38(5):235–236.
- Bethsass J, Colangelo A. 2006. European Union bans atrazine, while the United States negotiates continued use. Int J Occup Environ Health. 12(3):260–267.
- Blazer VS, Iwanowicz LR, Iwanowicz DD, Smith DR, Young JA, Hedrick JD, Foster SW, Reeser SJ. 2007. Intersex (Testicular oocytes) in smallmouth bass from the Potomac River and selected nearby drainages. J Aquat Anim Health. 19(4):242–253.
- Blazer VS, Iwanowicz DD, Walsh HL, Sperry AJ, Iwanowicz LR, Alvarez DA, Brightbill RA, Smith G, Foreman WT, Manning R. 2014. Reproductive health indicators of fishes from Pennsylvania watersheds: association with chemicals of emerging concern. Environ Monit Assess. 186(10):6471–6491.
- Bloodworth B, Marshall CD. 2005. Feeding kinematics of Kogia and Tursiops (Odontoceti: Cetacea): characterization of suction and ram feeding. J Experim Biol. 208(19):3721–3730.
- Dryden IL, Mardia KV. 1998. Statistical shape analysis. Hoboken (NJ): Wiley.
- Elmer KR, Fan S, Kusche H, Spreitzer ML, Kautt AF, Franchini P, Meyer A. 2014. Parallel evolution of Nicaraguan crater lake cichlid fishes via non-parallel routes. Nat Commun. 5(1):5168.
- Farruggia FT, Rossmeisl CM, Hetrick JA, Biscoe M, Branch MR. III. 2016. Refined ecological risk assessment for atrazine. US Environmental Protection Agency. Washington (DC): Office of Pesticide Programs.
- Franssen NR. 2011. Anthropogenic habitat alteration induces rapid morphological divergence in a native stream fish. Evolution Appl. 4(6):791–804.
- Gatz A, Adams S. 1994. Patterns of movement of centrarchids in two warmwater streams in eastern Tennessee. Ecol Freshwater Fish. 3(1):35–48.
- Gerber GP, Haynes JM. 1988. Movements and behavior of smallmouth bass, Micropterus dolomieui, and rock bass, Ambloplites rupestris, in southcentral Lake Ontario and two tributaries. J Freshwater Ecol. 4(4):425–440.
- Hall ES, Martin BE, Brubaker K, Grant CJ. 2018. Latitudinal variation in the geometric morphology of the largemouth bass, Micropterus salmoides. Mar Freshwater Res. 69(9):1480–1485.
- Hall LW, Anderson RD, Kilian J, Tierney DP. 1999. Concurrent exposure assessments of atrazine and metolachlor in the mainstem, major tributaries and small streams of the Chesapeake Bay watershed: indicators of ecological risk. Environ Monit Assess. 59:155–190.
- Hayes TB, Anderson LL, Beasley VR, de Solla SR, Iguchi T, Ingraham H, Kestemont P, Kniewald J, Kniewald Z, Langlois VS, et al. 2011. Demasculinization and feminization of male gonads by atrazine: consistent effects across vertebrate classes. J Steroid Biochem Molec Biol. 127(1–2):64–73.
- Hillis JJ, Garvey JE, Lydy MJ. 2015. Contaminants reduce male contribution to reproduction at the population scale. Ecosphere. 6(4):art66–12.
- Hiramatsu N, Matsubara T, Fujita T, Sullivan CV, Hara A. 2006. Multiple piscine vitellogenins: biomarkers of fish exposure to estrogenic endocrine disruptors in aquatic environments. Marine Biol. 149(1):35–47.
- Homer C, Dewitz J, Yang L, Jin S, Danielson P, Xian G, Coulston J, Herold N, Wickham J, Megown K. 2015. Completion of the 2011 National Land Cover Database for the conterminous United States—representing a decade of land cover change information. Photogramm Eng Remote Sens. 81:345–354.
- Humston R, BM, Priest WC, Hamilton PE, Bugas J. 2010. Dispersal between tributary and main‐stem rivers by Juvenile Smallmouth bass evaluated using otolith microchemistry. Trans Am Fisher Soc. 139(1):171–184.
- Iwanowicz LR, Blazer VS, Pinkney AE, Guy CP, Major AM, Munney K, Mierzykowski S, Lingenfelser S, Secord A, Patnode K, et al. 2016. Evidence of estrogenic endocrine disruption in smallmouth and largemouth bass inhabiting Northeast US national wildlife refuge waters: A reconnaissance study. Ecotoxic Environ Safe. 124:50–59.
- Iwanowicz LR, Pinkney AE, Guy CP, Major AM, Munney K, Blazer VS, Alvarez DA, Walsh HL, Sperry A, Braham R, et al. 2019. Temporal evaluation of estrogenic endocrine disruption markers in smallmouth bass (Micropterus dolomieu) reveals seasonal variability in intersex. Sci Tot Environ. 646:245–256.
- Klingenberg CP. 2011. MorphoJ: An integrated software package for geometric morphometrics. Mol Ecol Res. 11(2):353–357.
- Kolpin DW, Blazer VS, Gray JL, Focazio MJ, Young JA, Alvarez DA, Iwanowicz LR, Foreman WT, Furlong ET, Speiran GK, et al. 2013. Chemical contaminants in water and sediment near fish nesting sites in the Potomac River basin: Determining potential exposures to smallmouth bass (Micropterus dolomieu). Sci Tot Environ. 443:700–716.
- Kortenkamp A. 2007. Ten years of mixing cocktails: a review of combination effects of endocrine- disrupting chemicals. Environ Health Perspect. 115(Suppl. 1):98–S105.
- LeFevre SR. 2005. Juniata river subbasin survey: A water quality and biological assessment, June–November 2004 (Vol. 240). Susquehan River Basin Commission.
- Lee Pow CSD, Law JM, Kwak TJ, Cope WG, Rice JA, Kullman SW, Aday DD. 2017. Endocrine active contaminants in aquatic systems and intersex in common sport fishes. Environ Toxic Chem. 36(4):959–968.
- Matthiessen P, Wheeler JR, Weltje L. 2018. A review of the evidence for endocrine disrupting effects of current-use chemicals on wildlife populations. Critic Rev Toxic. 48(3):195–216.
- Mazzella N, Dubernet JF, Delmas F. 2007. Determination of kinetic and equilibrium regimes in the operation of polar organic chemical integrative samplers: application to the passive sampling of the polar herbicides in aquatic environments. J Chromatogr A. 1154(1-2):42–51.
- Mcllnay D. 2002. Juniata River of sorrow: one man’s journey into a River’s tragic past. Holidaysburg (PA): Seven Oaks Press.
- Norton SF. 1991. Capture success and diet of cottid fishes: the role of predator morphology and attack kinematics. Ecology. 72(5):1807–1819.
- Norton SF, Brainerd EL. 1993. Convergence in the feeding mechanics of ecomorphologically similar species in the Centrarchidae and Cichlidae. J Experim Biol. 176:11–29.
- Norton SF. 1995. A functional approach to ecomorphological patterns of feeding in cottid fishes. In: Ecomorphology of fishes. Dordrecht: Springer; p. 61–78).
- Orlando EF, Denslow ND, Folmar LC, Guillette LJ. Jr. 1999. A comparison of the reproductive physiology of largemouth bass, Micropterus salmoides, collected from the Escambia and Blackwater Rivers in Florida. Environ Health Perspect. 107:199.
- Pinkney AE, Myers MS, Rutter MA. 2017. Histopathology of brown bullhead (Ameiurus nebulosus), smallmouth bass (Micropterus dolomieu), and yellow perch (Perca flavescens) in relation to polychlorinated biphenyl (PCB) contamination in the Hudson River. Sci Tot Environ. 575:1325–1338.
- R Core Team. 2015. R: a language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing.
- Ries KG, III, Steeves PA, Coles JD, Rea AH, Stewart DW. 2004. StreamStats: a US Geological Survey web application for stream information. US Geol Surv Fact Sheet, 3115.
- Robinson BW, Wilson DS. 1994. Character release and displacement in fishes: a neglected literature. Am Natural. 144(4):596–627.
- Rohr JR, McCoy KA. 2010. A qualitative meta-analysis reveals consistent effects of atrazine on freshwater fish and amphibians. Environ Health Perspect. 118(1):20.
- Sass GG, Motta PJ. 2002. The effects of satiation on strike mode and prey capture kinematics in the largemouth bass, Micropterus salmoides. Environ Biol Fish. 65(4):441–454.
- Sepùlveda MS, Quinn BP, Denslow ND, Holm SE, Gross TS. 2003. Effects of pulp and paper mill effluents on reproductive success of largemouth bass. Environ Toxic Chem. 22(1):205–213.
- Shull D, Pulket M. 2015. Causal analysis of the smallmouth bass decline in the Susquehanna and Juniata Rivers. Harrisburg (PA): Pennsylvania Dep. of Environmental Protection, Bureau of Point and Non-Point Source Management.
- Solecki R, Kortenkamp A, Bergman Å, Chahoud I, Degen GH, Dietrich D, Greim H, Håkansson H, Hass U, Husoy T, et al. 2017. Scientific principles for the identification of endocrine-disrupting chemicals: a consensus statement. Arch Toxicol. 91(2):1001–1006.
- Solomon KR, Baker DB, Richards RP, Dixon KR, Klaine SJ, La Point TW, et al. 1996. Ecological risk assessment of atrazine in North American surface waters. Environ Toxicol Chem. 15:31–76.
- Stafford CP, McPhee MV, Eby LA, Allendorf FW. 2014. Introduced lake trout exhibit life history and morphological divergence with depth. Can J Fish Aquat Sci. 71(1):10–20.
- Svanbäck R, Eklöv P. 2004. Morphology in perch affects habitat specific feeding efficiency. Functional Ecology. 18:503–510.
- Van den Brink PJ, Bracewell SA, Bush A, Chariton A, Choung CB, Compson ZG, Dafforn KA, Korbel K, Lapen DR, Mayer-Pinto M, et al. 2019. Towards a general framework for the assessment of interactive effects of multiple stressors on aquatic ecosystems: Results from the Making Aquatic Ecosystems Great Again (MAEGA) workshop. Sci Tot Environ. 684:722–726.
- Wahlström E. 2001. Diet-dependent body morphology and ontogenetic reaction norms in eurasian perch. Oikos. 95:311–323.
- Wainwright PC, Richard BA. 1995. Predicting patterns of prey use from morphology of fishes. Environ Biol Fish. 44(1-–3):97–113.
- Walsh HL, Blazer VS, Smith GD, Lookenbill M, Alvarez DA, Smalling KL. 2018. Risk factors associated with mortality of age-0 Smallmouth Bass in the Susquehanna River Basin. J Aquat Anim Health. 30(1):65–80.
