Abstract
Temperature and food density are the most important factors influencing the population dynamics of rotifers. In the present study, the effects of temperature and food concentration on the developmental durations, egg ratio, and life-table demography in Brachionus angularis and Keratella valga were studied at four temperatures (15 °C, 20 °C, 25 °C and 30 °C) and four food levels (0.5 × 106, 1.0 × 106, 2.0 × 106 and 3.0 × 106 cells/mL Scenedesmus obliquus). The results showed significant effects of both temperature and food concentration, independently and interactively on the embryonic development (ED), juvenile period (JP), average lifespan (LS), generation time (T) and intrinsic rate of population increase (rm) in B. angularis, while the ED, life expectancy at hatching (e0), LS, T and rm in K. valga. In all conditions, the number of eggs per female and rm in B. angularis were higher than those in K. valga. These results suggested that B. angularis might be more suitable to mass culture in aquaculture than K. valga, and a potential prey for fish larvae in freshwater aquaculture.
Introduction
Rotifers are the most common invertebrates with small size (<200 μm) in freshwaters, estuaries and coast areas. They play important roles in the transportation of nutrient across food chains. Due to their vast availability and ease of cultivation, rotifers are useful model organisms for aquatic ecotoxicity assays and sensitive bioindicators of water quality changes (Dahms et al. Citation2011). In aquaculture, most of fish larvae are very small when they hatch, and need small preys during the early life stages (Conceição et al. Citation2010).
Environmental factors can affect the culture of rotifers, such as salinity (Sarma et al. Citation2006), pH (Yin and Niu Citation2008), food level (Kuefler et al. Citation2012) and temperature (Paraskevopoulou et al. Citation2020). Among them, temperature and food density have been ascribed as the most important and common factors influencing the occurrence, activities, development, reproduction, and population growth of rotifers because of their effects on the grazing rate of rotifers (Montagnes et al. Citation2001). Independently, temperature affects biological processes directly by altering the rate of chemical and biochemical reactions, and indirectly by altering viscosity and diffusion (Cossins and Bowler Citation1987). Food concentration influences the rate at which the rotifers encounter food items while grazing and the time required to food (Pickett et al. Citation1987). In addition, temperature and food level vary under natural conditions and can be controlled when maintaining rotifer cultures. Thus, knowledge of the independent and combined impact of these two factors on efficient culture of rotifers are needed.
Brachionus calyciflorus is considered as the most promising species as larval feed in aquaculture and receives the most attentions. In addition to B. calyciflorus, Brachionus angularis and Keratella valga are also common rotifer species worldwide, and generally dominant species in freshwater lakes. Compared with B. calyciflorus, B. angularis and K. valga are much smaller in body size, and may serve as supplementary feed to small larvae in addition to B. calyciflorus. Thus, investigating the combined effects of temperature and food level on the life history traits in these two species might provide useful data for the application of rotifers in aquaculture.
Life table demography is an effective method to reveal the tremendous variation of life histories within and among different groups of zooplanktons (Stelzer Citation2005). Life table demographic parameters, such as duration of different developmental stages, average lifespan, generation time and intrinsic population growth rate are closely related to survivorship and reproduction (Xiang et al. Citation2016). Quantification of these parameters provide valuable insights to understand the influences of environmental factors, which are important issues in ecological studies. In the present study, the responses of life-table demographic parameters to variations of temperature and food level were investigated in B. angularis and K. valga. The obtained data might contribute information to application of rotifers as larval fish feed in aquaculture.
Materials and methods
Rotifer collection and culture
B. angularis and K. valga individuals were collected from Lake Jing, Wuhu city, P. R. China and clonally cultured for three months under controlled laboratory conditions in EPA medium (pH = 7.5; oxygen saturation ≥ 90%; Peltier and Weber Citation1985). The culture temperature was set at 25 ± 1 °C and rotifers were fed daily with the algae Scenedesmus obliquus (2.0 × 106 cells/mL). S. obliquus was cultured in HB-4 medium (Li et al. Citation1959), precipitated by centrifugation at 4,000 rpm for 15 min and stored at 4 °C. Algal density was measured using a haemocytometer under a microscope. The illumination intensity was approximately 3000 lx and the photoperiod (light: dark) was 14 h: 10 h.
Determination of developmental stages and number of eggs (per female)
Life table demographic analyses were performed at four temperatures (15 °C, 20 °C, 25 °C and 30 °C) and four food levels (0.5 × 106, 1.0 × 106, 2.0 × 106 and 3.0 × 106 cells/mL, corresponding to 15, 30, 60 and 90 mg/L, dry weight of algal cells). The temperatures and food densities were set according to the annual changes of these indices in local water bodies (Xi et al. Citation2017). The photoperiod and light intensity were the same as described above. For each temperature and food level combination, three replicates were included. Each replicate contained 10 newly hatched rotifers (<2 h old) on one 24-well plate. Each animal was placed in one well containing 0.5 mL of EPA medium. Thus, a total of 480 rotifers (4 temperature × 4 algal densities × 3 replicates × 10 neonates) were assessed for each species. During the experimental period, the rotifers were observed every two hours until the first neonate was produced, and then observed every eight hours. The numbers of eggs, neonates produced, and original individuals alive were recorded. Dead rotifers and the produced neonates were removed at each observation. Every 24 h, 80% of the test solutions were changed with freshly prepared solutions containing designated concentrations of algae.
The durations of developmental stages, including embryonic development (ED, time from extrusion of the first egg to hatching of the first larva), juvenile period (JP, time from hatching to extrusion of the first egg), reproductive period (RP, time from extrusion of the first egg to extrusion of the last egg), and post-reproductive period (PP, time from extrusion of the last egg to death) were calculated, together with the number of eggs per female as described by Walz (Citation1983) and Korstad et al. (Citation1989). The life-table demographic parameters, including age-specific survivorship (lx) and fecundity (mx), net reproductive rate (R0), average lifespan, and generation time (T), and intrinsic rate of population growth (rm), were calculated following Krebs (Citation1978) and Pianka (Citation1988).
Data analysis
All data were tested for normality and homogeneity using the Kolmogorov Smirnov and Levene’s tests, respectively. Data are presented as mean ± SE. One-way analysis of variance (ANOVA) was used to test the effects of temperature at the same food density, or the effects of food density at the same temperature on each parameter in rotifers, following by the LSD tests for comparison between groups. Two-way ANOVA was conducted to test effects of temperature and food density, and their interaction on each life history parameter. P < 0.05 was used as the level of statistical significance. The pseudo values and 95% confidence interval of rm were calculated by the 1000 bootstrap method (Meyer et al. Citation1986). All statistical analyses were performed using the SPSS statistical package version 23.0.
Results
Effects of temperature and food density on developmental stages
One-way ANOVA demonstrated that, at the same food density, the durations of ED, JP, and RP in B. angularis tended to shorten with increasing temperature from 15 °C to 30 °C, with significantly differences between low temperature groups (15 °C and 20 °C) and high temperature groups (25 °C and 30 °C). There were no significant differences in ED, JP, RP, and PP between 25 °C and 30 °C. At 15 °C, 20 °C, and 30 °C, treatment with 0.5 × 106 cells/mL showed significantly longer JP than those with 2.0 and 3.0 × 106 cells/mL. Similarly, at 25 °C, treatment with 1.0 × 106 cells/mL showed longer JP than those with 2.0 and 3.0 × 106 cells/mL. Food level did not significantly affect PP at each temperature as well as RP at 25 °C and 30 °C (). Two-way ANOVA revealed that temperature significantly affected ED, JP, RP, and PP, while food density only significantly influenced JP. Their interaction significantly impacted ED, JP, and RP (Table S1).
Figure 1. Effects of temperature and food density on developmental stages in Brachionus angularis and Keratella valga. Data represent mean ± SE. Different lowercases represent significant statistical differences among different temperatures at the same food density (P < 0.05). Different capital letters represent significant statistical differences among different food density at the same temperature (P < 0.05).
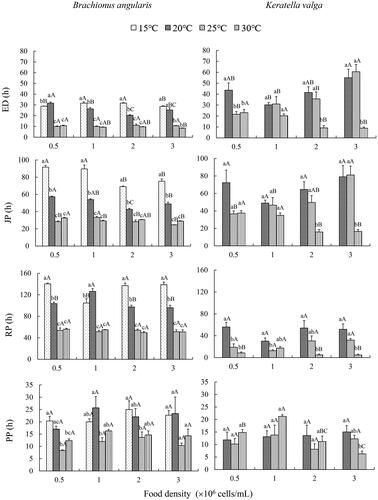
Table 1. Effects of temperature and food density on intrinsic growth rate (rm) in Brachionus angularis and Keratella valga.
At 15 °C, only a few eggs could hatch in K. valga. The developmental stages could not be calculated, and thus this group was ignored for analysis of developmental durations and number of eggs. Compared with 20 °C, treatments at 30 °C revealed significantly shorter ED, JP, and RP, except those at 1.0 × 106 cells/mL, and JP at 0.5 × 106 cells/mL. At 20 °C, ED, JP, and RP decreased first and then increased with the increase of food level, with the lowest values at 1.0 × 106 cells/mL. At 30 °C, ED, JP, and RP decreased with the elevation of food density, with the lowest values in treatments with 2.0 and 3.0 × 106 cells/mL (). Two-way ANOVA revealed that ED, JP, and RP were significantly affected by temperature. Food density and the interaction between temperature and food density only significantly influenced ED, but not JP and RP (Table S2).
Effects of temperature and food density on number of eggs in rotifers
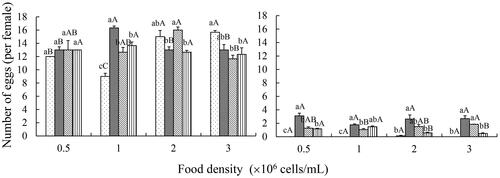
The number of eggs (per female) in B. angularis was always greatly higher than K. valga. Two-way ANOVA revealed that number of eggs was not affected by temperature or food density but significantly affected by their interaction in B. angularis. In K. valga, number of eggs was significantly affected by temperature, but not by food density independently and the interaction between temperature and food density (Table S3). Compared with 20 °C, treatment with 25 °C showed significantly lower number of eggs at 0.5, and 1.0 × 106 cells/mL, and treatment with 30 °C revealed significantly lower number of eggs at 0.5, 2.0 and 3.0 × 106 cells/mL in K. valga ().
Figure 2. Effects of temperature and food density on number of eggs (per female) in Brachionus angularis and Keratella valga. Data represent mean ± SE. Different lowercases represent significant statistical differences among different temperatures at the same food density (P < 0.05). Different capital letters represent significant statistical differences among different food density at the same temperature (P < 0.05).
Effects of temperature and food density on survivorship and fecundity of rotifers
In B. angularis, treatment at 15 °C showed greatly higher survivorship than 20 °C, and then than 25 °C and 30 °C at each food density. At all food levels, the fecundity tended to increase with a rise of temperature. At 15 °C, treatment with 2.0 × 106 and 3.0 × 106 cells/mL revealed higher fecundity than those with 0.5 and 1.0 × 106 cells/mL (Figure S1).
In K. valga, survivorship decreased gradually with the increasing temperature from 15 °C to 30 °C. At 15 °C, the fecundity was extremely low at all food densities. Fecundity decreased gradually with the increasing temperature from 20 °C to 30 °C (Figure S2).
Effects of temperature and food density on life table demographic parameters of rotifers
In B. angularis, at the same food density, the e0, LS and T of B. angularis had similar variation with the rise of temperature. These three parameters were significantly lower at 25 °C and 30 °C than at 20 °C, and then than at 15 °C (). As temperature increased, the trends of G0 and R0 was similar at the same food density. The maximum G0 and R0 were observed in treatment with 1.0 × 106 cells/mL at 20 °C. At each food level, rm significantly increased at 25 °C and 30 °C compared with 15 °C and 20 °C ().
Figure 3. Effects of temperature and food density on life-table demographic parameters in Brachionus angularis (A) and Keratella valga (B). Data represent mean ± SE. Different lowercases represent significant statistical differences among different temperature at the same food density (P < 0.05). Different capital letters represent significant statistical differences among different food density at the same temperature (P < 0.05).
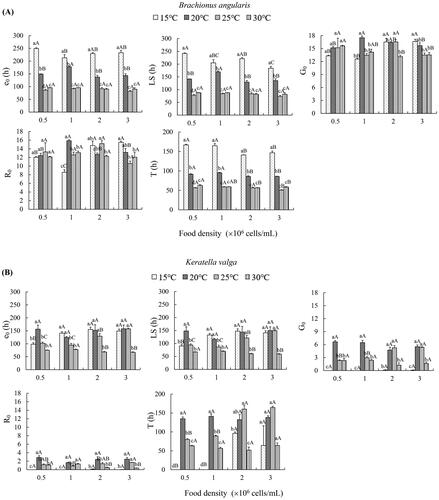
In K. valga, when the food density was 0.5 × 106 cells/mL, e0 and LS exhibited the highest value at 20 °C among all the tested temperatures. At 1.0 × 106 cells/mL, treatments at 25 °C and 30 °C revealed significantly lower e0 and LS than those at 15 °C and 20 °C. At 2.0 × 106 cells/mL and 3.0 × 106 cells/mL, e0 and LS showed the lowest values at 30 °C among the tested temperatures. G0, R0 and T showed significantly lower values at 30 °C than at 20 °C at all food levels, except R0 at 1.0 × 106 cells/mL. In treatments with 2.0 × 106 cells/mL and 3.0 × 106 cells/mL, K. valga revealed negative rm at 15 °C and 30 °C. At 20 °C, rm values were all positive at the four food levels, and did not significantly differ between different food levels ().
Two-way ANOVA indicated that e0, LS, G0, T and rm were significantly influenced by temperature, food density only significantly affected LS, T and rm, and the interaction between temperature and food density significantly affected all the tested life-table demographic parameters in B. angularis (Table S4). In K. valga, temperature, food level, and their interaction had significant effects on all tested life-table demographic parameters, except food level on G0 and R0 (Table S5).
Discussion
Development and reproduction of rotifers are directly impacted by temperature, food density and their interaction (Shurin et al. Citation2010), which will further influence the community structure of rotifers in field (Ma et al. Citation2010). In the present study, compared with B. angularis, all developmental stages and demographic parameters of K. valga were lower at 15 °C, suggesting that K. valga was relatively intolerant to low temperature than B. angularis. At the same food level, ED, JP, RP, e0 and LS of the two rotifer species decreased with the rise of temperature, which were in accordance to previous studies (Galkovskaja Citation1987, Sanoamuang Citation1993). This is a general effect in rotifers due to the increase of metabolic rate (Miracle and Serra Citation1989).
Food concentration is related to energy intake in rotifer, and rotifer populations are also controlled by the bottom-up regulation, depending on food quality and quantity (Walz Citation1995). When food is limited, the reduction of energy intake might leave much less energy to growth and development, and thus resulting in a prolonged juvenile period of rotifers (Dumont et al. Citation1995). Moreover, excessively high food concentration also decreases energy intake because of clogging of filtration apparatus (Xi et al. Citation2017). In the present study, food concentration had no significant effects on RP and PP in the two rotifer species as well as JP in K. valga (Tables S1 and S2). Similar results have been reported in B. plicatilis, Encentrum linnhei (Schmid-Araya Citation1991), and B. calyciflorus (Xi et al. Citation2017). In addition, G0 and R0 in both B. angularis and K. valga were not significantly affected by food density in this study. However, Sarma and Rao (Citation1991) reported that G0 and R0 of B. patulus increased with the increase of food density. Differently, increased food concentration significantly reduced R0 in Limnias ceratophylli and Limnias melicerta, whereas G0 were not significantly affected (Sarma et al. Citation2017). These differences suggested that the effects of food level on life history variables is species-dependent.
In aquaculture, rotifer populations occasionally crash for unexplained physiological stress, but egg ratio can be used as an indicator to assess the status of rotifer mass cultures (Korstad et al. Citation1995). Egg ratio is the ratio of the number of eggs in a given sample over the total number of females in that sample. The egg ratio indicates the growth stage of rotifer populations (exponential or stationary), and more importantly, it gives an estimation of the number of neonates that are expected in the next day (Snell et al. Citation1987). The egg ratio should not be lower than 0.13, as this may indicate population collapse in near future (Snell et al. Citation1987). In the present study, the number of eggs per female in B. angularis was higher than 0.13 in all cultures, indicating the potential to keep offspring productive and population stable. However, the egg ratios of K. valga ranged from 0 to 0.137 at 15 °C in all food concentrations, implying that the populations may crash soon.
Life table demographic parameters are age-specific summary of survival and reproduction. The rm is a comprehensive parameter including age-specific survival, fecundity, age at maturity, and reproductive interval, representing an ability of short-term fitness in a particular environment (Stelzer Citation2005). A reproductive pattern has already been pointed out by Snell and King (Citation1977) for Asplanchna brightwelli that long-lived individuals produced offspring at a low rate, whereas short-lived individuals produce offspring at a higher rate over a shorter time period. The reproductive pattern was conformed with the results of B. angularis in this study, for which the duration of LS at 25 °C and 30 °C were significantly lower than those at 15 °C and 20 °C, with higher rm at 25 °C and 30 °C than at 15 °C and 20 °C. Contrarily, no similar relationship between LS and rm was revealed in K. valga. Compared with K. valga, B. angularis had a higher rm in all treatments, representing higher fitness of B. angularis when facing environmental changes.
Theoretically, R0 of poikilothermal animals should not change significantly with temperature (Fanestil and Barrows Citation1965, Meadow and Barrows Citation1971). In the present study, similar results were observed, the R0 of B. angularis was not significantly affected by temperature (P = 0.388, Table S4). In contrast, temperature significantly affected R0 of K. valga (P < 0.001, Table S5), which was consistent with previous reports on B. calyciflorus (Xi et al. Citation2005) and B. havanaensis (Pavón-Meza et al. Citation2005). The underlying reason might be attributed to the low resistance to 15 °C, at which K. valga could not successively produce and hatch neonates (Figure S2).
Optimal culture temperature for rotifers is dependent on the strain tested, and mostly, the recommended temperature for rotifer culture is 25 °C (Snell and Carrillo Citation1984). In the present study, K. valga could survival but could not reproduce well at 15 °C (Figure S2). In addition, its rm in treatments with 2.0 × 106 cells/mL and 3.0 × 106 cells/mL at both 15 °C and 30 °C were all negative, indicating that K. valga could strictly adapt to moderate temperatures combined with low food levels. Obliviously, this species might not suit to be widely used as feed for aquaculture. The optimal temperature of B. angularis was between 25 °C and 30 °C due to its higher rm and fecundity at this temperature range. Currently, B. plicatilis is usually used as the food organism of marine fish and shrimp larvae (Lubzens Citation1987). However, in freshwater aquaculture, rotifers have not been widely used in fishery industries. The present results suggested that B. angularis might be used as an alternative feed for freshwater aquaculture.
Conclusion
The responses of developmental durations, egg ratio, and life-table demographic parameters to variations of temperature and food density were investigated in B. angularis and K. valga. Compared with K. valga, B. angularis had stable egg ratios and higher rm at all temperatures and food levels. The rm of B. angularis increased greatly with the elevation of temperature, but did not change significantly with food level variation at 20 °C, 25 °C, and 30 °C. Comparatively, K. valga population could grow well only at moderate temperature together with low food densities. These results suggested a higher fitness of B. angularis in nature and lower risk of collapses in mass cultures than K. valga.
Supplemental Material
Download Zip (1.6 MB)Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
All data have been included in this manuscript and supplementary materials.
Additional information
Funding
References
- Conceição LEC, Yúfera M, Makridis P, Morais S, Dinis MT. 2010. Live feeds for early stages of fish rearing. Aquac Res. 41(5):613–640.
- Cossins AR, Bowler K. 1987. Thermal energy and the thermal environment. In Temperature biology of animals. Dordrecht: Springer Netherlands; p. 1–22.
- Dahms HU, Hagiwara A, Lee JS. 2011. Ecotoxicology, ecophysiology, and mechanistic studies with rotifers. Aquat Toxicol. 101(1):1–12.
- Dumont HJ, Sarma SSS, Ali AJ. 1995. Laboratory studies on the population dynamics of Anuraeopsis fissa (Rotifera) in relation to food density. Freshwater Biol. 33(1):39–46.
- Fanestil DD, Barrows CH Jr. 1965. Aging in the rotifer. J Gerontol. 20(4):462–469.
- Galkovskaja GA. 1987. Planktonic rotifers and temperature. Hydrobiologia. 147(1):307–317.
- Korstad J, Neyts A, Danielsen T, Overrein I, Olsen Y. 1995. Use of swimming speed and egg ratio as predictors of the status of rotifer cultures in aquaculture. Hydrobiologia. 313(1):395–398.
- Korstad J, Olsen Y, Vadstein O. 1989. Life history characteristics of Brachionus plicatilis (rotifera) fed different algae. In Ricci C, Snell TW, King CE, editors. Springer Netherlands: Proceedings of the Rotifer Symposium V.
- Krebs CJ. 1978. Ecology: the experimental analysis of distribution and abundance. 2d ed. New York: Harper & Row. Bibliography; p. 630–665.
- Kuefler D, Avgar T, Fryxell JM. 2012. Rotifer population spread in relation to food, density and predation risk in an experimental system. J Anim Ecol. 81(2):323–329.
- Li S, Zhu H, Xia Y, Yu M, Liu K, Ye Z, Chen Y. 1959. The mass culture of unicellular green algae. Acta Hydrobiol Sin. 4:462–472. [In Chinese].
- Lubzens E. 1987. Raising rotifers for use in aquaculture. Hydrobiologia. 147(1):245–255.
- Ma Q, Xi YL, Zhang JY, Wen XL, Xiang XL. 2010. Differences in life table demography among eight geographic populations of Brachionus calyciflorus (Rotifera) from China. Limnologica. 40(1):16–22.
- Meadow ND, Barrows CH Jr. 1971. Studies on aging in a bdelloid rotifer. II. The effects of various environmental conditions and maternal age on longevity and fecundity. J Gerontol. 26(3):302–309.
- Meyer JS, Ingersoll CG, McDonald LL, Boyce MS. 1986. Estimating uncertainty in population growth rates: jackknife vs. bootstrap techniques. Ecology. 67(5):1156–1166.
- Miracle MR, Serra M. 1989. Salinity and temperature influence in rotifer life history characteristics. Hydrobiologia. 186(1):81–102.
- Montagnes D, Kimmance S, Tsounis G, Gumbs J. 2001. Combined effect of temperature and food concentration on the grazing rate of the rotifer Brachionus plicatilis. Mar Biol. 139(5):975–979.
- Paraskevopoulou S, Dennis AB, Weithoff G, Tiedemann R. 2020. Temperature-dependent life history and transcriptomic responses in heat-tolerant versus heat-sensitive Brachionus rotifers. Sci Rep. 10(1):13281.
- Pavón-Meza EL, Sarma SSS, Nandini S. 2005. Combined effects of algal (Chlorella vulgaris) food level and temperature on the demography of Brachionus havanaensis (Rotifera): a life table study. Hydrobiologia. 546(1):353–360.
- Peltier W, Weber C. 1985. Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms. Cincinnati, OH: United States Environmental Protection Agency.
- Pianka ER. 1988. Evolutionary ecology. 4th ed. New York, NY: Harper Collins; p. 387–458.
- Pickett STA, Carson WP, Begon M, Harper JL, Townsend CR. 1987. Ecology: individuals, populations and communities. Brittonia. 39(3):407–408.
- Sanoamuang LO. 1993. The effect of temperature on morphology, life history and growth rate of Filinia terminalis (Plate) and Filinia cf. pejleri Hutchinson in culture. Freshwater Biol. 30(2):257–267.
- Sarma SSS, Jiménez-Santos MA, Nandini S, Wallace RL. 2017. Demography of the sessile rotifers, Limnias ceratophylli and Limnias melicerta (Rotifera: Gnesiotrocha), in relation to food (Chlorella vulgaris Beijerinck, 1890) density. Hydrobiologia. 796(1):181–189.
- Sarma SSS, Nandini S, Morales-Ventura J, Delgado-Martínez I, González-Valverde L. 2006. Effects of NaCl salinity on the population dynamics of freshwater zooplankton (rotifers and cladocerans). Aquat Ecol. 40(3):349–360.
- Sarma SSS, Rao TR. 1991. The combined effects of food and temperature on the life history parameters of Brachionus patulus MULLER (Rotifera). Int Revue ges Hydrobiol Hydrogr. 76(2):225–239.
- Schmid-Araya JM. 1991. The effect of food concentration on the life histories of Brachionus plicatilis (O. F. M) and Encentrum linnhei Scott. archiv_hydrobiologie. 121(1):87–102.
- Shurin JB, Winder M, Adrian R, Keller W, Matthews B, Paterson AM, Paterson MJ, Pinel-Alloul B, Rusak JA, Yan ND. 2010. Environmental stability and lake zooplankton diversity – contrasting effects of chemical and thermal variability. Ecol Lett. 13(4):453–463.
- Snell TW, Carrillo K. 1984. Body size variation among strains of the rotifer Brachionus plicatilis. Aquaculture. 37(4):359–367.
- Snell TW, Childress MJ, Boyer EM, Hoff FH. 1987. Assessing the status of rotifer mass cultures. J World Aquaculture Soc. 18(4):270–277.
- Snell TW, King CE. 1977. Lifespan and fecundity patterns in rotifers: the cost of reproduction. Evolution. 31(4):882–890.
- Stelzer CP. 2005. Evolution of Rotifer Life Histories. Hydrobiologia. 546(1):335–346.
- Walz N. 1983. Individual culture and experimental population dynamics of Keratella cochlearis (Rotatoria). Hydrobiologia. 107(1):35–45.
- Walz N. 1995. Rotifer populations in plankton communities: Energetics and life history strategies. Experientia. 51(5):437–453.
- Xi YL, Ge YL, Chen F, Wen XL, Dong LL. 2005. Life history characteristics of three strains of Brachionus calyciflorus (Rotifera) at different temperatures. J Freshwater Ecol. 20(4):707–713.
- Xi YL, Ruan QC, Xu DD, Yu HY. 2017. The effects of food level on the life history variables of the two closely related rotifer species Keratella tropica and Keratella valga. Ann Limnol - Int J Lim. 53:153–160.
- Xiang XL, Chen YY, Han Y, Wang XL, Xi YL. 2016. Comparative studies on the life history characteristics of two Brachionus calyciflorus strains belonging to the same cryptic species. Biochem Syst Ecol. 69:138–144.
- Yin XW, Niu CJ. 2008. Effect of pH on survival, reproduction, egg viability and growth rate of five closely related rotifer species. Aquat Ecol. 42(4):607–616.
