 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Regulation of water flow by dams for flood control and power generation has changed the patterns of water level fluctuation in the lower Yangtze River. By investigating the branch and leaf traits of M. laxiflora plants at various growth recovery stages along the water fluctuation gradient, this study uncovered the spatiotemporal response in the relationships among branch and leaf traits during growth to the regulated water level fluctuations. Results indicated leaf number (LN) and leaf-and-branch volume (LV) rapidly increased during the first 30 d of growth recovery. Afterwards, the increase in LN gradually slowed, while LV continued to increase steadily and was isometric to the increase in leaf-and-branch dry mass (LM). The investment strategy of this plant species changed from ‘fast return’ to ‘slow return’ as plant growth was restored. In the upper hydro-fluctuation zone, the LN vs. LM and leaf-and-branch water content (LWC) vs. LM comparisons exhibited negative allometric growth, while the LV vs. LM comparison exhibited positive allometric growth. In the middle hydro-fluctuation zone, the LN vs. LM and LV vs. LM comparisons exhibited isometric growth patterns, while the LWC vs. LM comparison exhibited positive allometric growth. In the lower hydro-fluctuation zone, the LN vs. LM comparison exhibited positive allometric growth, while the LWC vs. LM comparison exhibited negative allometric growth. As water level decreased, the investment strategies of the plants switched from prioritizing LV and LWC to LN, and shifted from ‘slow return’ to ‘fast return’. The above results indicate that changes in water level fluctuation patterns have greatly affected branch and leaf growth, as well as the growth relationships among branch and leaf traits in the remnant populations of M. laxiflora. Growth of these remnant populations, especially those at the lower hydro-fluctuation zone, was seriously affected and exhibited signs of degradation as a result.
Regulation of water flow by dams for flood control and power generation has changed the patterns of water level fluctuation in the lower Yangtze River.
The human water flow regulation impacts the allometric growth of M. laxiflora in terms of branch and leaf traits, as well as the investment strategies during growth.
Clear differences were observed in the growth patterns of branches and leaves at different levels along the water fluctuation gradient.
HIGHLIGHTS
1. Introduction
Growth relationships among plant traits often change in heterogeneous environments (Bloomfield et al. Citation2018; Geng et al. Citation2019) . Such growth patterns and their variations are often used to characterize adaptation mechanisms of plants to environmental changes and investment strategies in heterogeneous environments (Vendramini et al. Citation2002; Poorter et al. Citation2018) . Allometric growth refers to the difference in the relative growth rate of a given plant trait from another trait. The allometric growth of plants under different environmental conditions will tend toward an optimal allometric growth curve (Voje et al. Citation2014; Wiebke et al. Citation2017) . Branches and leaves are important organs for plant photosynthesis and their synthesized nutrients form the basis of growth and reproduction (Inamoto et al. Citation2015). The allometric growth of branch and leaf traits reflect adaptation to environmental heterogeneity through biomass allocation and resource utilization (Lu et al. Citation2007; Liu et al. Citation2019) .
Water level fluctuation is a critical ecological process affecting the ecological environment of riparian plant habitats. The above-ground growth of riparian plants is mainly affected by water level fluctuation (de Assis et al. Citation2019). Under the influence of water level fluctuation, the allocation of biomass often exhibits spatial and temporal heterogeneity (Spencer et al., Citation2005; Li et al. Citation2017) . Allometric growth of branches and leaves in the riparian zone often respond to water level fluctuations in order to minimize the effect of changes in the environment on growth and development (Chen and Xie Citation2009; Li et al. Citation2013) . However, when the fluctuation patterns of river water levels are altered due to human water flow regulation, the aquatic ecosystem will also exhibit long-term changes, thereby affecting the pattern of allometric growth, ability to utilize resources, and investment strategies in plants (Li et al. Citation2013). In the long run, plant growth and reproduction are affected (Bijarchi et al. Citation2011).
Myricaria laxiflora ((Franch.) P. Y. Zhang et Y. J. Zhang) is a shrub of the Myricaria genus and Tamaricaceae family. It is native to the flood zones located along the upper-middle region of the Yangtze River from Zhijiang, Hubei Province to Yibin, Sichuan Province. The Three Gorges Reservoir Region comprises the central area of this species’ distribution (Chen et al. Citation2005). The construction of the Three Gorges Dam has caused substantial water level elevation in the Reservoir Region, submerging all of this species’ habitats in that area and endangering the species. Remnant populations are only found on a few islands in the mainstem of Yangtze River, downstream of the Xiangjiaba and Gezhouba Dams (Chen and Wang Citation2015). In order to prevent flooding and generate power, cascade hydropower stations, such as the Three Gorges-Gezhouba, imposes human river flow regulation, which has considerably altered water level fluctuations in the habitats of the remnant M. laxiflora populations. The exposure period has been delayed, and the entire growth period shortened. Water level recession has accelerated during the growth recovery stage after floods subside. The groundwater level during the dry season of winter has also been reduced significantly (Duan et al. Citation2016). Human water flow regulation severely impacted seedling regeneration, photosynthetic physiology and sexual reproductive efficiency of the remnant M. laxiflora populations (Bao et al. Citation2010; Chen et al. Citation2019D:\Praveen\TF-TJFECitation200012\7; Guan et al. Citation2020a, Citation2020b) .
The young branches and leaves of M. laxiflora wither and shed during plant dormancy every summer as an adaptation to flooding. After flooding, the branches and leaves promptly resume their growth to assimilate light (Chen et al. Citation2005). As growth is restarted, allometric growth among the traits of young branches and leaves of perennial plants is highly sensitive to changes in the external environment (Li et al. Citation2018). To understand the effects of human regulated water level fluctuations on the branch and leaf growth relationship, investment strategies in the remnant populations of M. laxiflora, we investigated the changes in branch and leaf traits in the remnant populations distributed in different parts of the hydro-fluctuation zone during the growth recovery stage after habitat exposure. Our goals were to compare the differences in branch and leaf traits and their relationships during different growth recovery stages along a water level gradient, and analyze the spatiotemporal responses of growth relationships among branch and leaf traits and the investment strategies to human regulated water level fluctuations. Further, the effects of human river flow regulation on the growth of remnant M. laxiflora populations were explored, taking into consideration the environmental changes downstream caused by the Three Gorges-Gezhouba cascaded hydropower stations, including water level fluctuation patterns and altered habitat exposure periods and the resulting timing.
2. Materials and methods
2.1. Study site profile and plot distribution
The remnant populations of M. laxiflora are located in the transition zone between central and northern subtropical China with an average annual temperature of 17.1–19.5 °C. The average temperatures in July and January are 28.0–29.6 °C and 5.0–7.1 °C, respectively. The average annual precipitation is 1000–1220 mm. The region remains frost-free for ≥ 300 d each year. The climate is mild with warm winters and cool summers, and has abundant precipitation (Chen and Wang Citation2015). In this study, Yanzhiba Island located in the Yichang section of mainstream Yangtze River downstream of the Three Gorges-Gezhouba cascade hydropower stations was selected as the study site (111°19’26′E, 30°38’57′N). This habitat is the home of a large community of remnant M. laxiflora populations of more than 10,000 plants (Chen and Wang Citation2015). The soil type of the habitat is sandy soil, with shrubs as the main vegetation type. The dominant species of the shrub layer includes M. laxiflora and Salix variegata, while the herbaceous layer includes Phragmitesaustralis and Cynodondactylon.
Due to seasonal fluctuations in water levels of the Yangtze River, the remnant populations of M. laxiflora are submerged by rising floods from June to September every year, which then grow and reproduce between September and June during the following year. At different elevations, the habitats experience diverse environments, such as submergence and exposure, caused by water level fluctuations. Therefore, timing varies in the growth and development of plants found at different levels along the water fluctuation gradient. The study site was divided into three sampling zones with respect to the water fluctuation gradients: upper (elevation ≥ 45.1 m), intermediate (45.1 m > elevation > 42.8 m), and lower (elevation ≤ 42.8 m) (Chen et al. Citation2019). Quadrats (5 m × 5 m) were set up in 10 m intervals so that each sampling zone consisted of a total of 10 quadrats; the entire study site consisted of 30 quadrats. Changes in the branch and leaf traits were measured on M. laxiflora plants in each quadrat. The temporal and spatial variations in the branch and leaf traits and variations in the growth relationships among these traits were investigated.
2.2. Fluctuation of water levels in the habitat and changes in the environment
From the beginning of habitat exposure, water levels in the selected habitat of the remnant populations of M. laxiflora were monitored and recorded daily based on data recorded at the Yichang section of the Yangtze River during the sampling period (www.cjh.com.cn). Field surveys were conducted to assess the degree of exposure of the sampling zones according to changes in Yangtze River water levels above sea level during the growth recovery period (). The upper hydro-fluctuation zone was initially exposed on September 9, 2018. The middle and lower parts of the hydro-fluctuation zone were exposed on September 19 and November 3, 2018, respectively. Between September 16 and October 14, 2018, the plants in the middle hydro-fluctuation zone were submerged twice due to the rise of Yangtze River water levels. The exposure periods of the upper, middle, and lower parts of the hydro-fluctuation zone were 250, 230, and 180 d, respectively (2018–2019).
Figure 1. Water level fluctuations and changes in exposure time and soil water content along sample transects. Soil water content in middle and upper parts of the fluctuation zone changes within a certain range with water level fluctuation, while soil water content in the lower part of the fluctuation zone decreases sharply with rapid decline of water level.
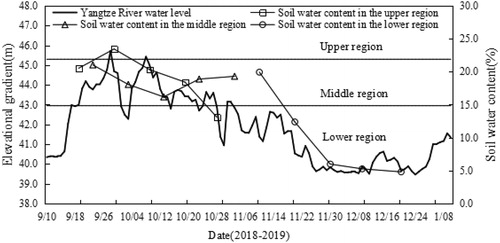
Water content of the soil was also measured as the branches and leaves were collected. For every sampling zone, three points were randomly selected in each quadrat. Soil samples were gathered with a ring sampler (15 cm depth) and brought back to the laboratory for drying (at 110 °C to a constant weight) and weighing in order to measure the water content. Thirty soil samples were collected each time from every sampling zone.
2.3. Quantification of growth traits for plant branches and leaves
In this study, the leaf number (LN), leaf-and-branch volume (LV), leaf-and-branch water content (LWC), and leaf-and-branch dry mass (LM) were measured. LN represents the light assimilation ability of the plant. LV and LM indicate the comprehensive investment of the plant. LWC reflects the metabolism level of branches and leaves (Roderick et al. Citation1999, Citation2000).
After 10, 20, 30, 40, and 50 d of exposure, one M. laxiflora plant with similar growth conditions was randomly selected from each quadrat as the study subject. For each plant, three secondary branches were picked at random to quantify the branch and leaf traits. Thirty secondary branches were retrieved from every sampling zone on each of the above sampling days. The collected samples were brought back to the laboratory in an ice box.
The LN on each secondary branch was counted, which was then placed in water for 12 hr in darkness at 5 °C. Upon removal, the surface of the branch was immediately dried with filter paper and the fresh weight of the saturated branch was measured on an electronic balance to a precision of 0.0001 g. LV was determined using the water displacement method by immersing the branch in a container of 0.01 mL precision (Zheng et al. Citation2014). Finally, the branch was heated at 105 °C for 15 min and dried to constant weight at 60 °C. The final weight was LM. LWC was calculated as follows:
2.4. Data analysis
The mean values of the branch and leaf traits were converted to common logarithms so that they exhibited a normal distribution. The equation for allometric growth (y = αxβ) was re-written as a logarithmic expression (logy = logα + βlogx), where x and y represent different leaf and branch traits, α is the constant of allometric growth, and β is the correlation slope (i.e., the scaling exponent of allometric growth). The data from each sampling zone was first used to find the relationships between LN, LV, LWC, and LM at different time points. Standardized major axis estimation (SMA) was utilized to estimate the parameters of trait correlations (Warton et al. Citation2006). The confidence interval for the correlation slope was determined following the methods described by Pitman (Citation1939). Slope heterogeneity was determined following the methods described by Warton and Weber (Citation2002). For slope homogeneity (differences in terms of branch and leaf traits are not significant(P > 0.05)), the common slope was computed and the significant differences between the slope and value (1.0) was analyzed. If the difference between β and 1.0 was significant, the leaf and branch traits exhibited an allometric growth relationship. Otherwise, the growth pattern of the traits was isometric. Upon detection of slope heterogeneity (differences in terms of branch and leaf traits are significant (P < 0.05)), multiple comparison tests followed by Tukey’s post-hoc test were conducted to analyze the relationships between growth traits at different time periods (10d periods between samplings) . The above procedure was repeated to determine the slopes for the growth relationships of the plant species across the entire study site during different time periods and at different elevations. All analyses were performed using R v3.4 software (http://www.r-project.org).
3. Results
3.1. Relationship between LN and LM during growth
A significant correlation was observed between LN and LM in the remnant populations of M. laxiflora across the whole hydro-fluctuation zone for different growth recovery stages (p < 0.05) (). The common slope of the LN–LM curve increased at first then decreased as growth resumed. The maximum slope (0.75) was reached after 30 d, and the minimum slope (0.46) occurred after 50 d (, ). No significant differences were detected in the slope of the LN–LM curve at different growth recovery stages. The common slope had a value of 0.66, which was significantly less than 1.0 (likelihood ratio, 44.61; degrees of freedom, 5; p < 0.01), suggesting positive allometric growth. As such, LN–LM exhibited distinct characteristics of allometric growth during the entire growth recovery stage and the growth rate of LN was smaller than LM.
Figure 2. Allometric relationships between leaf number, leaf-and-branch volume, and leaf-and-branch water content with leaf-and-branch dry mass of M. laxiflora at different growth recovery times. Allometric relationship between leaf traits differs among different plant recovery times.
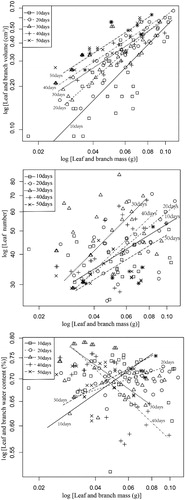
Table 1. Regression analysis of leaf trait relationships by standardized major axis estimation (SMA) at different recovery growths along elevation gradients. Relationship between leaf traits varies with recovery time and elevation gradient.
Varied patterns were observed among the growth relationships between LN and LM along the water fluctuation gradient (). The common slope of the LN–LM curve was −0.78 for the upper hydro-fluctuation zone, which was significantly different from 1.0 (p < 0.01), indicating negative allometric growth (i.e., LN of plants in the upper hydro-fluctuation zone decreased as LM increased). The common slope of the LN–LM curve was 0.79 for the middle hydro-fluctuation zone, which was not significantly different from 1.0 (p > 0.05), suggesting isometric growth (i.e., nearly identical increases in LN and LM). The common slope of the LN–LM curve was 0.53 for the lower hydro-fluctuation zone, which was significantly different from 1.0 (p < 0.01), indicating positive allometric growth (i.e., LN increased at a slower rate than LM) (, ).
Figure 3. Allometric relationships between leaf number, leaf-and-branch volume, and leaf-and-branch water content with leaf-and-branch dry mass of M. laxiflora along different water level gradients. Allometric relationship between leaf traits differs among water level gradients.
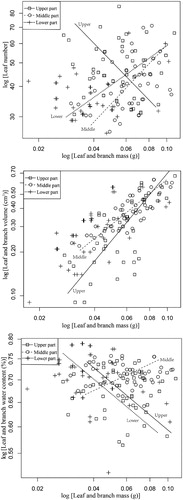
Table 2. Test for slope heterogeneity of leaf trait relationships and common slope for each water level gradient. Common slope varies among different water level gradients.
3.2. Relationship between LV and LM during growth
A significant correlation was detected between LV and LM across the growth recovery stages (p < 0.05). The common slope decreased as growth recovered (; ). The slope after 10 d of growth recovery was 1.29, which was significantly different from 1.0 (p < 0.05), indicating positive allometric growth (i.e., LV increased at a remarkably faster rate than LM). The slope after 20 d of growth recovery was 1.11, which was not significantly different from 1.0, indicating isometric growth (i.e., LV increased at a slightly faster rate than LM). The slopes after 30, 40, and 50 d of growth recovery were not significantly different (p < 0.05). The common slope was 0.88, which was not significantly different from 1.0, indicating isometric growth. However, LV increased at a slightly slower rate than LM. These results indicate a gradually reduced investment on LV as growth resumed. At the initial stages of growth recovery, the increase in LV was faster than LM, but slowed at later stages.
Significant differences were detected in the growth relationship between LV and LM along the water fluctuation gradient (p < 0.05). The common slope of the LV–LM curve decreased with the declining water fluctuation gradient (). The slope at the upper hydro-fluctuation zone was 1.51, which was significantly different from 1.0 (p < 0.00), indicating positive allometric growth (i.e., LV increased at a faster rate when compared to LM). The slope at the middle hydro-fluctuation zone was 1.02, which was not significantly different from 1.0, indicating isometric growth (i.e., LV increased at the same rate as LM). At the lower hydro-fluctuation zone, significant differences were detected in the slope of LV–LM for various growth recovery stages, while a common slope was not detected (). The slopes after 10, 20, and 50 d of growth recovery were not significantly different from 1.0, indicating isometric growth. The slopes after 30 and 40 d were significantly different from 1.0 (p < 0.05), indicating allometric growth (; ).
3.3. Relationship between LWC and LM during growth
A significant correlation was detected between LWC and LM across different growth recovery stages (p < 0.05). The common slope decreased at first then increased as growth resumed (). In the remnant populations, the common slope of the LWC–LM curve varied significantly among growth recovery stages (p < 0.05) (; ). The common slopes after 10, 20, and 50 d of growth recovery assumed positive values and exhibited significant differences from 1.0 (likelihood ratio, 222.00; degrees of freedom, 3; p < 0.01), indicating positive allometric growth. As the slopes were < 1, LWC increased at a slower rate than LM. The common slopes after 30 and 40 d were negative and significantly different from 1.0 (likelihood ratio, 105.50; degrees of freedom, 2; p < 0.01), indicating negative allometric growth, in which, LWC decreased as LM increased.
Going down the water fluctuation gradient, the common slope of the LWC–LM curve increased at first then decreased (; ). The common slopes at the upper and lower parts of the hydro-fluctuation zone were −0.22 and −0.23, respectively, and were significantly different from 1.0 (likelihood ratio, 218.40; degrees of freedom, 2; p < 0.01), indicating negative allometric growth (i.e., LWC in the upper and lower parts of the hydro-fluctuation zone decreased as LM increased). The common slope at the middle hydro-fluctuation zone was 0.12, which was significantly different from 1.0 (p < 0.01), indicating positive allometric growth. The increase in LWC was slower than LM ().
4. Discussion
4.1. Variations in allometric growth of leaves and branches with growth recovery stage
Changes in the growth relationships among leaf and branch traits are related to growth rhythm and signify the adaptation of resource utilization and investment strategies at different times. Growth and resource investment strategies of plants vary with growth stage, altering the growth relationships among traits and biomass allocation at different stages (Yao et al. Citation2013). In a study conducted by Yang et al. (Citation2016), variations in allometric growth among leaf traits of Populus euphratica with the leaf growth stage occurring in spring was investigated. Results revealed that the common slope between leaf area and petiole dry mass increased at first then decreased as leaves progressed from the young leaf stage to leaf development stage and eventually maturation. ‘Diminishing returns’ were observed in the plants based on dry matter investment.
The recovery growth of riparian plants also changes with the growth stage after the water level recedes. The branches and leaves of plants usually have a rapid growth process to compensate for the loss of biomass during flooding (Chen and Xie Citation2009), while the leaves thicken and leaf area increases (Rodriguez et al., Citation2015), resulting in changes in the growth relationship between branches and leaves. The growth relationships among branch and leaf traits of M. laxiflora also varied noticeably between growth recovery stages. LN and LM exhibited allometric growth patterns during the entirety of growth recovery. LV and LM exhibited an allometric growth pattern for the first 10 d and exhibited an isometric growth pattern after 20 d. LWC changed in a more complex manner and exhibited a positive allometric growth relationship with LM during the first 20 d, but switched to a negative allometric growth relationship after 30 d then changed back to a positive allometric growth relationship after 50 d. The above results indicate a rapid increase in LN and LV of M. laxiflora in the early stages of growth recovery. The increase in both LN and LV were higher than LM.
Leaf formation and development are central to the growth and investment strategies of this stage, as plants need to quickly reestablish photosynthesis and perform such functions by recovering and restoring younger branches and leaves lost during flooding (Chen et al. Citation2008a; Chen and Xie Citation2009) . This is a ‘fast return’ strategy. At the later stages of growth recovery, leaf development takes precedence. The increase in LV slows down and exhibits an isometric growth relationship with LM. However, the increase in LN drops faster, thereby increasing at a slower rate than LM. The steady rise in LV during this period indicates that the plant is still investing biomass into the construction of leaf vascular tissues in order to enhance water and nutrient transport ability (Garnier et al. Citation1999). The genesis of vascular tissues in new leaves also augments vascularization and leads to an increase in volume (Ishida et al. Citation2005). Leaf economics during this time are of the ‘slow return’ type.
4.2. Variation in allometric growth of leaves and branches with water fluctuation gradient
As water levels fluctuate, the environment in the hydro-fluctuation zone of the river bank often exhibits strong spatial heterogeneity. Significant differences were previously observed along water fluctuation gradients among different environmental factors, including exposure period, exposure time, and soil water content (Chen and Wang Citation2015). Spatial heterogeneity in the environment caused by water level fluctuations also affects the development of plant traits and investment strategies (Li et al. Citation2015; Hogg et al. Citation2017) . For example, Rood et al. (Citation2010) explored the effects of flooding on leaf traits in Populus angustifolia and found that prolonged submergence underwater reduced the number and area of leaves, impacting the plants’ investment strategy. Moreover, Huang et al. (Citation2017) studied growth patterns in plant populations along the Lijiang River riparian zone and discovered that the functional traits of plant leaves were significantly affected by water level fluctuation patterns, and that plants in the lower hydro-fluctuation zone have a smaller specific leaf area and higher leaf nitrogen content than those found in the upper hydro-fluctuation zone.
The branch and leaf growth and investment strategies of M. laxiflora were also affected by the fluctuation of water levels. In this study, the growth relationship between plant LN and LM varied along the water fluctuation gradient. Negative allometric growth, isometric growth, and positive allometric growth were observed in the upper, middle, and lower parts of the hydro-fluctuation zone, respectively. The growth relationship between plant LV and LM exhibited positive allometric growth in the upper hydro-fluctuation zone, but isometric growth in the middle hydro-fluctuation zone. This growth relationship assumed different patterns in the lower hydro-fluctuation zone at different time periods, lacking a common slope. The growth relationship between LWC and LM exhibited negative allometric growth in the upper and lower parts of hydro-fluctuation zone, but positive allometric growth in the middle hydro-fluctuation zone. Thus, the growth and investment strategies of plants in the upper hydro-fluctuation zone centers around LV. In this zone, plants grow branches and leaves with a strong photosynthetic ability by forming vascular tissues and storing large quantities of nutrients. The growth and investment focus of plants in the middle hydro-fluctuation zone was not clear, as LN increased and the development of leaf and branch structures occurred simultaneously. The growth and investment focus of plants in the lower hydro-fluctuation zone was LN, as plants strive to increase LN throughout the growth recovery stage. Down the water fluctuation gradient, the investment strategy of M. laxiflora diverged from a slow return to a fast return. The branches and leaves that grew in the lower hydro-fluctuation zone could not grow normally due to human water flow regulation.
Differences in the growth relationships among LN, LV, LWC, and LM at different elevations in the hydro-fluctuation zone could be attributed to spatial heterogeneity in the zone environment as water level fluctuated. The upper hydro-fluctuation zone was exposed early and exposed to intense light during the initial exposure period. The temperature was relatively high and the soil moisture content was desirable. These conditions ensured satisfactory plant growth. Plants found in this region quickly grew a large number of branches and leaves. The timely formation of well-developed vascular tissues and rich storage of nutrients was crucial to plants in this region. Thus, the investment of plant resources was focused on LV, while simultaneously reducing investments in LN and LWC. The middle hydro-fluctuation zone was exposed at a later time. The temperature and soil moisture content were suitable for plant growth. Plants found in this region had normal LN and experienced regular LV development. The lower hydro-fluctuation zone was exposed last. The temperature and soil moisture content were both low when plants finally emerged from underwater. Plants found in this region grew branches and leaves more rapidly. The increase in branches and LN was the emphasis of plant growth and investment in this region.
4.3. Impact of human water level regulation on plant growth in remnant populations
Most riparian plants have adapted to water level fluctuations and changes in the aquatic ecosystem over the course of their long evolutionary histories (Chen et al. Citation2008b; Jesús et al. Citation2009) . Normal fluctuations of water level affect the growth of riparian plants, but will not seriously harm their growth or development (Chen and Xie Citation2009). Some species rely on this unique environment to complete their own morphological development and subsequent growth and reproduction (Chen et al. Citation2008b; Joe et al. Citation2013) . However, as the patterns of water level fluctuation altered, long-term changes in the growth of plant branches and leaves will occur. This in turn affects biomass allocation and the investment strategies of plants, and their growth recovery and subsequent development (Chen et al. Citation2008a; Li et al. Citation2015) .
Case studies on the effects of water level fluctuations on plant growth exist in the literature (Kawano et al. Citation2008). Kate et al. (Citation2008) explored the effects of human water flow regulation on the growth of S. nigra Marshall at the Blowering Dam in Australia and found that the plants in the lower hydro-fluctuation zone had higher death mortality rates due to increased flooding depth and prolonged flooding. As the decline in water level accelerated, leaf shedding became more serious. Moreover, Jie et al. (Citation2012) studied the response of leaf functional traits to changes in water level fluctuations in 42 adaptable plants found in the hydro-fluctuation zone of the reservoir formed by the Three Gorges Dam. Results revealed that the leaf traits in the adaptive stage of hydro-fluctuation zone shrubs were all close to the low input–fast return end of the worldwide leaf economics spectrum. This may explain the lack of satisfactory growth observed in these shrubs under the influence of altered water level fluctuations.
M. laxiflora goes into spontaneous dormancy due to flooding every summer and sheds all young branches and leaves during this period. These plants grow branches and leaves at an accelerated rate after emerging from underwater to recover and reestablish photosynthesis, a vital process in plant growth and development (Chen and Wang Citation2015). However, construction of the Three Gorges-Gezhouba cascade hydropower stations has altered the downstream water level fluctuation patterns along the Yangtze River, which has substantially changed the environment during the growth recovery stages of the remnant M. laxiflora populations. Habitat exposure has been delayed from late August, before the construction of the dams, to mid-late September/early October. Water level recession has also accelerated. The entire recession process has been shortened to about one month. The water level of the habitat in the dry season decreased considerably to ∼1.29 m (Sun et al. Citation2007). Per the results of this study, these undeniable changes in the environment were caused by human river flow regulation, which has clearly altered the growth pattern among M. laxiflora branches and leaves, as well as the investment strategies of these plants. This is especially true for plants growing in the middle or lower parts of the hydro-fluctuation zone. Their LN, LV, LWC, and LM all exhibited signs of decline. The investment strategies changed from slow return to fast return and these trends were not favorable for the formation and development of branch and leaf morphology in the remnant populations, and as a result growth of the remnant populations have degenerated.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Shoupeng Guan
Shoupeng Guan is currently a PhD candidate for research on wetland restoration ecology at Hubei International Scientific and Technological Center of Ecological Conservation and Management in the Three Gorges Area, China Three Gorges University.
Fangqing Chen
Fangqing Chen is a professor of Hubei International Scientific and Technological Center of Ecological Conservation and Management in the Three Gorges Area, China Three Gorges University.
Jumei Zhou
Jumei Zhou is currently a M.A. candidate for research on wetland restoration ecology at Hubei International Scientific and Technological Center of Ecological Conservation and Management in the Three Gorges Area, China Three Gorges University.
Kun Lv
Kun Lv is an Assistant Professor of Hubei International Scientific and Technological Center of Ecological Conservation and Management in the Three Gorges Area, China Three Gorges University.
Yongwen Huang
Yongwen Huang is a program Professor of Hubei International Scientific and Technological Center of Ecological Conservation and Management in the Three Gorges Area, China Three Gorges University.
References
- Bao DC, Lu ZJ, Jiang MX, Xu SD, Yao Q, Liu QF, Wang Q. 2010. Population structure and dynamics of remanent Myricaria laxiflora downstream from the Three Gorges Dam. J Wuhan Bot. Res. 28:711–717.
- Bijarchi R, Sohrabi MR, Juibari MY, Yoonessi A. 2011. The ecology, effect of dams, and restoration of the black cottonwood (Populus trichocarpa T. & G.) forest community in the intermountain west. Dissertations Theses – Gradworks. 2:1–7.
- Bloomfield KJ, Cernusak LA, Eamus D, Ellsworth DS, Colin Prentice I, Wright IJ, Boer MM, Bradford MG, Cale P, Cleverly J, et al. 2018. A continental-scale assessment of variability in leaf traits: within species, across sites and between seasons. Funct Ecol. 32(6):1492–1506.,
- Chen FQ, Guan SP, Ma YR, Xie ZQ, Lv K, Huang YW, Jia GM. 2019. Impact of regulated water level fluctuations on the sexual reproduction of remnant Myricaria laxiflora populations. Glob Ecol Conser. 18:e00628.
- Chen FQ, Guo CY, Wang CH, Xu WN, Fan DY, Xie ZQ. 2008a. Effects of water logging on ecophysiological characteristics of Salix variegate seedlings. Chinese J Appl Ecol. 19:1229–1233.
- Chen FQ, Li Y, Qie GW, Xu WN. 2008b. The morphological responses and endurance of Polygonum hydropiper to flooding stress. J Wuhan Bot Res. 26:142–146.
- Chen FQ, Wang CH. 2015. Ecological protection of the rare and endangered plant Myricaria laxiflora in the Three Gorges Area. Beijing: Science Press.
- Chen FQ, Xie ZQ. 2009. Survival and growth responses of Myricaria laxiflora seedlings to summer flooding. Aquat Bot. 90(4):333–338.
- Chen FQ, Xie ZQ, Xiong GM, Liu YM, Yang HY. 2005. Reintroduction and population reconstruction of an endangered plant Myricaria laxiflora in the Three Gorges Reservoir area. China. Acta Ecol. Sin. 27:1811–1817.
- de Assis RL, Wittmann F, Bredin YK, Schöngart J, Quesada CAN, Piedade MTF, Haugaasen T. 2019. Above-ground woody biomass distribution in Amazonian floodplain forests: Effects of hydroperiod and substrate properties. Forest Ecol Manag. 432:365–375.
- Duan WX, Guo LS, Wang J. 2016. Impact of upper Yangtze river large-scale cascade reservoirs on flow regime at Yichang station. Resour Environ Yangtze Basin. 25:120–130.
- Garnier E, Salager JL, Laurent G, Sonié L. 1999. Relationships between photosynthesis, nitrogen and leaf structure in 14 grass species and their dependence on the basis of expression. New Phytol. 143(1):119–129.
- Geng MY, Chen FQ, Lv K, Wang YB, Guan SP, Liu YY. 2019. Changes of allometric relationships among leaf traits of Disanthus cercidifolius var. longipes in different ontogenetic stages and altitude gradients. Guihahia. 39:1387–1397.
- Guan SP, Chen FQ, Lv K, Zhou JM, Huang YW. 2020a. Effects of soil water conditions on seedling regeneration in Myricaria laxiflora remnant populations. Ecol Res. 35(3):524–532.
- Guan SP, Chen FQ, Zhou JM, Xie ZQ, Hunag YW. 2020b. Spatiotemporal photosynthetic physiology responses of remnant Myricaria laxiflora populations to regulated water level fluctuations. Conser Physiol. 8(1):coaa020. http://doi.org/10.1093/conphys/coaa020
- Hogg EH, Michaelian M, Hook TI, Undershultz ME. 2017. Recent climatic drying leads to age-independent growth reductions of white spruce stands in western Canada. Glob Chang Biol. 23(12):5297–5308.
- Huang D, Wang DM, Ren Y, Qin YB, Wu LC. 2017. Responses of leaf traits to submergence stress and analysis of the economic spectrum of plant species in an aquatic-terrestrial ecotone, the Li River. Acta Ecol Sin. 37:750–759.
- Inamoto K, Nagasuga K, Yano T, Yamazaki H. 2015. Influence of light intensity on the rate of photosynthesis and dry matter accumulation in oriental hybrid lily siberi at different developmental stages. J Pomol Hortic Sci. 90(3):259–266.
- Ishida A, Yazaki K, Hoe AL. 2005. Ontogenetic transition of leaf physiology and anatomy from seedlings to mature trees of a rain forest pioneer tree, Macaranga gigantea. Tree Physiol. 25(5):513–522.
- Jesús SBM, Sara A, Alejandra N, Sebastián BÓ. 2009. Changes in leaf water relations, gas exchange, growth and flowering quality in potted geranium plants irrigated with different water regimes. J Plant Physiol. 166:467–476.
- Jie SL, Fan DY, Xie ZQ, Zhang XY, Xiong GM. 2012. Features of leaf photosynthesis and leaf nutrient traits in reservoir riparian region of Three Gorges Reservoir. China Acta Ecol Sin. 32:1723–1733.
- Joe G, Roger DC, Angus W. 2013. Seasonal timing of inundation affects riparian plant growth and flowering: implications for riparian vegetation composition. Plant Ecol. 214:87–101.
- Kate ES. 2008. Exotic invasive black willow (Salix nigra) in Australia: influence of hydrological regimes on population dynamics. Plant Ecol. 197:91–105.
- Kawano N, Ito O, Sakagami JI. 2008. Relationship between shoot elongation and dry matter weight during submergence in Oryza sativa L. and O. glaberrima Steud. rice cultivars. Plant Prod. Sci. 11(3):316–323.
- Li L, Bonser SP, Lan Z, Xu L, Chen J, Song Z. 2017. Water depth affects reproductive allocation and reproductive allometry in the submerged macrophyte Vallisneria natans. Sci Rep. 7(1):16842.
- Li ZJ, Fan DY, Chen FQ, Yuan QY, Chow WS, Xie ZQ. 2015. Physiological integration enhanced the tolerance of Cynodon dactylon to flooding. Plant Biol (Stuttg)). 17(2):459–465.
- Li L, Lan ZC, Chen JK, Song ZP. 2018. Allocation to clonal and sexual reproduction and its plasticity in Vallisneria spinulosa along a water‐depth gradient. Ecosphere. 9(1):e02070.
- Li W, Zhou H, Fu A, Chen Y. 2013. Ecological response and hydrological mechanism of desert riparian forest in inland river. Ecohydrol. 6(6):949–955.
- Liu Z, Jiang F, Li F, Jin G. 2019. Coordination of intra and inter-species leaf traits according to leaf phenology and plant age for three temperate broadleaf species with different shade tolerances. Forest Ecol. Manag. 434:63–75.
- Lu XM, Zhou CF, An SQ, Fang C, Zhao H, Yang Q, Yan C. 2007. Phenotypic plasticity, allometry and invasiveness of plants. Chinese J. Ecol. 9:1438–1444.
- Pitman E. 1939. A note on normal correlation. Biometrika. 31(1/2):9–12.
- Poorter L, Castilho CV, Schietti J, Oliveira RS, Costa FRC. 2018. Can traits predict individual growth performance? A test in a hyperdiverse tropical forest. New Phytol. 219(1):109–121.
- Roderick ML, Berry SL, Noble IR. 2000. A framework for understanding the relationship between environment and vegetation based on the surface area to volume ratio of leaves. Funct Ecol. 14(4):423–437.
- Roderick ML, Berry SL, Noble IR, Farquhar GD. 1999. A theoretical approach to linking the composition and morphology with the function of leaves. Funct Ecol. 13(5):683–695.
- Rodrıguez ME, Achinelli FG, Luquez VMC. 2015. Leaf traits related to productivity in Populus deltoides during the post-flooding period. Trees. 29(3):953–960.
- Rood S, Nielsen J, Shenton L, Gill K, Letts M. 2010. Effects of flooding on leaf development, transpiration, and photosynthesis in narrowleaf cottonwood, a willow-like poplar. Photosyn Res. 104(1):31–39.
- Spencer DF, Ksander GG, Whitehand LC. 2005. Spatial and temporal variation in RGR and leaf quality of a clonal riparian plant: Arundo donax. Aquat Bot. 81(1):27–36.
- Sun ZH, Li YT, Li M, Ge H. 2007. Effect of channel degradation on lower level in Yichang-Shashi reach in the Yangtze River. Hydro-Sci Eng. (4):14–20.
- Vendramini F, Díaz S, Gurvich DE, Wilson PJ, Thompson K, Hodgson JG. 2002. Leaf traits as indicators of resource use strategy in floras with succulent species. New Phytol. 154(1):147–157.
- Voje KL, Hansen TF, Egset CK, Bolstad GH, Christophe P. 2014. Allometric constraints and the evolution of allometry. Evolution. 68(3):866–885.
- Warton D, Weber N. 2002. Common slope tests for bivariate errors-in-variables models. Biom J. 44(2):161–174.
- Warton DI, Wright IJ, Falster DS, Westoby M. 2006. Bivariate line-fitting methods for allometry. Biol Rev Camb Philos Soc. 81(2):259–291.
- Wiebke W, Klaus S, Henning K. 2017. Organ-specific approaches describing crop growth of winter oilseed rape under optimal and N-limited conditions. Eur J Agron. 82(Part A):71–79.
- Yang Q, Li ZZ, Fu Q, Feng JC. 2016. Relationship among leaf trait and developing progress in Populus euphratica. J Desert Res. 36:659–665.
- Yao J, Li Y, Wei LP, Jiang SS, Yang S, Hou JH. 2013. Changes of allometric relationships among leaf traits in different ontogenetic stages of Acer mono from different types of forests in Donglingshan of Beijing. Acta Ecol Sin. 33:3907–3391.
- Zheng CJ, Wang Y, Yu JD, Dai LF, Bai XL. 2014. Measurements of plant volume and leaf area of Platycladus orintalis and their relations to plant height and crown width. B Soil Water Conserv. 34:182–185.
