Abstract
This study aims to assess the physicochemical characteristics regulating the distribution and abundance of M. marcella larvae in six wetlands in the northern Colombia. Standardized techniques for collecting invertebrate and physicochemical data were used in 29 sampling points in an intraannual period. Mean pH and temperature oscillated in narrow ranges within wetlands (7.7 ± 0.09–8.6 ± 0.07; 28.1 ± 0.29–32.8 ± 0.17 °C, respectively), whereas ammonium concentrations and conductivity exhibited a wide variation (0.2 ± 0.03–2.8 ± 0.54 mg NH4 L−1; 861 ± 30.7–19254 ± 1706 µS cm−1, respectively). A total of 2586 individual M. marcella larvae were collected. Abundance was greater in wetlands influenced by the Magdalena River, with 19.4 ± 1.7 and 9.3 ± 1.4 individuals; followed by wetlands hydrologically influenced by seasonal runoff, with 8.1 ± 0.4 and 6.4 ± 0.4 individuals; and lowest in wetlands with influence of the Caribbean Sea, with 3.9 ± 0.3 and 0.3 ± 0.1 individuals. Abundances of M. marcella larvae exhibited similar variations at different months during the sampling period. Abundance and distribution of M. marcella larvae in wetlands of northern Colombia is strongly dependent on water conductivity, transparency and alkalinity. This study evidence that Odonata larvae are a valuable tool as bioindicators for wetland assessment and monitoring.
Introduction
Freshwater bodies and coastal lagoons are among the most threatened ecosystems in the world (Esteves et al. Citation2008; Campbell Citation2020; Lubanga et al. Citation2021). Human activities represent a major threat to ecosystem ecological integrity, as they generate impacts and cause irreparable diversity loss, pollution, substrate disturbance, and water quality deterioration (Miguel et al. Citation2017; Vanacker et al. Citation2018; Silva et al. Citation2021). In addition, riparian and aquatic communities are the most affected by human water use, as their structure and composition deteriorates due to physicochemical changes related to water quality loss (Vanacker et al. Citation2018). Therefore, there is a need to recognize the importance of these ecosystems and prioritize the monitoring of water bodies. The use of organisms, in particular macroinvertebrates, as bioindicators, is an efficient tool for assessing water quality, as well as anthropogenic disturbance and its impact on the ecological integrity of aquatic species (Miguel et al. Citation2017; Mendes et al. Citation2018; Adu et al. Citation2019; Perron and Pick Citation2020; López-Díaz et al. Citation2021). One of these bioindicators are odonates (dragonflies and damselflies), an insect order with approximately 6500 described species worldwide (Kohli et al. Citation2021). Previous works have shown the high sensitivity of this group to minimal disturbances in water, expressed in all life stages through changes in physical traits, species structure and composition (Petersen et al. Citation2018; Adu et al. Citation2019). The use of this type of bioindicators has become important due to their sampling accessibility, rapid response to environmental change, differing degrees of tolerance to aquatic pollution, and characteristics associated to each ecosystem (Adu et al. Citation2019).
Research and applications of odonates as water quality proxies have been developed around the world, focusing on the analysis of different water bodies. Miguel et al. (Citation2017) assessed the effects of anthropogenic disturbance on aquatic ecosystems using bioindicators, demonstrating that species composition, taxonomic diversity, and community distribution allow to assess ecological integrity and the relationship between odonates and environmental impacts. Miathyria marcella is a dragonfly widely distributed in the Caribbean region of Colombia, inhabiting wetlands (Ciénagas), and other lentic habitats. Adult specimens form swarms, whereas aquatic larvae occupy diverse microhabitats (González Citation2007). The taxonomic description of M. marcella is well documented (Von Ellenrieder and Garrison Citation2007); as well as some ecological aspects such as distribution, habitat (Beckemeyer Citation2009), and adult behavior (Von Ellenrieder and Garrison Citation2007). There are several references on population variation within lotic environments, leading to several hypotheses related to physicochemical variables of water, as well as the drag effect caused by flood pulse (González Citation2007). Other hypotheses involve a population decrease effect caused by flow increase (Poi and Bruquetas Citation1989). However, the populational variation of this species within wetlands remains unstudied, mainly due to the different behavior of water conditions in these ecosystems.
Lentic wetlands of the Colombian Caribbean basin, known as Ciénagas, are currently affected by agriculture, extensive livestock farming, drought, filling for housing construction, and pollution. Sedimentation and construction also disrupt natural fluxes and communication between wetlands (CRA Citation2017). Ciénagas are directly influenced by natural flood pulses, which generate recurrent disturbance (Neiff Citation1999). The physical and biotic environment has a deep influence in the ecology and behavior of odonates (Corbet Citation1999). Physicochemical characteristics are related to spatial and temporal patterns of larvae distribution and abundance. Therefore, the use of bioindicators plays a key role for evaluation ecological condition in water bodies. Considering all the above, this research aims to assess the physicochemical characteristics regulating the abundance of M. marcella larvae. Lastly, this research contributes to the understanding, use, and management of water bodies in the Colombian Caribbean, and to generate knowledge on optimal conditions of wetlands and the effect of their deterioration on the preservation of associated biological diversity.
Materials and methods
Study area
This study was conducted in the Atlántico department of northern Colombia, particularly in the wetlands Totumo (TM), Mallorquín (MQ), Sabanagrande (SG), La Larga-Luisa (LL), Tocagua (TG), and Luruaco (LU) (Supplementary Material, Table S1). In this regard, two wetlands with influence of the Magdalena River were selected, together with two influenced by seasonal runoff, and two influenced by the Caribbean Sea (coastal lagoons). Sampling sites were selected for each wetland considering the affluents, effluents, and macrophyte distribution (). Monthly sampling were performed in the period between July of 2013 and June of 2014.
Figure 1. Geographical location of wetlands and sampling points in the Atlántico Department. A. Totumo (TM); B. Mallorquín(MQ); C. Sabanagrande (SG); D. La Larga-Luisa (LL); E. Tocagua (TG); F. Luruaco (LU).
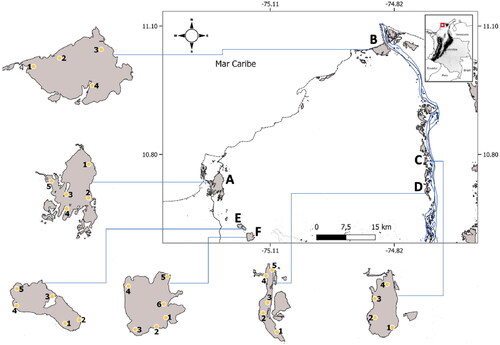
Table 1. Physicochemical characteristics of wetlands with different types of water influence.
Physicochemical characteristics
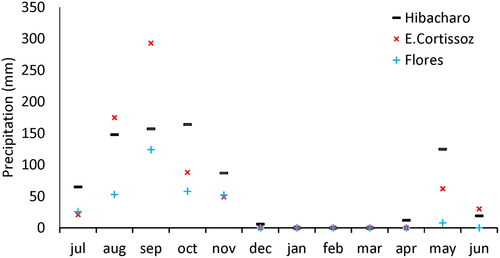
Physicochemical characteristics were measured in situ using a Hanna multiparameter probe, model HI98199 including conductivity (µS cm−1), temperature (°C), dissolved oxygen (DO, mg L−1) and pH. Transparency (cm) was measured using a Secchi disk. Ammonium concentration (mg NH4 L−1), was measured in situ using an Aquamerck colorimetric test kit with a range of 0.5 − 10 mg L−1. Alkalinity (mg CaCO3 L−1) was measured using a MolLabs titration Kit with a range of 0 − 500 mg L−1. Monthly measurements were performed in the established sampling points. Precipitation records during the sampling months were obtained from the Colombian Institute of Hydrology, Meteorology, and Environmental Studies (IDEAM, http://www.ideam.gov.co/) through the climatological stations located closer to the sampled wetlands (Hibacharo, E. Cortissoz y Flores).
Sample collection
Standardized techniques for macroinvertebrate collection (Correa-Araneda et al. Citation2021) were used in this study. Odonates larvae associated to macrophytes were collected using a conical net with a diameter of 40 cm, 65 cm long, a mesh size of 250 μm, and a metallic handle of 90 cm. The collection method was standardized in each sampling point, five minutes in a 1 m2 area (Correa-Araneda et al. Citation2021; Cortés-Guzmán et al. Citation2021). Samples were deposited in Ziploc plastic bags in order to be separated in the laboratory.
Laboratory analysis
Samples were washed, stored in 70% alcohol, processed and identified. Odonata larvae were identified under a dissection microscope, using taxonomic keys (Heckman Citation2006; Neiss et al. Citation2018) and their abundances were quantified for each wetland.
Data analysis
The biological and environmental data were summarized and analyzed using descriptive statistics. Time series were made to compare M. marcella abundances in sampled points at the north and south ends of each wetland. In addition, significance was evaluated using the Student’s t-test. On the other side, a hierarchical cluster analysis (UPGMA method, Euclidian coefficient analysis) was performed to evaluate similarity between wetlands in agreement with M. marcella abundances. The physicochemical characteristics and M. marcella abundances were z-scored standardized prior multiple linear regression analysis. Multiple linear regression is a method used to predict a response variable Y as a function of more than one predictor Xp, assigning a coefficient for each predictor variable (Fernández del Castillo et al. Citation2022). Coefficient is a reference of how significant the parameter influence is over M. marcella. To develop this model, abundance of M. marcella was considered as Y; physicochemical parameters, were used as predictor variables Xp. All statistical analyses and graphs were performed using the R software package (R Core Team Citation2020).
Results
Physicochemical and environmental characteristics
Temperature and pH oscillated within narrow ranges in the wetlands, whereas ammonium concentration and conductivity displayed a wide variation. Depth was between 0 and 233 cm, zero values represent points which dried due to wetland dynamics during prolonged drought intervals. Transparency values ranged between 7 and 122 cm. Minimum values were similar in all sampled wetlands ranging between 7 and 15 cm, whereas the maximum values were found in wetland LU with 122 cm, followed by TG and LL (). Conductivity ranged from 346 to 37900 µS cm−1. The highest average value was observed in wetland MQ, with 19254 µS cm−1. Surface temperatures varied between 24.1 and 38.5 °C. pH values oscillated from 6.99 to 9.98. Dissolved oxygen ranged between 0.25 y 14.68 mg L−1, with average values between 5.1 y 5.8 mg L−1 in most wetlands. Oxygen value values in MQ had a lower average value (3.8 mg L−1) than that of other wetlands. Alkalinity varied between 40 and 380 mg L−1, with the greatest average values in TG and LL (268.8 mg L−1 and 230.6 mg L−1, respectively). Ammonium ranged between 0 and 10 mg L−1 ().
In the study area two rainy periods (August-November and May) were observed. During the sampled year the greatest precipitation occurred between August and October. In 2014 the warm phase of the El Niño Southern Oscillation (ENSO) was recorded, with dry periods and low precipitation volumes, decreasing water levels or completely drying some sampling points between February and June ().
Abundance of M. marcella larvae
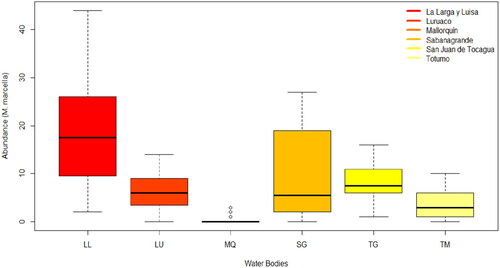
A total of 2586 individual (ind) M. marcella larvae were collected. Larvae were distributed in the following way: 1011 ind in LL, 486 ind in TG, 465 ind in LU, 372 ind in SG, 239 ind in TM and 13 ind in MQ. Mean and standard deviation were higher in wetlands with influence of the Magdalena River (19.4 ± 1.7 ind and 9.3 ± 1.4 ind in LL and SG, respectively), followed by wetlands with influence of seasonal runoff (8.1 ± 0.4 ind and 6.4 ± 0.4 ind in TG and LU, respectively). Lastly, the abundance was lower in those wetlands influenced by the Caribbean Sea (3.9 ± 0.3 ind and 0.3 ± 0.1 ind for TM and MQ, respectively) ().
Spatial and intraannual variation of M. marcella larvae
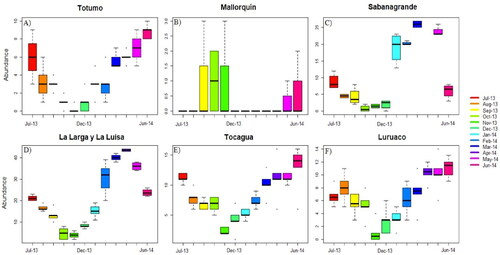
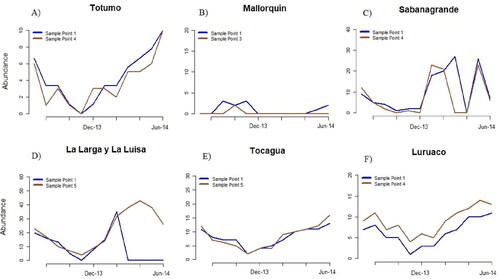
Abundance of M. marcella larvae showed similar changes during the months of sampling, except for the wetland MQ. Abundance was lowest in the months of highest precipitation (September to November), and increased during the months with precipitation close, or equal, to zero (). On the other hand, comparisons between sampling points indicate that abundance variation was similar for most wetlands studied. The greatest differences were found in the points of wetlands SG and LL, in which abundance concentrated in the points of lowest depth (). The t-test revealed significant differences between sampling points in wetlands LL and LU (p-value ≤ 0.05). When drought extended, the water column of wetlands lowered rapidly, and some sampling points disappeared in wetlands SG, LL, and LU. In April, wetland SG dried completely, and abundance of M. marcella larvae was zero.
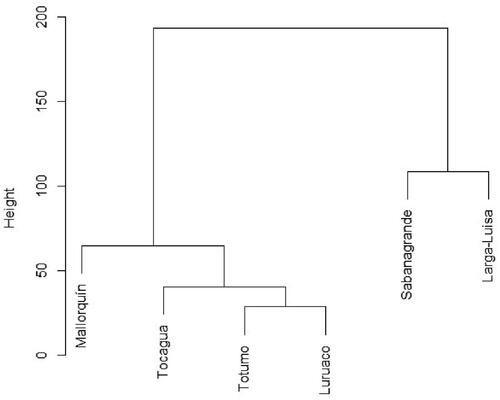
The conglomerate analysis showed two main groups, one including SG and LL, and another comprised by MQ, TG, TM, and LU. The latter group is exhibited further divisions, particularly that formed by TM and LU. The first group comprised those wetlands influenced by the Magdalena River, whereas the second group is a mixture of wetlands influenced by the Caribbean Sea and by local streams, being Mallorquín the most dissimilar among the wetlands studied ().
Relationship between physicochemical variables and abundance of M. marcella larvae
The multiple linear regression model indicated that the abundance of M. marcella can be explained based on the parameter’s transparency, conductivity, pH, DO and alkalinity. However, there was variation among the wetlands studied. The coefficients indicated which parameters have the greatest influence on the abundance of M. marcella. In the TM, LL, TG and LU wetlands, conductivity presented the highest coefficient. In the SG wetland, transparency presented the highest coefficient. In MQ, the parameters did not explain the abundance of M. marcella ().
Table 2. Multiple linear regression models’ coefficients and p-values.
Discussion
In this study, the abundance distribution of M. marcella larvae responds to the type of hydric influence in the wetland. Additionally, distribution patterns of M. marcella showed the influence of water quality changes. Abundance and distribution of M. marcella larvae in wetlands of northern Colombia is strongly dependent on water conductivity, transparency and alkalinity. In Colombia, there are at least 1900 Ciénagas below 1000 meters above sea level. These water bodies occupy more than 7800 km2 along large rivers (e.g. Cauca, Magdalena, Atrato and Sinú). Despite their importance and abundance, knowledge on these ecosystems is still limited (Roldán Citation2020). Depth in the assessed Ciénagas varied according to meteorological conditions (rainy and drought seasons), as well as to solar radiation, sedimentation, hydric source input, and modification due to human water use (Roldán and Ramírez Citation2008; Torres and Pinilla Citation2011). The wetland with the greatest depth decrease was MQ, likely due to the high levels of sedimentation, coastal erosion, and human disturbance due to landfill construction (Torres-Bejarano et al. Citation2020). On the other hand, in the LU, LL and SG wetlands there were totally dry sampling points, possibly related to the ENSO (El Niño, Southern Oscillation) of 2014 (DNP Citation2018). Many Odonate species are organisms that are highly tolerant to habitat changes and are widely distributed, even with the effects of ENSO events (Niba and Samways Citation2006).
In terms of physicochemical characteristics, conductivity was very closely related to salinity. This relationship can be explained by different dissolved salts in water bodies (Zheng et al. Citation2018). In continental waters, conductivity values greater than 2000 µS cm−1 indicate high mineralization levels (Roldán and Ramírez Citation2008; Ndong et al. Citation2022). Average conductivity surpassed 2000 µS cm−1 in MQ and TM, both marine-coastal ecosystems where conductivity is strongly related to salinity (NaCl). In addition, surface waters in all studied wetlands can be considered as slightly warm (Lewis Citation1995; García et al. Citation1998; Roldán and Ramírez Citation2008), with temperatures between 24 °C and 32 °C. This behavior may be associated to low depth, high solar radiation, wind patterns, and low water input through the year. All the above are characteristics typical of tropical ecosystems (Roldán and Ramírez Citation2008). Likewise, temperature patterns are related to rain and drought periods in the Atlántico department. Temperatures close to 32 °C represent values not suitable for the survival of aquatic organisms (Roldán and Ramírez Citation2008; Dallas et al. Citation2015). In Ciénagas and coastal lagoons, pH ranges from 5 to 9, depending on its alkalinity and degree of eutrophication (Roldán and Ramírez Citation2008; Verspagen et al. Citation2014). Studied wetlands lied within this range, with values typical of these ecosystems, which tend to have alkaline waters. Reported values of pH are optimal for flora and fauna development (Dirisu et al. Citation2016; Dodds and Whiles Citation2020). On the other side, average dissolved oxygen was > 4 mg L−1 in all assessed wetlands excepting MQ. This value lies within permitted limits for flora and fauna conservation in aquatic ecosystems (Gupta and Gupta Citation2020). Low DO concentrations found in wetland MQ are attributed to a local runoff stream (Arroyo León) coming from the city of Barranquilla. This stream is continuously spilling waste waters, with a high content of suspended solids that decrease oxygen concentrations, leading to hypoxic conditions and continuous decay of organic matter (Mangones and León Citation2014). Hutchins et al. (Citation2020) reported that variations in DO concentration are typical in wetlands due to hydrodynamics, weather conditions, and pollution sources.
The ammonium ion (NH4) is a source of nitrogen for aquatic plants and algae (Lachmann et al. Citation2019). However, elevated ammonium levels may be a problem for water (Rusydi et al. Citation2020). In this study, MQ was the ecosystem with the highest (10 mg L−1) ammonium levels, particularly in field stations closer to the local stream (Arroyo León) runoff. High ammonium levels indicate anthropogenic pollution from sources such as fertilizers, manure, septic tanks, and wastewaters (WHO. Citation2017). In tropical ecosystems such as Ciénagas and coastal lagoons, alkalinity may reach values greater than 100 mg L−1 (Roldán and Ramírez Citation2008). Studied wetlands exhibited alkalinity values above 100 mg L−1. In these ecosystems, alkalinity is associated to bicarbonates and may bring an increase in primary activity (Khan et al. Citation2020).
Regarding M. marcella larvae abundances, this varied between different months. In the months of greatest precipitation (July-November), abundance of larvae was lower, and increased during the dry period months, when precipitation was close or equal to zero (December-April). Several studies have shown that odonates species respond to the greater availability of water and substrate during rainy months by increasing in numbers, whereas in drought months populations decrease (Barbán Citation2015). However, M. marcella larvae showed the opposite pattern in the wetlands studied. This pattern is likely related to the fact that the only available space for oviposition is that offered by the retreating water body, facilitating a larger concentration of larvae (Rache Citation2015). In the same way, the presence of macrophytes at this time of the year provides a habitat with shelter and food available for population development (Barbán Citation2015). Gómez-Tolosa et al. (Citation2015), have pointed out in their studies that M. marcella is an opportunistic species, since they can adapt to different aquatic environments and can establish themselves in environments disturbed by human action or climatological changes in the environment. Nevertheless, the results indicated a spatial difference, marked in wetlands that are influenced by the Magdalena River, followed by wetlands with local runoff LU, TG and TM (it is influenced by the Caribbean Sea, but in times of high rainfall water arrives from the TG wetlands). MQ was excluded due to the characteristics of a coastal lagoon and high anthropic intervention. The few individuals found are possibly associated with the entrance to the wetland through floating macrophytes in the rainy season. In the multiple linear regression model, it was evidenced that the parameters that have the greatest influence on the abundance of M. marcella varied according to the wetland and may be related to the fact that each wetland presents characteristics of the physicochemical variables. However, the distribution depends mainly on conductivity, alkalinity and transparency in the aquatic ecosystems assessed.
The distribution and abundance of aquatic organisms is related to the number of ions dissolved in water. In this case M. marcella displayed low abundance in sites with high conductivity. This pattern is also described by Rache (Citation2015) for other odonates species. The relationship with alkalinity is probably due to the high productivity of these waters, which makes them favorable for feeding, larval development, and shelter (Khan et al. Citation2020). The relationship between the abundance of M. marcella and transparency was present in most of the wetlands. Raebel et al. (Citation2012) found that greater transparency increases the abundance of odonates. This may be due to better water quality (Zhou et al. Citation2021), the relationships may be due to the presence of aquatic macrophytes, which provide resources for odonates and their prey (Brito et al. Citation2021).
Conclusions
The results suggest that abundance distribution of M. marcella larvae responds to the type of hydric influence in the wetland. This species, considered as a generalist, is more common in wetlands influenced by the Magdalena River, followed by those influenced by seasonal runoff. Few individuals were found in those wetlands influenced by the Caribbean Sea. Distribution patterns of M. marcella showed the influence of water quality changes. Abundance and distribution of M. marcella larvae in wetlands of northern Colombia is strongly dependent on water conductivity, alkalinity and transparency. In the case of MQ (wetland influenced by the Caribbean Sea) the results indicate that low abundance is related to high conductivity, high ammonium concentration, and low DO, all due to anthropogenic activity. Therefore, the study of physicochemical variables on odonates larvae populations may be a useful tool for the assessing and monitoring anthropogenic impacts in tropical wetlands. Lastly, the use of macroinvertebrates as bioindicators is important for the scientific community, governments, and policies directed to the improvement of wetland management.
Supplemental Material
Download MS Word (14.5 KB)Acknowledgments
We thank the Colombian Caribbean Biodiversity research group of the Universidad del Atlántico. Also, to all the staff of the National University of Colombia, who contributed to help finish this study.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author, Moreno M. The data are not publicly available due to privacy/ethical restrictions.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Adu BW, Amusan BO, Oke TO. 2019. Assessment of the water quality and Odonata assemblages in three waterbodies in Ilara-Mokin, south-western Nigeria. Int J Odonatol. 22(2):101–114. https://doi.org/10.1080/13887890.2019.1593889
- Barbán L. 2015. Larvas de odonatos asociadas a las raíces de Eichhornia Crassipes (Pontederiaceae), en la represa Halons, Santiago de Cuba, Cuba. Rev Colomb ciencia animal, 7(2), p. 130–138. https://doi.org/10.24188/recia.v7.n2.2015.242
- Beckemeyer RJ. 2009. First record of the dragonfly Miathyria marcella (selys) for kansas (odonata: Anisoptera: Libellulidae). Trans Kansas Acad Sci. 112(1–2):130–132. https://www.jstor.org/stable/40588232
- Brito JS, Michelan TS, Juen L. 2021. Aquatic macrophytes are important substrates for Libellulidae (Odonata) larvae and adults. Limnology. 22(1):139–149. https://doi.org/10.1007/s10201-020-00643-x
- Campbell D. 2020. Wetlands. In: Goldstein MI, DellaSala DA, editors. Encyclopedia of the world’s biomes. Oxford: Elsevier; p. 99–113. https://doi.org/10.1016/B978-0-12-409548-9.11810-X
- Corbet PS. 1999. Dragonflies, behaviour and ecology of Odonata. Colchester: Harley Books.
- Corporación Autónoma Regional Del Atlántico (CRA). 2017. Plan de ordenamiento y manejo de las cuencas hidrográficas. Colombia: Barranquilla.
- Correa-Araneda F, Núñez D, Díaz ME, Gómez-Capponi F, Figueroa R, Acuña J, Boyero L, Esse C. 2021. Comparison of sampling methods for benthic macroinvertebrates in forested wetlands. Ecol Indic. 125:107551. https://doi.org/10.1016/j.ecolind.2021.107551
- Cortés-Guzmán D, Alcocer J, Cummins KW. 2021. Benthic macroinvertebrates of tropical streams: Functional and trophic diversity of the Lacantún River, Mexico. Limnology. 22(3):313–328. https://doi.org/10.1007/s10201-021-00658-y
- Dallas HF, Ross-Gillespie V, Dallas HF. 2015. Sublethal effects of temperature on freshwater organisms, with special reference to aquatic insects: review. Water SA. 41(5):712–726. http://dx.doi.org/10.4314/wsa.v41i5.15
- Dirisu CG, Mafiana MO, Dirisu GB. 2016. Level of pH in drinking water of an oil and gas producing community and perceived biological and health implications. Eur J Basic Appl Sci. 3(3):53–60.
- DNP. 2018. Panorámica Regional. Impactos económicos del fenómeno El Niño del 2015–2016. Bogotá, Colombia.
- Dodds WK, Whiles MR. 2020. Freshwater ecology. Academic Press. London; San Diego, CA; Cambridge, MA; Oxford.
- Esteves FA, Caliman A, Santangelo JM, Guariento RD, Farjalla VF, Bozelli RL. 2008. Neotropical coastal lagoons: an appraisal of their biodiversity, functioning, threats and conservation management. Braz J Biol. 68(4 Suppl):967–981. https://doi.org/10.1590/S1519-69842008000500006
- Fernández del Castillo A, Yebra-Montes C, Verduzco Garibay M, de Anda J, Garcia-Gonzalez A, Gradilla-Hernández MS. 2022. Simple prediction of an ecosystem-specific water quality index and the water quality classification of a highly polluted river through supervised machine learning. Water. 14(8):1235–1224. https://doi.org/10.3390/w14081235
- García M, Sánchez F, Marín R, Guzmán H. 1998. El agua. In: Leyva P, editor. El medio ambiente en Colombia. Colombia: Ideam; p. 115–189.
- Gómez-Tolosa MdL, Mendoza-Cuenca LF, Rioja-Paradela TM, Espinoza-Medinilla EE, Edith Alonso-Eguía-Lis P, Rivera-Velázquez G, Penagos-García FE, Pérez-Munguía RM, Ortega-Salas H, Gómez-Cristiani M, et al. 2015. Odonata (Insecta) de tres cuencas en la costa de Chiapas: Lista de especies y registro nuevo. Rev Mex Biodivers. 86(1):41–47. https://dx.doi.org/10.7550/rmb.48665
- González B. 2007. Los Odonata (Insecta) del Río San Pedro, Parque Nacional Laguna del Tigre (San Andrés, Petén): Taxonomía, Diversidad e Historia Natural [Tesis de pregrado]. Ciudad de Guatemala, Guatemala: Universidad de San Carlos de Guatemala.
- Gupta SK, Gupta IC. 2020. Drinking water quality assessment and management. Jodhpur, India: Scientific Publishers.
- Heckman CW. 2006. Encyclopedia of South American aquatic insects: Odonata–Anisoptera: Illustrated keys to known families, genera, and species in South America. 1. Aufl. ed. Berlín, Alemania: Springer-Verlag.
- Hutchins MG, Harding G, Jarvie HP, Marsh TJ, Bowes MJ, Loewenthal M. 2020. Intense summer floods may induce prolonged increases in benthic respiration rates of more than one year leading to low river dissolved oxygen. J Hydrol X. 8:100056. https://doi.org/10.1016/j.hydroa.2020.100056
- Khan H, Laas A, Marcé R, Obrador B. 2020. Major effects of alkalinity on the relationship between metabolism and dissolved inorganic carbon dynamics in lakes. Ecosystems. 23(8):1566–1580. https://doi.org/10.1007/s10021-020-00488-6
- Kohli M, Letsch H, Greve C, Béthoux O, Deregnaucourt I, Liu S, Zhou X, Donath A, Mayer C, Podsiadlowski L, et al. 2021. Evolutionary history and divergence times of Odonata (dragonflies and damselflies) revealed through transcriptomics. iScience. 24(11):103324. https://doi.org/10.1016/j.isci.2021.103324
- Lachmann SC, Mettler Altmann T, Wacker A, Spijkerman E. ‐ 2019. Nitrate or ammonium: Influences of nitrogen source on the physiology of a green alga. Ecol Evol. 9(3):1070–1082. https://doi.org/10.1002/ece3.4790
- Lewis W. 1995. Tropical lakes: how latitude makes a difference. In: Schiemer F, Boland KT, editors. Perspectives in tropical limnology. Amsterdam: SPB Academic Publishing; p. 43–64.
- López-Díaz JA, Gómez B, González-Soriano E, Gómez-Tolosa M. 2021. Odonata (insecta) como indicador de la calidad ambiental en humedales de montaña neotropicales. Acta Zool Mex. 37(1):1–17. https://doi.org/10.21829/azm.2021.3712379
- Lubanga HL, Manyala JO, Sitati A, Yegon MJ, Masese FO. 2021. Spatial variability in water quality and macroinvertebrate assemblages across a disturbance gradient in the Mara River Basin, Kenya. Ecohydrol Hydrobiol. 21(4):718–730. https://doi.org/10.1016/j.ecohyd.2021.03.001
- Mangones A, León I. 2014. Elementos nutritivos la clorofila a y su relación con las variables físico químicas en la Ciénaga Mallorquín, Colombia. Bol Del Inst Oceanográfico Venezuela. 53(2):127–141.
- Mendes TP, Luiza-Andrade A, Cabette H, Juen L. 2018. How does environmental variation affect the distribution of Dragonfly Larvae (Odonata) in the Amazon-Cerrado Transition Zone in Central Brazil? Neotrop Entomol. 47(1):37–45. https://doi.org/10.1007/s13744-017-0506-2
- Miguel TB, Oliveira-Junior JMB, Ligeiro R, Juen L. 2017. Odonata (insecta) as a tool for the biomonitoring of environmental quality. Ecol Indic. 81(1):555–566. https://doi.org/10.1016/j.ecolind.2017.06.010
- Ndong S, Ngom B, Ndao S, Ly A, Ngom S, Tamba S. 2022. Contribution to the Hydrochemical Study of Groundwater from the Continental Terminal in Saloum. J Water Resour Prot. 14(02):51–71. https://doi.org/10.4236/jwarp.2022.142004
- Neiff JJ. 1999. El régimen de pulsos en ríos y grandes humedales de Sudamérica. In: Malvárez AI, Kandus P, editors. Tópicos sobre grandes humedales sudamericanos. Universidad de Buenos Aires. Oficina Regional de Ciencia y Tecnología de la UNESCO para América Latina y el Caribe, ORCYT-UNESCO. Montevideo, Uruguay.
- Neiss UG, Fleck G, Pessacq P, Tennessen KJ. 2018. Chapter 14.3 - Odonata: Superfamily Libelluloidea. In: Hamada N, Thorp JH, Rogers DC, editors. Thorp and Covich’s freshwater invertebrates. 4th ed. London; San Diego, CA: Academic Press. p. 399–447. https://doi.org/10.1016/B978-0-12-804223-6.00017-2
- Niba AS, Samways MJ. 2006. Remarkable elevational tolerance in an African Odonata larval assemblage. Odonatologica. 35(3):265–280.
- Perron MAC, Pick FR. 2020. Water quality effects on dragonfly and damselfly nymph communities: A comparison of urban and natural ponds. Environ Pollut. 263(Pt B):114472. https://doi.org/10.1016/j.envpol.2020.114472
- Petersen CR, Jovanovic NZ, Grenfell MC, Oberholster PJ, Cheng P. 2018. Responses of aquatic communities to physical and chemical parameters in agriculturally impacted coastal river systems. Hydrobiologia. 813(1):157–175. https://doi.org/10.1007/s10750-018-3518-y
- Poi A, Bruquetas IY. 1989. Efecto de las crecidas sobre las poblaciones de invertebrados que habitan macrófitas emergentes en islas del río Paraná. Rev D'hydrobiologie Trop. 22(1):13–20.
- R Core Team. 2020. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
- Rache L. 2015. Caracterización de hábitat y morfología de algunas especies del género Perithemis (Odonata: Anisoptera) presentes en la cordillera oriental [Tesis de maestría]. Bogotá, Colombia: Facultad de ciencias, Departamento de Biología, Universidad Nacional de Colombia.
- Raebel EM, Merckx T, Feber RE, Riordan P, Macdonald DW, Thompson DJ. 2012. Identifying high-quality pond habitats for Odonata in lowland England: implications for agri-environment schemes. Insect Conserv Divers. 5(6):422–432. https://doi.org/10.1111/j.1752-4598.2011.00178.x
- Roldán G, Ramírez JJ. 2008. Fundamentos de limnología neotropical. Segunda Edición. Medellín, Colombia: Editorial Universidad de Antioquia, Universidad Católica de Oriente y Academia Colombiana de Ciencias-ACCEFYN. p. 440.
- Roldán G. 2020. Revisión histórica de la limnología en Colombia. Rev Acad Colomb Cienc Exactas Físicas Naturales. 44(171):303–328. https://doi.org/10.18257/raccefyn.1056
- Rusydi AF, Onodera SI, Saito M, Hyodo F, Maeda M, Sugianti K, Wibawa S. 2020. Potential sources of ammonium-nitrogen in the coastal groundwater determined from a combined analysis of nitrogen isotope, biological and geological parameters, and land use. Water. 13(1):25. https://doi.org/10.3390/w13010025
- Silva LFR, Castro DMP, Juen L, Callisto M, Hughes RM, Hermes MG. 2021. Functional responses of odonata larvae to human disturbances in neotropical savanna headwater streams. Ecol Indic. 133:108367. https://doi.org/10.1016/j.ecolind.2021.108367
- Torres J, Pinilla G. 2011. Revisión de las características limnológicas de los sistemas acuáticos de la región de la Mojana. Universidad Nacional de Colombia. Bogotá, Colombia.
- Torres-Bejarano FM, Torregroza-Espinosa AC, Martinez-Mera E, Castaneda-Valbuena D, Tejera-Gonzalez MP. 2020. Hydrodynamics and water quality assessment of a coastal lagoon using environmental fluid dynamics code explorer modeling system. Glob J Environ Sci Manage. 6(3):289–308. https://doi.org/10.22034/gjesm.2020.03.02
- Vanacker M, Wezel A, Oertli B, Robin J. 2018. Water quality parameters and tipping points of dragonfly diversity and abundance in fishponds. Limnology. 19(3):321–333. https://doi.org/10.1007/s10201-018-0549-z
- Verspagen JMH, Van de Waal DB, Finke JF, Visser PM, Van Donk E, Huisman J. 2014. Rising CO2 levels will intensify phytoplankton blooms in eutrophic and hypertrophic lakes. PLoS One. 9(8):e104325. https://doi.org/10.1371/journal.pone.0104325
- Von Ellenrieder N, Garrison R. 2007. Dragonflies and Damselflies (insecta: odonata) of the Argentine Yungas: species composition and identification. Sci Rep. 7(1):1–103.
- WHO. 2017. Guidelines for drinking-water quality: fourth edition incorporating the first addendum. Geneva, Switzerland: World Health Organization.
- Zheng Z, Fu Y, Liu K, Xiao R, Wang X, Shi H. 2018. Three-stage vertical distribution of seawater conductivity. Sci Rep. 8(1):9916–9910. https://doi.org/10.1038/s41598-018-27931-y
- Zhou Y, Yu D, Yang Q, Pan S, Gai Y, Cheng W, Liu X, Tang S. 2021. Variations of water transparency and impact factors in the Bohai and Yellow Seas from satellite observations. Remote Sens. 13(3):514–519. https://doi.org/10.3390/rs13030514