Abstract
Microplastics are contaminants of emerging concern. In particular, research on the ecological effects of microplastics on microorganisms has attracted widespread attention. In this study, the role of characteristics of microplastics and environmental factors on microorganisms in sediments along the Daihai Lakeshore were investigated. Microscopic identification of microplastics revealed that the most common characteristics of microplastics were fiber, black color and particle size <0.5 mm. The results of Pearson correlation analysis verified that the characteristics of microplastics with particle size <0.5 mm and the main physicochemical characteristics of the sediments (pH, salinity) were positively correlated with the abundance of dominant microorganisms (P < 0.05). In addition, Kyoto Encyclopedia of Genes and Genomes (KEGG) functional pathway analysis that the proportion of functional metabolic pathways related to DNA replication, recombination and reparation was higher in the sampling sites with higher microplastics abundance. This study will provide valuable information for assessing the microbial ecological effects of microplastics contamination in sediments.
1. Introduction
Plastics are widely used in many different fields due to its light weight, low price and durability. Approximately 400 million tons of plastic waste were generated globally by 2019, and about 12,000 tons of plastic waste will end up in landfills or the natural environment by 2050 if current production and waste management patterns continue, according to the Comprehensive Report on Global Plastic Production (Hou Citation2020). Large amounts of plastics fragment are continuously released into the environment, directly or indirectly, and then broken into smaller particles, collectively known as microplastics (<5mm in diameter). Microplastics are widely distributed in the world, whether in the ocean, on land, even in polar glaciers with less human activities (Law and Thompson Citation2014; Obbard et al. Citation2014), which are considered as an emerging threat to ecological and environmental security (Ivleva et al. Citation2017). And the research on microplastics in aquatic ecological environment had been valued in recent years, with the aquatic microplastics pollution even has been listed as one of the top ten environmental problems to be solved globally (Yang et al. Citation2020), due to the negative impact of microplastics on a series of aquatic organisms such as phytoplankton, zooplankton and fish (Ogonowski et al. Citation2018). Meanwhile, the lakeshores in offshore ecosystem have become a hotspot for microplastics research, which are an important source of microplastics due to the intensive human activities (Zhao et al. Citation2014). Especially, it is generally believed that the microplastics with greater density than seawater would accumulate in sediments, which are the ultimate collection places for microplastics waste (Harrison et al. Citation2014). Moreover, microplastics in sediments cannot be photolyzed by ultraviolet light and can only be decomposed by microorganisms (Nava and Leoni Citation2021). And the sediments in lakeshores offer a unique opportunity to evaluate microplastics contamination because the dense mat of grass roots and rhizomes confers substantial stability to sediment columns, a feature that may diminish potential bioturbation and hydrodynamic sediment disturbances that commonly take place in bare sediments (Näkki et al. Citation2017). So microplastics have a relatively longer duration which is related to the health and safety of aquatic organisms and aquatic ecosystems (Shao et al. Citation2019). Thus, microplastics in sediments of lakeshores are a worthy subject which should to be studied urgently.
The buoyancy, hydrophobic hard surfaces, novel polymer carbon sources and long-distance transport make microplastics a new niche for microbial growth, potentially settled by a variety of microorganisms (Nava and Leoni Citation2021). In addition, environmental factors and microplastics surface features also affect the microbial community structure and function, therefore the ecological effects of microplastics on microorganism characteristics has become an important ecological problem and has increasingly attracted the attention of many researchers (Rogers et al. Citation2020; Chai et al. Citation2021). Studies had shown that microbial biofilm formation of microplastics surface was rapid during 1–2 weeks in the aquatic environment, and the microbial combination on biofilm was different from that in the surrounding water body (De Tender et al. Citation2017). The colonization characteristics and species diversity of microbial communities on microplastics in sediments have been extensively studied by high-throughput sequencing methods (Chai et al. Citation2021). Harrison et al. (Citation2014) investigated the surface colonization of low-density PE in estuarine sediments by using 16S rRNA gene clone libraries, and they found that different sediment types (fine sand, medium sand and silt) had different bacterial compositions, with two genera (Arco-bacter and Colwellia) accounting for 84%∼93% of the total sequences. Furthermore, Nauendorfet al. (Citation2016) studied the surface colonization of plastic bags (polyethylene) in organic-rich marine sediments and they observed rapid bacterial colonization of the polymer surfaces, but the community composition was not characterized. Several studies have shown that environmental factors also play an important role in the composition of microorganism communities. Among these, temperature plays a role in the settlement and growth of the colonizing community, as higher temperatures increase cell metabolism, resulting in the rapid development of the attached organisms. Oberbeckmann et al. (Citation2014) highlighted that the overall average diversity of PET plastisphere communities in a marine environment was highest and lowest in summer and winter, respectively. A pivotal role of salinity has also been recognized, which is known to shape communities in aquatic environments (Dussud et al. Citation2018; Oberbeckmann et al. Citation2018; Kesy et al. Citation2019). Exposure of five types of plastic debris (polyvinyl chloride, polypropylene, polyethylene, polystyrene, and polyurethane) in the Haihe Estuary highlighted that salinity was negatively correlated with the average growth rate of the biofilm and positively correlated with the diversity of the colonizing community (Li et al. Citation2019). Besides temperature and salinity, nutrients may also influence plastic colonization, where increased nutrients are usually associated with greater biodiversity on plastic-attached communities (Oberbeckmann et al. Citation2018; Li et al. Citation2019). However, there is still debate among researchers as to whether it is the occurrence characteristics of microplastics or environmental factors prevail in affecting microorganisms.
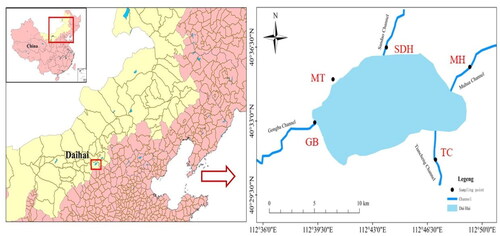
Daihai Lake, one of the three inland lakes in Inner Mongolia Autonomous Region, is important ecological barriers of the green Great Wall in the north of China. There are more than 20 rivers entering the lake in the Daihai basin, most of which are intermittent. Among them, the rivers with high flow and frequent water into the lake are Gongba River, Muhua River, Tiancheng River, etc. (Zhang et al. Citation2020). The discharge of pollutants from human activities along the Dahai Lakeshore exceeds the self-purification capacity of the channel and rivers, leading to the discharge and accumulation of pollutants into the lake, and the decrease in water volume in the lake area further causes the concentration of pollutants. Given the lakeshore is the key input flux to Daihai Lake, the output of plastics there could be one of the most important sources of microplastics in the Daihai Lake Basin. Therefore, this study was carried out in lakeshore of Daihai Lake Basin.
The specific objectives of the present study are: (1) to study the occurrence characteristics of microplastics in sediments of the Daihai Lakeshore; (2) to do further biological information analysis about composition and structure of microbial community in sediments; (3) to clarity the correlation between characteristics of microplastics, environment factors and microorganisms in sediments of the Daihai Lakeshore; (4) to analyze the responses of microbial function to microplastics in sediments by using metagenome.
2. Materials and methods
2.1. Study area and sampling sites
In this study, the Daihai Basin (40°30′–40°45'N, 112°30′–112°52'E) is located in Ulanqab city, Inner Mongolia autonomous region, China. The sediment samples were collected from the Daihai Lakeshore at five sites in September 2020: Muhua channel (MH), Tiancheng channel (TC), Gongba channel (GB), Matou (MT) and Sandaohe channel (SDH) (). The position of sampling points was located by global positioning system (GPS), and three replicate sediment samples were collected at each sampling site. During the sampling process, surface sediment (5 cm) was collected from the bottom of each site using a stainless steel mud picker (Li et al. Citation2020). All sediment samples were packed into aluminum foil sealing bags. After removing impurities such as gravels, shells and plant residues, the samples were placed in a dark place for natural air drying. All the samples were divided into two equal parts, one of which was ground in an agate mortar and screened with a sieve of over 60 meshes for physical and chemical analyses. The other part was not screened and only used for flotation separation of microplastics.
2.2. Physicochemical analyses
The lake area of Daihai Basin has been shrinking dramatically and the salinization degree deepening continuously in recent years. Therefore, the effects of microplastics on the structure and function of microorganisms in sediment may be associated with the special physicochemical characteristics of Daihai Lake. Sediment pH was measured with a pH meter after shaking a sediment: water (1:5 wt/vol) suspension for 30 min (Shen et al. Citation2013). The salinity in sediments was determined by leaching, filtration, steaming and constant weight. Total nitrogen (TN) was measured by using a CN Analyzer (Vario Max CN, Elementar, Germany). Total organic carbon (TOC) and total phosphorus (TP) were measured as described previously (Walkley and Black Citation1934).
2.3. Separation and extraction of microplastics
Microplastics and sediment particles were separated according to the principle of density separation. The samples were dried at 70 °C for 24 h to reach a constant weight. Then, the samples were moved into a clean glass beaker and soaked in a 30% hydrogen peroxide solution to digest the organic and inorganic impurities. Samples were filtered through a glass microfiber membrane with a pore size of 0.45 μm (GF/F, 47 mm Ø, Whatman). Finally, the filter paper was dried at 40 °C for 24 h prior to extraction. To avoid contamination by airborne impurities, samples were covered with the foil before being tested; the beakers containing samples were washed and eluted with a prefiltered saturated salt solution (NaCl with ρ = 1.20 g/mL) to avoid the loss of microplastics particles that might adhere to the beaker walls after each extraction be; the residual solids were washed with pure water, dried at 40 °C and placed in a clean petri dish for microscope observation (Zheng et al. Citation2019).
2.4. Observation and identification of microplastics
The features of microplastics were identified by using stereo microscope (M65FC, Leica, Germany) and laser confocal microscope (Olympus, OLS4000), and the abundance of microplastics in various points was measured using Nano Measurer 1.2 software. The unit for calculating the abundance of microplastics in sediments was items/kg. The particle size of the microplastics was counted by the length of the longest side. If the particle size of the microplastics was less than 500 μm or difficult to determine, the composition of the microplastics particles was identified by the Fourier transform infrared (FTIR) spectroscope (Wang et al. Citation2020). During sampling and experiment, the use of plastic containers should be avoided. The prepared solution should be filtered in advance and sealed for use. The environment in the laboratory should be kept clean to reduce the influence of plastic components in the air on the experimental results (Su et al. Citation2016).
2.5. DNA extraction
DNA was extracted from 0.5 g of wet sediments from each sample using the A.E.Z.N.A.™ soil DNA kit (Omega Biotek, Inc., Norcross, GA) according to the manufacturer's instructions. Ten aliquots of DNA were extracted per sample. The extracted DNA samples were dissolved in Tris-EDTA buffer of 30 μL, and then quantified using a NanoDrop™2000 spectrophotometer (Thermo, USA). DNA extractions with OD260/280 nm around 1.80, and OD260/230 nm above 1.80, were pooled for metagenome sequencing. All DNA samples were stored at −80 °C until sequencing.
2.6. Metagenome sequencing and annotation
Metagenomic sequencing libraries were prepared and then sequenced by Majorbio, Inc. (Shanghai, China) using the HiSeq2000 platform. Sequences were cleaned and assembled using Seqprep, Sickle, BWA, and SOAPdenovo (Version 1.06), and the length of contigs >500 bp were retained for further bioinformatics analyses. MetaGene software was used for ORF prediction, and CD-HIT was used to build a non-redundant gene catalog. Taxonomic assignment of the predicted genes was carried out using BLASTP alignment against the integrated non-redundant (NR) database of the National Center for Biotechnology Information (NCBI) and Kyoto Encyclopedia of Genes and Genomes (KEGG) database (Version 2.2.28+).
2.7. Statistical analyses
Redundancy analysis (RDA) was performed using Canoco version 5.0 software (Microcomputer Power) to explore the correlation between the occurrence characteristics of microplastics and the abundance of dominant microorganisms. Heatmap were performed using R version 3.5.1. Statistical analyses were performed using SPSS 22.0 and drawing using Origin 9.0. All data results were tested at significance level P = 0.05.
3. Results and discussion
3.1. Abundances and occurrence characteristics of microplastics in sediments
The results of the microplastics abundance in each sampling site are presented in . The average microplastics abundance in sediments of the Daihai Lakeshore was 397 ± 20 items/kg, which was similar to that of Ulansu Lake and Laizhou Bay (Teng et al. Citation2020; Wang et al. Citation2020). The lowest abundance in SDH (Sandaohe channel) was 236 items/kg, while the highest abundance in MH (Muhu channel) was 612 items/kg. The scene investigation showed that there were many residential areas in the villages near the MH, and there were large-scale agricultural mulch planting areas, which were aggregation regions of discharge of household garbage and agricultural sewage, resulting in significant increase of microplastics. In contrast, the abundance of microplastics in SDH may be relatively low because of the relatively loose population distribution and the low impact of human activities there. The sample size of the sediment collected in this study may also have some influence on the microplastics abundance statistics.
Figure 2. The abundance distribution characteristics (a), size (b), shape (c) and color (d) proportion of microplastics in the lakeshore of Daihai Basin. It depicts the variance of size, shape and color in all sampling sites.
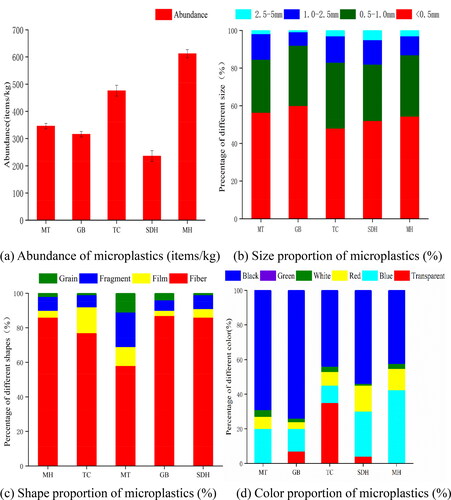
The particle size distribution of microplastics at each sampling site is shown in . In the lakeshore, the particles with size less than 0.5 mm accounted for 55.32–62.01%, followed by 0.5–1 mm, which was consistent with previous studies showing that the abundance of microplastics was negatively correlated with their particles size (Zhang et al. Citation2017). This is because microplastics with larger particle size are more likely to be decomposed into smaller particle size under long-term immersion, mechanical friction and hydraulic corrosion in lakeshore (Zhang et al. Citation2017). According to the observation by laser confocal microscope, the main forms of microplastics were fibers, fragments, films and grains. Among them, fibrous microplastics accounted for the largest proportion, up to 58.50–89.71%, followed by fragment and film (). The higher surface area volume ratio of fiber allows for higher aggregation and deposition rates of microplastics, so the larger plastic debris sink faster into the sediments in a short time (Ryan Citation2015). Microplastics in lakeshore sediments were rich in color, which could be divided into black, green, white, red, blue and transparent colors, as shown in . Black microplastics accounted for 43.10–68.28%, followed by blue. The reason for the color difference is that pigment molecules with small particle sizes are often added as colorants in the plastic production process, and colorants and resins are mostly mechanically bonded, which can move gradually from the inside of the resin to the surface during the aging process, forming new pollution (Sahin and Yayla Citation2005).
3.2. Composition and structure of microbial community in sediments
It can be seen that the dominant phyla (relative abundance more than 1%) across all samples were Proteobacteria (58.00%), Actinobacteria (22.80%), Chloroflexi (5.80%), Bacteroidetes (5.62%), Gemmatimonadetes (2.71%), Acidobacteria (2.34%) and Firmicutes (2.64%) (). In addition, the abundance of bacteria in different sampling sites was diverse according to the detailed information of the abundance of dominant phyla (). This result was consistent to the previous reported by Jiang et al. (Citation2018) who observed that Proteobacteria, Chloroflexi and Acidobacteria were the dominant microorganisms on the microflora of the ‘plastic circle’ in the intertidal zone of the Yangtze River. However, the higher abundance of Acidobacteria and Actinobacter at each sampling site in this study compared to the results of the studies on the microflora of microplastics in seawater might be explained by the fact that the lake shore as a transitional zone was influenced by both terrestrial and aquatic environments (Elifantz et al. Citation2013). The complex exchange of lakeshore materials contributed to the formation of microbial community structure in the lakeshore sediments of the Daihai Basin, and physical and chemical factors also contributed to the effect on microorganisms, especially the salinization characteristics of the Daihai Basin, such as pH and salinity. Hou (Citation2020) indicated that the addition of microplastics significantly altered the dominant phyla in soil aggregates, with Actinobacteria replacing Proteobacteria as the dominant phyla after 150 days of soil incubation experiments, consistent with the finding that Actinobacteria were the dominant flora in the sediments of Daihai Lakeshore. Many studies have shown that polyethylene plastics can be degraded by Streptomyces, which is the most abundant Actinomyces (Sen and Raut Citation2015; Huerta Lwanga et al. Citation2018). Huang et al. (Citation2019) found that the abundance of Actinobacteria was increased and suggested that microplastics had an obvious enrichment effect on Actinobacteria, leading to the speculation that Actinobacteria have the ability to degrade and synthesize polymers. In previous studies on microorganisms in saline-alkali environments, it was also written that Acidobacteria were a key group of bacteria in saline-alkali soil, which might be related to their ability to decompose organic matter (Banerjee et al. Citation2016). Meanwhile, such microorganisms might establish associations with other groups and controlled the dynamics of carbon in saline-alkali soil (Zhao et al. Citation2020). The relative abundance of different species in the microbial community was often used to indicate different stages of biofilm formation. Proteobacteria were usually early colonizers of unnatural substrate biofilms in the aquatic environment, accounting for the highest proportion of total abundance among all sampling sites. It also has been well documented that Proteobacteria were widely distributed in various ecosystems and were considered as a specific phylum for degrading complex organic matter in the biosphere (Wolińska et al. Citation2017). In addition, the differences in bacterial abundance at different sampling sites suggested that microbial growth in biofilms might be at different stages.
Figure 3. Abundance of microorganism of sediments in the lakeshore of Daihai Basin. Cluster with abundances >0.3% in samples are shown. (a) Phylum level and (b) Genus level.
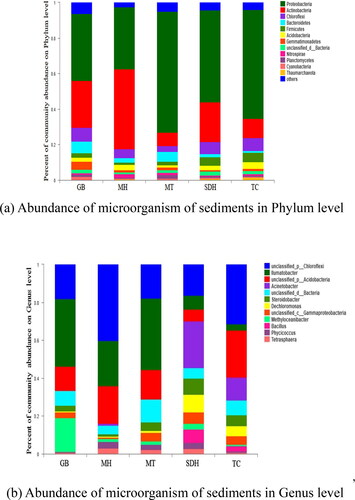
Table 1. Abundance of the top seven dominant microorganism (phylum level).
The dominant genus of microbial communities were similar across sampling sites, including unclassified _ p_ Chloroflexi (16.28–40.12%), llumatobacber, unclassified _P_ Acidobacteria and Acinetobacter, which all accounted for more than 60% of the total level. Among them, unclassified _ p_ Chloroflexi accounted for 18.0%, 40.11%, 17.9%, 16.3% and 31.4% in the five sample points, respectively (). It was clearly found that the MH site with the highest microplastics abundance (612 items/kg) had the highest abundance of Chloroflexi (40.11%) among the five sites, while the SDH site with the lowest microplastics abundance (236 items/kg) corresponded to a relatively low abundance of Chloroflexi (16.3%). This also showed a positive correlation between Chloroflexi and microplastics abundance and the degree of environmental pollution. In previous research on Chloroflexi, it also reported that the bacterium can absorb and assimilate many kinds of organic substances of the biological and non-biological sources from environments and it was considered to be used for solving the increasingly serious environmental pollution (Xian et al. Citation2020). Furthermore, it can also be seen from the results of this study that the microbial community structures between GB and MT were similar, with unclassified _ p_ Chloroflexi (18.01%, 17.91%), llumatobacber (35.67%, 37.65%), and unclassified _P_ Acidobacteria (12.80%, 15.57%) being common dominant genus in both sample sites with similar proportions. The community composition and structure of MH and SDH were different. The unclassified _ p _ Chloroflexi (40.12%) was significantly higher in MH than in SDH (16.27%), While Acinetobacter as the dominant genus in SDH (24.6%) was also much higher than in MH (0.96%). According to the abundance of microplastics, it could be found that the abundance of microplastics in GB and MT were 316 and 346 items/kg, respectively, which were one of the largest and smallest microplastics abundance points, and also indicated that microplastics might play a role in the structure and composition of microbial communities.
3.3. Correlation between microplastics, physicochemical characteristics and dominant microorganisms
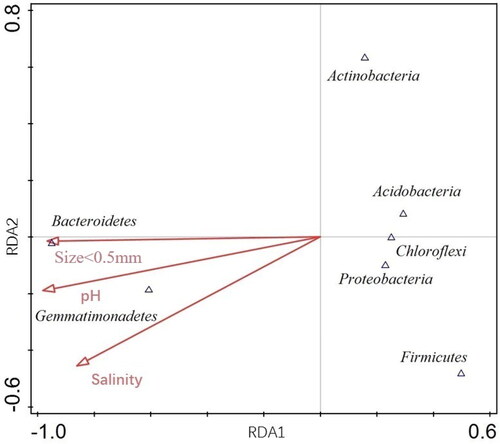
In this study, there were differences and similarities in the microbial community structure at the sampling sites, which may be related to the properties of plastics and plastic additives, while differences in environmental factors may also have directly influenced the microbial community (Nava and Leoni Citation2021). The wide variety of physicochemical characteristics of GB, MT, SDH, MH and TC, including pH, air temperature, concentrations of TOC, TP and TN, were summarized in . The six physicochemical characteristics and the four occurrence characteristics of microplastics were taken into consideration to evaluate the relative contributions to the microbial community, and the parameters with high contribution and their Pearson correlation were listed in . This research results showed that the microplastics of black, of fibrous and with particle size <0.5 mm were predominant, and pH has become a significant physicochemical environmental indicator according to the current situation of Daihai Basin. Among them, the particle size <0.5 mm was positively correlated with the dominant microorganism (P < 0.05), probably because microplastics are hydrophobic particles of exogenous origin at the water surface, and the smaller the particles obtain a larger specific surface area, which can adsorb more organic matter and thus provide a richer living environment for microorganisms. For correlation between environmental indicators and phyla, the results showed that parameters regarding pH and salinity contributed positively to the dominant bacteria (P < 0.05), which was also in line with the findings of previous study on saline agricultural soil, showing that salinity was the sharpest force to shape the composition and diversity of bacterial community (Zhao et al. Citation2020). Previous studies on five microplastics debris exposures in the Haihe Estuary also showed that salinity was negatively correlated with the average growth rate of biofilms on microplastics, while positively correlated with the community diversity of colonized microorganisms (Nava and Leoni Citation2021).
Table 2. Physicochemical profile of the sediment samples.
Table 3. Correlation between micropalstics, physicochemical properties and dominant microorganism.
Three parameters with high contributions were listed in RDA (). According to redundancy analysis, it was further showed the pH of sediments was the key factor affecting the abundance of dominant microorganism, and salinity of sediments was also a significant factor. Notably, among the top seven dominant phylum, only Bacteroidetes and Gemmatimonadetes exhibited significant positive correlation with pH. It has been well documented that Bacteroidetes is widely distributed in various ecosystems and is considered as a phylum dedicated to the degradation of complex organisms in the biosphere (Wolińska et al. Citation2017). Moreover, in order to investigate the diversity and distribution characteristics of PET hydrolase in different marine and terrestrial environment worldwide, the group of Professor Streit at the University of Hamburg conducted a Metagenomic analysis of 5–108 marine and terrestrial sources and identified 504 potential new PET degrading enzymes, which mainly come from the phylum Bacteroidetes, Proteobacteria and Actinobacteria phylum (Danso et al. Citation2018). Bauer's study also showed that the representative of Bacteroidetes could be adapted to degrade the polymer carbon source (Debroas et al. Citation2017). These findings are consistent with our study results and verify the possibility of biodegradation due to the production of microplastics degrading enzyme in Bacteroidetes. Salinity is the main factor affecting the bacterial community structure in salinized agricultural ecosystem and influencing the bacterial symbiosis mode. The latest research on the bacterial community of salinized agricultural soil have also shown that Bacteroidetes and Gemmatimonadetes are the dominant microorganisms in the salinized soil environment, which is consistent with our results that Bacteroidetes and Gemmatimonadetes are positively related with pH and salinity. Potential plastic-degrading bacteria have been observed to colonize the surface of microplastics in both marine and freshwater environment, suggesting that microplastics can serve as a special aggregation to enrich potential plastic-degrading bacteria (Debroas et al. Citation2017; Frère et al. Citation2018; Huerta Lwanga et al. Citation2018). In conclusion, more extensive studies are needed in the future to identify relevant microorganisms with the ability to break down microplastics and synthesize polymers.
3.4. Responses of microbial function to microplastics in sediments
In this study, the sequence information obtained from metagenomic sequencing was annotated in KEGG database to obtain the abundance of each sampling site corresponding to level 1 and level 2. The similarity and difference between different sampling sites can be studied by cluster analysis. Based on the KEGG functional abundance, it showed the functional abundance of sampling sites at level 1 (). As can be seen from the figure, the biological Metabolism pathways of all samples were classified into five categories, including metabolism, genetic information processing, human diseases and cellular processes. According to the heatmap of functional abundance, all sampling sites were positively correlated with the metabolism function that had the highest abundance among all functions.
Figure 5. The abundance of KEGG pathways present at the sediments samples in level 1 (a) and level 2 (b).
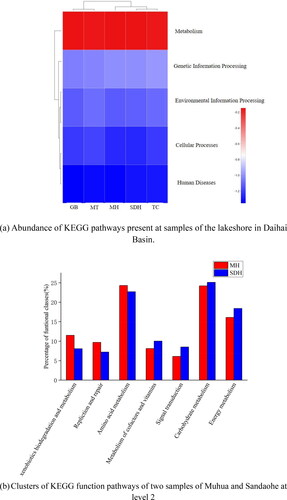
To deeply analyze the responses of microbial function to microplastics in sediments, Metagenomic sequencing was used to detect the functional pathways between the Sandaohe channel (SDH) and the Muhua channel (MH) at level 2, as these two sampling sites showed the greatest differences in microplastics abundance. The results revealed a generally high abundance of functions related to development and metabolism, such as carbohydrate metabolism (24.65%), energy metabolism (17.25%) and amino acid metabolism (23.51%). The high abundance of amino acid metabolism functional pathways may affect the carbon and nitrogen cycle in sediments, which is closely related to the degradation of alanine, aspartic acid and other carbohydrates (Neis et al. Citation2015). Therefore, these finding are consistent with the lifestyle formed by microorganisms in the presence of microplastics. The abundance of some special functional pathways worthy of our attention was also correlated with the sampling sites: Compared with SDH, samples from MH exhibited higher relative abundance of gene functions regarding DNA replication, recombination and repair. For example, the abundance of ‘replication and repair’ and ‘xenobiotics biodegradation and metabolism’ were 9.70% and 11.50%, respectively, in MH samples compared to 7.20% and 8.06%, respectively, in SDH. The metabolic pathways of xenobiotics biodegradation may be related to genes involved in the degradation of artificial substrates or contaminants, which may indicate that bacteria enriched on microplastics can use plastic polymers or additives as carbon sources (Debroas et al. Citation2017). In addition, the metabolic pathways of replication and repair is closely related to repair the damaged DNA gene, which is consistent with previous studies that the hydrophobic and large specific surface area characteristics of microplastics make them contaminant carriers, and the colorants and plasticizers added during their production process are gradually released in the environment, leading to microbial DNA damage (Ghosal et al. Citation2016). Therefore, genes of the DNA repair can be exchanged through plasmids or gene transfer (Chen et al. Citation2018). All indications prove that microbial communities have potentially acquired resistance to plastic pollution as well as repair and recombination with long-term microplastics pollution, which as a novel pollutant may alter microbial survival strategies and affect ecological functions, thus influencing biogeochemical processes. Studies have shown that many bacteria, fungi and algae have the ability to degrade organic pollutants, which also demonstrates that microorganisms may remove their associated chemicals through functions such as metabolism, recombination and remediation, and may affect microbial bioavailability due to the long-term accumulation of microplastics (Banerjee et al. Citation2016).
4. Conclusion
The results indicated that the occurrence characteristics of microplastics and environmental factors both have an ecological role in the microbial characteristics in the sediments of the Daihai Lakeshore Basin. The formation of these specific microbial communities was significantly associated with the occurrence characteristics of microplastics and also influenced by salinity and alkaline environmental factors. Among them, both Chloroflexi and Bacteroidetes as the dominant microorganisms in sediment samples were associated with the biodegradation of organic matter, and the abundance of microplastics with particle size less than 0.5 mm was positively correlated with the abundance of the dominant microorganisms, which indicated that microplastics influenced the microbial community structure in sediments. In addition, the proportion of functional metabolic pathways related to DNA replication, recombination and reparation was higher in the sampling sites with higher microplastics abundance, which indicated the response of microbial functions to microplastics in sediments. To further elucidate the mechanisms of ecological effects of microplastics on microorganisms, future work is planned to design an indoor sediment-microplastics incubation experiment.
Acknowledgments
We thank editors and reviewers for their helpful suggestions on the paper.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Zhichao Wang
Zhichao Wang is a lecturer at Inner Mongolia University of Science and Technology. His research interests include river and lake water environment treatment.
Wenlu Li
Wenlu Li is a graduate student at Inner Mongolia University of Science and Technology, and her research topic is microplastics pollution.
Wenhuan Yang
Wenhuan Yang, a teacher at Inner Mongolia University of Science and Technology, has a research topic on wastewater treatment.
Weiping Li
Weiping Li is the Dean of the Graduate School of Inner Mongolia University of Science. Her research focuses on waste water treatment.
Jianlin Yang
Jianlin Yang is a graduate student at Inner Mongolia University of Science and Technology, and his research topic is microplastics.
Fan Yang
Fan Yang is a graduate student at Inner Mongolia University of Science and Technology, and his research topic is microplastics.
References
- Banerjee S, Kirkby CA, Schmutter D, Bissett A, Kirkegaard JA, Richardson AE. 2016. Network analysis reveals functional redundancy and keystone taxa amongst bacterial and fungal communities during organic matter decomposition in an arable soil. Soil Biol Biochem. 97:188–198.
- Chai BW, Yin H, Wei Q, Lu JN, Dange Z. 2021. The relationship between microplastics and the surrounding soil environment in the e-waste disassembly area. Environ Sci. 42:1073–1080.
- Chen Y, Jiang YM, Huang HY, Mou L, Ru J, Zhao J, Xiao S. 2018. Long-term and high-concentration heavy-metal contamination strongly influences the microbiome and functional genes in Yellow River sediments. Sci Total Environ. 637:1400–1412.
- Danso D, Schmeisser C, Chow J, Zimmermann W, Wei R, Leggewie C, Li X, Hazen T, Streit WR. 2018. New insights into the function and global distribution of polyethylene terephthalate (PET)-degrading bacteria and enzymes in marine and terrestrial metagenomes. Appl Environ Microbiol. 84:e02773-17.
- De Tender C, Devriese LI, Haegeman A, Maes S, Vangeyte J, Cattrijsse A, Dawyndt P, Ruttink T. 2017. Temporal dynamics of bacterial and fungal colonization on plastic debris in the North Sea. Environ Sci Technol. 51(13):7350–7360.
- Debroas D, Mone A, Ter Halle A. 2017. Plastics in the North Atlantic garbage patch: a boat-microbe for hitchhikers and plastic degraders. Sci Total Environ. 599–600:1222–1232.
- Dussud C, Meistertzheim AL, Conan P, et al. 2018. Evidence of niche partitioning among bacteria living on plastics, organic particles and surrounding seawaters. Environ Pollut. 236:807–816.
- Elifantz H, Horn G, Ayon M, Cohen Y, Minz D. 2013. Rhodobacteraceae are the key members of the microbial community of the initial biofilm formed in Eastern Mediterranean coastal seawater. FEMS Microbiol Ecol. 85(2):348–357.
- Frère L, Maignien L, Chalopin M, et al. 2018. Microplastics bacterial communities in the Bay of Brest: influence of polymer type and size. Environ Pollut. 242(Pt A):614–625.
- Ghosal D, Ghosh S, Dutta TK, Ahn Y. 2016. Current state of knowledge in microbial degradation of polycyclic aromatic hydrocarbons (PAHs): a review. Front Microbiol. 7:1369.
- Harrison JP, Schratzberger M, Sapp M, Osborn AM. 2014. Rapid bacterial colonization of low-density polyethylene microplastics in coastal sediment microcosms. BMC Microbiol. 14(1):2–15.
- Hou JH. 2020. Effects of polyethylene microplastics on the properties of soil aggregates and microbial diversity [dissertation]. Lanzhou Jiaotong University, Lanzhou, China.
- Huang Y, Zhao Y, Wang J, Zhang M, Jia W, Qin X. 2019. LDPE microplastic films alter microbial community composition and enzymatic activities in soil. Environ Pollut. 254(PtA):112983.
- Huerta Lwanga E, Thapa B, Yang X, Gertsen H, Salánki T, Geissen V, Garbeva P. 2018. Decay of low-density polyethylene by bacteria extracted from earthworm's guts: a potential for soil restoration. Sci Total Environ. 624:753–757.
- Ivleva NP, Wiesheu AC, Niessner R. 2017. Microplastic in aquatic ecosystems. Angew Chem Int Ed Engl. 56(7):1720–1739.
- Jiang P, Zhao S, Zhu LX, Li DJ. 2018. Microplastic-associated bacterial assemblages in the intertidal zone of the Yangtze Estuary. Sci Total Environ. 624:48–54.
- Kesy K, Oberbeckmann S, Kreikemeyer B, Labrenz M. 2019. Spatial environmental heterogeneity determines young biofilm assemblages on microplastics in Baltic Sea mesocosms. Front Microbiol. 10:1665.
- Law KL, Thompson RC. 2014. Microplastics in the seas. Science. 345(6193):144–145.
- Li W, Zhang Y, Wu N, Zhao Z, Xu W, Ma Y, Niu Z. 2019. Colonization characteristics of bacterial communities on plastic debris influenced by environmental factors and polymer types in the Haihe Estuary of Bohai Bay, China. Environ Sci Technol. 53(18):10763–10773.
- Li WH, Jian MF, Liu SL, et al. 2020. The occurrence relationship between microplastics and heavy metal pollutants in sediments of Poyang Lake Estuary and Yangtze River. Environ Sci. 41(1):242–252.
- Näkki P, Setälä O, Lehtiniemi M. 2017. Bioturbation transports secondary microplastics to deeper layers in soft marine sediments of the northern Baltic Sea. Mar Pollut Bull. 119(1):255–261.
- Nauendorf A, Krause S, Bigalke NK, Gorb EV, Gorb SN, Haeckel M, Wahl M, Treude T. 2016. Microbial colonization and degradation of polyethylene and biodegradable plastic bags in temperate fine-grained organic-rich marine sediments. Mar Pollut Bull. 103(1–2):168–178.
- Nava V, Leoni B. 2021. A critical review of interactions between microplastics, microalgae and aquatic ecosystem function. Water Res. 188:116476.
- Neis EP, Dejong CH, Rensen SS. 2015. The role of microbial amino acid metabolism in host metabolism. Nutrients. 7(4):2930–2946.
- Obbard RW, Sadri S, Wong YQ, Khitun AA, Baker I, Thompson RC. 2014. Global warming releases microplastics legacy frozen in Arctic Sea ice. Earths Future. 2(6):315–320.
- Oberbeckmann S, Kreikemeyer B, Labrenz M. 2018. Environmental factors support the formation of specific bacterial assemblages on microplastics. Front Microbiol. 8:2709.
- Oberbeckmann S, Loeder MGJ, Gerdts G, Osborn AM. 2014. Spatial and seasonal variation in diversity and structure of microbial biofilms on marine plastics in Northern European waters. FEMS Microbiol Ecol. 90(2):478–492.
- Ogonowski M, Motiei A, Ininbergs K, Hell E, Gerdes Z, Udekwu KI, Bacsik Z, Gorokhova E. 2018. Evidence for selective bacterial community structuring on microplastics. Environ Microbiol. 20(8):2796–2808.
- Rogers KL, Carreres‐Calabuig JA, Gorokhova E, Posth NR. 2020. Micro‐by‐micro interactions: how microorganisms influence the fate of marine microplastics. Limnol Oceanogr. 5(1):18–36.
- Ryan PG. 2015. Does size and buoyancy affect the long-distance transport of floating debris? Environ Res Lett. 10(8):084019.
- Sahin S, Yayla S. 2005. Effects of processing parameters on the mechanical properties of polypropylene random copolymer. Polym Test. 24(8):1012–1021.
- Sen SK, Raut S. 2015. Microbial degradation of lowdensity polyethylene (LDPE): a review. J Environ Chem Eng. 3(1):462–473.
- Shao ZZ, Dong CM, Guo WB, Luo ZH. 2019. Research progress on marine microplastic pollution and plastic degradation microorganisms. J Appl Oceanogr. 38(4):490–501.
- Shen C, Xiong J, Zhang H, Feng Y, Lin X, Li X, Liang W, Chu H. 2013. Soil pH drives the spatial distribution of bacterial communities along elevation on Changbai Mountain. Soil Biol Biochem. 57:204–211.
- Su L, Xue Y, Li L, Yang D, Kolandhasamy P, Li D, Shi H. 2016. Microplastics in Taihu Lake, China. Environ Pollut. 216:711–719.
- Teng J, Zhao J, Zhang C, et al. 2020. A systems analysis of microplastics pollution in Laizhou Bay, China. Sci Total Environ. 745(25):140815.
- Walkley A, Black IA. 1934. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 37:29–38.
- Wang ZC, Yang F, Yang WH, Li WP, Yang JL, Qin YM, Li H. 2020. Occurrence characteristics and quality estimation of microplastics in drainage ditches in Hetao irrigated Area, Inner Mongolia. Environ Sci. 41(10):4590–4598.
- Wolińska A, Kuźniar A, Zielenkiewicz U, Izak D, Szafranek-Nakonieczna A, Banach A, Błaszczyk M. 2017. Bacteroidetes as a sensitive biological indicator of agricultural soil usage revealed by a culture-independent approach. Appl Soil Ecol. 119:128–137.
- Xian WD, Zhang XT, Li WJ. 2020. Research status and prospect of C. aeruginosa. J Microbiol. 60(9):1801–1820.
- Yang Y, Chen L, Xue L. 2020. China's solution to global problems: problems and countermeasures of marine plastic waste and microplastics pollution control system. Popul Resour Environ China. 30(10):45–52.
- Zhang W, Zhang S, Wang J, Wang Y, Mu J, Wang P, Lin X, Ma D. 2017. Microplastic pollution in the surface waters of the Bohai Sea, China. Environ Pollut. 231(Pt 1):541–548.
- Zhang YF, Liao ZL, Han ZH, et al. 2020. Analysis on the change law of water quantity in Daihai, Inner Mongolia. Water Conserv. 2020(7):8–9.
- Zhao S, Liu J, Banerjee S, Zhou N, Zhao Z, Zhang K, Hu M, Tian C. 2020. Biogeographical distribution of bacterial communities in saline agricultural soil. Geoderma. 361(C):114095.
- Zhao S, Zhu L, Wang T, Li D. 2014. Suspended microplastics in the surface water of the Yangtze Estuary System, China: first observations on occurrence, distribution. Mar Pollut Bull. 86(1–2):562–568.
- Zheng YF, Li JX, Cao W, Liu X, Jiang F, Ding J, Yin X, Sun C. 2019. Distribution characteristics of microplastics in the seawater and sediment: a case study in Jiaozhou Bay, China. Sci Total Environ. 674:27–35.