Abstract
Invasive species are a major and growing threat to China’s native freshwater biodiversity. They have caused species extinctions, biodiversity loss, and environmental changes. Mrigal carp (Cirrhinus mrigala), one of the most widespread and invasive of freshwater fish species, now occurs in major rivers throughout southern China. We report the current (to 2017) distribution of mrigal carp throughout southern China, and experiments with temperature, water quality and food availability, that assess possible impacts of this species on populations of native mud carp (C. molitorella)”. In the wild, the mud carp to mrigal carp weight ratio was 1:1.65. Mrigal carp were a dominant species in some rivers of southern China, such as Liuxi River, Zengjiang River and the Zhaoqing section of Xijiang River. Manipulative experiments reveal mrigal carp to be more tolerant of colder temperature, more eutrophic conditions, and lower dissolved oxygen levels than mud carp. In food-limited experiments, mrigal carp displayed no direct aggression towards mud carp, but they did snatch food from them, and excluded them from eating. Mud carp growth was curtailed in the presence of mrigal carp in food-limited treatments, with a growth rate 2.71%, only 17.02% of that in treatments where carp were fed to satiation. In satiation treatments, mrigal carp also grew up to 2.21 times faster than mud carp. Our results indicate that mrigal carp will outcompete mud carp for resources and habitat, and that both its distribution and population will increase in the future.
1. Introduction
Invasive species present a great threat to aquatic ecosystems (Kolar and Lodge Citation2000; Moyle et al. Citation1999). As both intentional and unintentional introductions of species have increased, some invasive species have established and affected native biodiversity and ecosystem function (Simberloff et al. Citation2013) or posed threats to human health (McMichael and Beaglehole Citation2000). Invasions can render the conservation of natural resources more difficult, and even cause serious economic losses (Pimentel et al. Citation2005). The impact of invasive species on aquatic ecosystems has been ranked the first or second-most’ significant impact on global biodiversity (Kolar and Lodge Citation2000).
Fierce competition as a consequence of niche overlap has been described between native and invasive species (Qin Citation2005). Invasive species often displace native fauna with similar niches by competing for resources and habitat (Taniguchi et al. Citation1998; Jia Citation2005; Blanchet et al. Citation2007; Martin et al. Citation2010; Gu et al. Citation2016). Interspecific relationships between fishes with niche overlaps have been the focus of a number of studies (Wu Citation2001; Li et al. Citation2012). Examples include the Mississippi river basin primitive paddlefish (Polyodon spathula), which has been reduced more seriously in abundance because of competitive interactions with invasive silver and bighead carps, which have the same nutritional niche (Sampson et al. Citation2009), and native whitespotted charr (Salvelinus leucomaenis), which, because of fierce competition and habitat overlap, has been confined to deeper water by invasive brown trout (Salmo trutta) (Morita et al. Citation2004). Biotic interactions such as competition, predation, or exclusion, are major processes structuring communities and influencing populations (Begon et al. Citation1996).
China has experienced numerous biological invasions, deliberate or accidental, with invasive species now occurring in many ecosystems (Wan et al. Citation2015). Mrigal carp (Cirrhinus mrigala) is a successful invader of river systems throughout Guangdong Province (Gu et al. Citation2018), where it has regularly occurred in field surveys from 2011 to 2015. Mrigal carp was initially introduced to Guangdong province, China, for aquaculture in 1982 (Li et al. Citation2007; Fang et al. Citation2017); since 1985 it has been successfully bred in China (Li et al. Citation2007; Fang et al. Citation2017). Owing to its hardy nature and wide range of trophic and ecological adaptations (Yao Citation1999; Ye and Liu Citation1999; Jin et al. Citation2009), mrigal carp was farmed throughout Guangdong province in the 1990s. Cultured mrigal carp then entered natural waters through flooding events, breeding escape, breeding discard, and so on (Yao Citation1999; Jin et al. Citation2009), where it developed large overwintering populations, and in places came to dominate (Gu et al. Citation2012, Citation2018; Hu et al. Citation2015; Wan et al. Citation2017), it has been reported to prey on the fertilized eggs of other fish species (Li et al. Citation2007).
The four major economy carp species within Guangdong province are the mud (Cirrhinus molitorella), grass (Ctenopharyngodon idellus), silver (Hypophthalmichthys molitrix) and bighead (Hypophthalmichthys nobilis) carps (Pan et al. Citation1991). Mud carp are economically important in southern China river systems, especially the Pearl River. Given the congeneric mrigal and mud carp shave similar niches—in that they have similar behaviors (group living, like jumping),similar diets (organic debris, plant debris, and algae), and occupy similar habitat (lower water layer) (Fang et al. Citation2017; Yao Citation1999; Jin et al. Citation2009; Ye and Liu Citation1999). We predict that competitive relationships between them might affect their populations. Although several reports have detailed biological characteristics of mrigal carp (Fang et al. Citation2017; Yao Citation1999; Jin et al. Citation2009; Ye and Liu Citation1999), no study has examined competitive interactions between this species and mud carp, or any mechanism mediating any interactions. Though the distribution of mrigal carp throughout Guangdong Province has been detailed (Hu et al. Citation2015), its wider distribution throughout southern China has not.
We ascertain the distribution of mrigal carp throughout southern China river systems based on field surveys from 2015 to 2017, and report competitive interactions between mrigal and mud carps to understand the effects one might have on the other in series of experiments that manipulate temperature, water quality and food availability. On the bases of these data, we make a prediction regarding the future abundance and distribution of mrigal carp in southern Chinese waters.
2. Materials and methods
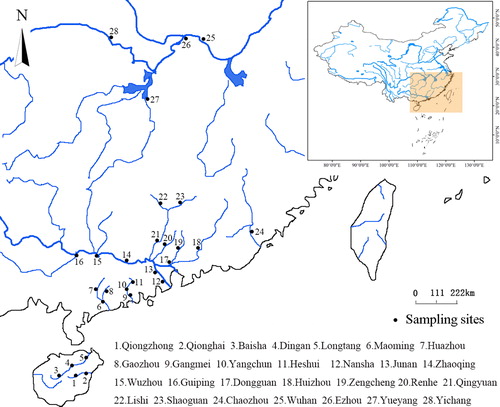
2.1. Field surveys
Surveys were carried out at 28 sites located along the primary rivers of South China: the Xijiang River, Beijiang River, Dongjiang River, Jianjiang River, Moyangjiang River, Hanjiang River, Zengjiang River, Liuxi River, Nandujiang River, Wanquanhe River and Yangtze River, in Hunan, Hubei, Guangdong, Guangxi and Hainan provinces of southern China (). Within different river sections, we selected sampling sites that had been traditional fishing grounds for generations. All samples were collected from commercial fishing boats. In addition, the 28 sampling points were distributed over a narrow longitudinal range (110–116°E), so that relationships between the distributions of the two carp species at different latitudes could be better explored. Fish were collected from June to October in 2015–2017 using licenses provided by the local Oceanic and Fishery Administration. During the survey period, the average minimum and maximum temperatures at sampling sites were 12.5 ± 1 °C, and 32.5 ± 0.5 °C, respectively. (China Meteorological Service network) Sampling at each site occurred over 2 days and involved all fishing boats at each site; no protected species were taken (Gu et al. Citation2018). Fish were caught with the assistance of fishermen using gillnets (3 cm mesh) and shrimp pots, both set for 24 h. Carp were weighed using an electronic scale (± 0.01 g).
As latitude was closely related to minimum temperature at our sampling sites, latitude was used as an indicator of temperature in the wild. A numerical ratio (Rm) was used to represent relationships between latitude and the distribution of mrigal carp (Rm = total number of all mrigal carp from a site/total number of both carp species from a site).
2.2. Experiments
2.2.1. Experimental fishes
Mrigal and mud carps hatched in June 2017 were purchased from the Hegang Fish Farm of Yangchun, Guangdong Province. For acclimation, fish were held in tanks for 10 days, and fed to satiation twice daily (morning and afternoon) using commercial fishing baits (also used in subsequent experimentation). Mortality during acclimation was low (2%).
According to field survey data, the weight ratio of individual mud carp to mrigal carp was 1:1.65. We imitated this weight ratio during experimentation. Carps were individually weighed before experimentation, and then two carps of appropriate size (in accordance with this ratio) were placed into the same tank. Only healthy, brightly coloured, intact fish were chosen from available stocks for subsequent experiments. Selected fish comprised 390 mrigal carp (average size 0.0106 kg) and 390 mud carp (average size 0.0063 kg), of a comparable size to the ratio experienced in the wild (mud/mrigal carp weight 1:1.65). Animal ethics permits were approved by the Aquatic Animal Research Committee of the Pearl River Fisheries Research Institute; experimentation was performed according to the Chinese Association for Laboratory Animal Sciences guidelines for the care and use of laboratory animals.
2.2.2. Experiment 1: temperature tolerance
To determine cold resistance in mrigal and mud carps, we performed lethal temperature (TLD50) experiments. Lethal temperature is conventionally the temperature at which 50% of individuals die due to experimentation (Sun Citation2014). For each treatment, 10 mrigal carp and 10 mud carp (n = 20 total/tank) were combined in one rectangular 10.8 L (30 × 20 × 18 cm high) tank, covered with an anti-skipping dense mesh, filled with water to 13 cm depth, and held in constant temperature chambers (Shanghai Yiheng Science Corporation). Water temperature in treatment tanks was modified gradually to achieve the desired treatment temperature, after which each experiment ran for four hours. There were six treatments at different temperatures (10, 9, 8, 7, 6 and 5 °C); each treatment replicated three times (3 × 6 = 18 treatments in total). Survival rates were averages of the three replicates for each treatment.
2.2.3. Experiment 2: water-quality tolerance
To determine water-quality tolerances of mrigal and mud carps, fishes were exposed to four different soluble phosphate (P) and inorganic nitrogen (N, comprising nitrates, nitrites, total nitrogen) concentrations, with tank concentrations of TN and TP at 2, 3, 4 and 5 times that of eutrophic water (referring to the water eutrophication rating standard). The soluble phosphate and inorganic nitrogen concentrations of eutrophic water are TP = 1.3 mg/L, TN = 16 mg/L (Jin et al. Citation2011). Three replicate experiments of each of four water-quality treatments (2, 3, 4 and 5 times that of eutrophic water) were conducted in 12 adjacent plastic tanks, each filled with 7 L water, with algae, using appropriate amounts of sodium phosphate monobasic dehydrate (NaH2PO4·2H2O) and ammonium nitrate (NH4NO3) to raise TN and TP in tanks to treatment concentrations. Water was first cultured in sunlight for three days before soluble and then total phosphate and inorganic nitrogen (nitrates, nitrites, total nitrogen) were measured using a Multiskan FC Microplate reader. Finally, carps (10 mrigal, 10 mud) were placed into each tank, where their survival was monitored for seven days. For the first three days after the carp were admitted to tanks, dissolved oxygen (DO) was measured in each tank every 2 h from 4:00 to 22:00 using an SX836 multi-parameter water quality analyzer (Shanghai Sanxin instrument factory).
2.2.4. Experiment 3: food competition
To assess competitive interaction for food between mrigal and mud carps, three replicates of each of three food-trial treatments were carried in 9 adjacent glass tanks (40 × 40 cm, 40 cm high). Each tank contained 2 cm of natural gravel, was individually filtered with air-powered foam filters, and was maintained at an experimental water temperature (26 ± 1 °C). Initial fish body weight (m0) was measured using an electronic scale to the nearest 0.1 g. Commercial fishing baits were supplied as food. Each replicate tank contained 10 mrigal and 10 mud carp of weight comparable to their wild-weight ratio (1:1.65). Control treatment (Treatment A) fish were not fed. Treatment B fish were fed baits weighing 3% of the total weight of fish in each tank daily, creating a within-tank food shortage (a competitive environment). Treatment C fish were fed baits weighing 9% of the total weight of fish in each tank daily, satiating them (a non-competitive environment with regard to food). Relative growth rate (%) was calculated as [(m1−m0)/m0 × 100], where m0 represents the average weight of mrigal carp or mud carp in each tank at the beginning of the experiment, and m1 represents the average weight in each tank at the end of the experiment, 20 days later. Interactions between these two carp species were also recorded during 15-minute observation periods every two days.
2.3. Statistical analyses
Preliminary analysis of field survey and experimental data was first conducted in Excel2007. Statistical tests were performed using SPSS (version 19.0), with significance levels p = 0.05, and 0.01. Differences in temperature-related mortality, water-quality-related mortality, and fish growth under different feeding regimes, were analysed by way of two-way ANOVA. Relationships between latitude and mrigal carp numerical ratio (Rm) were analysed using linear regressions. Bar and line charts were developed in SPSS, the survey map was painted in Adobe Photoshop CS6 and Surfer (v 11), and image post-processing was performed in Adobe Illustrator CS5.
3. Results
3.1. Carp biological parameters and distribution
In total, 1070 mrigal carp weighing 257.46 kg, ranging 0.025–3.5 kg individual weight, of average size 0.241 ± 0.013 kg, and 1857 mud carp weighing 271.18 kg, ranging 0.020–0.7 kg individual weight, averaging 0.146 ± 0.005 kg, were caught. The weight ratio of individual wild mud to mrigal carp was 1:1.65. The largest wild mrigal and mud carp individuals were 3.5 kg and 0.7 kg, respectively. The numbers and weights of these two carps at each of the 28 sampling sites were presented in .
Table 1. Field parameters of mrigal and mud carps.
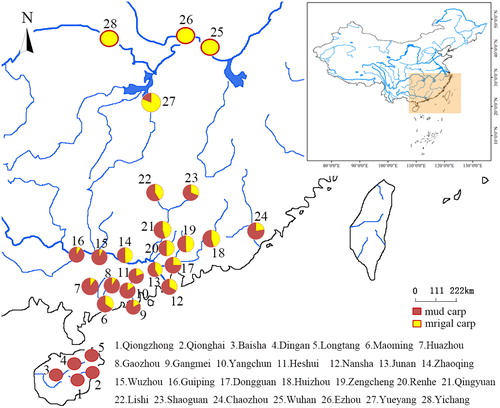
The numerical proportion and distribution of these two carps in southern China were shown in . Mrigal carp were captured in all but Hainan sampling sites; mud carp occurred everywhere, although few were caught in Hubei sites. In the sites located in Guangdong and Guangxi Provinces, mud carp was more abundant than mrigal carp in terms of individual numbers.
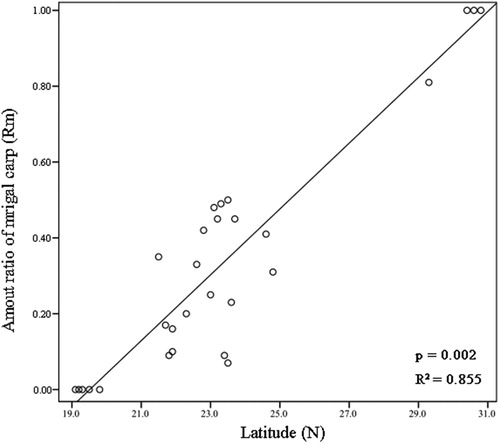
Relationships between latitude and numerical ratios (Rm) of mrigal carp were shown in . Rm values decreased as latitude increased, with the relationship between strong and linear. At the Wuhan, Ezhou and Yichang sampling sites, Rm was 1; at Yueyang sites, Rm was 0.81; Rm values ranged 0–0.49 at lower-latitude sites.
3.2. Experiments
3.2.1. Temperature tolerance
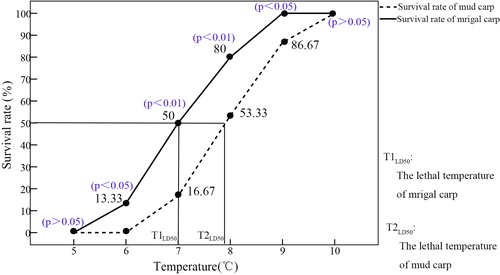
Mrigal and mud carps survival at 10 °C was 100% (p > 0.05). There was no change in mrigal carp survival rate at 9 °C, but mud carp survival rate decreased to 86.67% (p < 0.05). Survival rate decreased for both mud and mrigal carp as temperatures decreased from 8 °C to 7 °C, with the latter noticeably swimming more slowly (p < 0.01). At 6 °C, all mud carp had perished, while mrigal carp became dully and had an average survival rate of 13.33% (p < 0.05). At 5 °C, all mrigal carp perished (p > 0.05). The lethal temperature (TLD50) for mrigal carp (T1LD50 = 7 °C) was 0.8 °C lower than that for mud carp (T2LD50); the lowest temperature at which mrigal carp survived (5 °C) was 1 °C lower than mud carp (). (These p value meant that the different significance levels between survival of mrigal and mud carp at the same temperature).
3.2.2. Water-quality tolerance
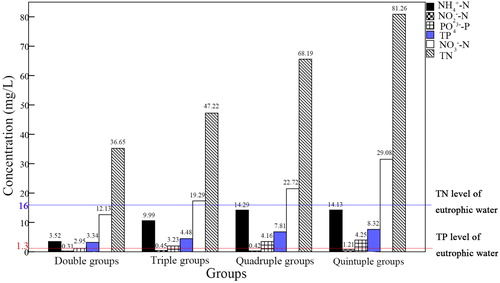
Differences in P and N measured three days after cultivation are shown in ; maximum values occurred in treatments containing 5 × TN and TP levels; minimum values occurred in treatments containing 2 × TN and TP levels. In all treatments, TN and TP ranged 40.21–80.88 mg/L, and 9.22–15.63 mg/L, respectively ().
Two-way ANOVA () revealed extremely significant differences in DO throughout the day (p < 0.01), that different levels of eutrophication (multiple treatments) had significant effects on DO (p = 0.022), and that the interaction between levels of eutrophication and time had an extremely significant impact on DO (p < 0.01). The lowest DO values appeared in the most eutrophic treatment (TN and TP × 5) at 04:00 h, with the three replicate values ranging 1.71–1.8 mg/L. Maximum DO values were experienced in moderately eutrophic conditions (TN, TP × 2) at 16:00 h, with replicate values ranging 11.7–12.6 mg/L. Significant differences in DO among all groups were not apparent between 12:00 and 14:00 h (ANOVA, p > 0.05). DO in each treatment first increased, then decreased from 04:00 to 22:00 h each day.
Table 2. Tests of between-subject effects.
Mean survival rates of mrigal carp were 100%, 100%, 83.33% and 33.33% in the 2, 3, 4 and 5 × TN and TP treatments; corresponding mean survival rates for mud carp were 100%, 86.67%, 43.33% and 10%. Survival rates of mrigal carp in all treatments were higher than mud carp. Differences between survival rates of the two species were significantly different at treatment concentrations of: double groups (p > 0.05), triple groups (p < 0.05), quadruple groups (p < 0.01), and quintuple groups (p < 0.01).
3.2.3. Food competition
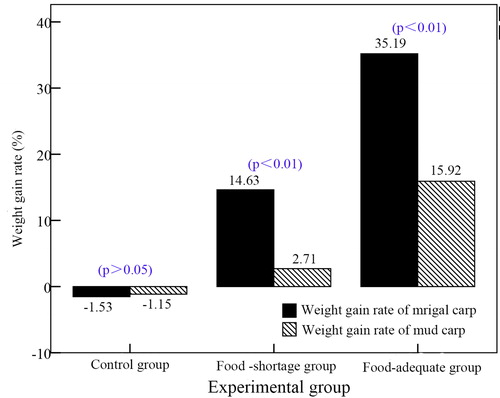
No mortality was observed over the 20-day experiment duration. Weight of mrigal carp in treatment A (control) decreased by 1.53%, while that of mud carp decreased by 1.15% (p > 0.05). As food was limited in treatment B, all food was consumed during the experiment and water quality remained clear; during feeding, mrigal carp rushed to eat bait, and snatched it, whereas mud carp reached and ate only floating debris. Weight of mrigal carp in treatment B increased by 14.63%, whereas that of mud carp increased by only 2.71% (p < 0.01). Fish in treatment C were fed to satiation, and the water became cloudy and had to be periodically changed (every four days) to prevent fish mortality. In this treatment, both carp species ate bait and increased in weight (mrigal carp in treatment C by 35.19%, mud carp by 15.92%) (p < 0.01). Growth rates are depicted in . (These p value meant that the different significance levels between weight change rates of mrigal and mud carp at the same treatment).
Multiple comparison tests demonstrated: a significant difference existed between the weight change rates of mrigal carp in treatments A and B (p < 0.05), but not between mud carp in these two treatments (p > 0.05), and a significant difference in the growth rates of mrigal carp in treatments B and C (p < 0.05), and an extremely significant difference in growth rates of mud carp between these two treatments (p < 0.01).
4. Discussion
4.1. Field surveys
The largest wild mrigal carp we caught (3.5 kg) was far larger than the largest mud carp (0.7 kg); the average mrigal carp weight was 1.65 times that of mud carp. After consulting some fishermen, despite their larger size, wild-caught mrigal carp retail at about 7 yuan/kg, which renders them economically unattractive; no fishermen deliberately target this species on a large scale. To make matters worse, mrigal carp is also relatively unpalatable, rendering its meat unpopular, which are described by customers. Unlike in the 1990s (Fang et al. Citation2017; Jin et al. Citation2009; Ye and Liu Citation1999), few farms today specialize in the culture of adult mrigal carp, with the larvae of these fish today basically used as live baits for other species, such as Chinese perch (Siniperca chuatsi). In contrast, fishermen said that the meat of mud carp is delicious and profitable (16 yuan/kg), and they have increased effort to target this species, which will naturally impact their wild populations.
In recent years, studies have reported mrigal carp throughout Xijiang, Dongjiang, and Beijiang rivers (Li et al. Citation2010; Liu Citation2011; Gao et al. Citation2017); this species has also been recognized as a dominant species in Beijiang River during summer and autumn (Gao et al. Citation2017), and to have invaded Guangdong Province (Gu et al. Citation2018). We now recognize this species to occur in most parts of southern China: they are abundant in Guangdong Province, especially in the Beijiang River, Zeng jiang River and Liuxihe River, and the Zhaoqing section of the Xijiang River. Although not yet a dominant species in the Xijiang River basin, Guangxi Province, mrigal carp are common. There are also significantly more mrigal carp than mud carp in Dongting Lake (lower Yangtze River reaches), Yueyang, in the northern Hunan Province. In the higher latitude (cooler area) Hubei Province (middle reaches of Yangtze River), few mud carp occurred, but mrigal carp occasionally occurred in catches. Why mrigal carp are absent from Nandujiang River and Wanquanhe River, Hainan Island, as also reported by Yu et al. (Citation2018), is unknown.
4.2. Manipulative experiments
Temperature is an important ecological factor that works everywhere, and no organism can be completely free from it (Sun Citation2014). As mrigal carp are more tolerant of colder temperatures than mud carp, they might be expected to have greater geographic and altitudinal distributions. This may explain why there are fewer mud than mrigal carp at higher latitudes, such as sampling point in Hunan and Hubei Province.
Mrigal carp not only tolerate more eutrophic conditions than mud carp, but also have greater survival rate (33.33%) in the lowest DO eutrophic conditions compared with mud carp (10%). However, one reality is that many river basins in China are subject to varying degrees of pollution (Gu et al. Citation2012; Liu Citation2011; Yu et al. Citation2018). Therefore we suspect that this factor will mediate in the competition between these two species, and mrigal carp will eventually win in the wild without any management measures. This requires further research in the future.
Our food experiments reveal the nature of competition between mrigal and mud carps to be ‘exploitative’ (Wan et al. Citation2015)—a type of competition in which mrigal carp will likely outcompete mud carp by reducing resources, without any aggressive behavior. Mrigal carp took less time to search for, and snatched and acquired more food than mud carp; mrigal carp also ate clumps of food, whereas mud carp tended to eat excess scraps. Mud carp growth was severely curtailed in the presence of mrigal carp, and in food-limited treatments their weight increased by only 2.71% over 20 days, only 17.02% of their growth rate in treatments where they were fed to satiation. When both carps were fed to satiation for 20 days, mrigal carp growth rate (35.19%) was 2.21 times that of mud carp (15.92%). Mrigal carp appear more capable of acquiring limited resources and habitat, and in a competitive environment, both grow faster and attain a larger size. Accordingly, we predict that increases in the distribution and abundance of mrigal carp might lead to declines in populations of mud carp.
4.3. Potential risks
Temperature, TN and TP tolerance, tolerance to low DO, and the competitive advantage mrigal carp have over mud carp for limited food resources, in addition to their greater growth rates and size of the former, are all characteristics that facilitate their invasion in southern China rivers. Invasive species can disrupt ecological balances and cause extinction of native species (Stone and Sunnucks Citation1993; Tsutsui and Case Citation2001; Bryant and Meffert Citation1990; Britton-Davidian et al. Citation2000), particularly when one species is capable of acquiring more resources than another, and is more tolerant of environmental conditions (Wan et al. Citation2015). When sympatric, mrigal carp prove to be more successful than mud carp in acquisition of limited food resources. Because omnivorous fish prey on the eggs of other fish (Maezono and Miyashita Citation2003), and mrigal carp is no exception (Li et al. Citation2007), this carp may also affect populations of mud carp by consuming its eggs.
The ability of a species to invade a new habitat is often, related to its invasive power and the health of the ecosystem (San and Brown Citation2000; Elton Citation1958), with low biodiversity and frequent disturbance being two features typical of ecosystems prone to invasion (Townsend Citation1996; Doyle and Light Citation1996). In terms of invasive power, invasive species with wider ecological valence, higher tolerance and r-selection strategy, generally also find it easier to invade (Qin Citation2005). In China, frequent disturbance and overfishing of native species have affected environmental quality, and the richness and abundance of native fish (San and Brown Citation2000), which may all facilitate the invasion of mrigal carp. Although mud carp have large populations in Guangdong and Guangxi provinces (simply because this species was historically cultured in these areas), the lack of co-evolution of this species with mrigal carp means it might be disturbed or even replaced by the latter without appropriate management. We predict (if water quality is not better managed) that mrigal carp will likely continue to expand their range and population size throughout southern China as they can survive at higher latitude (cooler temperatures) and are more tolerant of polluted waters and lower DO than mud carp.
To reduce the impact of mrigal carp, the breeding and breeding-areas of invasive species should be better managed, water pollution and the maintenance of water quality need to be addressed, the biodiversity of rivers needs to be better protected, and human interference on the environment should be reduced (Gu et al. Citation2018). Additionally, research on and development of specific drugs and assist in control of mrigal carp in the wild should be explored. Such as ‘Mie fei ling’ were used to control wild invasive Nile tilapia in small water bodies (Ma et al. Citation2014).
Notes on contributors
Fan Dong Yu is a master student who is studying in Pearl River Fisheries Research Institute, Chinese Academy of Fishery Sciences and Shanghai Ocean University.
Dang En Gu is a researcher of Pearl River Fisheries Research Institute, Chinese Academy of Fishery Sciences with interests in the Monitoring and management of alien fish species.
Yan Nan Tong is an assistant researcher of Hainan Academy of Ocean and Fisheries Sciences, China, where he worked as a fisheries scientist.
Gao Jun Li is an assistant researcher of Hainan Academy of Ocean and Fisheries Sciences, China, where he worked as a fisheries scientist.
Hui Wei is an assistant researcher of Pearl River Fisheries Research Institute, Chinese Academy of Fishery Sciences, where she worked as an ecologist.
Xi Dong Mu is a researcher of Pearl River Fisheries Research Institute, Chinese Academy of Fishery Sciences, where he worked as a fisheries scientist.
Meng Xu is an assistant researcher of Pearl River Fisheries Research Institute, Chinese Academy of Fishery Sciences, where he worked as an ecologist.
Ye Xin Yang is an assistant researcher of Pearl River Fisheries Research Institute, Chinese Academy of Fishery Sciences, where she worked as a fisheries scientist.
Du Luo is an assistant researcher of Pearl River Fisheries Research Institute, Chinese Academy of Fishery Sciences, where he worked as an ecologist.
Fang Yuan Li is an assistant researcher of Hainan Academy of Ocean and Fisheries Sciences, China, where he worked as a fisheries scientist.
Yin Chang Hu is a professor of Pearl River Fisheries Research Institute, Chinese Academy of Fishery Sciences, where he worked as an ecologist.
Disclosure statement
The authors declared that they have no conflicts of interest to this work.
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Additional information
Funding
References
- Bryant EH, Meffert LM. 1990. Multivariate phenotypic differentiation among bottleneck lines of the housefly. Evolution. 44(3):660–668.
- Begon M, Harper JL, Townsend CR. 1996. Ecology. Individuals, populations and communities. 3rd ed. Oxford (UK): Blackwell Scientific Publishers.
- Britton-Davidian J, Catalan J, da Graça Ramalhinho M, Ganem G, Auffray JC, Capela R, Biscoito M, Searle JB, da Luz Mathias M. 2000. Rapid chromosomal evolution in island mice. Nature (London). 403(6766):158–158.
- Blanchet S, Loot G, Grenouillet G, Brosse S. 2007. Competitive interactions between native and exotic salmonids: a combined field and laboratory demonstration. Ecol Freshwater Fish. 16(2):133–143.
- Moyle PE, Claudi R, Leach JH. 1999. Non-indigenous freshwater organisms: vectors, biology and impacts. Quart Rev Biol. 81(10):244.
- Doyle PB, Light T. 1996. Fish invasions in California: do abiotic factors determine success? Ecology. 77(6):1666–1670.
- Elton CS. 1958. The ecology of invasions by animals and plants. London, U.K.: Methuen.
- Fang L, Liang XF, Li J, Yi TL, Xu QQ, Fang JC, Zhang J, Yang ZC. 2017. Comparative study on growth, water quality and gastrointestinal bait biological structure of mud carp in two farming m odes (Cirrhinus mrigola). Guangdong Agric Sci. 44(1):143–148. (in Chinese).
- Gu DE, Mu XD, Luo D, Li YY, Wang XJ, Hu YC. 2012. The distribution of alien aquatic animals in the main rivers of Guangdong Province, China. J Biosafe. 21(4):272–276. (in Chinese).
- Gu DE, Mu XD, Xu M, Luo D, Wei H, Li YY, Zhu YJ, Luo JR, Hu YC. 2016. Identification of wild tilapia species in the main rivers of south China using mitochondrial control region sequence and morphology. Biochem Syst Ecol. 65:100–107.
- Gu DE, Hu YC, Xu M, Wei H, Luo D, Yu FD. 2018. Fish invasion in the river systems of Guangdong Province, South China: Possible indicators of their success. Fish Manag Ecol. 25(1):44–53.
- Gao TY, Xie D, Peng ND, Chao LI, Zhang SP. 2017. Fish community structure and its relationships with environmental factors in the Beijiang River. Freshw Fish. 47(6):47–55.
- Hu YC, Gu DE, Mu XD. 2015. The common alien aquatic species in China. Beijing, China: Science Press. (in Chinese).
- Jin C, Zhang WT, Sun Z. 2011. Discussion on evaluation method and grading standard of eutrophication of lakes. Guizhou Water Power. 25(5):4–6. (in Chinese).
- Jia YJ. 2005. Comparative studies on the physiological ecology of exotic and native fishes in lakes of the Yunnan Plateau [Doctoral dissertation]. Exotic Fish. (in Chinese).
- Jin G, Yu ZH, Yan Y. 2009. P. Pond culture technology of mrigal carp. Fish Sci Technol Ind. 2009(4):12–14. (in Chinese).
- Kolar CS, Lodge DM. 2000. Freshwater nonindigenous species: interactions with other global changes. In: Mooney, H.A. and Hobbs, R.J. editors, Invasive species in a changing world. Washington, D. C.: Island Press; p. 3–30.
- Li JL, Dong ZG, Li YS, Wang CH. 2007. Chinese exotic aquatic animals and plants. Shanghai: Shanghai Science Press. (in Chinese).
- Li J, Li XH, Jia XP, Li YF, He MF, Tan XC, Wang C, Jiang WX. 2010. Evolvement and diversity of fish community in Xijiang River. Journal of Fishery Sciences of China. 17(2):298–311. (in Chinese).
- Liu Y. 2011. Fish community changes and the evaluating the biotic integrity in Dongjiang River mainstream (Master's dissertation). Guangzhou: Jinan University. (in Chinese).
- Li SF, Liu WD, Su JR, Lang XD, Zhang ZJ. 2012. Niche and interspecific association of species of Taxus Yunnan ensis communities in Northwest Yunnan Province. Plant Sci. J. 30(6):568–576.
- McMichael AJ, Beaglehole R. 2000. The changing global context of public health. Lancet. 356(9228):495–499.
- Maezono Y, Miyashita T. 2003. Community-level impacts induced by introduced largemouth bass and bluegill in farm ponds in Japan. Biol. Conserv. 109(1):111–121.
- Morita K, Tsuboi J-I, Matsuda H. 2004. The impact of exotic trout on native charr in a Japanese stream. J Appl Ecol. 41(5):962–972.
- Martin CW, Valentine MM, Valentine JF. 2010. Competitive interactions between invasive Nile tilapia and native fish: the potential for altered trophic exchange and modification of food webs. Plos One. 5(12):e14395.
- Ma GM, Gu DE, Mu XD, Luo JR, Hu YC. 2014. Poison effect of “Mie fei ling” on the alien Nile tilapia (Oreochromis niloticus). Chin J Ecol. 33(9):2442–2447. (in Chinese).
- Pan JH, Zhong L, &Zheng CY. 1991. The freshwater fishes of Guangdong Province. Guangdong: Guangdong Science and Technology Press. (in Chinese).
- Pimentel D, Zuniga R, Morrison D. 2005. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol Econ. 52(3):273–288.
- Qin JH. 2005. Ecological effects of the invasive Icefish Neosalanx taihuensis in a deep oligotrophic plateau lake, Lake Fuxian [Doctoral dissertation]. Graduate School of Chinese Academy of Sciences Institute of Hydrobiology. Chin Acad Sci Wuhan, Hubei, P. R. China. (in Chinese).
- Stone GN, Sunnucks P. 1993. Genetic consequences of an invasion through a patchy environment—the Cynipid gallwasp and Ricus quercuscalicis (Hymenoptera: Cynipidae). Mol Ecol. 2(4):251–268.
- San DF, Brown JH. 2000. The paradox of invasion. Global Ecol Biogeography. 9:363–371.
- Sampson SJ, Chick JH, Pegg MA. 2009. Diet overlap among two Asian carp and three native fishes in backwater lakes on the Illinois and Mississippi rivers. Biol Invasions. 11(3):483–496. doi:10.1007/s10530-008-9265-7
- Simberloff D, Martin JL, Genovesi P, Maris V, Wardle DA. 2013. Impacts of biological invasions: What’s what and the way forward. Trends Ecol Evol. 28(1):58–66.
- Sun RY. 2014. Animal ecology. Beijing: Beijing Normal University Press. (in Chinese).
- Taniguchi Y, Rahel FJ, Novinger DC, Gerow KG. 1998. Temperature mediation of competitive interactions among three fish species that replace each other along longitudinal stream gradients. Can J Fish Aquat Sci. 55(8):1894–1901.
- Townsend CR. 1996. Invasion biology and ecological impacts of brown trout (Salmo trutta) in New Zealand. Biol Conserv. 78(1-2):13–22.
- Tsutsui ND, Case TJ. 2001. Population genetics and colony structure of the Argentine ant (Linepithema humile) in its native and introduced ranges. Evolution. 55(5):976–985.
- Wu DR. 2001. A study on the niche of dominant species in Phoebe bournei forests in Luoboyan Nature Reserve of Fujian. Acta Ecol Sin. 21(5):851–855.
- Wan FH, Hou YM, Jiang MX. 2015. Invasion biology. Beijing: Science Press. (in Chinese).
- Wan FH, Jiang MX, Zhan AB. 2017. Biological invasions and its management in china. The Netherlands: Invading Nature - Springer Series in Invasion Ecology.
- Yu FD, Wang DQ, Gu DE, Hu YC. 2018. Species composition and distributional status of fishes in Nandujiang River, Hainan Island, China. Freshw Fish. 48(2):56–67. (in Chinese).
- Yao GC. 1999. The monster in the water “mrigal carp. Rural Knowl. 99(24):22. (in Chinese).
- Ye X, Liu JZ. 1999. Comparisons of culture effects, cold-resistance and nutrient contents in muscles between Cirrhinus mrigala and C. molitorella. J Fish Sci China. 6(4):126–128. (in Chinese).