Abstract
Because of the pivotal role of zooplankton in most aquatic ecosystems; there is constant need to explore the effect of stressors (such as physicochemical properties of freshwater) on their abundance. We documented the effect of temperature, pH, turbidity, dissolved oxygen (DO), nitrate (NO3-), and phosphate (PO4³-) on the abundance of zooplankton of the River Adada, Nigeria. Using a manual pump, zooplankton were collected from three strategic stations from January to April (dry season) and June to September (rainy season). A total of 2,219 (dry = 945; rainy = 1,274) zooplankton occurred in the following descending order of abundance: copepods < rotifers < cladocerans < ostracods < insect larva. Whereas based on species number, cladocerans (n = 10) < copepods (n = 8) < rotifers (n = 7) < insect larva (n = 4) < ostracods (n = 2) occurred in this descending order. The downstream (station C) had more zooplankton (dry = 427; rainy = 391), followed by the mid-stream (station B; dry = 216; rainy = 591) and upstream (station A; dry = 302; rainy = 292). There was also a significant (P < 0.05) joint interactive effect of thermal condition on turbidity, pH, DO, PO4³- and NO3- to influence zooplankton abundance. Indicating that temperature is an important factor determining the assemblage of zooplankton in freshwater. Nevertheless, to assure an accurate evaluation of the effect of physicochemical parameters on zooplankton abundance in freshwater, future studies should include extended study periods and make use of more sophisticated trapping technique that would ensure the collection of a larger sample size with more species of zooplankton.
Introduction
Freshwater ecosystems provide unique habitats that enhance ecological services and the survival of different life forms (Johnson and Pflugh Citation2008). Systematic monitoring of a freshwater resource (such as the river) is necessary to sustain its socioeconomic function. But currently, factors such as anthropogenic activities and climate change are obstructing the stability of most freshwater ecosystems (Dudgeon et al. Citation2006), thereby, causing a loss in diversity of zooplankton (Geist Citation2011; Alahuhta et al. Citation2019). Zooplankton consist of mainly microscopic organisms such as protists, rotifers, copepods, and cladocerans. These organisms are indicators and fast responders to environmental stressors (Pawlowski et al. Citation2016) such as nutrients (Xiong et al. Citation2019) and pesticides accumulation (Hanazato Citation2001). Zooplankton contribute to the biodiversity of aquatic ecosystems. Thus, the assessment of factors that affect the distribution of zooplankton is necessary (Hemlata et al. Citation2013; Mimouni et al. Citation2018; Xiong et al. Citation2020), because zooplankton are widely accepted and irreplaceable bioindicators in the ecological conservation and management of aquatic ecosystems (Xiong et al. Citation2020). The importance of zooplankton includes being used as a biological tool, studying the fate of biogeochemical cycling of potentially toxic metals (Fernández-Severini et al. Citation2013; Snell and Marcial Citation2017), predicting ecological thresholds of ammonia nitrogen (Yang et al. Citation2017), and controlling undesirable phytoplankton biomass (Teissier et al. Citation2012).
Despite their ecological importance, the richness and diversity of zooplankton are at risk (Oertli et al. Citation2005). This becomes worse when factors influencing communities of zooplankton remain undescribed (Mimouni et al. Citation2018) especially in an area where zooplankton are considered organisms with low ecological value (Noble and Hassall Citation2015). As such, the conservation value of zooplankton is mostly unknown and rarely estimated in a developing country such as Nigeria. Indeed, zooplankton occupy a key position in aquatic food webs, impacting the overall cycling of materials and energy (Finlay et al. Citation2007). Yet, the macroinvertebrate communities are getting more attention (Azevedo et al. Citation2015) even when zooplankton play a complementary role to macroinvertebrates in assessing the environmental quality of freshwater ecosystems (Payne Citation2013; Azevedo et al. Citation2015). Zooplankton are predators of phytoplankton and are very sensitive to changes in environmental conditions (Eisner et al. Citation2014; Xiong et al. Citation2016). Understanding the behavior of the zooplankton community under seasonal environmental conditions is necessary to support a healthy and productive aquatic ecosystem (Lacerot et al. Citation2013). A freshwater such as the River Adada is one of the main revenue emolument, a resource for fishermen and biodiversity in Southeast Nigeria; however, there are limited records on its pollution status (Aganigbo and Ifediegwu Citation2021) including the knowledge about the joint interactive effect of physicochemical properties on the distribution of zooplankton of the river. It is debatable whether nutrients, temperature or the joint interactive effect of both properties (nutrients and temperature) are responsible for shaping the abundance of zooplankton community in the water (Işkın et al. Citation2020).
We investigated the effect of physicochemical parameters on zooplankton abundance of the River Adada. Also, the joint interactive effect of thermal condition on the chemical properties of the river was explored, to test if zooplankton abundance is predicted (determined) by the interactive effect of temperature with the indices of chemical parameters of the water. Therefore, the physicochemical parameters we measured in this study are: temperature, turbidity (transparency), pH, dissolved oxygen (DO), phosphate (PO4³-), and nitrate (NO3-). The seasonal changes, especially in thermal condition, could influence the heterogeneity of the chemical indices of water. For instance, a study suggested that thermal condition is capable of influencing dissolved oxygen and metabolic activities (Roman et al. Citation2019), consequently bringing about differences in zooplankton communities (Carvalho et al. Citation2001; De Paggi and Paggi Citation2008; Roman et al. Citation2019). Thus, we hypothesized that temperature which is seasonally driven and a strong physical property of water will significantly interact with the chemical properties of the river to influence zooplankton abundance. We also hypothesized that the portion of the river with the highest temperature, algal bloom, and zooplankton should experience a low level of DO and pH (Diaz and Rosenberg Citation2008). Because of the transparent lorica and small size that makes them unlikely to be predated by fish, we predicted that rotifers should be the most abundant species of zooplankton in the river. Besides, rotifers have been reported to outnumber other zooplankton found in Afrotropical freshwaters (e.g. Mustapha Citation2009).
Materials and methods
Study area
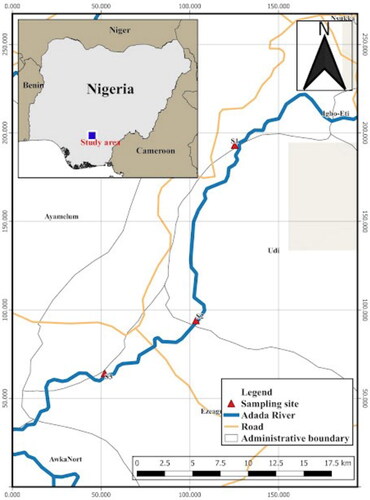
River Adada () has an entire length of 876 km but 314 km is covered by Enugu State (Aganigbo and Ifediegwu Citation2021). The river is located in the following latitude (6° 67′ 0” N and 6° 45′ 0” N) and longitude (7° 0′ 0” E and 7° 24′ 0” E); passing through Nsukka, Lejja hills, then flowing through several communities such as Uzo-uwani, Ogbosu and Anambra state before emptying into the River Niger. It drains a catchment area of about 43,187 km2, and rivers such as Awwa, Adogwu, Ukpotu are the principal tributaries of the river. The elevation of the region where the river is situated varies between 201 − 470 m above sea level. The mean annual precipitation also varies between 1890 − 2000 mm. During the rainy season, the river overflows its banks, causing flooding and water logging. Consequently, this promotes the cultivation of rice in this area of the river (Aganigbo and Ifediegwu Citation2021). The climatic condition includes a mean temperature of 26 and 30 °C in the rainy and dry seasons. The rainy season occurs between April to mid-July and early October, while the dry season begins from ending October to April in the subsequent year. Between November and early March, harmattan wind, accompanied by cold, and very dry air sets in. To date, anthropogenic activities such as logging, mining, swimming, fishing, yachting, fermenting of cassava, and washing of clothes occur at the river (Aganigbo and Ifediegwu Citation2021).
Selection of sampling stations
The current study lasted from January to April (dry season) and June to September (rainy season), 2018. The river was divided into 3 stations (∼500 m apart) based on the morphology, algal bloom, and anthropogenic activities. The upstream was labeled station A (longitude 7° 18′ 54” N and latitude 6° 67′ 26” N), some of the characteristic features observed here are some vegetation that consists of macrophytes, floating plants, and shrubs. Algal bloom and anthropogenic activities were not much in this station compared with the mid-stream (station B; longitude 7° 15′ 38” N and latitude 6° 52′ 06” N). The mid-stream water is relatively stable, thus, we witnessed more agricultural activities such as farming, vegetation such as palm trees, and the occurrence of more algal bloom. The downstream of the river (station C; longitude 7° 07′ 96” N and latitude 6° 48′ 78” N) consist of grasses, floating plants, macrophytes, and the shrinking channels caused by erosion. Because of variation in the composition of zooplankton with the time of the day (Das et al. Citation2020), and the diel periodicity that triggers the migration of zooplankton to the surface of the water before daylight hours (Rusconi-Rodrigues Citation2016), the river was traversed using a boat (twice weekly) and screened in the early hours before sunrise (0600 to 0730 hours).
Collection and identification of zooplankton
For effectiveness and to avoid damage to target species, we used a manually driven bilge pump for collection (Sluss et al. Citation2011). Besides, this device has been effectively used for sampling the community of zooplankton at different thermo strata of water resource (Patil and More Citation2017). The pump, having an inlet of 6.0 cm and a capacity of 1-L, was purchased from JOECHEM® Mechanical Store, Nsukka. The pump was lowered to the required depth of 2 m, then zooplankton were collected by pumping 20 L of the water through a 63-μm net into a large calibrated container. Collected samples were rinsed, preserved in a 250 mL Nalgene bottle, and fixed using 10% sugared formalin (Sluss et al. Citation2011). Standardization for each sample was conducted using a 63-μm mesh, before identification and counting of samples on Olympus® microscope (x10 objective) in the Department of Zoology and Environmental Biology, the University of Nigeria, Nsukka.
Quantification of temperature and turbidity of the river
Water temperature was measured every sampling day using the mercury-in-bulb thermometer (having a graduation of 0 − 360 °C). The probe was lowered (2 m) into the river for ∼5 min, then read immediately while the instrument was still in the water to avoid interference from ambient temperature (Andong et al. Citation2019). The measure of the clarity or cloudiness of the river (turbidity) was conducted in the field by filling the calibrated standard cuvettes of the meter with water from the sampling stations, then placed on the Hanna® portable field nephelometer, the values obtained were recorded in nephelometric turbidity units (NTU).
Quantification of pH, DO, (PO4³-), and (NO3-)
The pH and DO were quantified based on standard procedures in the field. In brief: the probe (Hanna Field pH meter, Model PHS 25) for measuring pH was inserted (2 m) into the river, allowed to attain a stable value, then recorded (Andong et al. Citation2019). The DO of the river was measured based on procedures outlined by Winkler’s method (Langdon, 2010). Conversely, the nutrient content (PO4³-and NO3-) of the river were quantified by ion chromatography (Dionex) (APHA Citation1998; Dirican Citation2015).
Statistical design and data analyses
The data were sub-set based on stations to test, using a general linear model (GLM) with Gaussian errors and an identity link function, if the zooplankton abundance in the individual stations will relate to seasons, DO, pH, PO4³-, NO3-, and turbidity of the water. Amid the analyses, interactions added to the model on the explanatory axis were to test if the joint interactive effect of physical and chemical indices would significantly impact the zooplankton abundance. These interactions consisted of temperature and DO, temperature and pH, temperature and NO3-, and temperature and PO4³- from the different stations. Using a step-wise single deletion of least-significant terms (P < 0.05), we removed the non-significant terms starting with the interactions.
Results
We encountered a total of 2,219 zooplankton and seasonality in abundance indicates more zooplankton in the rainy season (n = 1,274) when compared with the dry season (n = 945). Station C had more zooplankton (n = 818; dry = 427; rainy = 391), followed by B (n = 807; dry = 216; rainy = 591) and A (n = 594; dry = 302; rainy = 292) (). The relationships between zooplankton abundance with seasons () and the multiple pairwise-comparison of zooplankton abundance with the stations () was not statistically significant. The copepods were more abundant (n = 798), compared with rotifers (n = 564), cladocerans (n = 476), ostracods (n = 274) and insect larva (n = 107) (). Conversely, the cladocerans had more species of zooplankton (n = 10) identified as: Moina reticulate, Euryalona orientalis, Chydorus sphaericus, Diaphanosoma excisum, Moina micrura, Bythosrephes longimanus, Simocephalus expinous, Bosmina longirostris, Acroperus africanus sp. nov, Alona costata. Followed by the copepods (n = 8 species) identified as: Macrosetella gracilis, Setella gracillis, Diaptomus africanus, Cryptocyclops bicolor, Thermocyclops neglectus, Centropages furcatus, Copepod nauplii, Harpacticoid nauplii. The rotifers (n = 7 species) encountered are Keratella cochlearis, Brachionus angularis, Lecane leontina, Trichocerca cylindrical, Keratella tropica, Lophocharis oxysternon, Brachionus caudatus. The insect larva (n = 4 species) consisted of Chironomid imicola larva, Parachironomus biannulatus larva, Tanytarsus curticornis, Chaoborus sp. larva, and ostracods (n = 2 species) Parastenocypris unispinosa, Cytheridella tepida sp. nov respectively.
Table 1. From the total number of zooplankton, the rainy season had the highest number of encountered individuals. By stations and seasons, C had more number of individuals in the dry season, while station B in the rainy season (Table 1a). There was no significant difference in zooplankton abundance with seasons (Table 1 b). The multiple pairwise-comparison (Tukey contrasts) between different stations also found no significant difference in zooplankton abundance (table 1c). Significant term in bold.
Table 2. Indicating that copepods (n = 798) were the most encountered zooplankton, followed by the rotifers (n = 564), cladocerans (n = 476) and Ostracods (n = 381). However, the cladocerans had more number of life forms (n = 10 species), followed by copepods (n = 8), rotifers (n = 7) and ostracods (n = 2).
Results from the physicochemical analyses of the water indicates temperature (station A) was higher in the dry season. In the rainy season, turbidity was higher in station B, while DO in station B, pH in station C, PO4³- in station B and NO3- in station A ().
Table 3. Comparing values from the dry and rainy seasons, the mean water temperature in station A was high during dry season. Meanwhile, turbidity in station B, DO (station B), pH (station C), PO4³⁻ (station B) and NO3- (station A) were all high in the rainy season.
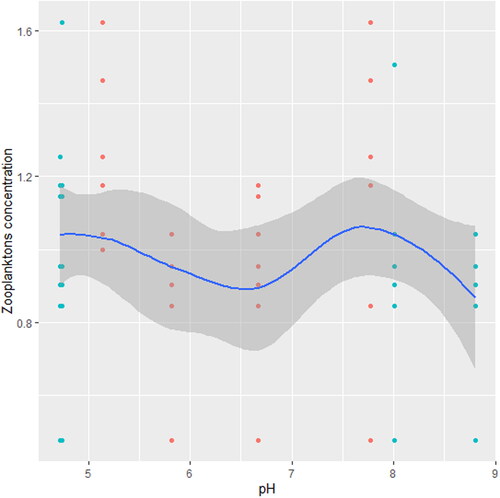
There was a significant effect of seasons, pH, temperature, turbidity, PO4³- and DO on Zooplankton. There was a significant joint interactive effect between temperature and DO, PO4³-, pH on zooplankton (site A; ).
Table 4. Zooplankton abundance in station A in relation to physical and chemical indices of River Adada. Indicating that zooplankton abundance in the river was not only affected by seasons, pH, turbidity, PO4³⁻, and DO but the joint interactive effect of temperature on DO, PO4³⁻, and pH as well. Significant terms are in bold, while insignificant terms are not included in the table. Insignificant predictors may not be statistically significant, but had a joint significant effect in the presence of another predictor (temperature), thus, included in the table.
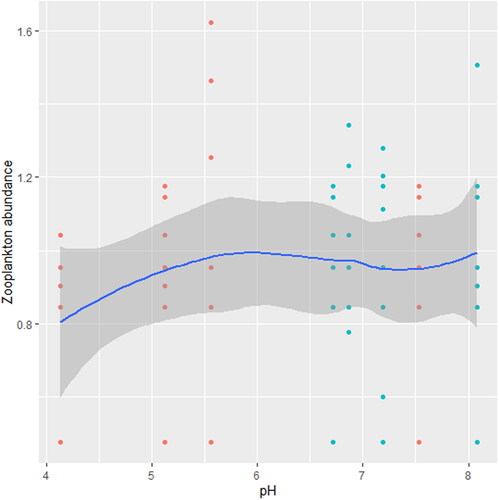
indicates the significant effect of seasons, turbidity, PO4³-, NO3- and DO on Zooplankton. There was a significant joint interactive effect between temperature and DO, NO3- on zooplankton (station B; ).
Table 5. Zooplankton abundance in station B in relation to physical and chemical indices of River Adada. Indicating that zooplankton abundance in the river is not only affected by seasons, turbidity, PO4³⁻, NO3-, and DO but the joint effect of temperature on DO, and NO3-. Significant terms are in bold, while insignificant terms are not included in the table. Insignificant predictors may not be statiscally significant, but had a joint significant effect in the presence of another predictor (temperature), thus, included in the table.
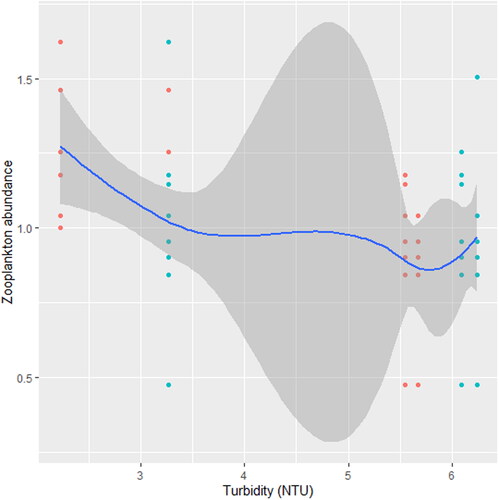
indicates the significant effect of turbidity, PO4³-, NO3- and DO on Zooplankton. There was a significant joint interactive effect between temperature and PO4³- on zooplankton (station C; ).
Table 6. Zooplankton abundance in station C in relation to physical and chemical indices of River Adada. Indicating that zooplankton abundance in the river is not only affected by temperature, turbidity, PO4³⁻, NO3-, and DO but the joint effect of temperature on DO, and PO4³⁻. Significant terms are in bold, while insignificant terms are not included in the table. Insignificant predictors may not be statiscally significant, but had a joint significant effect in the presence of another predictor (temperature), thus, included in the table.
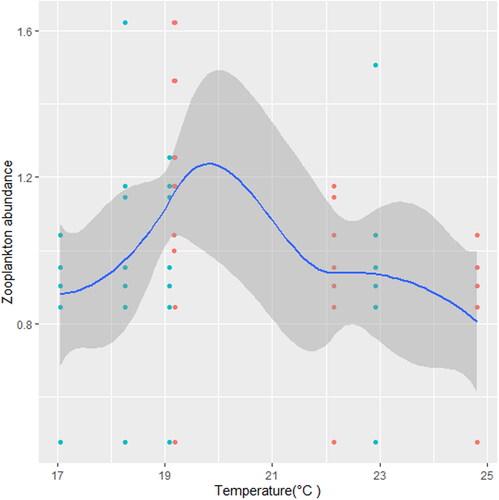
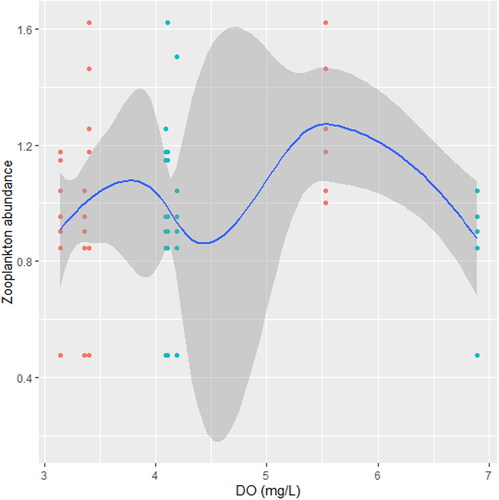
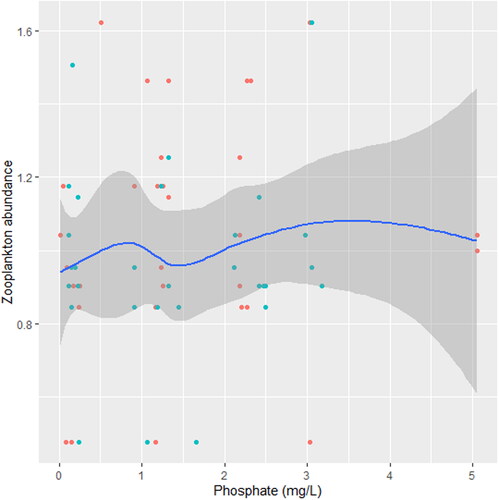
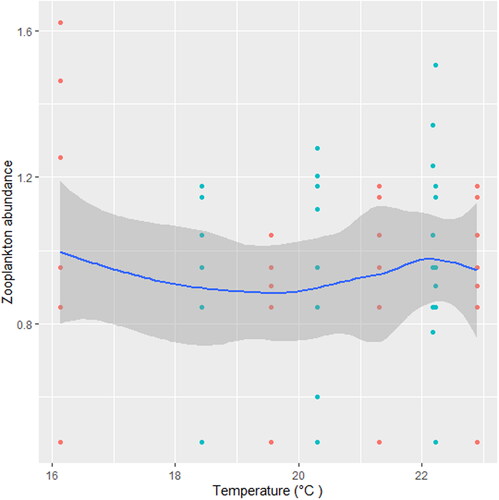
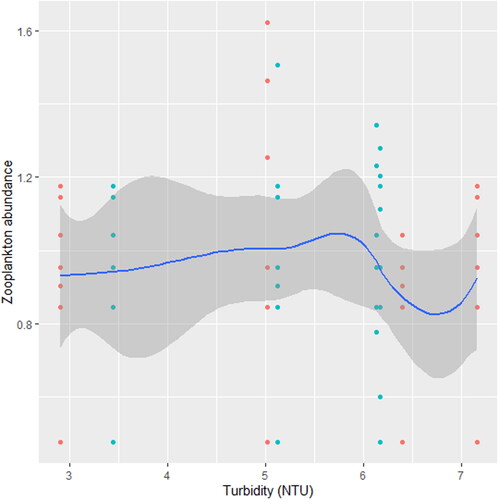
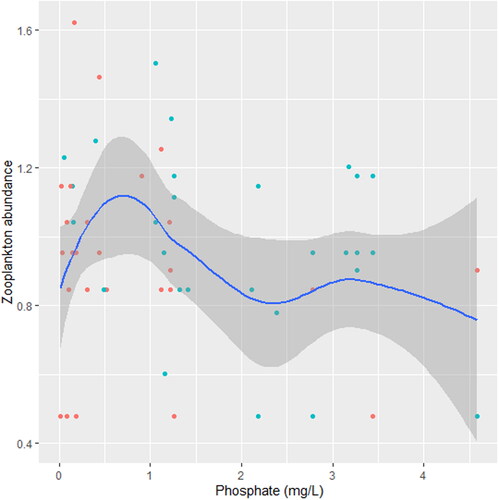
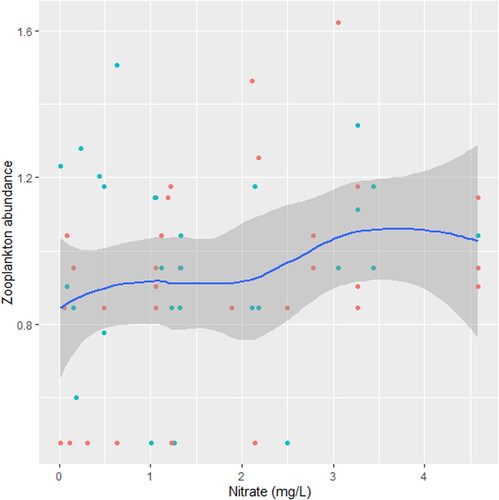
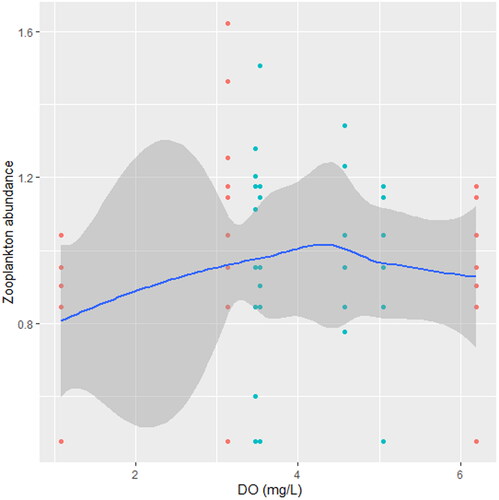
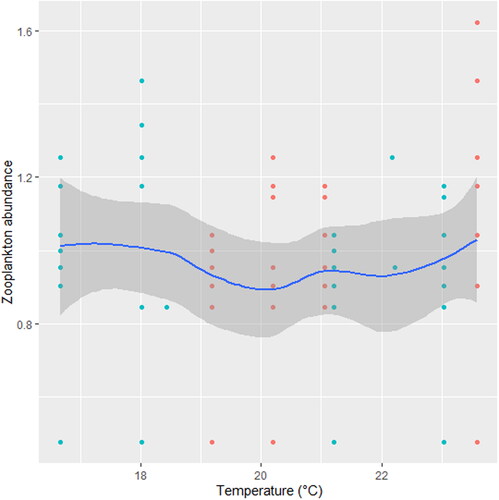
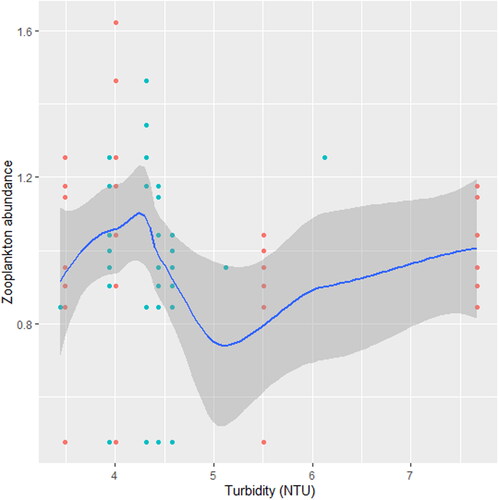
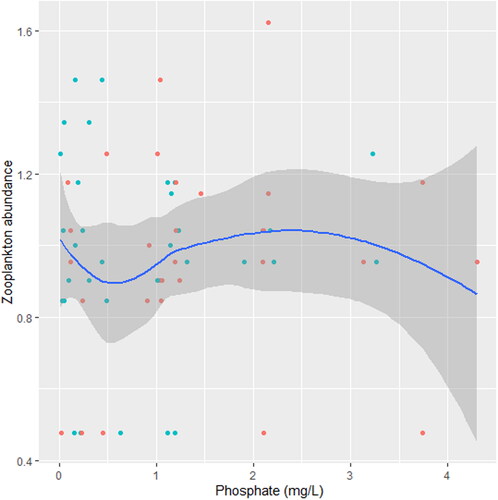
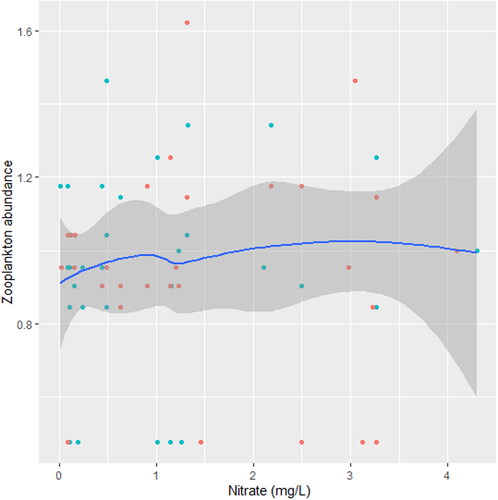
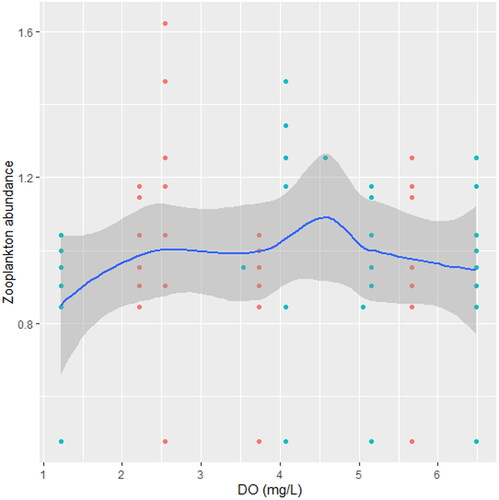
We found that the relationship between temperature and zooplankton abundance significantly fluctuated. The variability was more pronounced in station A () when compared with station B () and C (). Also, DO had a highly variable relationship that was more pronounced in station A () when compared with station B () and C (). Phosphate significantly declined with zooplankton abundance in station B () when compared with station A () and C (). Conversely, NO3- unsteadily increased significantly with zooplankton abundance in station B () when compared with station C (). In station A, pH unsteadily related to zooplankton abundance (), while in station B, relationship between pH and zooplankton abundance unsteadily declined (). Zooplankton abundance unsteadily declined with turbidity in station A (), when compared with the marginal steady increase which then declined in station B (). In station C (), there was a significant relationship between turbidity and zooplankton abundance.
Discussion
Statistically, we found no seasonal variation in zooplankton abundance with seasons and the stations sampled, except that there are indications that the rainy season and the downstream of the river (station C) had more zooplankton. In line with our prediction, we found more zooplankton in the mid-stream (station B) of the river during the rainy season. The mid-stream had more algal bloom and we initially hypothesized that this station should attract more zooplankton. Our result is consistent with a contemporary study that found zooplankton abundance positively correlating with algae and algal abundance in a freshwater of Western Poland (Goldyn and Kowalczewska-Madura Citation2007). Also, the more zooplankton encountered in the rainy season was consistent with a previous study in the Niger Delta region of Nigeria. Zooplankton distribution could provide ideas on the prevailing physical and chemical conditions of freshwater (Jakhar Citation2013). Thus, we encountered more copepods when compared with rotifers, cladocerans, ostracods and insect larva. Based on lifeforms (number of species), we found more cladocerans, when compared with copepods, rotifers, ostracods and insect larva. In some previous studies for instance, Zabbey et al. (Citation2008) and Obot et al. (Citation2020) found more copepods in the water sampled from South East Nigeria. While it may be difficult to explain why copepods were dominant in the water, we suspect that their success could be as a result of mere chance in the evolutionary process of natural selection which makes them competitively superior to other, similarly sized organisms (Kiørboe Citation2011). For instance, some species of copepods share a viscous, nutritionally dilute and dangerous habitat with other zooplankters and for these reasons, have in many cases evolved unique and efficient solutions to the main challenges of feeding and surviving in water. For instance, the special body shape of copepods allows for an efficient pursuit for prey at a very high speed and acceleration. Secondly, the ability to avoid predation (from e.g. fish) through jumping is facilitated by the dual propulsion mechanisms that many copepods possess (Kiørboe Citation2011). By this, the copepods dominated the invertebrate fauna of many inland freshwaters in high densities (Williamson and Reid Citation2001). Meanwhile, rotifers are zooplankton that are usually abundant in most Afrotropical freshwaters (e.g. Mustapha Citation2009; Echoke et al. Citation2018). However, the low abundance of rotifers in this study could be due to predation. In fact , the low abundance of rotifers was once linked to planktivorous fishes (Stemberger and Lazorchak Citation1994) and chaoborus larvae (Saunders and Lewis Citation1988), a carnivorous zooplankton found in this study.
The physicochemical properties of River Adada and zooplankton abundance varied across stations. Results from the upstream (station A) indicates that seasons, pH, DO, turbidity, PO4³- and the joint interactive effect of temperature on DO, PO4³-, and pH significantly influenced zooplankton abundance. In the mid-stream (station B), seasons, turbidity, PO4³-, NO3-, DO and the joint interactive effect of temperature on DO and NO3- signifcantly correlated to zooplankton abundance, while turbidity, PO4³-, NO3-, DO and the joint interactive effect of temperature on NO3- related significantly to zooplankton abundance of the downstream (station C). The mid-stream of the river experienced the highest mean turbidity level in the rainy season. However, this could be attributed to the silt, mud, algae, plant pieces, wood ashes or chemicals arising from anthropogenic activities such as mining, logging or dredging of the river. High turbidity could suppress photosynthesis by obstructing sunlight. Halted or reduced photosynthesis hampers the survivability of plants, including other aquatic animals through the reduction in DO output (Andrulewicz et al. Citation2008; USEPA Citation2012). Dissolved oxygen including biological oxygen demand in water are essential in measuring the pollution status of water. In this study, DO was high at the mid-stream in the rainy season and low in the dry season at the downstream. The increase in DO could be attributed to the high algal bloom encountered in this station (mid-stream) of the river. Conversely, algal bloom may result in hypoxic or anoxic conditions during decomposition. Low DO experienced at the downstream of the river could be as result of the increase in the metabolic activities or the increase in concentration of chemicals at this section of the water. We found that the mean temperature was a low in both the up-and-downstream of the river during the rainy season. Notably, seasonal differences in water temperature across an aquatic ecosystem can dramatically impact the metabolic activities of aquatic organisms in numerous ways. An increase or fall in temperature could kill zooplankton that are sensitive to temperature changes in water (Andrulewicz et al. Citation2008; Tunowski Citation2009). Thus, a major factor affecting the distribution of zooplankton (Chen Citation2020), and a determinant of oxygen level in the water is temperature. At a very high temperature, zooplankton tend to avoid the surface of water to avoid hypoxia (Roman et al. Citation2019).
In this study, the joint interactive effect of temperature on pH and nutrients related significantly to zooplankton abundance. The mean pH was high in the rainy season, at both the mid-and-downstream of the river. A fall in pH level decreases abundance/diversity of most aquatic organisms (Jeffries and Mills Citation1990; Ivanova and Kazantseva Citation2006; Andrulewicz et al. Citation2008). On the other hand, high pH and moderate temperature promotes primary productivity which favors growth and survival of zooplankton (Bednarz et al. Citation2002; Mustapha Citation2009). We found that the mean PO4³- was high in the mid-stream in the rainy season, while NO3- was also high in the rainy season but at the upstream portion of the river. The availability of nutrients determines zooplankton structure and abundance (Paturej Citation2006; Yildiz et al. Citation2007; Kudari and Kanamadi Citation2008). An increase in the concentration of PO4³- and NO3- in water has been linked to anthropogenic activities, by this, eutrophication is bound to occur (Andong et al. Citation2019). Consequently, a previous study found that nutrients have a greater impact than temperature on most biota of a freshwater (Birk et al. Citation2020), while another study found that the effect of temperature on aquatic invertebrates was stronger under nutrient limitation (Pomati et al. Citation2019). In this study, we found that temperature interacts significantly with nutrients to influence zooplankton abundance. Besides, we found that both PO4³- and NO3- were significantly related to zooplankton abundance of the mid-and downstream of the river. Other factors (not measured in this study) that may have influenced zooplankton abundance in River Adada could be predation (Roman et al. Citation2019). For instance, a study suggests that juveniles fish such as Oreochromis niloticus and Clarias sp. prowl most freshwaters during the dry or post-rainy season in search of microinvertebrates such as zooplankton (Ovie and Ovie Citation2002; Okogwu Citation2008; Citation2010). Or probably, salinity which was not measured in this study, may have played a role in influencing or reducing zooplankton abundance (Yuan et al. Citation2020). Compared with other contemporary studies from other regions. We encountered more groups of zooplankton when compared with Nkwoji et al. (Citation2010) and Bashini et al. (Citation2017) from Nigeria and India. Contrarily, studies by Shi et al. (Citation2020) in China, Montoya-Maya and Strydom (Citation2009) in South Africa, while Ayodele and Adeniyi (Citation2006) in Southwest, Akindele and Adeniyi (Citation2013) in Northern Nigeria found more species number of zooplankton. Nevertheless, to assure an accurate evaluation of the physicochemical parameters on zooplankton abundance, future studies should include extended study periods and make use of a more sophisticated trapping techniques that would ensure the collection of larger sample size/more species of zooplankton.
Data accessibility statement
Data will be available on request
Authors’ contributions
The study was conceived and designed by NFO, JCE, IAN, FAA, ESO, and JCN were involved in study design, search in databases, quality assessment, study selection, data extraction, manuscript drafting, and critical discussion. Laboratory analysis was conducted by NFO, IAN, JCE, ESO, and JCN. The statistical design, data analysis, and interpretation by FAA. Manuscript was drafted, revised, and approved for submission by NFO, JCE, IAN, FAA, ESO, and JCN.
Ethics statement
All fieldwork and laboratory work performed complied with local ethical regulations and agreements
Acknowledgements
The study was supported with facilities from the Department of Zoology and Environmental Biology, and the National Center for Energy Research and Development, University of Nigeria, Nsukka, Enugu State. We also thank the following people for their help with various aspects of this project: Dr. NS. Oluah. Mr. EC. Odii, Dr. IE. Onah. Mrs. TD. Melefa, Mrs. EA. Orji, and Mrs. FF. Hinmikaiye.
Disclosure statement
No potential conflicts of interest were reported by the author(s)
Additional information
Notes on contributors
Nkiruka F. Oparaku
Nkiruka F. Oparaku (Ph.D.) is a onetime affiliate of the Lioning Institute of Energy, Yingkou City, China. Currently, she is with the Department of Zoology and Environmental Biology, the University of Nigeria, Nsukka. Her research currently focuses on aquatic sciences and bio-energy research management.
Felix A. Andong
Felix A. Anodng (M. Sc.) is an affiliate of A.P. Leventis Ornithological Research Institute, the University of Jos, and the Department of Zoology and Environmental Biology, the University of Nigeria. His research currently focuses on Conservation Biology.
Ijem A. Nnachi
Ijem A. Nnachi (M. Sc.) is an affiliate of the Department of Zoology and Environmental Biology, the University of Nigeria, Nsukka. His research focuses on parasites, especially the soil transmitted helminths.
Elijah S. Okwuonu
Elijah S. Okwuonu (M. Sc.) is an affiliate of the Department of Zoology and Environmental Biology, the University of Nigeria, Nsukka. His research focuses on parasites, especially the blood parasites from vertebrates and the risk factors in South East Nigeria.
James C. Ezeukwu
James C. Ezeukwu (B. Sc.) is an affiliate of the Department of Zoology and Environmental Biology, the University of Nigeria, Nsukka. His research focuses on animal welfare and management.
Joseph C. Ndefo
Joseph C. Ndefo (Ph.D.) is a Biochemist with the Department of Science Laboratory Technology, the University of Nigeria, Nsukka. His area of research currently focuses on Medical Biochemistry, Pharmacological Biochemistry, Nutritional Biochemistry and Environmental Toxicology. Specifically, he focuses on understanding how natural products and food–derived compounds affect the body condition, and how environmental alterations exposes humans to diseases.
References
- Aganigbo IC, Ifediegwu SI. 2021. Investigation of baseline metal pollution in soils from the Adada River Bank, Enugu State, Nigeria. Int J Environ Anal Chem. 101:1–19.
- Akindele EO, Adeniyi IF. 2013. Zooplankton composition and community structure in Lake Tiga, Kano, Nigeria. Afr J Aquatic Sci. 38 (3):279–286.
- Alahuhta J, Erős T, Kärnä O-M, Soininen J, Wang J, Heino J. 2019. Understanding environmental change through the lens of trait-based, functional, and phylogenetic biodiversity in freshwater ecosystems. Environ Rev. 27 (2):263–273.
- Andong FA, Ezenwaji NE, Melefa TD, Hinmikaiye FF, Nnadi OV, Oluwafemi O. 2019. Assessment of the physical and chemical properties of Lake Oguta (Nigeria) in relation to the water quality standard established by the Nigerian Federal Ministry of Water Resources. Adv Oceanogr Limnol. 10:74–78.
- Andrulewicz E, Szymelfenig M, Urbański J, Węsławski JM. 2008. The Baltic Sea − what is worth knowing. Astra Print Shop, Gdynia.
- APHA 1998. Standard methods for the examination of water and wastewater. Washington, DC: American Public Health Association.
- Ayodele HA, Adeniyi IF. 2006. The zooplankton fauna of six impoundments on River Osun, Southwest, Nigeria. Zoologist. 1:49–67.
- Azevedo DJS, Barbosa JEL, Gomes WIA, Porto DE, Marques JC, Molozzi J. 2015. Diversity measures in macroinvertebrate and zooplankton communities related to the trophic status of subtropical reservoirs: contradictory or complementary responses? Ecol Indicat. 50:135–149.
- Bashini JM,Pandiammal S, Senthilkumaar P. 2017. Seasonal variations in zooplankton with reference to physico- chemical conditions in temple pond at Thiruvottiyur, Chennai. J Environ Sci Toxicol Food Technol. 11 (7):1–5.
- Bednarz T, Starzecka A, Mazurkiewicz-Boroń G. 2002. Microbio-logical processes accompanying the blooming of algae and cyanobacteria. Wiad Botan. 46:45–55.
- Birk S, Chapman D, Carvalho L, Spears BM, Andersen HE, Argillier C, Auer S, Baattrup-Pedersen A, Banin L, Beklioğlu M, et al. 2020. Impacts of multiple stressors on freshwater biota across spatial scales and ecosystems. Nat Ecol Evol. 4(8):1060–1068.
- Carvalho PD, Bini LM, Thomaz SM, Oliveira LGD, Robertson B, Tavechio WLG, Darwisch AJ. 2001. Comparative limnology of South American flood-plain Lakes and Lagoons. Acta Sci. 23(2):265–273.
- Chen G. 2020. Effects of physical and chemical factors on zooplankton in tropical shallow urban lakes. conference Series: Earth and Environ Sci. 526:1–4.
- Das P, Kar S, Das U, Bimola M, Kar D, Aditya G. 2020. Day time variations of zooplankton species composition: observations from the wetlands of Assam, India. Acta Limnol Bras. 32:1–14.
- De Paggi SBJ, Paggi JC. 2008. Hydrological connectivity as a shaping force in the zooplankton community of two lakes in the Paraná River floodplain. Internat Rev Hydrobiol. 93(6):659–678.
- Diaz RJ, Rosenberg R. 2008. Spreading dead zones and consequences for marine ecosystems. Science. 321(5891):926–929.
- Dirican S. 2015. Assessment of water quality using physicochemical parameters of Çamlıgöze Dam Lake in Sivas. Turkey. Ecologia. 5:1–7.
- Dudgeon D, Arthington AH, Gessner MO, Kawabata Z-I, Knowler DJ, Leveque C, Naiman RJ, Prieur-Richard A-H, Soto D, Stiassny MLJ, et al. 2006. Freshwater biodiversity: importance, threats, status and conservation challenges. Biol Rev Camb Philos Soc. 81(2):163–182.
- Echoke JC, Nadana RW, Idowu RT. 2018. Zooplankton assessment of Usuma Reservoir in federal capital territory Abuja Nigeria. Direct J Public Health Environ Technol. 3(7):87–101.
- Eisner LB, Napp JM, Mier KL, Pinchuk AI, Andrews AG. 2014. Climate-mediated changes in zooplankton community structure for the Eastern Bering Sea. Deep-Sea Res. 109:157–171.
- Fernández-Severini MD, Hoffmeyer MS, Marcovecchio JE. 2013. Heavy metals concentrations in zooplankton and suspended particulate matter in a southwestern Atlantic temperate estuary (Argentina). Environ Monit Assess. 185(2):1495–1513.
- Finlay K, Beisner BE, Patoine A, Pinel-Alloul B. 2007. Regional ecosystem variability drives the relative importance of bottom-up and top-down factors for zooplankton size spectra. Can J Fish Aquat Sci. 64(3):516–529.
- Geist J. 2011. Integrative freshwater ecology and biodiversity conservation. Ecol Indicat. 11 (6):1507–1516.
- Goldyn R, Kowalczewska-Madura K. 2007. Interactions between phytoplankton and zooplankton in the hypertrophic Swarzędzkie Lake in Western Poland. J Plankton Res. 30(1):33–42.
- Hanazato T. 2001. Pesticide effects on freshwater zooplankton: an ecological perspective. Environ Pollut. 112(1):1–10.
- Hemlata V, Davendra NP, Sandeep KS. 2013. Monthly variations of zooplankton in a freshwater body, Futera anthropogenic pond of Damoh District (M.P.). Int J Innov Res Sci Engine Technol. 2(9):4781–4788.
- Işkın U, Filiz N, Cao Y, Neif ÉM, Öğlü B, Lauridsen TL, Davidson TA, Søndergaard M, Tavşanoğlu ÜN, Beklioğlu M, et al. 2020. Impact of nutrients, temperatures, and a heat wave on zooplankton community structure: an experimental approach. Water. 12(12):3416–3435.
- Ivanova MB, Kazantseva TI. 2006. Effect of water pH and total dissolved solids on the species diversity of pelagic zooplankton in Lakes: a statistical analysis. Russ J Ecol. 37(4):264–270.
- Jakhar P. 2013. Role of phytoplankton and zooplankton as health indicators of aquatic ecosystem: a review. Int J Innov Res Stud. 2:490–500.
- Jeffries M, Mills D. 1990. Freshwater Ecology. London: Belhaven Press; p. 285.
- Johnson BB, Pflugh KK. 2008. Local officials’ and citizens’ views on freshwater wetlands. Soc Nat Res. 21(5):387–403.
- Kiørboe T. 2011. What makes pelagic copepods so successful? J Plankton Res. 33 (5):677–685.
- Kudari VA, Kanamadi RD. 2008. Impact of changed trophic status on the zooplankton composition in six water bodies of Dharwad district, Karnataka state (South India. Environ Monit Assess. 144(1-3):301–313.
- Lacerot G, Kruk C, Lürling M, Scheffer M. 2013. The role of subtropical zooplankton as grazers of phytoplankton under different predation levels. Freshw Biol. 58(3):494–503.
- Mimouni E-A, Pinel-Alloul B, Beisner BE, Legendre P. 2018. Summer assessment of zooplankton biodiversity and environmental control in urban waterbodies on the Island of Montreal. Ecosphere. 9(7):e02277–19.
- Montoya-Maya PH, Strydom NA. 2009. Zooplankton composition, abundance and distribution in selected south and west coast estuaries in South Africa. Afr J Aquatic Sci. 34(2):147–157.
- Mustapha MK. 2009. Zooplankton assemblage of Oyun Reservoir, Offa, Nigeria. Rev Biol Trop. 57(4):1027–1047.
- Nkwoji JA, Onyema IC, Igbo JK. 2010. Wet season spatial occurrence of phytoplankton and zooplankton in Lagos Lagoon. Nigeria. Sci World J. 5 (2):7–14.
- Noble A, Hassall C. 2015. Poor ecological quality of urban ponds in Northern England: causes and consequences. Urban Ecosyst. 18(2):649–662.
- Obot OI, David GS, Ekpo IE. 2020. Zooplankton assemblages of a tropical coastal creek. South-Eastern Nigeria. Ecologia. 10:63–70.
- Oertli B, Biggs J, Céréghino R, Grillas P, Joly P, Lachavanne J-B. 2005. Conservation and monitoring of pond biodiversity: introduction. Aquatic Conserv: Mar Freshw Ecosyst. 15(6):535–540.
- Okogwu OI. 2010. Seasonal variations of species composition and abundance of zooplankton in Ehoma Lake, a floodplain lake in Nigeria. Int J Trop Biol. 15(1):171–182.
- Okogwu OI. 2008. The physicochemical parameters, plankton and ichthofauna of the mid-Cross River floodplain ecosystem, South-Eastern Nigeria [Ph.D. Thesis]. Nigeria: Department of Zoology, University of Ibadan.
- Ovie SI, Ovie SO. 2002. Fish-larval rearing: The effect of pure/mixed zooplankton and artificial diet on the growth and survival of Clarias anguillaris (Linnaeus, 1758) larvae. Afr J Aquat Sci. 17:69–73.
- Patil SS, More VR. 2017. Zooplankton sampling methods in freshwater lakes. Rev Res. 6 (12):1–9.
- Paturej E. 2006. Assessment of the trophic state of the coastal Lake Gardno based on community structure and zooplankton-related indices. Electr J Polish Agricult Univ. 9 (2):1–17.
- Pawlowski J, Lejzerowicz F, Apotheloz-Perret-Gentil L, Visco J, Esling P. 2016. Protist metabarcoding and environmental biomonitoring: time for change. Eur J Protistol. 55(Pt A):12–25.
- Payne RJ. 2013. Seven reasons why protists make useful bioindicators. Acta Protozool. 52 (3):105–113.
- Pomati F, Shurin JB, Andersen KH, Tellenbach C, Barton AD. 2019. Interacting temperature, nutrients and zooplankton grazing control phytoplankton size-abundance relationships in eight Swiss Lakes. Front Microbiol. 10:3155–3172.
- Roman MR, Brandt SB, Houde ED, Pierson JJ. 2019. Interactive effects of hypoxia and temperature on coastal pelagic zooplankton and fish. Front Mar Sci. 6:1–18.
- Rusconi-Rodrigues G. 2016. Understanding diurnal vertical migration of zooplankton in Perialpine freshwater lakes. Spring. 16:1–16.
- Saunders JF, Lewis WM. 1988. Zooplankton abundance and transport in a tropical whitewater river. Hydrobiologia. 162(2):147–155.
- Shi Y, Wang J, Zuo T, Shan X, Jin X, Sun J, Yuan W, Pakhomov EA. 2020. Seasonal changes in zooplankton community structure and distribution pattern in the Yellow Sea. China. Front Mar Sci. 7:1–14.
- Sluss TD, Jack JD, Thorp JH. 2011. A comparison of sampling methods for riverine zooplankton. rs. 19(4):315–326.
- Snell TW, Marcial HS. 2017. Using rotifers to diagnose the ecological impacts of toxicants. In: Hagiwara A, Yoshinaga T, editors. Rotifers, fisheries science series. Singapore: Springer Nature Singapore; p. 129–147.
- Stemberger RS, Lazorchak JM. 1994. Zooplankton assemblage responses to disturbance gradients. Can J Fish Aquat Sci. 51(11):2435–2447.
- Teissier S, Peretyatko A, De Backer S, Triest L. 2012. Strength of phytoplankton–nutrient relationship: evidence from 13 biomanipulated ponds. Hydrobiologia. 689(1):147–159.
- Tunowski J. 2009. Zooplankton structure in heated lakes with differing thermal regimes and water retention. Arch Polish Fish. 17(4):291–303.
- USEPA 2012. United States Environmental Protection Agency: Channel Processes: Suspended Sediment Transport. In Water: Science and Technology. http://water.epa.Gov/scitech/datait/tools/warsss/suspend.cfm.
- Williamson CE, Reid JW. 2001. Copepods, ecology and classification of North American freshwater invertebrates (eds). Pp. 915–954.
- Xiong W, Huang X, Chen Y, Fu R, Du X, Chen X, Zhan A. 2020. Zooplankton biodiversity monitoring in polluted freshwater ecosystems: a technical review. Environ Sci Ecotechnol. 1:1–11.
- Xiong W, Li J, Chen Y, Shan B, Wang W, Zhan A. 2016. Determinants of community structure of zooplankton in heavily polluted river ecosystems. Sci Rep. 6(1):22043–22243.
- Xiong W, Ni P, Chen Y, Gao Y, Li S, Zhan A. 2019. Biological consequences of environmental pollution in running water ecosystems: a case study in zooplankton. Environ Pollut. 252:1483–1490.
- Yang J, Zhang X, Xie Y, Song C, Sun J, Zhang Y, Giesy JP, Yu H. 2017. Ecogenomics of zooplankton community reveals ecological threshold of ammonia nitrogen. Environ Sci Technol. 51(5):3057–3064.
- Yildiz S, Altindağ A, Ergönül MB. 2007. Seasonal fluctuations in the zooplankton composition of a eutrophic lake: Lake Marmara (Manisa, Turkey). Turk J Zool. 31 (2):121–126.
- Yuan D, Chen L, Luan L, Wang Q, Yang Y. 2020. Effect of salinity on the zooplankton community in the Pearl River Estuary. J Ocean Univ China. 19(6):1389–1398.
- Zabbey N, Sikoki FD, Edoghotu J. 2008. Plankton assemblages and environmental gradients in the middle reaches of the Imo river, Niger Delta, Nigeria. Afr J Aquatic Sci. 33(3):241–248.