Abstract
Freshwater ecosystems are facing degradation from human activities in the form of alterations of flow regimes, changes in land-use, and intensive water abstraction, in turn generating adverse effects on biodiversity and ecological functionality. Efficient assessment of river/stream ecosystem response to such anthropogenic changes is urgently needed. Many human activities that have an effect on water quality and river health are increasing along the Mohlapitsi River, which used to be one of the rivers with good water quality in South Africa. The aim of the study was to assess spatial variation in macroinvertebrate assemblages in relation to water quality of the Mohlapitsi River. A combination of canonical correspondence analysis and univariate analyses were used to examine the macroinvertebrate assemblages at six different sites of the Mohlapitsi River. The spatial difference in macroinvertebrate abundance was mostly related to changes in water quality. The number of taxa and the diversity of macroinvertebrates remained relatively high across the river as compared to other rivers in the Olifants River System. The midstream and downstream sites were mostly dominated by macroinvertebrates tolerant to disturbance/pollution. The most abundant family was the Thiaridae (about 60%) and mainly occurred in the midstream and downstream sites of the river (more disturbed sites). The study showed a higher evenness index in the upstream than the midstream and downstream sites which is an indication of good water quality at the upstream sites of the river. The results suggest that the river is being subjected to a moderate environmental pollution, which requires continuous assessment and monitoring to determine causes and initiate remedial measures.
Introduction
Globally, the deterioration of freshwater ecosystems has been attributed to increasing anthropogenic activities and impacts of climate change (Jun et al. Citation2016; Ferreira et al. Citation2017; Addo-Bediako et al. Citation2018). Freshwater ecosystems are threatened by pollution, habitat loss and degradation, resulting in species population declines (Niba and Sakwe Citation2018). Biological monitoring is one of the methods used to determine the effects of anthropogenic activities on water quality. It is considered an effective tool for monitoring water quality and very useful to obtain ecological information about rivers (Merritt et al. Citation2017).
Many South African rivers are facing degradation due to pollution and other human disturbances that are affecting the natural habitats and nutrients of macroinvertebrates, thus causing a decline in their abundance and diversity (Matlou et al. Citation2017; Rasifudi et al. Citation2018). Furthermore, changes in the climate, such as variation in precipitation and high temperatures, can be major drivers affecting the hydrologic regime and geomorphology in stream environments, thereby affecting the diversity and abundance of macroinvertebrates (Dudgeon Citation2000). It is predicted that mean land surface temperature in southern Africa is likely to exceed the global mean land surface temperature increase in all seasons (IPCC Citation2021). In many southern Africa countries, including South Africa, there are changes in total or mean summer rainfall, seasonal rainfall onset and duration, dry spell frequencies, and rainfall intensity (IPCC Citation2021). These can cause changes in the life cycle of macroinvertebrates.
Benthic macroinvertebrates provide a more precise understanding of changes in aquatic conditions when compared to chemical and microbiological data, which rather present short term fluctuations (Ghasemi and Kamali Citation2014). Benthic macroinvertebrates are a highly diverse faunal group that inhabit many niches and habitats in freshwater systems (Obade and Moore Citation2018). The distribution of macroinvertebrate taxa across a river system is mainly dependent on the availability of microhabitats and food resources. In addition, their distribution also is influenced by the interactions among habitat characteristics, physicochemical variables, structural and hydrological characteristics, and by human activities (Resh and Rosenberg Citation1984). Therefore, changes in water body characteristics, habitat, and environmental resources can strongly influence patterns of distribution in benthic communities (Buss et al. Citation2002). Many studies have used macroinvertebrate abundance and richness (e.g. family richness) to detect environmental responses because of their variable sensitivity towards multiple disturbances (e.g. Ferreira et al. Citation2011; Friberg et al. Citation2011; Rasifudi et al. Citation2018). Thus, changes in the macroinvertebrate composition and community structures can be used to establish environmental alterations in rivers.
Many rivers in the Olifants River Basin are threatened by the growing human population and increasing water demands for economic development (Matlou et al. Citation2017). Studies have reported declining water quality and quantity due to increased pollution caused by industrialization, urbanization, afforestation, mining, agriculture and power generation (Gerber et al. Citation2015; Matlou et al. Citation2017; Chetty and Pillay Citation2019; Addo-Bediako Citation2020). The Mohlapitsi River is known to supply water of good quality to the Olifants River, however, the increasing human activities such as agriculture, sand mining and human settlements along the midstream and downstream of the river is affecting the water quality adversely. The river serves as a source of water for agriculture and drinking water for some communities in the area. To date, no scientific research regarding pollution from the various human activities along the river and its impact on aquatic biota has been conducted. The aim of the present study was to assess the composition and diversity of aquatic benthic macroinvertebrates in the Mohlapitsi River.
Variations in the community structure of macroinvertebrates were compared at different sites of the river and we assessed the influence of the environmental variables on the community structure. We hypothesized that the community structure of macroinvertebrates in the Mohlapitsi River is a function of changes in water quality due to land-use changes in the catchment.
Method and materials
Study area
The Mohlapitse River is a perennial river in the Limpopo Province. The source of the river is in the protected Wolkberg Wilderness area. The area is about 22 000 ha and it is dominated by yellowwood Podocarpus latifolius, wild figs Ficus sp., wild beeches Fagus sp and waterberry trees Syzygium cordatum. This area is characterized by vertical quartzite krantzes, countless cliffs, cool, deep and densely forested ravines, massive buttresses and folded and interlocking spurs. From its source, the Mohlapitsi River flows downstream passing through various agricultural fields and human settlements before converging with the Olifants River. The communities in the area depend on the river for domestic use, irrigation and livestock. Six sampling sites were selected along the river (, ).
Figure 1. Map of the study area, showing the locations of the six sampling sites of the Mohlapitsi River.
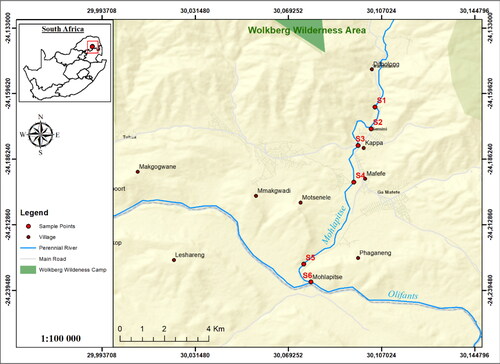
Table 1. Description and geo-reference of the sampling sites of the Mohlapitsi River.
Water sampling
Water samples were collected at six sites along the Mohlapitse River in March (autumn), June (winter), October (spring) and December (summer) of 2019. The water samples were collected in 1000 ml acid pre-treated polyethylene bottles. The water was stored at 4 °C prior to chemical analysis. The pH, water temperature, dissolved oxygen (DO), total dissolved solids (TDS), electrical conductivity (EC) and salinity were recorded in situ at sampling sites using the YSI Model 554TM Datalogger multiprobe. The water samples were analyzed for nutrients (nitrate, nitrite, total nitrogen, ammonia and ortho-phosphate), and turbidity at an accredited (ISO 17025) chemical laboratory (WATERLAB (PTY) LTD). Chlorophyll-a was determined in situ using an AquaFluor™ handheld fluorometer to determine eutrophication level. Flow velocity was measured using a Flo-mate portable flowmeter Model 2000 (Marsh McBirney, Maryland, US). The width and water depth were measured using a measuring tape and graduated measuring rod, respectively. The physicochemical variables measured were compared with the South African water quality guideline for aquatic ecosystems (DWAF Citation1996).
Macroinvertebrate sampling
Benthic macroinvertebrates were collected at the selected sites using a standard net of 300 mm × 300 mm with the mesh size of approximately 500 µm. The kick-stir-sweep methodology following Dickens and Graham (Citation2002) was used. At each site, approximately 6 min was spent sampling all aquatic habitats (i.e. riffles, pools and vegetated margins) and the samples were combined to form one composite sample. The macroinvertebrate samples collected were identified to the family level in the field using an Invertebrate Field Guide Manual (Gerber and Gabriel Citation2002), with the aid of magnifying glass. However, macroinvertebrates which could not be identified in the field were preserved in 70% ethanol in one liter polypropylene buckets and transported to the laboratory for further identification using a stereomicroscope (Leica EZ4).
Statistical analysis
The mean and the standard deviation of the physicochemical variables were calculated. Analysis of variance (ANOVA) was used to determine the differences among the sampled sites of water quality and macroinvertebrate distribution, using Statistica Version 10 (2007). The Shannon Weiner index was used to compare the macroinvertebrate diversity at the selected sites. The following macroinvertebrate metrics were also used to assess the environmental integrity: Ephemeroptera–Plecoptera–Trichoptera (EPT), Shannon–Wiener diversity index and Shannon evenness index. In this study, the South African Scoring System version 5 (SASS5) was used to assess the river health. It is a qualitative, multi-habitat, rapid field-based method that requires identification of macroinvertebrates to family level (Dalla). It is envisioned to be a rapid, inexpensive technique for the detection of water quality deterioration and to assess the ecological integrity of river ecosystems (Dickens and Graham Citation2002). The SASS score, which is the sum of the sensitivity weightings for taxa present at a site, and Average Score Per Taxon (ASPT), which is the SASS score divided by the number of at each site (Dickens and Graham Citation2002) were used to assess the water quality. The SASS and ASPT scores were used as a measure of the condition at each site: excellent (SASS5 score > 100 and ASPT score > 7), good (80–100 and 5–7), fair (60–80 and 3–5), poor (40–60 and 2–3) and very poor (< 40 and < 2) (Thirion et al. Citation1995). Cluster analysis, a non-metric multi-dimensional scaling (NMDS) was used to identify grouping of sampling sites with similar macroinvertebrate assemblages using Euclidean distance (Kruskal Citation1964), based on the Bray-Curtis dissimilarity clustering method (Bray and Curtis Citation1957) using Statistica version 10 (2011). This analysis was also used to examine the degree to which sites clustered according to macroinvertebrate assemblages. Canonical Correspondence Analysis (CCA) was performed to determine the relationship between the physicochemical parameters and the macroinvertebrates using statistical package CANOCO version 5.1 software (ter Braak and Šmilauer Citation2012). Canonical Correspondence Analysis was then used to show how the significant variables jointly influenced macroinvertebrates in the different site categories. The macroinvertebrate and physico-chemical data were log (x + 1) transformed to reduce the effects of extreme values.
Results
Physicochemical variables
Mean current velocity ranged from 0.84 ms−1 at S4 to 0.42 ms−1 at S5. Mean water depth was highest at S6 (0.64 m) and lowest at S2 (0.31 m). Generally, the width of the river increased from upstream to downstream sites, ranging from 6.12 at S1 to 15.8 at S6 (). The pH values observed at the sites ranged from 7.6 at S1 to 8.5 at S6, thus, the water was slightly alkaline at all sites. Mean temperature ranged from 18.1 °C at S1 to 20.4 °C at S3. Mean dissolved oxygen ranged from 7.6 mg/l at S6 to 10.5 mg/l at S1. Mean conductivity ranged from 140.3 (µS/cm) at S1 to 275.3 (µS/cm) at S6. Mean TDS ranged from 104.6 at S1 to 296.8 at S6, mean turbidity ranged from 1.6 NTU (nephelometric turbidity unit) at S3 to 5.78 NTU at S6, and mean salinity ranged from 0.07 at S1 to 0.34 at S3. There was no significant difference in any of the physicochemical parameters among the sites (p > 0.05), however there were significant seasonal differences in temperature (F = 36.07, p < 0.0001) and turbidity (F = 8.64, p < 0.01). Salinity exceeded the guideline value (DWAF Citation1996) at all sites. Mean chlorophyll concentration ranged from 19.3 µg/l at S2 to 28.3 µg/l at S6. The highest mean nitrate concentration of 0.25 mg/l was at S5 and the lowest at S1, S3, and S4 (0.15 mg/l). Mean nitrite concentration was similar at all the sites except at S2. The highest mean concentration of ammonium concentration was at S1 and the lowest mean at S3 and S4. The highest mean total nitrogen was at S5 and the lowest mean at S3 and S4. While phosphate concentration was below detection level at all sites (). Conductivity and DO were significantly different across the sites (ANOVA, p < 0.05). The measured salinity exceeded the guideline value and ammonium also exceeded the guideline values for aquatic ecosystems.
Table 2. Mean water physical variables (± standard deviation) recorded across six sites of the Mohlapitsi River.
Macroinvertebrate community structure
A total of 26,243 individual macroinvertebrates belonging to 63 families, 10 orders, six classes, Annelida: Oligochaeta and Hirudinea; Mollusca: Arthropoda: Crustacea and Insecta; Gastropoda and Pelecypoda. were collected in the Mohlapitsi River (Appendix 1). The families with the highest abundance were the Thiaridae (16,661), Baetidae (2,527), Planorbidae (2,096), Hydropsychidae (700), and Gomphidae (587). S3 had the highest number of individuals of 7,554 (28.78%), followed by S5 with 6 292 (23.98%), S4 with 5,042 (19.21%), S6 with 4,482 (17.08%), S1 with 1,469 (5.60%) and S2 with 1404 (5.35%). The Thiaridae contributed more than 60% of the total macroinvertebrate abundance and mainly occurred at the midstream and downstream sites, S3, S4, S5 and S6 (Appendix 1). The abundance of macroinvertebrates did not differ significantly among the sites (F = 0.534, p > 0.05).
Taxa richness ranged from 32 at S3 to 44 at S5. The EPT index ranged from 6 at S3 to 12 at S1 and S5. The SASS5 scores at all the sites were greater than 100 and the ASPT scores were between 5 and 7. The Shannon diversity index varied from 2.25 to 2.46 in the upstream sites (S2 and S1), 1.60 to 1.62 in the downstream sites (S6 and S5), and 0.96 to 1.06 in the midstream sites (S3 and S4). The highest evenness (0.67) was recorded at S2 followed by S 1 (0.63) and then S6 (0.46), while the lowest evenness was recorded at S3 (0.27) ().
Table 3. Number of taxa, EPT, SASS5 (Dickens and Graham Citation2002) indices calculated (SASS score, number of taxa and ASPT), Shannon-Weiner diversity and evenness indices for the different sites in the Mohalpitsi River.
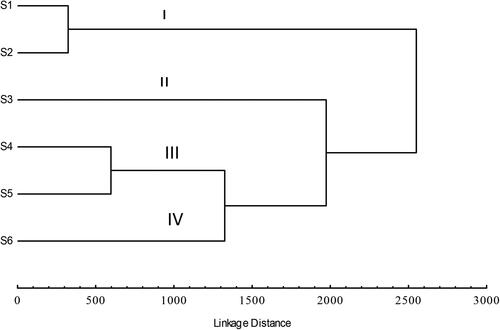
Hierarchical cluster analysis used to evaluate the faunal similarities among the study sites showed four groups (). Cluster I was composed of S1 and S2, cluster II was S3, cluster III wasS4 and S5, and cluster IV wasS6. The macroinvertebrate assemblage in cluster I was related to the upstream sites, S1 and S2. In this assemblage, EPT were the dominant group in terms of richness and abundance. The physicochemical parameters at these sites had relatively higher DO and lower TDS, EC, temperature, turbidity and salinity as compared to midstream and downstream sites (S3 to S6). In cluster II (S3), the dominant taxa were the Gastropods, mainly Planorbidae and Thiaridae. Cluster III (S4 and S5), has high anthropogenic disturbances and high abundance of tolerant taxa, such as Thiaridae and Oligochaetae. Cluster IV (S6), at the confluence with the Olifants River, had relatively higher turbidity, conductivity and temperature, but a high spatial heterogeneity (diverse microhabitats).
Figure 2. Dendrogram showing sites belonging to four clusters, representing macroinvertebrate assemblages based on the Bray-Curtis dissimilarity clustering method.
Seasonally, the highest abundance of macroinvertebrates was in autumn (8,351), followed by spring (7,976), summer (5,098) and winter (4,818) (Appendix 2). Taxa richness, was highest during winter (54), followed by autumn (51), spring (49) and summer (35). There were no significant differences in number of individuals and taxa richness among the seasons. The highest EPT taxa count was recorded during autumn (1,397), followed by winter (1,352), spring (916) and summer (455).
Relationship between macroinvertebrates and physicochemical variables
The first four CCA axes accounted for 87.6% variation, with the first two axes explaining 52.3% of the total variation () in the macroinvertebrates (Monte Carlo test; p < 0.05). Based on CCA analysis, pH, EC, TDS, DO, temperature, salinity and turbidity had significant associations with macroinvertebrate community composition (). The macroinvertebrate families associated with S1 included sensitive taxa Blepharoceridae, Dipseudopsidae and Perlidae, moderately tolerant Caenidae and Dytiscidae. S2 was associated with sensitive Psychomyiidae, moderately tolerant Naucoridae and tolerant Psychodidae. S3 was associated with moderately tolerant Ceratopogonidae, tolerant Ephydridae and Platycnemidae. S4 was associated with sensitive Oligoneuridae and moderately tolerant Hydrometridae. S5 was characterized by temperature and turbidity and was associated with sensitive Atyidae, Synlestidae, Helodidae and tolerant Ephemeridae. S6 was characterized by TDS and was associated with sensitive Chlorocyphidae, moderately tolerant taxa Hydrophilidae and tolerant Belostomatidae.
Figure 3. Canonical Correspondence Analysis of the macroinvertebrates, physicochemical variables at the sampling sites (S1-S6). Family code names are given in Appendix 1. Temp = temperature, Condu = electrical conductivity, DO = dissolved oxygen, Chloro-a = Chlorophyll-a, Turbi = turbidity and Velo = velocity, (open triangle and open circle represent families and sites respectively).
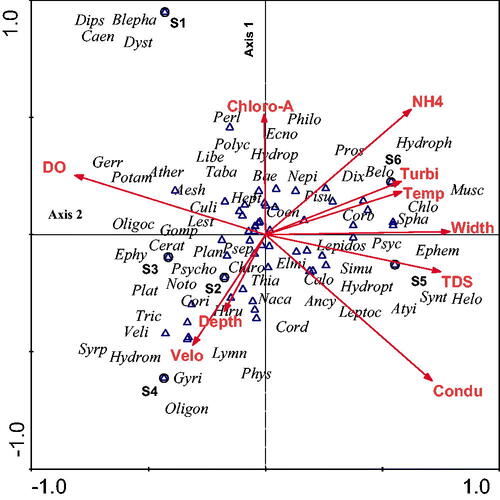
Table 4. CCA results indicating the correlation between physico-chemical parameters and macroinvertebrates communities.
Discussion
Physicochemical variables
Current velocity and depth did not show any increasing trend from upstream to downstream, and this could be due to the various disturbances, such as weir construction at S3 and sand mining at S4. Generally, the levels of most of the physicochemical parameters were within the standard guideline values. There was a concentration gradient of TDS and conductivity from upstream to downstream, while DO decreased from upstream to downstream. This observed pattern could be due to pollution from human activities such as agriculture and sand mining in the midstream flowing downstream in the river. The recorded chlorophyll-a (19.3 − 28.3 µg/l) values were similar to values recorded in other rivers in South Africa (Modley et al. Citation2020). High concentration of chlorophyll-a is an indication of a rich food source and contributes considerably to high production of macroinvertebrates. Chlorophyll-a is an important indicator of algae and it is also a source of oxygen, but it is often considered as an important factor for assessing eutrophication (Zhou et al. Citation2014).
Macroinvertebrate community structure
The structural composition of macroinvertebrates in the Mohlapitsi River varied among the sites. The physicochemical variables such as dissolved oxygen, temperature, water velocity, river depth and width food resources and different land cover characteristics are usually responsible for determining macroinvertebrate assemblages (Al-Shami et al. Citation2013; Lamouroux et al. Citation2014). The highest diversity of sensitive families was found at S1 and S5 in the upstream and downstream respectively, S1 was in a forested area with minimal human impacts. The upstream sites had well-protected banks with vegetation cover that offered a wider habitat diversity to aquatic biota. It is known that riparian vegetation with higher plant surface area are likely to support large macroinvertebrate populations (Elias et al. Citation2014; Kaaya et al. Citation2015). Similar studies have found higher taxon richness of macroinvertebrates in upstream shaded sites (with high canopy cover) than in open sites (low canopy cover) (Heino and Peckarsky Citation2014; Tonkin et al. Citation2016). There was also a high taxa diversity at S5, a downstream site, and could be attributed to the habitat heterogeneity that may attract a diversity of taxa at different times (Braccia and Voshell Citation2007). Many studies have reported a wide distribution of EPT taxa in areas with high substrate heterogeneity (Oi Edia et al. Citation2007; Camara et al. Citation2012). The low number of sensitive taxa at S3, S4 and S6 could be attributed to habitat disturbances caused by sand mining, domestic and agricultural practices in the area.
The high abundance of Baetidae throughout the Mohlaptisi River could be due to nutrient enrichment and the fact that this family is often associated with moderate levels of pollution (Armitage et al. Citation1983; Azrina et al. Citation2006). High abundance of Baetidae has also been reported in some tropical rivers in Malaysia (Ghani et al. Citation2016). The high abundance of Gastropods, especially Thiaridae at S3, S5 and S6 is an indication that the water quality in the midstream and downstream is deteriorating. They have the ability to tolerate habitat disturbance and variability due to their extra ordinary structural organization (Santhosh et al. Citation2011). They are also good colonizers and the presence of parthonogenetic females enables them to multiply in large numbers within a short time.
The different activities in the catchment area, such as sand mining, construction of reservoirs, dumping of wastes, and agricultural runoff usually affect water quality and habitat availability, which reflects on the community structure of macroinvertebrates. The high abundance at S3 and S4 does not necessarily depict better environment but rather might be due to minor disturbance that favours some tolerant taxa with consequent decrease in the abundance of sensitive taxa (Elias et al. Citation2014). When the environment is disturbed, usually, the abundance of sensitive taxa is reduced while that of more tolerant taxa is increased and therefore the assemblages become more homogeneous (Sánchez-Fernández et al. Citation2010). This could be the situation at S3 and S4, where the Thiridae contributed to 74% and 78.5% at S3 and S4 respectively, to the total macroinvertebrate abundance. This could be attributed to degradation of the sites due to the various human activities. However, the fact that sensitive taxa were recorded in the disturbed areas is an indication that the Mohlapitsi River is still in good condition. This is supported by the SASS5 scores which were greater than 100 at all sites, an indication that the river had good water quality. Similarly, the ASPT scores (between 5 and 7) indicated that all the sites had good water quality. Thus, based on the SASS5 (> 100) and ASPT (5–7) scores, all the sites had good water quality during sampling period, though the river shows signs of degradation at midstream and downstream sites.
The Shannon–Wiener diversity index and Shannon evenness index were highest at the upstream sites (S1, S2) and lowest at the midstream (S3, S4) and rose again at the downstream sites (S5, S6) (thus with improvementat downstream sites). The slight improvement at the downstream sites could be due to self-purification. The diversity and evenness indices at the various sampling sites seemed to reflect the water quality conditions at each site. High species diversity at the upstream sites was associated with less unimpacted or unpolluted conditions, while a lower diversity at S3 and S4 signified environmental pollution due to increasing human activities, such as sand mining and agriculture (Esenowo and Ugwumba Citation2010). Sand mining removes benthic macroinvertebrates living in the sediment (sand) and thereafter only species which have higher fecundity and can tolerate disturbances survive, i.e. species like those in the Thiaridae (Appleton et al. Citation2009; Karatayev et al. Citation2009; López-López et al. Citation2009). The diversity index >1 at all the sites except Site 3 (0.96), is an indication that the river is moderately polluted.
The macroinvertebrate assemblage in the cluster analysis showed groupings of the upstream sites (S1 and S2) in cluster I. In this assemblage, EPT taxa were the dominant group in terms of richness and abundance (). The assemblages related to these sites were characterized by relatively higher DO and lower TDS, EC, temperature, turbidity and salinity as compared to midstream and downstream sites (S3 to S6). The assemblage in cluster II consists of S3, the dominant taxa were the Gastropods, mainly the Planorbidae and Thiaridae. Cluster III consists of S4 and S5, with high anthropogenic disturbances and high abundance of tolerant taxa, such as the Thiaridae and Oligochaetae. The Thiaridae is the most abundant family and represented by Tarebia granifera, which is highly invasive in South Africa. Studies in South Africa have reported the presence of T. granifera in a number of habitats in which it was not previously found and it has the ability to invade a variety of habitats in a relatively short period (Wolmarans and de Kock Citation2006), and there is evidence to suggest that it reduces benthic macroinvertebrate biodiversity (Jones et al. Citation2017). If the species is not controlled, it is likely to change the local community structure and alter the ecosystem functions.
The low abundance of macroinvertebrates in summer could be due to high precipitation that washed effluents from urban or agricultural areas could also wash away benthic macroinvertebrates. Seasonal changes in the benthic community occur as a result of different life history parameters of species such as the growth rate, numbers of generations per year, emergence patterns, birth and death rates among others (Ravera Citation2001). The EPT taxa showed a decrease from low flow (winter and autumn) to high flow (summer and spring) seasons. This could be attributed to better water quality or less runoff from the catchment during autumn and winter than spring and summer.
The difference in composition and diversity for the four clusters were related to environmental differences at the sites and concur with other studies, that is, both spatial and temporal variations at sampling sites cause variation amongst the macroinvertebrate communities (e.g. Beyene et al. Citation2009; Heino and Peckarsky Citation2014; Baker and Greenfield Citation2019). The CCA shows that physicochemical variables and nutrients are important in structuring macroinvertebrate assemblages. The poorer water quality in the midstream and to a certain extent downstream sites affected macroinvertebrate assemblages.
Conclusion
The Mohlapitsi River remains one of the few rivers in the area which is not seriously degraded. However, this study shows that the water quality in the Mohlapitsi River is deteriorating from the midstream to downstream and this was mainly due to the different type of anthropogenic activities in the catchment area. The benthic macroinvertebrate abundance and diversity followed the observed water quality differences at the study sites. The study provides information on the present status of water quality and baseline data of macroinvertebrate assemblages of the Mohlapitsi River. The study forms the foundation for a long-term assessment and monitoring of the river for management purposes. It is envisaged that a more comprehensive study using the lowest macroinvertebrate taxonomic level (genus or species) is required, though, moving to genus/species level will consume enormous amounts of time, whiles using family level saves time and with better technology for physicochemical measurements can provide a rapid water quality and river health assessment. Furthermore, the authorities need to take corrective measures to regulate agricultural and sand mining activities along the river.
Supplemental Material
Download MS Word (34.2 KB)Acknowledgements
The research was supported financially by the Flemish Inter-University Council (VLIR-UOS), Belgium and National Research Foundation (NRF).
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
Data used in this study are available upon request to the corresponding author.
Additional information
Funding
Notes on contributors
M. E. Raphahlelo
ME Raphahlelo is holds MSc from University of Limpopo, South Africa. His work is centered on freshwater Ecology.
A. Addo-Bediako
Abraham Addo-Bediako has expertise in the field of Entomology, Ecology, Ecotoxicology and risk analysis. He is particularly interested in understanding the processes underlying patterns in the distribution of biodiversity, especially macroinvertebrates. This is very important given the rapid global climate change which is likely to present profound physiological challenges to many organisms and may alter their pattern of distribution.
W. J. Luus-Powell
Wilmien Luus-Powell is DSI-NRF SARChI Chair (Ecosystem Health) at University of Limpopo. Her main research interests include water quality, ecology, distribution morphology and biology of freshwater fish parasites.
References
- Addo-Bediako A, Matlou K, Makushu E. 2018. Heavy metal concentrations in water and sediment of the Steelpoort River, Olifants River System, South Africa. Afr J Aquat Sci. 43(4):413–416.
- Addo-Bediako A. 2020. Assessment of heavy metal pollution in the blyde and steelpoort rivers of the olifants river system, South Africa. Pol J Environ Stud. 29 (5):3023–3039.
- Al-Shami S, CheSalmah MR, Hassan AA, Madrus MR. 2013. Biodiversity of stream insects in the Malaysian Peninsula: Spatial patterns and environmental constraints. Ecol Entomol. 38:238–249.
- Appleton CC, Forbes AT, Demetriades NT. 2009. The occurrence, bionomics and potential impacts of the invasive freshwater snail Tarebia granifera (Lamarck, 1822) (Gastropoda: Thiaridae) in South Africa. Zool Med Leiden. 83:525–536.
- Armitage P, Moss D, Wright J, Furse M. 1983. The performance of a new biological water quality score system based on macroinvertebrates over a wide range of unpolluted running water sites. Water Res. 17(3):333–347.
- Azrina MZ, Yap CK, Ismail RA, Ismail A, Tan SG. 2006. Anthropogenic impacts on the distribution and biodiversity of benthic macro-invertebrates and water quality of the Langat River, Peninsular Malaysia. Ecotoxicol Environ. Saf. 64(3):337–347.
- Baker NJ, Greenfield R. 2019. Shift happens: Changes to the diversity of riverine aquatic macroinvertebrate communities in response to sewage effluent runoff. Ecol Indic. 102:813–821.
- Beyene A, Addis T, Kifle D, Legesse W, Kloos H, Triest L. 2009. Comparative study of diatoms and macroinvertebrates as indicators of severe water pollution: Case study of the Kebena and Akaki rivers in Addis Ababa, Ethiopia. Ecol Indic. 9(2):381–392.
- Braccia A, Voshell JR. 2007. Benthic macroinvertebrate responses to increasing levels of cattle grazing in Blue Ridge Mountain Streams, Virginia, USA. Environ Monit Assess. 131(1–3):185–200.
- Bray JR, Curtis JT. 1957. An ordination of the upland forest communities of Southern Wisconsin. Ecol Monogr. 27(4):325–349.
- Buss DF, Baptista DF, Silveira MP, Nessimian JL, Dorvillé LFM. 2002. Influence of water chemistry and environmental degradation on macroinvertebrate assemblages in a river basin in South-East Brazil. Hydrobiologia. 481(1/3):125–136.
- Camara IA, Diomande D, Bony YK, Ouattara A, Franquet E, Gourene G. 2012. Diversity assessment of benthic macroinvertebrate communities in Banco National Park (Banco Stream, Côte d’Ivoire). Afr J Ecol. 50(2):205–217.
- Chetty S, Pillay L. 2019. Assessing the influence of human activities on river health: a case for two South African rivers with differing pollutant sources. Environ Monit Assess. 191(3):168.
- Dickens CW, Graham P. 2002. The South African Scoring System (SASS) version 5 rapid bio-assessment method for rivers. Afr J Aquat. Sci. 27(1):1–10.
- Dudgeon D. 2000. The ecology of tropical Asian rivers and streams in relation to biodiversity conservation. Annu Rev Ecol Syst. 31(1):239–263.
- DWAF. 1996. South African Water Quality Guidelines, Volume 7: Aquatic Ecosystems, Department of Water Affairs and Forestry, Pretoria, South Africa.
- Elias JD, Ijumba JN, Mgaya YD, Mamboya FA. 2014. Study on freshwater macroinvertebrates of some Tanzanian rivers as a basis for developing biomonitoring index for assessing pollution in tropical African regions. J Ecosyst. 2014:1–8.
- Esenowo IK, Ugwumba AAA. 2010. Composition and abundance of macro-benthos in Majidun River Ikorodu Lagos State, Nigeria. Res J Biol Sci. 5(8):560–556.
- Ferreira ARL, Sanches Fernandes LF, Cortes RMV, Pacheco FAL. 2017. Assessing anthropogenic impacts on riverine ecosystems using nested partial least squares regression. Sci Total Environ. 583:466–477.
- Ferreira WR, Paiva LT, Callisto M. 2011. Development of a benthic multimetric index for biomonitoring of a neotropical watershed. Braz J Biol. 71(1):15–25.
- Friberg N, Bonada N, Bradley DC, Dunbar MJ, Edwards FK, Grey J, Hayes R, Hildrew A, Lamouroux N, Trimmer M, et al. 2011. Biomonitoring of human impacts in freshwater ecosystems: the good, the bad and the ugly. Adv Ecol Res. 44:1–68.
- Gerber A, Gabriel MJM. 2002. Aquatic invertebrates of South African Rivers: Field guide. Resource Quality Services, Department of Water Affairs and Forestry, Pretoria.
- Gerber R, Wepener V, Smit NJ. 2015. Application of multivariate statistics and toxicity indices to evaluate the water quality suitability for fish of three rivers in the Kruger National Park, South Africa. Afr J Aquat Sci. 40(3):247–259.
- Ghani WMH, Rawi CMD, Hamid SA, Al-Shami SA, Ahmad AH, Hassan ANN. 2016. Variation in environmental conditions influences diversity and abundance of Ephemeroptera in forest streams of northern Peninsular Malaysia. Trop Ecol. 57(3):489–501.
- Ghasemi AF, Kamali M. 2014. Benthic macroinvertebrates along the haraz downstream in Southern Caspian Sea Basin: In gradient of the physicochemical parameters. Int J Zool. 2014:1–7.
- Heino H, Peckarsky BL. 2014. Integrating behavioral, population and large-scale approach for understanding stream insect communities. Curr Opin Insect Sci. 2:7–13.
- Intergovernmental Panel on climate change (IPCC). 2021. AR6 Climate change 2021. The Physical Science Basis. [accessed 2021 November 11]. https://www.ipcc.ch/report/sixth-assessment-report-workinggroup-i/.
- Jones RW, Hill JM, Coetzee JA, Avery TS, Weyl OLF, Hill MP. 2017. The abundance of an invasive freshwater snail Tarebia granifera (Lamarck, 1822) in the Nseleni River, South Africa. Afr J Aquat Sci. 42(1):75–81.
- Jun Y-C, Kim N-Y, Kim S-H, Park Y-S, Kong D-S, Hwang S-J. 2016. Spatial distribution of benthic macroinvertebrate assemblages in relation to environmental variables in Korean Nationwide streams. Water. 8(1):27.
- Kaaya LT, Day JA, Dallas HF. 2015. Tanzania River Scoring System (TARISS): a macroinvertebrate-based biotic index for rapid bioassessment of rivers. Afr J Aquat Sci. 40(2):109–117.
- Karatayev AY, Burlakova LE, Karatayev VA, Padilla DK. 2009. Introduction, distribution, spread, and impacts of exotic freshwater gastropods in Texas. Hydrobiologia. 619(1):181–194.
- Kruskal JB. 1964. Multidimensional scaling by optimising goodness of fit to a nonmetric hypothesis. Psychometrica. 29(1):1–27.
- Lamouroux N, Trimmer M, Woodward G. 2014. Biomonitoring of human impacts in freshwater ecosystems: the good, the bad and the ugly. Adv Ecol Res. 44:1–68.
- López-López E, Sedeño-Díaz JE, Vega PT, Oliveros E. 2009. Invasive mollusks Tarebia granifera Lamarck, 1822 and Corbicula fluminea Müller, 1774 in the Tuxpam and Tecolutla rivers, Mexico: spatial and seasonal distribution patterns. AI. 4(3):435–450.
- Matlou K, Addo-Bediako A, Jooste A. 2017. Benthic macroinvertebrate assemblage along a pollution gradient in the Steelpoort River, Olifants River System. Afr Entomol. 25(2):445–453.
- Merritt DM, Manning ME, Hough-Snee N. 2017. The National Riparian Core Protocol: A riparian vegetation monitoring protocol for wadeable streams of the conterminous United States. Gen. Tech. Rep. RMRS-GTR-367. Fort Collins (CO): U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. p. 37. p.
- Modley LAS, Rampedi IT, Avenant-Oldewage A, Van Dyk C. 2020. A comparative study on the biotic integrity of the rivers supplying a polluted, hyper-eutrophic freshwater system: A multi-indicator approach. Ecol Indic. 111(105940):105940.
- Niba A, Sakwe S. 2018. Turnover of benthic macroinvertebrates along the Mthatha River, Eastern Cape, South Africa: implications for water quality bio-monitoring using indicator species. J Freshw Ecol. 33(1):157–171.
- Obade VD, Moore R. 2018. Synthesizing water quality indicators from standardized geospatial information to remedy water security challenges: a review. Environ Int. 119:220–231.
- Oi Edia E, Brosse S, Ouattara A, Gourene G, Winterton P, Lek-Ang S. 2007. Aquatic insect assemblage patterns in four West-African coastal rivers. J of Biological Sciences. 7(7):1130–1138.
- Rasifudi L, Addo-Bediako A, Swemmer A, Bal K. 2018. Distribution and diversity of benthic macroinvertebrates in the Selati River of Olifants River System, South Africa. Afr Entomol. 26(2):398–406.
- Ravera O. 2001. A comparison between diversity, similarity and biotic indices applied to macroinvertebrate community of a small, Ravella River (Como Province, Northern, Italy. Aquat Ecol. 35(2):97–107.
- Resh VH, Rosenberg DM. 1984. The ecology of aquatic insects. Praeger Publishers, New York, 625p.
- Sánchez-Fernández D, Calosi P, Atfield A, Arribas P, Velasco J, Spicer JI, Millán A, Bilton DT. 2010. Reduced salinities compromise the thermal tolerance of hypersaline specialist diving beetles. Physiol Entomol. 35(3):265–273.
- Santhosh S, KrishnaMohan C, Dhanesh NR, Akolkar P. 2011. Water quality assessment of river Karamana by using Benthic macroinvertebrates, Southern Kerala, India. Ecoscan. 5:135–140.
- ter Braak CJF, Šmilauer P. 2012. Canoco reference manual and user’s guide: Software for ordination, version 5.0. Microcomputer Power, Ithaca (NY). p. 118.
- Thirion C, Mocke A, Woest R. 1995. Biological monitoring of streams and rivers using SASS4: a user manual. Institute for Water Quality Studies, Department of Water Affairs and Forestry, Pretoria.
- Tonkin JD, Arimoro FO, Haase P. 2016. Exploring stream communities in a tropical biodiversity hotspot: biodiversity, regional occupancy, niche characteristics and environmental correlates. Biodivers Conserv. 25(5):975–993.
- Wolmarans CT, de Kock KN. 2006. The current status of freshwater molluscs in the Kruger National Park. Koedoe. 49(2):39–44.
- Zhou J, Liu Y, Guo H, He D. 2014. Combining the SWAT model with sequential uncertainty fitting algorithm for streamflow prediction and uncertainty analysis for the Lake Dianchi Basin, China. Hydrol Processes 28(3):521–533.
Appendix 1.
Abundance of macroinvertebrates recorded at different sites along the Mohlapitsi River
Appendix 2.
Seasonal abundance of macroinvertebrates recorded in the Mohlapitsi River
