?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Plasticity of spine morphology is a universal phenomenon in rotifers. Some Brachionus calyciflorus clones consistently develop two posterolateral spines (PS) with similar length (Type A), but others (Type B) not. The underlying mechanisms remain unknown. In the present study, the resting eggs were collected from the same B. calyciflorus strain, their hatchlings (stem rotifers) were categorized into types A and B, and then, clonally cultured for eight generations at different algal densities or temperatures. The results showed that the PS varied in length among different temperatures and food levels, but inherent differences between types A and B were always observed, indicating that differentiation between types A and B might not associate with temperature or food availability. Next, we compared the population growth and PS length between types A and B at the general condition (control), in responses to Asplanchna kairomone, Keratella tropica and Moina macrocopa. The results showed that type B had higher population growth rate than type A, suggesting the higher adaptability of type B in the general condition. In response to Asplanchna kairomone, the PS length of both types A and B was significantly induced, and their PS length was similar, but type B showed higher population growth rate than type A. Coculture with M. macrocopa significantly lengthened the PS of type B but not type A, and types A and B showed similar population growth curves. Coculture with K. tropica did not affect significantly the PS length of types A and B. However, type A showed significantly and greatly higher population density and population growth rate than type B, indicating a much stronger competition ability of type A than type B against K. tropica. Overall, these results indicated that consistent development of PS might facilitate B. calyciflorus to defend against small competitors.
Introduction
Rotifers are important components of aquatic ecosystems, and play important roles in maintaining the structure and function of zooplankton community, e.g. transferring energy and materials from alga to fish (Moreira et al. Citation2016). In response to environmental changes, the morphology of rotifers, especially body length, width and spine length may alter directly. For example, temperature, turbulence or food availability (Bogdan and Gilbert Citation1982; Stelzer Citation2002; Gilbert and McPeek Citation2013; Gilbert Citation2018) were all reported to affect the morphological characteristics of rotifers. This phenotypic plasticity might be the comprehensive outcome of the tradeoff between survival, development and reproduction, usually accompanied with changes of physiology, life history and behavior of rotifers (Stemberger and Gilbert Citation1984), which is ecologically important and influences the population dynamics of rotifers in natural environments.
The spines, especially the posterolateral spine (PS), are highly plastic traits in rotifers. As reported, the PS of Brachionus and the caudal spine of Keratella exhibit considerable environmentally controlled variation, especially in response to competition and predation pressure caused by cladocerans, cyclopoid copepods and Asplanchna (Gilbert and Waage Citation1967; Stemberger and Gilbert Citation1984; Zagarese and Marinone Citation1992; Guo et al. Citation2011; Gilbert Citation2011a; Huang et al. Citation2014; Ge et al. Citation2020a, Citation2020b). Under the competition or predation pressure, rotifers elongate their spines greatly and significantly, which could decrease the possibility of being captured, damaged and/or ingested, and thus, protect themselves against predators or competitors (Gilbert Citation2011a; Citation2013; Zhang et al. Citation2017; Yin et al. Citation2020). In addition, both temperature and food availability have been reported to significantly affect spine length of Brachionus havanaensis (Pavón-Meza et al. Citation2007), Keratella tropica (Gilbert Citation2011b) and Brachionus calyciflorus (Stemberger Citation1990). The underlying mechanisms were thought to be related to the adaptation to the buoyancy of media, since long spines may increase the buoyancy of rotifers and decrease sinking rate (Carlin Citation1943; Pejler Citation1962; Stemberger Citation1988), which help them stay in the desired water layer, for example the phototrophic zone where the food resource was relatively more abundant (Green Citation2005).
In addition to environmental changes, some internal factors also influence the spine length of rotifers. With the increasing maternal age, spine length of offspring increases, suggesting the effects of maternal age on spine length of offspring (Gilbert and McPeek Citation2013). Schröder and Gilbert (Citation2009) compared transgenerational variation of spine development between B. calyciflorus hatched from different resting eggs produced by amictic females of the same strain, and found that generation significantly influenced spine length of some clones, but other clones may keep the similar spine length across generations. The underlying mechanism remains unclear. The morphological differences between the hatchlings of resting eggs (namely stem rotifer) and the parthenogenetic generations were thought to be related to the environmental adaptability of individuals (Yin et al. Citation2015a). However, no experimental evidence has been revealed. Development of long PS should cost more energy and materials. Under natural selection, rotifer should prefer to develop short or none spine in the absence of predation and competition stress. Thus, it is hard to explain why some rotifer clones consistently develop long PS. Considering the known effects of temperature, food availability, competition and predation pressures on spine development, we hypothesized that consistent development of long PS in some rotifer strains may be an adaptative strategy to certain conditions, avoiding population extinction if the environmental conditions suddenly changed.
In the present study, a B. calyciflorus strain was cultured clonally to harvest resting eggs. The resting eggs were hatched and then stem rotifers were separately cultured at different temperatures and algal densities. Next, the stem rotifers were clonally cultured for up to eight generations. The populations from different stem rotifers represented different clones. The morphologies of the stem rotifers and their offspring were observed. Based on the PS morphology, the rotifer clones were divided into two types. Type A: the stem rotifer (F1 generation) and all its offspring (F2–F8) consistently developed two PS (TPS) with similar length. Type B: the stem rotifer had TPS, whereas their F2–F8 offspring had two short PS or even had none PS (NPS). The effects of temperature and food density on transgenerational variations in PS were compared between types A and B. Next, the population dynamics of rotifer and changes of PS length were compared between types A and B under the competition stress of Moina macrocopa and K. tropica, and the predation stress of A. brightwelli kairomone. These experiments aimed to compare the effects of temperature and food on the transgenerational variation of PS development between rotifers with different inherent spine characters, and their responses to competition and predation pressure. Based on these results, ecological benefits of consistent development of two PS were identified, contributing new knowledge to the understanding of polymorphism and dynamics of rotifer morphological types in aquatic environments.
Materials and methods
Ethics statement
No specific permit is required for research on rotifers in P. R. China. The sampling location Lake Jing is a public lake and sampling can be performed without special permits. No protected species has been reported in this lake. All local laws and regulations were carefully followed during the entire study.
Animal collection and culture
B. calyciflorus, K. tropica and A. brightwelli were originally isolated from Lake Jing (Wuhu City, Anhui Province, China, N31°20′, E118°22′) in July, 2017. M. macrocopa were purchased from the Shenzhen GenProMetab Biotechnology Company Limited (Shenzhen, China). B. calyciflorus, K. tropica and M. macrocopa were clonally cultured at 25 ± 1 °C and fed with the green alga Scenedesmus obliquus at the densities of 2.0 × 106, 1.0 × 106 and 0.5 × 106 cells/mL, respectively. A. brightwelli were fed with 5 ind./mL B. calyciflorus daily. The illumination intensity was approximately 130 lux and the photoperiod was 16 h: 8 h (light: dark). The Environmental Protection Agency (EPA) solution (96 mg/L of NaHCO3, 60 mg/L of CaSO4, 60 mg/L of MgSO4 and 4 mg/L of KCl; pH 7.5) was used as the culture medium and was refreshed daily (Peltier and Weber Citation1985). S. obliquus was cultured in the HB-4 medium, harvested by centrifugation at 4000 rpm for 15 min and then stored at 4 °C. Algal density was measured using a haemocytometer under a microscope.
Stimulation and collection of resting eggs
After cultivation in the laboratory for two months, one strain of B. calyciflorus was retained for stimulation of resting eggs according to the published method (Gilbert Citation2003). Resting eggs were collected using a micropipette and then stored in dark at 4 °C. Resting eggs were stored for at least one month before commencement of hatching experiments.
Effects of food density and temperature on morphological characteristics of offspring from different stem rotifers
To test the effects of food density and temperature on morphological characteristics of offspring from different rotifer types, 50 resting eggs were individually placed in each well of 24-well plastic plates and then hatched at 25 °C. The photoperiod was 14 h:10 h (light: dark) with the illumination intensity of 130 lux. Each well contained 0.5 mL of EPA media and 3.0 × 106 cells/mL S. obliquus. Next, the plates were observed every 12 h. The stem rotifers were labeled as F1 and the next generations were labeled from F2 to F8. After the stem rotifers entered the post-reproductive period (stop to produce newborns for 24 h), they were fixed in 4% formalin solution for measurement of morphological indices. Meanwhile, all produced offspring of the same clone were randomly assigned to one treatment. Overall, six treatments were performed, including three temperatures (20 °C, 25 °C and 30 °C at 3.0 × 106 cells/mL of S. obliquus) and three food densities (1.0 × 106, 3.0 × 106, 6.0 × 106 cells/mL S. obliquus at 25 °C). At the same condition, rotifers were further cultured until the eighth generation (F8). At the beginning, each treatment contained eight clones (hatched from eight resting eggs). From the offspring of each generation, approximately 100 individuals carrying eggs (except the first three generations due to the low numbers of offspring) were transferred to new culture cups to harvest the subsequent generation. During the culture period, to ensure the accuracy of algal density, the culture media were renewed every 24 h. At each time for changing media, 90% of the old media were removed using a filter sucker (cover a 22 μm pore-sized net on the opening of glass sucker), and then, freshly prepared culture media containing certain densities of algae were added. In addition, the culture media were resuspended every 12 h using a glass sucker. From each generation, 30 individuals at the post-reproductive period were collected and fixed in 4% formalin solution for measurement of morphological indices.
Based on the morphology of stem rotifers and offspring, all the clones were categorized into two types. Type A consistently grew two PS for individuals at all generations. Type B had two PS for rotifers at the F1 generation, but had both TPS and NPS morphotypes in F2 to F8 offspring. The morphologies of two B. calyciflorus types were showed in Supporting Information . Since most clones died before the eighth generation, finally the number of clones in each treatment was not the same. Type B in treatment at 30 °C had three clones, type B in treatments at 25 °C and 3.0 × 106 cells/mL had two clones. The other treatments only had one clone.
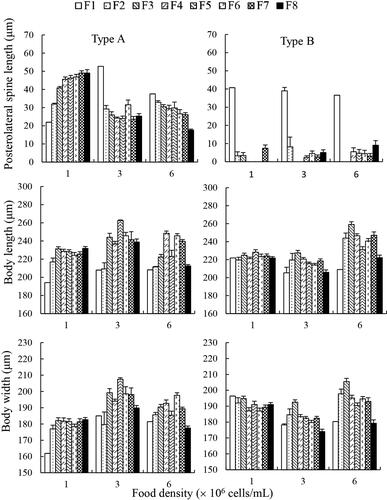
Figure 1. Posterolateral spine length, body length and width of the stem females and their parthenogenetic offspring of two Brachionus calyciflorus types cultured at three temperatures (mean ± SE).
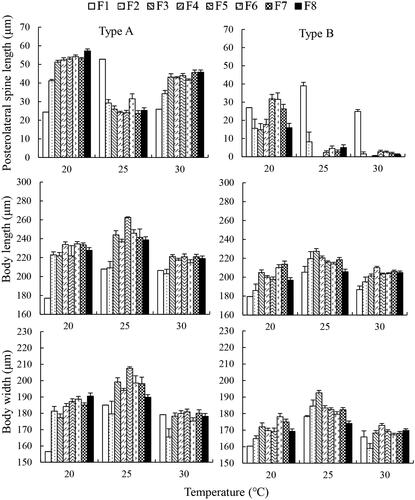
From each generation in each treatment, 10–20 rotifers at the post reproductive stage were subjected to the measurement of body length, width and PS length using the Image J software after taking photos under a microscope (Olympus BX61, China). The number of rotifers at the F2 generation were limited, and only 1–8 individuals were measured for each type.
Competition experiments
The competition abilities of B. calyciflorus against K. tropica or M. macrocopa were compared between the two types (A and B). First, B. calyciflorus (from the F8 generation), K. tropica and M. macrocopa were pre-cultured at 25 ± 1 °C (photoperiod 16: 8) and food density of 1.0 × 106 cells/mL S. obliquus for more than one week. Next, animals were transferred to 25 mL glass beakers containing 20 mL of EPA media. Each beaker contained 4 ind./mL B. calyciflorus and 25 ind./mL K. tropica (competition with K. tropica) or 4 ind./mL B. calyciflorus and 0.2 ind./mL M. macropcopa (competition with M. macropcopa) or only 4 ind./mL B. calyciflorus (control). Each treatment repeated four times independently. Rotifers and M. macropcopa were counted every 24 h. For each counting, three aliquots of 1–3 mL were sampled to count rotifers under a microscope and the average values were used to calculate densities. M. macropcopa were counted by eyes directly during transferring from one tube to another using a glass sucker. After counting, all living individuals were transferred to clean beakers containing fresh media and algal food. In addition, five adult rotifers were randomly selected from each treatment to measure posterolateral spine length. The population growth experiments continued for 15 days.
Based on the density data, population growth rates (r) at each day were calculated using the following equation (Nandini et al. Citation2019):
where N0 and Nt are the initial and final population densities, respectively, and t is the time (days).
Predation pressure experiments
A. brightwelli adults were cultured in fresh EPA media without food for 24 h at the densities of 0.5, 1.0 and 2.0 ind./L. After 24 h, the media were harvested and filtered through 0.2 µm polyethylene microfiltration membranes, which were called Asplanchna-conditioned medium (Yin et al. Citation2015a). Asplanchna-conditioned media at different Asplanchna densities represented different intensities of predation pressure.
To test the effects of predation pressure on the two types of B. calyciflorus, B. calyciflorus (2.0 ind./mL) were cultured in Asplanchna-conditioned media for population growth assays. The other culture conditions were the same as those for competition assays. Four replicates were performed for each predation pressure. Population density and PS length were determined daily as described for the competition experiment.
Data analysis
Data were first tested for homoscedasticity and normality using the Levene's test and the Kolmogorov–Smirnoff test, respectively. Next, three-way ANOVA was conducted to test the effects of genetic type, generation and algal density or temperature on morphological indices (including body length, width and PS length). Two-way analysis of variance (ANOVA) was performed to identify significant differences in competition and predation pressure assays. Multiple comparisons were carried out to compare the differences between treatments. All statistical analyses were performed using SPSS 20.
Results
Effects of temperature on the PS length of different generations between types A and B
Three-way ANOVA revealed that temperature, clone and generation all significantly affected body length, width and PS length independently. Meanwhile, the interactions between each two of the three parameters and among the three parameters also significantly affected body length, width and PS length (p < .05), except the effects of the interaction between temperature and clone on body length and width (p > .05, ).
Table 1. Three-way ANOVA of temperature, type and generation on posterolateral spine length in Brachionus calyciflorus.
Compared to F1 generation, both types A and B showed longer body length from F3 to F7 at each temperature. Similarly, both types A and B showed longer body width from F3 to F7 generations than those of F1 at 20 °C and 25 °C, but not at 30 °C (). In comparison to F1 generation, type A displayed significantly longer PS from F2 to F8 at 20 °C and 30 °C, but significantly shorter PS at 25 °C. Type B grew significantly shorter PS at generations F2–F8 than F1 at both 25 °C and 30 °C ().
At each temperature, the offspring of type A showed 15–30% longer body length and width than type B, and great differences in PS length were observed between types A and B. At 20 °C, the PS length of type A was nearly two times longer than type B. At 25 °C and 30 °C, the offspring of type A still grew long PS, ranging from 20 to 50 μm. However, type B only had very short PS, with all of them less than 10 μm from F2 to F8, and some of them even did not grow observable PS ().
Effects of food density on the PS length at different generations between types A and B
Three-way ANOVA revealed significant effects of food density and generation on body length and width (p < .05), but the effects of type were not significant (p > .05). Differently, food density, generation and type all significantly affected PS length (p < .05). The interactions between each two of the three parameters and among the three parameters all significantly affected body length, width and PS length ().
Table 2. Three-way ANOVA of food density, type and generation on posterolateral spine length, body length, and width in Brachionus calyciflorus.
Compared to treatment with 1.0 × 106 cells/mL, type A showed longer body length and width at 3.0 × 106 cells/mL and 6.0 × 106 cells/mL, and type B showed longer body length and width only at 6.0 × 106 cells/mL. At all food levels, type A always grew long PS at all generations, ranging from 20 to 55 μm. In comparison, the F1 generation of clone B grew long PS, approximately 40 μm, but its F2–F8 offspring showed very short PS, with all shorter than 10 μm at all food densities ().
Effects of Asplanchna kairomone on population dynamics and PS length of types A and B
In the control group (without Asplanchna kairomone), type B grew faster than type A. Its population growth rates were significantly higher than those of type A at the initial stage. Moreover, type A always showed significantly longer PS than type B during the whole experimental period ().
Figure 3. Population growth and posterolateral spine length of two Brachionus calyciflorus types cultured at different concentrations of Asplanchna brightwelli kairomone (mean ± SE).
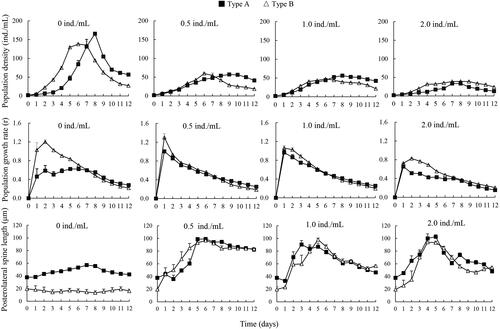
In treatments with Asplanchna kairomone, population densities of both type A and type B were greatly suppressed, in comparison to the control. Under low (0.5 ind./mL) and medium (1.0 ind./mL) predation pressure, type A displayed higher population density than type B after day 8. Differently, under high predation pressure (2.0 ind./mL), population density of type A never exceeded type B during the entire experiments. In all treatments with Asplanchna kairomone, both type A and type B elongated their PS, and the PS length of type B arrived the similar level of type A ( and Supporting Information ).
Two-way ANVOA displayed that type, predation pressure and their interaction all significantly influenced maximum population density, maximum population growth rate and PS length ().
Table 3. Effects of type and Asplanchna brightwelli kairomone on maximum population density, maximum population growth rate and posterolateral spine length of Brachionus calyciflorus (Two-way ANOVA).
Effects of K. tropica and M. macropcopa on population dynamics and PS length of types A and B
When co-cultured with K. tropica, type A showed significantly higher population density and population growth rate than type B after day 5. On day 9, the population of type B became extinct, but type A still maintained a high population density. During the culture period, the PS length of type A almost maintained constant, but PS length of type B changed dynamically, increasing at day 1 but, then, decreasing. Type A showed significantly longer PS than type B from days 5 to 8 (). The maximum population density of type A was significantly higher than that of type B (Supporting Information). However, the maximum population growth rate of type A was lower than that of type B. Two-way ANOVA indicated that type, presence of K. tropica and their interaction all significantly affected maximum population density, but only type significantly influenced maximum population growth rate, and PS length ().
Figure 4. Population growth and posterolateral spine length of two Brachionus calyciflorus types cocultured with Keratella tropica and Moina macrocopa (mean ± SE).
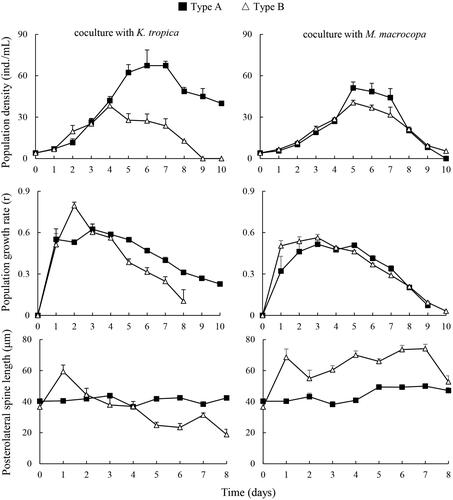
Table 4. Two-way ANOVA of competitive pressure (Keratella tropica) and type of Brachionus calyciflorus on maximum population density, maximum population growth rate and posterolateral spine length of B. calyciflorus.
Under the competition stress of M. macropcopa, type A and type B showed similar population growth rate (). From day 1 to day 7, type B grew significantly longer PS than type A. Two-way ANOVA revealed that type, presence of M. macropcopa, and their interaction all significantly affected maximum population density and PS length. However, type, and presence of M. macropcopa both significantly regulated maximum population growth rate, but their interaction did not ().
Table 5. Two-way ANOVA of competitive pressure (Moina macrocopa) and type of Brachionus calyciflorus on maximum population density, maximum population growth rate and posterolateral spine length of B. calyciflorus.
Discussion
Three morphotypes of B. calyciflorus, including TPS, NPS and single posterolateral spine (SPS), are widely present in natural lakes (Yin and Niu Citation2007). Among them, SPS showed a less ratio than TPS and NPS (Yin and Niu Citation2007). These morphotypes could be found in both sexual and asexual hatchlings. In the offspring of stem rotifers, TPS, SPS and NPS were all observed (Yin and Niu Citation2008), and their ratios were associated with strain (Liu and Niu Citation2010). Next, in the parthenogenetic process, stem rotifers of TPS, SPS and NPS could produce offspring with all the three morphotypes (Yin and Niu Citation2008). In addition, these three types could respond to Asplanchna-conditioned medium well and then develop long spines (Yin and Niu Citation2007). In the present study, two types of rotifers were compared. One consistently expressed two PS and another produced both TPS and NPS offspring in the seven parthenogenetic generations from resting eggs, which were originated from the same strain. Thus, the potential influence of cryptic species could be excluded, which is a universal phenomenon in B. calyciflorus (Gilbert and Walsh 2005; Xiang et al. Citation2011) and a significant factor affecting morphology of rotifers (Xi et al. Citation2010). Moreover, we identified stem rotifers consistently developing two PS in the next seven generations of offspring, which is an extreme representative of defense morphotype and provides materials to study differences in life-history characters and responses to competition and predation pressures between rotifers with different morphological strategies. To the best of our knowledge, studies on this kind of consistent two PS morphotype have not been reported yet.
Three-way ANOVA showed significant effects of generation on spine length in single-factor assays of temperature and food level. In treatments at 20 °C, 30 °C and 1.0 × 106 cells/mL, spine length of type A showed an increasing tendency with generation, and a decreasing tendency was observed in treatment at 6.0 × 106 cells/mL. These phenomena were described as transgenerational changes (Schröder and Gilbert Citation2009). As proposed by Yin et al. (Citation2015a), stem females may transmit adaptation strategies to successive offspring.
Temperature (Halbach Citation1970) and food level (Stemberger Citation1990) are two environmental factors affecting rotifer spine morphology. Hillbricht-Ilkowska (Citation1983) revealed that the long-spined individuals dominate in oligotrophic and cold habitats, but the short-spined forms prevail in warm and eutrophic lakes. In the present study, the offspring of both types A and B showed significantly longer PS at 20 °C than at 25 °C (), consitent with Athibai and Sanoamuang (Citation2008), which might increase fitness by reducing the rotifer’s sinking rate at low temperature (Gilbert Citation2018). However, compared with 25 °C, type A developed longer PS at 30 °C, but type B did not, indicating type A might use the water temperature as a proxy signaling change of some risks before defense is needed (Ramos-Rodriguez et al. Citation2020). At optimal food levels, very low or very high temperatures resulted in decreased body size in B. havanaensis (Pavón-Meza et al. Citation2007). The different trends in response to low temperature between spine length and body length might indicate a trade-off of material and energy investment between body growth and spine development.
Compared to treatments with 3.0 × 106 and 6.0 × 106 cells/mL, type A rotifer displayed longer PS in treatment with 1.0 × 106 cells/mL, consistent with the general conclusion that low food level stimulates long spines, since long-spined individuals have lower food thresholds than individuals with no or short spines (Stemberger Citation1990). It is an energy-economic life cycle strategy in the development of prolonged PS. The long-spined individuals with lower food thresholds may consume less energy than the reduced spine one (Yin and Niu Citation2008). However, type B did not reveal significant changes of spine in response to food level variation. These results indicated that types A and B had different responses of spine length to changes of temperature and food level, suggesting an inherent difference in ecological strategy between these two types. Considering that long spine is a defense mechanism, to explore the potential mechanisms, the responses of types A and B to competition and predation pressures were compared.
Compared with B. calyciflorus (150–300 μm), K. tropica is smaller (85 μm), but M. macrocopa (830–1200 μm) and A. brightwelli (500–800 μm) are larger in body size. B. calyciflorus, K. tropica and M. macrocopa have a similar ecological niche (Walz Citation1995). They compete for algal food. Compared with K. tropica, M. macrocopa not only shows greater filter-feeding capacity (de Bernardi et al. Citation1987), but also may incidentally ingest, mechanically kill or damage B. calyciflorus (Gilbert and Slemberger Citation1985; Gilbert Citation1988). Thus, M. macrocopa is a stronger competitor to B. calyciflorus than K. tropica. A. brightwelli is carnivorous and can prey on B. calyciflorus. A. brightwelli shows a stronger threat to B. calyciflorus than M. macrocopa, and then, than K. tropica. In the present study, the PS of type A was significantly induced by Asplanchna kairomone, and not by K. tropica or M. macrocopa. In comparison, the PS of type B could be induced by all these three enemies, and the length of PS followed the sequence: exposure to Asplanchna kairomone > cocultured with M. macrocopa > cocultured with K. tropica > cultured alone. This sequence was consistent with the threat level among these three enemies, indicating different sensitivities of type B to different enemies. Similarly, Gilbert (Citation2011b) reported that Asplanchna induces a strong lengthening, but Daphnia induces a moderate elongation of the right posterior spine in K. tropica. Thus, spine length might be a qualitative trait determined by the threat intensity of enemy in rotifers.
In the absence of competition and predation pressure, type B showed a higher population growth rate than type A, suggesting that development of long PS might cost materials and energy (Yin et al. Citation2015b), and thus, decreasing the investment to reproduction. Similarly, Aránguiz-Acuña et al. (Citation2010), Xiang et al. (Citation2010) and Sarma et al. (Citation2011) also reported reduction of reproduction due to development of long spines in B. calyciflorus. In the presence of Asplanchna kairomone, the population growth of both type A and type B was suppressed greatly and significantly (). Generally, rotifers can adopt life-history strategies of low reproduction and high survivorship in response to predators (García et al. Citation2007; Guo et al. Citation2011). Under the conditions of low and medium predation pressure (0.5 and 1.0 ind./L), the PS length was similar between types A and B, but type A revealed significantly higher population densities than type B from day 8 to day 12, which was similar to the results in the control (without Asplanchna kairomone). Comparatively, under high predation pressure (2.0 ind./L), type B revealed higher population densities than type A at most time points (), indicating a stronger resistance of type A to Asplanchna predation than type B. This difference might be associated with the shorter PS length of type B than type A at the early (from day 1 to day 2) and later (from day 8 to day 11) exposure stage. Protection from ingestion by Asplanchna requires long PS for herbivorous rotifers (Marinone and Zagarese Citation1991), accompanying with reduced reproduction (Stemberger and Gilbert Citation1987; Yin et al. Citation2015b). Overall, these results indicated that consistent expression of long PS (type A) might be an adaptative mechanism to avoid population extinction if a low predation pressure emerged suddenly.
When co-cultured with K. tropica, type A showed better population growth and longer PS than type B, indicating that B. calyciflorus with long PS may have a better defense ability to K. tropica. In response to M. macrocopa, significantly longer PS was induced in type B. Similar to Ge et al. (Citation2012), the PS length of Brachionus forficula was positively correlated with copepod and cladocera density in lakes. However, the protective effects of long PS against M. macrocopa were limited, evidenced by the similar population growth curves between types A and B. These data suggested that the long PS may not an effective tool to large competitors. Development of long spine may decrease rotifer swimming speed, food gathering capacity and finally population growth.
In the present study, types A and B showed different growth ability in the presence or absence of K. tropica, M. macrocopa or Asplanchna kairomone. The results showed that type A might grow better than type B in the presence of K. tropica and at a low level of Asplanchna kairomone, suggesting that consistent expression of long PS is a beneficial strategy for B. calyciflorus to defend weak competitors or low predation pressure. In natural waterbodies, the environmental conditions change dynamically. Competitors and predators to B. calyciflorus may hatch from resting eggs or from other waters. These two types of rotifers may alternatively dominate in the water in response to different competition and/or predation stresses. In the absence of competitors or predators, type B grows faster and can rapidly establish a population. If competitors or predators suddenly emerged, the ecological strategy of type A helps B. calyciflorus avoid population extinction.
Conclusions
We identified two types of B. calyciflorus. One consistently expressed long PS (consistent type, type A) and the other expressed short or none PS (inducible type, type B) in offspring. For both types, food density, temperature and generation all affected their PS length. Without predation or competition stress, type A showed longer PS but lower population growth rate than type B, indicating that development of long spines costs energy. In response to Asplanchna kairomone, type B was induced to develop long PS and revealed better population growth than type A. Coculture with K. tropica did not induce long spines in type B, which showed lower competition ability with K. tropica than type A. Exposure to M. macrocopa promoted PS length in type B, which did not greatly enhance its competition ability with M. macrocopa in comparison to type A. Overall, consistent expression of long PS might benefit B. calyciflorus to overcome small, weak competitions, but was not effective to compete with strong competitors or predators.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
All data have been included in this manuscript and supplementary materials.
Additional information
Funding
Notes on contributors
Ya-Li Ge
Dr. Ya-Li Ge is an associate professor at School of Ecology and Environment, Anhui Normal University. She is interested in freshwater zooplankton ecology.
Yi-Long Xi
Dr. Yi-Long Xi is a professor at School of Ecology and Environment, Anhui Normal University, and his work focuses on freshwater ecology, rapid evolution and ecotoxicology.
Gen Zhang
Dr. Gen Zhang is a guest professor at School of Ecology and Environment, Anhui Normal Univeristy. He works on toxicology, molecular mechanisms underlying development and natural product chemistry.
References
- Aránguiz-Acuña A, Ramos-Jiliberto R, Sarma N, Sarma SSS, Bustamante RO, Toledo V. 2010. Benefits, costs and reactivity of inducible defences: an experimental test with rotifers. Freshwater Biol. 55(10):2114–2122.
- Athibai S, Sanoamuang LO. 2008. Effect of temperature on fecundity, life span and morphology of long- and short-spined clones of Brachionus caudatus f. apsteini (Rotifera). Internat Rev Hydrobiol. 93(6):690–699.
- Bogdan KG, Gilbert JJ. 1982. The effects of posterolateral spine length and body length on feeding rate in the rotifer, Brachionus calyciflorus. Hydrobiologia. 89(3):263–268.
- Carlin B. 1943. Die Planktonrotatorien des Motalastrom. Zur Taxonomie Und Ökologie Der Planktonrotatorien. Medd Lunds Univ Limnol Inst. 5:1–260.
- de Bernardi R, Giussani G, Manca M. 1987. Cladocera: predators and prey. Hydrobiologia. 145(1):225–243.
- García CE, De Jesús Chaparro-Herrera D, Nandini S, Sarma SSS. 2007. Life-history strategies of Brachionus havanaensis subject to kairomones of vertebrate and invertebrate predators. Chem Ecol. 23(4):303–313.
- Ge Y, Xi Y, Ma J, Xu D. 2012. Spatio-temporal variation of morphometric characteristics of Brachionus forficula in relation to ecological factors. Acta Ecol Sin. 32(16):5034–5042.
- Ge YL, Ge CC, Zhan R, Yu JH, Tong-Luo Xi YL, Zhang G. 2020a. Influence of two Keratella tropica morphs on the population dynamics of the predator Asplanchna brightwelli. J Plankton Res. 42(2):203–209.
- Ge YL, Yu XJ, Zhan R, Yu JH, Xi YL, Zhang G. 2020b. Comparison of life-history parameters and effectiveness in competition with Moina macrocopa between two Keratella tropica morphs. Limnologica. 85:125823.
- Gilbert JJ. 1988. Suppression of rotifer populations by Daphnia: a review of the evidence, the mechanisms, and the effects on zooplankton community structure1. Limnol Oceanogr. 33(6):1286–1303.
- Gilbert JJ. 2003. Specificity of crowding response that induces sexuality in the rotifer Brachionus. Limnol Oceanogr. 48(3):1297–1303.
- Gilbert JJ. 2011a. Induction of different defences by two enemies in the rotifer Keratella tropica: response priority and sensitivity to enemy density. Freshwater Biol. 56(5):926–938.
- Gilbert JJ. 2011b. Temperature, kairomones, and phenotypic plasticity in the rotifer Keratella tropica (Apstein, 1907). Hydrobiologia. 678(1):179–190.
- Gilbert JJ. 2013. The cost of predator-induced morphological defense in rotifers: experimental studies and synthesis. J Plankton Res. 35(3):461–472.
- Gilbert JJ. 2018. Morphological variation and its significance in a polymorphic rotifer: environmental, endogenous, and genetic controls. Bioscience. 68(3):169–181.
- Gilbert JJ, McPeek MA. 2013. Maternal age and spine development in a rotifer: ecological implications and evolution. Ecology. 94(10):2166–2172.
- Gilbert JJ, Slemberger RS. 1985. Control of Keratella populations by interference competition from Daphnia. Limnol Oceanogr. 30(1):180–188.
- Gilbert JJ, Waage JK. 1967. Asplanchna, Asplanchna-substance, and posterolateral spine length variation of the rotifer Brachionus calyciflorus in a natural environment. Ecology. 48(6):1027–1031.
- Gilbert JJ, Walsh EJ. 2005. Brachionus calyciflorus is a species complex: mating behavior and genetic differentiation among four geographically isolated strains. In: Herzig A, Gulati RD, Jersabek CD, May L, editors. Rotifera X: developments in hydrobiology. Dordrecht: Springer Netherlands; p. 257–265.
- Green J. 2005. Morphological variation of Keratella cochlearis (Gosse) in a backwater of the River Thames. Hydrobiologia. 546(1):189–196.
- Guo R, Snell TW, Yang J. 2011. Ecological strategy of rotifer (Brachionus calyciflorus) exposed to predator- and competitor-conditioned media. Hydrobiologia. 658(1):163–171.
- Halbach U. 1970. The factors determining temporal variation in Brachionus calyciflorus pallas (rotatoria). Oecologia. 4(3):262–318.
- Hillbricht-Ilkowska A. 1983. Morphological variation of Keratella cochlearis (Gosse) in Lake Biwa, Japan. Hydrobiologia. 104(1):297–305.
- Huang L, Xi Y, Wang X, Xia M, Han Y, Wen X. 2014. Competitive outcome between the rotifer Brachionus calyciflorus and the cladoceran Moina macrocopa depends on algal density but not temperature. Ann Limnol - Int J Lim. 50(2):109–119.
- Liu W, Niu C. 2010. Polymorphism in resting egg size and hatching strategy in the rotifer Brachionus calyciflorus pallas. Zoolog Sci. 27(4):330–337.
- Marinone MC, Zagarese HE. 1991. A field and laboratory study on factors affecting polymorphism in the rotifer Keratella tropica. Oecologia. 86(3):372–377.
- Moreira RA, da Silva Mansano A, Rocha O, Daam MA. 2016. The use of rotifers as test species in the aquatic effect assessment of pesticides in the tropics. Hydrobiologia. 773(1):1–9.
- Nandini S, Sanchez-Zamora C, Sarma SSS. 2019. Toxicity of cyanobacterial blooms from the reservoir Valle de Bravo (Mexico): a case study on the rotifer Brachionus calyciflorus. Sci Total Environ. 688:1348–1358.
- Pavón-Meza EL, Sarma SSS, Nandini S. 2007. Combined effects of temperature, food (Chlorella vulgaris) concentration and predation (Asplanchna girodi) on the morphology of Brachionus havanaensis (Rotifera). Hydrobiologia. 593(1):95–101.
- Pejler B. 1962. On the variation of the rotifer Keratella cochlearis (Gosse). Zoologiske Bidrag Fra ˚n Uppsala. 35:1–17.
- Peltier WH, Weber CI. 1985. Methods for measuring the acute toxicity of effluents to freshwater and marine organisms: environmental Monitoring and Support Laboratory. Ohio: Office of Research and Development, US Environmental Protection Agency Cincinnati.
- Ramos-Rodriguez E, Moreno E, Conde-Porcuna JM. 2020. Intraspecific variation in sensitivity to food availability and temperature-induced phenotypic plasticity in the rotifer Keratella cochlearis. J Exp Biol. 223(7):jeb209676.
- Sarma SSS, Resendiz RAL, Nandini S. 2011. Morphometric and demographic responses of brachionid prey (Brachionus calyciflorus Pallas and Plationus macracanthus (Daday)) in the presence of different densities of the predator Asplanchna brightwellii (Rotifera: Asplanchnidae). Hydrobiologia. 662(1):179–187.
- Schröder T, Gilbert JJ. 2009. Maternal age and spine development in the rotifer Brachionus calyciflorus: increase of spine length with birth orders. Freshwater Biol. 54(5):1054–1065.
- Stelzer CP. 2002. Phenotypic plasticity of body size at different temperatures in a planktonic rotifer: mechanisms and adaptive significance. Funct Ecol. 16(6):835–841.
- Stemberger RS. 1988. Reproductive costs and hydrodynamic benefits of chemically induced defenses in Keratella testudo. Limnol Oceanogr. 33(4):593–606.
- Stemberger RS. 1990. Food limitation. spination, and reproduction in Brachionus calyciflorus. Limnol Oceanogr. 35(1):33–44.
- Stemberger RS, Gilbert JJ. 1984. Spine development in the rotifer Keratella cochlearis: induction by cyclopoid copepods and Asplanchna. Freshwater Biol. 14(6):639–647.
- Stemberger RS, Gilbert JJ. 1987. Multiple-species induction of morphological defenses in the rotifer Keratella Testudo. Ecology. 68(2):370–378.
- Walz N. 1995. Rotifer populations in plankton communities: energetics and life history strategies. Experientia. 51(5):437–453.
- Xi Y, Li HB, Cheng XF. 2010. Morphometric differences among three sibling species in Brachionus calyciflorus species complex. Acta Ecol Sin. 30(13):3645–3653.
- Xiang XL, Xi YL, Wen XL, Zhang G, Wang JX, Hu K. 2011. Genetic differentiation and phylogeographical structure of the Brachionus calyciflorus complex in eastern China. Mol Ecol. 20(14):3027–3044.
- Xiang XL, Xi YL, Zhang JY, Ma Q, Wen XL. 2010. Effects of temperature on survival, reproduction, and morphotype in offspring of two Brachionus calyciflorus (Rotifera) morphotypes. J Freshwater Ecol. 25(1):9–18.
- Yin XW, Niu CJ. 2007. Polymorphism and morphotype transformations in the rotifer (Brachionus calyciflorus). Zoolog Res. 28(1):68–72.
- Yin XW, Yin HY, Wang JJ, Xu XY, Ruan YJ. 2020. Joint effects of predation risk and food nutrient on sexual and asexual reproductions, and morphological defenses of freshwater rotifer Brachionus calyciflorus. Aquat Ecol. 54(1):35–44.
- Yin XW, Niu CJ. 2008. Polymorphism in stem females and successive parthenogenetic generations in Brachionus calyciflorus Pallas. Aquat Ecol. 42(3):415–420.
- Yin XW, Zhao NX, Wang BH, Li WJ, Zhang ZN. 2015a. Transgenerational and within-generational induction of defensive morphology in Brachionus calyciflorus (Rotifera): importance of maternal effect. Hydrobiologia. 742(1):313–325.
- Yin XW, Zhou YC, Li XC, Li WX. 2015b. Reduced investment in sex as a cost of inducible defence in Brachionus calyciflorus (Rotifera). Freshw Biol. 60(1):89–100.
- Zagarese HE, Marinone MC. 1992. Induction and inhibition of spine development in the rotifer Keratella tropica. Freshwater Biol. 28(3):289–300.
- Zhang H, Hollander J, Hansson LA. 2017. Bi-directional plasticity: rotifer prey adjust spine length to different predator regimes. Sci Rep. 7(1):10254.