Abstract
The problem of seasonal drought has intensified in recent years. Unfortunately, dry seasons can lead to poor water quality due to nitrogen (N) accumulation, which, in turn, may result in the gradual decline or even disappearance of submerged plants. This outdoor study was conducted to determine the morphological, biomass responses and C/N metabolism of Vallisneria spinulosa to the high N content and low water level. We used two N contents (i.e., low and high) and three simulated water levels (i.e., gradual water level increase from 60 cm to 150 cm over four periods (C), low water level of 60 cm maintained for three periods followed by an abrupt increase to 150 cm in the fourth period (CE) and low water levels of 60 ± 30 cm maintained for three periods followed by an abrupt increase to 150 cm in the fourth period (E)). For the low N content, low water levels for CE and E treatments caused increased ramet number and biomass of V. spinulosa. However, for the high N content, the low water levels did not cause such big changes in V. spinulosa. High N contents demonstrated a remarkably negative influence on the biomass of each organ (included leaf, stolon, root and tuber) of V. spinulosa and flowering ramet number. For C water level, the high N content caused an increase in free-amino acid (FAA) content in stolons and leaves, and no difference in soluble carbohydrate (SC) content. For E water level, there was no significant increase in FAA content of V. spinulosa under high N treatment; furthermore, there was no significant change in SC content under high N treatment except for leaves. In conclusion, low water levels exacerbated the damage to V. spinulosa caused by high nitrogen level.
1. Introduction
Eutrophication due to excessive nutrient loading is a serious environmental problem that could lead to the degradation of lake ecosystems (Codd Citation1995). The disappearance of aquatic plants in eutrophic water bodies, especially submerged macrophytes, is caused by various factors (Xie et al. Citation2005; Chu et al. Citation2006). It is well known that NO3- and NH4+ are the main components of inorganic nitrogen (N) in the water of eutrophic lakes. Excessive NO3--N will be stored in vacuoles so as to be harmless for submerged macrophytes (Surya et al. Citation2007). Submerged macrophytes are highly sensitive to toxicity of N in form of NH4+-N at high concentrations. Under NH4+-N stress, most plants show slow growth rates and oxidative damages (Nimptsch and Pflugmacher Citation2007). The adverse effects of nitrogen (N) on submerged macrophytes could be partly explained by toxic stress, which manifests as disrupted carbon (C) and N metabolism via free-amino acid (FAA) accumulation and soluble carbohydrate (SC) loss (Cao et al. Citation2009, Yuan et al. Citation2016), and photosynthesis inhibition (Su et al. Citation2012). The accumulation of N could result in an excessive growth of phytoplankton in water and epiphytic algae attached on leaves of V. spinulosa. This undesirable overgrowth of phytoplankton and periphyton promotes the shading effects of algae, which could inhibit the photosynthesis of submerged macrophytes (Zhi et al. Citation2018). The TN concentration in Poyang Lake has become high recent years, even with a peak of 4 mg·L−1, and the NH4+-N concentration may sometimes reach 1.5 mg·L−1 (Wu et al. Citation2018). Thus, the high N content may be an important cause for degradation of aquatic macrophytes in Poyang Lake.
The change in water level is an important ecological factor affecting the growth and reproduction of submerged macrophytes (Zhang et al. Citation2015). Due to environmental changes, Lake Poyang, which is located in the middle reach of the Yangtze River (China), has experienced frequent hydrological drought events in past decades. The lowest water level is becoming lower, the dry season in coming earlier, and the drought period in lasting longer (Hu et al. Citation2015). The hydrological drought events of Poyang Lake lead to the earlier and longer period of low water level to submerged macrophytes in Poyang Lake Basin (Li et al. Citation2019). Several researchers have found that submerged macrophytes are able to adapt to changes in water depth by adjusting their life history period (Brock and Rogers Citation1998). Yuan et al. (Citation2016) found that water depth influences C/N metabolic levels and carbohydrate storage in Vallisneria natans. Therefore, the mechanisms behind variations in the morphology, biomass and C/N metabolism of submersed macrophytes growing under high-N content and low-water level conditions in spring are of great interest.
In this work, a whole-ecosystem experiment lasting approximately 3 months was carried out in six experimental ponds to explore the effects of N and water level on submersed macrophytes under conditions close to those of natural systems. The objectives of this study are twofold: 1) to explore the growth and C/N metabolism of submersed macrophytes under high N concentrations and low water levels in spring and 2) to test whether low water levels in spring alleviate the harm caused by NH4+-N to V. spinulosa.
2. Materials and methods
2.1. Materials
Sediments were collected from the shore of Lake Poyang in Xingzi City, China. The samples were dried, sieved (mesh size, 5.0 mm) and mixed to ensure homogeneity. The sediment contained 2.41 mg·g−1 total nitrogen (TN), 0.75 mg·g−1 total phosphorus (TP) and 5.82% organic matter.
2.2. Experimental design
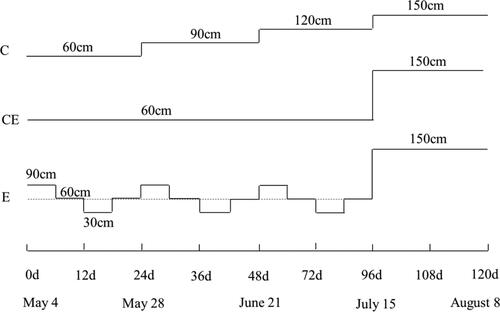
The experiments were carried out at the Laboratory of Poyang Lake and Wetland Ecosystem Research, Chinese Academy of Sciences (116°03′E, 29°26′N), which is located at the north-western region of Poyang Lake Basin, from March 23 to August 8, 2019. Six outdoor mesocosms (2 m in length × 2 m in wide × 1.5 m in depth) in the experimental platform were used. Evenly sized V. spinulosa tubers were planted in pots (diameter, 26 cm; height, 25 cm) with the sediments obtained from the shore of Poyang Lake on March 23. Tap water (dissolved total nitrogen (DTN), 2.00 mg·L−1; dissolved total phosphorus (DTP), 0.076 mg·L−1) was added to the mesocosms to maintain the V. spinulosa. 12 pots were hung from 3 iron frames above each mesocosm to the water depth of 30 cm. Seedlings of even size (26.2 ± 2.0 cm) were selected for further treatment on May 4, with 1 shoot per pot for V.spinulosa. Three mesocosms treated only with tap water were used as low N content controls. Another three mesocosms were used for high N treatment. The average TN concentration in Poyang Lake is 2.0 mg·L−1, with a peak of 4 mg·L−1, and the NH4+-N concentration may sometimes reach 1.5 mg·L−1 (Wu et al. Citation2018). Thus, 2.0 mg·L−1 NH4+-N and 1.0 mg·L−1 NO3−-N (from NH4Cl and KNO3) were added to tap water of another three mesocosms to maintain a high DTN concentration of 5 mg·L−1 on May 4. Phosphorus (P) concentrations were maintained at a controlled level without artificial fertilisation. Poyang Lake undergoes seasonal water level fluctuations of 11 m, and water levels may range from 9 m to 20 m (Zhao and Liu Citation2017). Water level fluctuations of 0.5-1 m and water level change rates of 5-10 cm·d−1 are frequent in the middle and lower reaches of the Yangtze River (Xu et al. Citation2016). Thus, a fluctuation of 30 cm within a 24 d period was designed to simulate the natural water level fluctuation in Poyang Lake. Three water level treatments were established for each mesocosm: normal (C), extreme control (CE) and extreme (E). In the C treatment, the water level was gradually increased from 60 cm to 150 cm over four periods (30 cm per period). In the CE treatment, the water level was maintained at 60 cm for three periods and then abruptly increased to 150 cm in the fourth period. In the E treatment, a low water level of 60 ± 30 cm was maintained for three periods and then abruptly increased to 150 cm in the fourth period to simulate initial low water level. The specific treatments for water levels have been shown in . Additional tap water was added to each mesocosm every 12 d to maintain a consistent water level. During the experiment, prior to water level adjustment, the nutrient concentrations (DTN, DTP, NO3−-N, NH4+-N, PO43−-P) of the water were measured every 12 d and then appropriate content of NH4+-N and NO3−-N (from NH4Cl and KNO3) were added in the water for the high N treatment to obtain a relatively constant high DTN concentration of 5 mg·L−1, that contained 2.0 mg·L−1 NH4+-N. Light intensity in the water column was recorded in each mesocosm using a Hydrolab DS5X Multi-parameter sonde with PAR sensor (Hach Company, Loveland, Colorado, USA) at noon at the end of the experiment, at noon on 8 August. Light intensities with low N content and high N content were 127.4 µmol·m−2·s−1 and 87.3 µmol·m−2·s−1 (mean ± standard error, n = 3), respectively.
2.3. Sampling procedures and measurements
Phytoplankton and periphyton biomass (Chla) was measured at the end of the second to fourth period. Phytoplankton biomass was represented by the water chlorophyll a. The water chlorophyll a (Chlaphyt) content was determined by filtering 1 L of water through a Whatman GF/C filter after ethanol extraction (Huang et al. Citation1999). We randomly selected a ramet in one pot and removed the second mature leaf of the ramet each time. Afterwards, the second leaf was stored and shook gently in a plastic bag at 4 °C for determination of epiphyton biomass in terms of chlorophyll a (Chlaperi) (Yu et al. Citation2015).
At the end of the experiment, all pots with healthy and undamaged plants were removed from the mesocosms. Some pots that had been damaged by aquatic insects or sampling for measuring epiphyton biomass during the experiment were excluded from analyses. Ultimately, six replication plants (pots) were obtained for each treatment. There were a total of 36 pots. All the plants in each pot were collected, washed and dried with tissue paper. We counted the number of reproductive (flowering) and vegetative (mature but not flowering) ramets in each pot (each ramet was defined as a single shoot with roots). The mother plant was selected, and its leaves were measured by a meter ruler to determine the plant height and leaf width.
Three replication plants (pots) were used for the biomass measurements for V. spinulosa. All the plants of three replication pots were partitioned into leaves, stolons, roots and tubers, separately dried at 70 °C in paper bags for 72 h and immediately weighed after drying. For another three pots, the plants were partitioned into leaves, stolon and roots, and were placed in −40 °C refrigerator for further biochemical analysis. The frozen samples were ground into a fine powder in liquid N2 with a mortar and a pestle. Approximately 0.5 g of each powder sample was extracted in 5 mL of 80% ethanol at 80 °C for 20 min and centrifuged at 10,000× g for 15 min. The supernatant was used for FAA and SC analyses (Yemm and Willis Citation1954; Yemm et al. Citation1955).
2.4. Data analysis
SPSS software was used for statistical analysis, and values were expressed as mean ± standard error (SE). The data were tested for normality and homogeneity prior to analysis. If the normality and homogeneity was not met, transformations were used to meet analysis of variance (ANOVA) assumptions. Differences were considered significant at P < 0.05. Spearman Rank Correlations analysis were used to test relationships between environmental and macrophyte growth variables at P < 0.05 and P < 0.01. Two-way ANOVA was used to determine whether N content and water level exerted joint effects on the growth and physiological variation of V. spinulosa (P < 0.05 and P < 0.01).
3. Results
3.1. Phosphate content, phytoplankton and epiphyton biomass in the experimental mesocosms
During the experiment, the DTN concentration in the water was kept about 5·L−1. The concentration of PO43--P was always kept a low level and increased at the 4th period (). The high N content in water promoted the growth of phytoplankton and periphyton, as manifested by the high values of Chlaphyt and Chlaperi obtained ().
Table 1. Phosphate content, phytoplankton and epiphyton biomass in the experimental mesocosms.
3.2. Spearman rank correlations (r values) between algae (include phytoplankton and periphyton) and growth indicators of V. asiatica
No relationship was found between phytoplankton and growth of V. spinulosa. In the E treatment, there was also no relationship between periphyton and growth of V. spinulosa. In the C static treatment, an obvious relationship was found between periphyton and weights of root and leaf. In the CE static treatment, there was obvious relationship between periphyton and stolon weights and tuber weights ().
Table 2. Spearman Rank Correlations (r values) between algae and growth indicators of V. asiatica.
3.3. Morphological variation of V. spinulosa
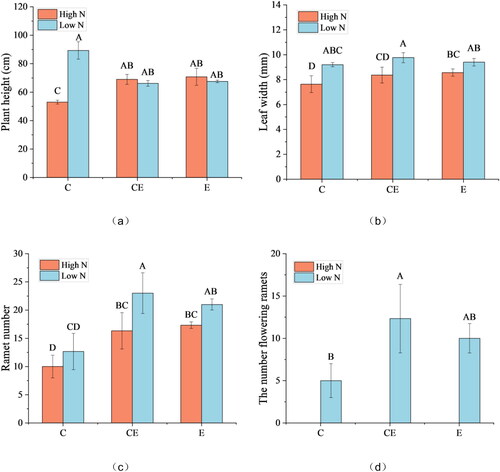
N content was correlated with plant height, leaf width, ramet number and flowering ramet number of V. spinulosa. Water level was correlated only with the number of ramet and flowering ramet. There was an interaction occurred between N content and water level in plant height and number of flowering ramets (). Plant height and leaf width decreased significantly under the dual conditions of high N concentration and low water levels of CE and E. Regardless of high and low N, low water levels CE and E led to an increase in the ramet number, essentially no plants flowered at the high N content ().
Table 3. ANOVA of the effects of high N content and low water level on the morphology and growth traits of V. asiatica.
3.4. Biomass of V. spinulosa
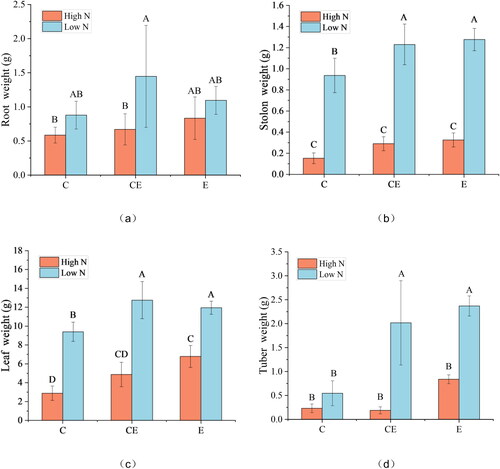
N content was correlated with the biomass of each organ of V. spinulosa (). Water level was correlated with the stolon, leaf and tuber biomasses of V. spinulosa, and an obvious interaction occurred between N content and water depth in tubers (). The stolon and leaf biomass of V. spinulosa grown in water with high N contents was obviously lower than those of plants grown in water with low N contents for each studied water level (). At low N content, the low water levels of CE and E caused an increase in the biomass of stolon, leaf and tuber. However, at high N content, the water level treatments did not cause an increase in biomass except leaf ().
3.5. C/N Metabolism of V. spinulosa
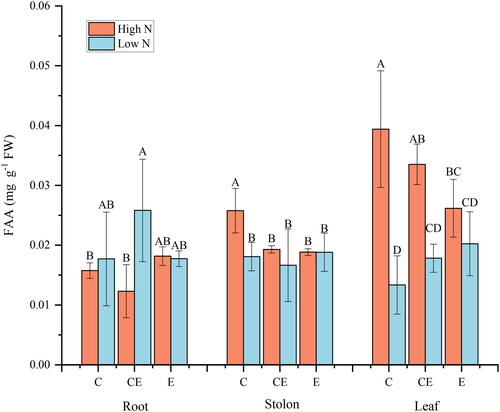
There was a correlation between N content and FAA content in leaf and stolon of V. spinulosa, and no correlation between water level and FAA content. A significant interaction between N content and water level was noted only on FAA content in leaf (). At C water level, high N content caused an increase in stolon FAA content. The high N content did not cause an increase in FAA content of stolon under low water levels of CE and E ().
Table 4. ANOVA of the effects of high N content and low water level on the physiological traits of V. asiatica.
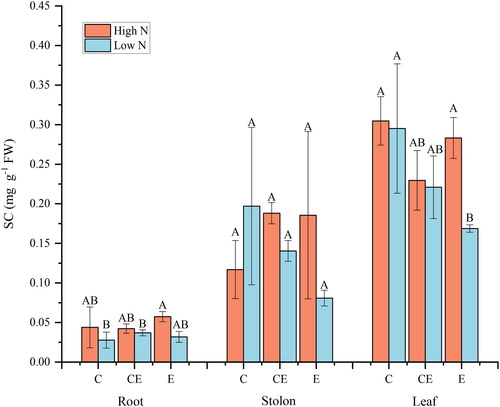
N content was correlated with root SC contents, whilst water level was correlated with leaf SC contents (). Water level and N content caused no changes in SC content of root and stem. At E water level, the high N content caused an increase in leaf SC content of V. spinulosa ().
4. Discussion
The results indicate that water level and N content influence the growth and reproduction of V. spinulosa. At low N content, low water level from May to July decrease plant heights because V. spinulosa is a type of submerged macrophyte that survives under the water surface. However, the ramet number and weights of stolons, leaves and tubers increased at low water levels maybe because the plant increases its ramet number to capture light near the water surface and colonise the available water column. Some researchers have found that submerged macrophytes are able to adapt to changes in water depth, flood time and flood frequency by adjusting their life history period (Brock and Rogers Citation1998), which supports our results. V. spinulosa breed offspring mainly through asexual reproduction (tubers) but can produce a small number of seeds. Increases in tuber weights indicate that declines in water level have a strong positive impact on V. spinulosa breeding. V. spinulosa could show certain morphological responses to changes in water level to achieve optimal growth and reproduction. The weight of each organ under high N contents was significantly lower than that under low N contents, which indicates that high N levels severely impact the growth and reproduction of V. spinulosa.
At high N content, water level did not cause changes in the biomass of root, stolon and tuber; in fact, only the leaf weight of plants in the E treatment was higher than that of plants in the C treatment. In addition, low water level caused increased flowering ramet number under low N content, whereas few V. spinulosa flowered under high N content. The results indicated that the high N content badly impacted the adaptability of V. spinulosa to low water depths. Several authors have determined that the adverse effects of N on submerged macrophytes may be attributed, at least in part, to toxic stress (Cao et al. Citation2009, Yuan et al. Citation2016) and promotion of the shading effect of algae, which inhibits the photosynthesis of submerged macrophytes (Zhi et al. Citation2018). In our experiment, high N contents increased the growth of phytoplankton and periphyton in water (). However, no relationship was found between phytoplankton and growth of V. spinulosa. There was also no relationship between periphyton and growth of V. spinulosa under the E treatment (). As known, when cyanobacterial concentrations increase to a certain level, they may affect light conditions, produce toxins, exhaust dissolved oxygen and release organic matter, all of which are detrimental to the growth of aquatic plants (Li et al. Citation2009). In another acute aquarium experiment, V. asiatica undergoes oxidative stress when the cyanobacterial concentration exceeds 109 g·m−3 (Kang et al. Citation2015). In the E treatment, periphytons showed no relationship with growth of V. asiatica maybe because of low periphyton biomass resulting from the water level fluctuation of 60 ± 30 cm. Thus, it can be inferred that it was possibly that the concentration of 44.7 mg·m−3 phytoplankton and 0.094 ug·m−2 periphyton was not high enough to form great shading effects on V. spinulosa.
In the present study, N concentrations were considered high at a level of 5.0 mg·L−1 DTN (NO3−-N, 3.0 mg·L−1). A NO3−-N concentration of 3 mg·L−1 caused only a slightly negative impact on V. spinulosa because excessive NO3−-N can be stored in vacuoles and, thus, presents limited harm to submerged macrophytes (Surya et al. Citation2007). Many researchers have found that high concentrations of NH4+-N have a direct toxic effect on submerged macrophytes, which mainly manifests as a slow growth rate, C-N metabolism disorder and oxidative damage (Cao et al. Citation2004; Nimptsch and Pflugmacher Citation2007). Nimptsch and Pflugmacher (Citation2007) found that above 1 mg·L−1 NH4+-N generated oxidative stress on aquatic macrophyte Myriophyllum mattogrossense. Thus, in our experiment, it was supposed that an NH4+-N concentration of 2.0 mg·L−1 inhibited the growth of V. spinulosa. In water with high N content, NH4+-N is converted into FAAs and amines to reduce its accumulation in plants or transferred out of plant cells, which requires energy and carbohydrates, to alleviate its toxicity (Smolders et al. Citation1996). Thus, in response to NH4+-N stress, plant detoxification mechanisms are mainly manifested by the production of more FAA and lower SC/FAA. It was found that high concentration of ammoniacal nitrogen (1-20mg/L) caused the accumulation of large amount of FAA in minnow grass, and the SC content decreased sharply (Cao et al. Citation2009). However, in our study, at high N content, the increased FAA content of stolon and leaf for water level C, and decreased SC content in leaf for water level E were observed. The concentration of PO43−-P was very low during the experiment. Because phosphate is important for ATP recycling and energy transfer within cells, V. spinulosa may be unable to transport excess NH4+-N out of its cells because of the energy shortage caused by P deficiency (Cao et al. Citation2009). In this study, high N contents were the primary factor affecting FAA contents in the stolon and leaf of V. spinulosa. No obvious increase in stolon FAA content caused by NH4+-N was found in the CE and E treatments, and no increase in leaf FAA content in the E treatment with high N content was observed. These results indicate that declines in water level limit plants’ ability to transform NH4+ into FAAs and amines, consistent with the relatively constant stolon and leaf biomass observed in different water level treatments with high N content.
Cao et al. (Citation2009) also found that fertile sediment treatment and NH4+ fertilisation of the water column without shading does not cause FAA accumulation in Potamogeton crispus shoots; however, the NH4+ content in shoots is positively related to FAA content, thereby indicating that internal NH4+ content, rather than external NH4+ availability, directly affects the FAA synthesis of the plants. In the present study, the combination of N content and water level exerted extremely significant influences (P < 0.05) on the leaf FAA contents of V. spinulosa. High radiation intensity often induces photoinhibition and results in decreased photosynthetic capacity in many plants (Gerganova et al. Citation2016; Huang et al. Citation2019). Thus, long-term exposure to strong sunlight aggravates the harm caused by high N content to V. spinulosa, possibly because V. spinulosa at water level E lacks the ability to assimilate NH4+-N in water on account of its photoinhibition.
Many of the shallow lakes in the middle and lower reaches of the Yangtze River in China are eutrophic, and submerged macrophytes in these lakes frequently disappear (Wang et al. Citation2012). Environmental changes have intensified the occurrence of drought from 2003 to 2013 compared with that observed from 1960 to 2003. Such changes have led to the degeneration of the Poyang Lake Wetland (Zhang et al. Citation2017). Thus, establishing a dual strategy to control N nutrients and water levels is necessary to restore V. spinulosa in eutrophic lakes, such as Poyang Lake.
5. Conclusion
In conclusion, low water levels and high N contents affect the growth and reproduction of V. spinulosa. At low N content, V. spinulosa can well adapt to low water levels by increasing their ramet number, biomass and flowering ramet number. Although V. spinulosa could also convert excessive NH4+-N into FAAs to reduce its accumulation in plants or transfer the same out of plant cells to alleviate the toxicity of NH4+-N. High N content badly impacted the growth and reproduction of V. spinulosa, and inhibited the adaptability of V. spinulosa to low water level.
Acknowledgements
The study was processed in the Laboratory of Poyang Lake and Wetland Ecosystem Research Chinese Ecosystem Research Network research platform.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data presented in this study are available on request from the corresponding author.
Additional information
Funding
References
- Brock MA, Rogers KH. 1998. The regeneration potential of the seed bank of an ephemeral floodplain in South Africa. Aquat Bot. 61(2):123–135.
- Cao T, Ni LY, Xie P. 2004. Acute biochemical responses of a submersed macrophyte, Potamogeton crispus L., to high ammonium in an aquarium experiment. J Freshwater Ecol. 19(2):279–284.
- Cao T, Xie P, Ni LY, Zhang M, Xu J. 2009. Carbon and nitrogen metabolism of an eutrophication tolerative macrophyte, Potamogeton crispus, under NH4+ stress and low light availability. Environ Exp Bot. 66(1):74–78.
- Chu JZH, Wang SR, Jin XC. 2006. Effects of sediments nutrition condition on the growth and the photosynthesis of Hydrilla verticillata. Ecol Environ. 15:702–707. (In Chinese)
- Codd GA. 1995. Cyanobacterial toxins: occurrence, properties and biological significance. Water Sci Technol. 32(4):149–156.
- Gerganova M, Antoaneta VP, Stanoeva D, Velitchkova M. 2016. Tomato plants acclimate better to elevated temperature and high light than to treatment with each factor separately. Plant Physiol Biochem. 104:234–241.
- Hu ZP, Ge G, Liu CL. 2015. Cause analysis and early warning for wetland vegetation degradation in poyang lake. Resourc Environ Yangtze Basin. 24(3):381–386. (in Chinese).
- Huang XF, Chen WM, Cai QM. 1999. Survey, observation and analysis of lake ecology. In Standard methods for observation and analysis in Chinese Ecosystem Research Network, Series V. Standards Press of China, Beijing (in Chinese).
- Huang W, Yang JJ, Zhang SB. 2019. Photoinhibition of photosystem I under fluctuating light is linked to the insufficient ΔpH upon a sudden transition from low to high light. Environ Exp Bot. 160:112–119.
- Kang CX, Kuba T, Hao AM, Iseri Y, Li CJ, Zhang ZJ. 2015. Oxidative stress responses of submerged macrophyte Vallisneria asiatica to different concentrations of cyanobacteria. Chin J Ocean Limnol. 33(2):364–371.
- Li DH, Li GB, Chen WX, Liu YD. 2009. Interactions between a cyanobacterial bloom (Microcystis) and the submerged aquatic plant ceratophyllum oryzetorum Kom. Chin J Ocean Limnol. 27(1):38–42.
- Li YK, Qian FW, Silbernagel J, Larson H. 2019. Community structure, abundance variation and population trends of waterbirds in relation to water level fluctuation in Poyang Lake. J Great Lakes Res. 45(5):976–985.
- Nimptsch J, Pflugmacher S. 2007. Ammonia triggers the promotion of oxidative stress in the aquatic macrophyte Myriophyllum mattogrossense. Chemosphere. 66(4):708–714.
- Smolders AJP, Hartog CD, Gestel CBV, Roelof JGM. 1996. The effects of ammonium on growth, accumulation of free amino acid and nutritional status of yang phosphorus deficient Stratiotes aloides plants. Aquat Bot. 53(1–2):85–96.
- Su SQ, Zhou YM, Qin JG, Wang W, Yao WZ, Song L. 2012. Physiological responses of Egeriadensa to high ammonium concentration and nitrogen deficiency. Chemosphere. 86(5):538–545.
- Surya K, Pragya K, Herman L. 2007. Partial substitution of NO3- by NH4+ fertilization increases ammonium assimilating enzyme activities and reduces the deleterious effects of salinity on the growth of barley. Plant Physiol. 164:308–309.
- Wang L, Dronova I, Gong P, Yang WB, Li YR, Liu Q. 2012. A new time series vegetation–water index of phenological–hydrological trait across species and functional types for Poyang Lake wetland ecosystem. Remote Sens Environ. 125:49–63.
- Wu ZS, Cai YJ, Zhang L, Chen YW. 2018. Spatial and temporal heterogeneities in water quality and their potential drivers in Lake Poyang (China) from 2009 to 2015. Limnologica. 69:115–124.
- Xie YH, An S, Wu B. 2005. Resource allocation in the submerged plant Vallisneria natans related to sediment type, rather than water-column nutrients. Freshwater Biol. 50(3):391–402.
- Xu WW, Hu WP, Deng JC, Zhu JG, Zhou NN, Liu X. 2016. Impacts of water depth and substrate type on Vallisneria natans at wave-exposed and sheltered sites in a eutrophic large lake. Ecol Eng. 97:344–354.
- Yemm EW, Cocking EC, Ricketts RE. 1955. The determination of amino-acids with ninhydrin. Analyst. 80(948):209–214.
- Yemm EW, Willis AJ. 1954. The estimation of carbohydrates in plant extracts by anthrone. Biochem J. 57(3):508–514.
- Yu Q, Wang HZ, Li Y, Liang XM, Shao JC, Jeppesen E, Wang HJ. 2015. Effects of high nitrogen concentrations on the growth of submersed macrophytes at moderate phosphorus concentrations. Water Res. 83:385–395.
- Yuan G, Fu H, Zhong J, Lou Q, Ni L, Cao T. 2016. Growth and C/N metabolism of three submersed macrophytes in response to water depths. Environ Exp Bot. 122:94–99.
- Zhang X, Liu X, Wang H. 2015. Effects of water level fluctuations on lakeshore vegetation of three subtropical floodplain lakes. China. Hydrobiologia. 747(1):43–52.
- Zhang XL, Zhang Q, Werner AD, Tan ZQ. 2017. Characteristics and causal factors of hysteresis in the hydrodynamics of a large floodplain system: Poyang Lake (China). J Hydrol. 553:574–583.
- Zhao XS, Liu YB. 2017. Phase transition of surface energy exchange in China’s largest freshwater lake. Agric For Meteorol. 244–245:98–110.
- Zhi YW, Cao Y, Sun YY, Li W, Jeppesen E. 2018. Indirect effects of extreme precipitation on the growth of Vallisneria denseserrulata Makino. Environ Exp Bot. 153:229–235.