Abstract
Understanding habitat use and nursery areas of larval fish is a key component to managing and conserving riverine fishes. Yet, freshwater researchers often focus only on adult fishes, resulting in a limited understanding of the habitat requirements for the early life stages of freshwater fishes. The goal of this study was to quantify the larval fish microhabitat use of three fish families in Twelvemile Creek, a fifth-order tributary of Lake Hartwell (Savannah River basin) in the Piedmont ecoregion of South Carolina, USA. We used handheld dipnets to sample larval fishes along 20 equidistant transects spaced 10 m apart weekly from May to July 2021 along a 200 m stream reach. We also collected microhabitat data at each larval fish capture location. Most captured individuals were in the metalarval stage and were identified to the family level. A partial distance-based redundancy analysis indicated that water velocity contributed to changes in larval fish assemblage structure. Larval fishes occupied a subset of the available habitat that was characterized by low water velocity, non-Podostemum substrate, and shallow habitats close to the shore or bed rock structure. We also detected temporal patterns in larval fish counts, with peak Percidae and Leuciscidae counts in late July and the highest Catostomidae counts in late May–early June. Our results suggest that larval fishes select habitats with low water velocity and shallow habitats close to shore microhabitat characteristics, and that riffle-pool sequences may serve as a nursery habitat for Percidae, Catostomidae and Leuciscidae metalarvae.
Graphical Abstract
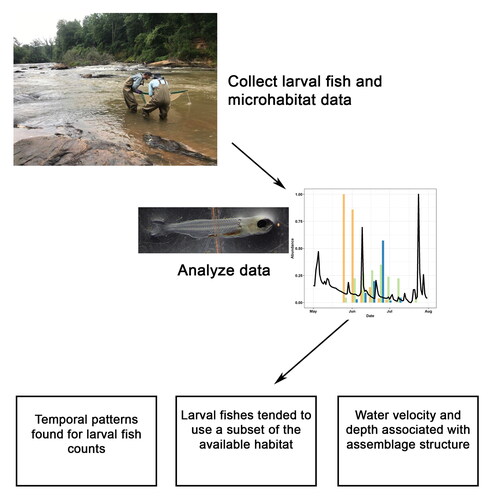
Introduction
The management and conservation of riverine fishes have focused primarily on adult life stages, generally excluding the early life stages of many fishes (Snyder Citation1990; Raborn et al. Citation2001). Yet, the population dynamics, assemblage structure and year-class strength of adult fishes are strongly influenced by events in early life stages (Harvey Citation1987; Houde Citation1987; Sogard Citation1997). Riverine larval fishes often have habitat requirements, diets and responses to environmental stresses distinct from those of adult and juvenile fishes (Floyd et al. Citation1984; Snyder Citation1990; Scheidegger and Bain Citation1995). Studies of larval fishes have focused predominantly on marine, lake, or large riverine systems, with very little larval fish research conducted in smaller streams. Yet, streams contain the highest fish diversity by volume compared to other aquatic habitats (Helfman Citation2007). The lack of studies on larval stream fishes likely stems from the absence of keys for most larval stream fish and the difficulty of identifying individuals to species (Snyder Citation1990). Regardless of the cause, this lack of studies has led to serious gaps in our understanding of the ecology and natural history of critical life stages, preventing managers from adequately protecting fish populations and assemblages.
Fish habitat use varies across life stages and among species (Schlosser Citation1991). For example, larvae of more precocial species (sensu (Balon Citation1981) emerge from the egg well developed and closely resembling adults and often remain near the spawning location or use the same habitat types throughout their development cycle (Simon and Wallus Citation2003). In contrast, larvae of more altricial species emerge poorly developed and can drift long distances or actively migrate to more suitable habitats (Balon Citation1984; Snyder Citation1990; Hoagstrom and Turner Citation2015). Larval fish habitat use can also be affected by the development time required for larvae to become free-swimming and can differ among species. For example, Hypentelium nigricans (Catostomidae) can require 8 days after hatching to become free-swimming (Buynak and Mohr Citation1978), while Leuciscus leuciscus (Cyprinidae) can start actively swimming only hours after hatching (Kupren et al. Citation2016). This development time creates a time lag between spawning and movement to nursery habitats. Larval fishes are often more sensitive to abiotic and biotic stresses than adults (Balon Citation1984; Schlosser Citation1985; Sogard Citation1997). For example, some larval fish have lower larval survival rates during high flows as a result of increased stress, abrasion, or inability to feed (Raborn et al. Citation2001). Given that development time and spawning length and timing varies among species (Floyd et al. Citation1984), understanding the temporal patterns of larval fish abundance can provide insights on when fish populations may be most sensitive to instream flow alterations and other environmental stressors (Marchetti and Moyle Citation2000; Rodger et al. Citation2016).
Habitat requirements and specialization have been reported for some riverine larval fishes (Dewey and Jennings Citation1992; Scheidegger and Bain Citation1995; Childs et al. Citation1998; Pusey et al. Citation2002). For example, leuciscid larvae tend to occupy shallow microhabitats near to shore and cover, indicating these habitats are a preferred nursery habitat for some larval stream fish and important for species survival (Scheidegger and Bain Citation1995; Childs et al. Citation1998). Larval fish habitat requirements and specialization can also vary among taxa and regions (Scheidegger and Bain Citation1995; Childs et al. Citation1998). Therefore, site-specific information on larval fish habitat requirements and specialization is needed to better understand how larval and adult fish assemblages will respond to environmental alteration. Quantifying microhabitat use can also aid in identifying potential nursery habitats that are needed for larval fish survival (Scheidegger and Bain Citation1995; Pusey et al. Citation2002). However, information of the habitat requirements and specialization of riverine larval fish is lacking, and quantitative analysis of microhabitat preference remains limited (Floyd et al. Citation1984; Scheidegger and Bain Citation1995; Pusey et al. Citation2002).
In this study, we conducted single-season, spatially intensive larval fish surveys in a rocky shoal complex of a mid-sized river in the Piedmont ecoregion of South Carolina, USA. Our goal was to quantify microhabitat associations and temporal changes in metalarvae abundance of three freshwater fish families: Catostomidae, Leuciscidae and Percidae. Specifically, our objectives were to: (1) identify the timing of peak larval fish counts; (2) quantify the microhabitat preference of larval fish to identify potential nursery habitats; and (3) quantify the influence of microhabitat characteristics on larval fish assemblage structure. While this work is limited in spatiotemporal extent and taxonomic specificity, it is intended as an initial step toward a better understanding of larval fish microhabitat associations in streams.
Methods
Twelvemile Creek is a tributary to Lake Harwell located in the Piedmont ecoregion of northwestern South Carolina (; Omernik Citation1987). This small river has a mean annual discharge of 6.03 m3/sec and drains roughly 352 km2. Land cover in the Twelvemile Creek watershed is made up largely of forest (57%) and agriculture (23%) with some urban development (19%). Our sample site is located approximately 500 m downstream of a small dam with a hydraulic height of 6 m. Adult fish assemblages at this site have been sampled extensively for a previous study (Marion Citation2014), and ongoing routine sampling provides of a list common species present at this sampling site (Supplemental Table 1). The sampling site included two shoal sequences made up of riffles, runs and pools divided by large bedrock outcroppings (; henceforth riffle-pool sequence), creating a complex habitat network with varying water velocities. The primary substrates in the riffle-pool sequences were bedrock and sand.
Figure 1. A) A map showing the study site on Twelvemile Creek in northwestern South Carolina. Layers were collected from ArcGIS. B) An image of the sampling site taken by Bower, LM.
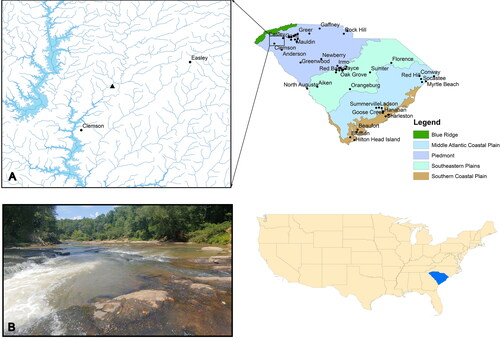
Table 1. The mean, weighted average, standard error (SE), and range of water depth, velocity, and distance weighted by abundance from shore for each fish family in Twelvemile Creek, South Carolina.
We actively collected larval fishes along a 200 m stream reach of Twelvemile Creek below the U.S. Geological Survey gage 02186000. We created transects every 10 m along the 200 m stream reach, sampling for fish in all microhabitats along these transects with velocities less than 0.5 m/sec. We limited our sampling from the highest velocities because larval fish generally cannot persist for long periods in habitats with water velocities more than 3 to 7 times their body lengths per second (Webb Citation1975). Microhabitats were designated as areas of relatively homogeneous depth, current velocity, and substrate composition and were generally less than 2 × 2 m. We sampled the stream reach weekly from late May to mid-July 2020. However, we excluded the mid-July sample from analyses because too few larval fish specimens were collected. Owing to the complex nature of the riffle-pool sequence habitat, traditional collection methods such as drift nets or ichthyoplankton pulls would be ineffective since many of the larval fish were located in small, shallow rocky pools. All active sampling was done during daylight hours with either a 1- × 1-m seine (500-µm mesh) or a 250-µm-mesh dip net. We used the seine in larger, deeper habitats, whereas the dip nets were used in smaller, shallower habitats. Dipnets and seining are effective methods for collecting larval fish in clear shallow streams (Pusey et al. Citation2002; Hanks and Hartman Citation2019). A collection of a microhabitat was considered complete after three samples produced no new fish individuals. All individuals collected from a given microhabitat were placed in a labeled Whirlpak to be later identified and counted in the lab. Once fishes were captured from a given microhabitat, we marked this point with labeled flagging tape attached to 5-ounce pyramid fishing weights to allow for microhabitat data to be collected at the point of fish capture. All individuals were immediately preserved in 10% buffered formalin. All specimens were identified to the family level (Hogue et al. Citation1976; Auer Citation1982; Holland-Bartels et al. Citation1990). Analysis at the family level can reduce the possibility of inaccurate conclusions from misidentifications. No keys exist for many species of stream fishes with most keys focusing on marine, lake, or large river species. Identification of fish larvae to species often requires costly molecular tools such as DNA barcoding (Peoples et al. Citation2017).
To estimate the microhabitat occupied by larval fishes (hereafter, occupied microhabitat data), we recorded the following microhabitat data at each sample point containing larval fish: distance from shore (cm), water velocity (cm/sec), depth (cm), and percentage of substrate in the sample area. All microhabitat variables were measured at each flagged point after sampling for fish to minimize disturbing the sampling points. Current velocity was measured at 0.6 depth to the nearest 0.01 m/s with an electromagnetic flow meter using a Hach FH950. Distance from shore was measured from the point of capture to the closest large bedrock outcroppings or shoreline. We estimated the percentage of substrate composition within a 1 m2 quadrant centered at each sampling point. Substrate types were categorized based on a coarsened Wentworth scale: mud (<1 mm), sand (2–6 mm), fine gravel (7–32 mm), gravel (33–64 mm), cobble (65–128 mm), rock (128–512 mm), bedrock (>512 mm), large woody debris (diameter > 20 cm), small woody debris (diameter < 20 cm) and small aquatic vegetation (Podostemum sp.). Microhabitat data were collected during the first three samples. In addition, we collected the same microhabitat variables every 1 m along each transect after the collection of larval fish specimens in a grid design, providing a representative sample of available microhabitat space within the sampling site (hereafter, available microhabitat data). Points falling on dry bed rock were not included in the analysis of the available microhabitat data.
We used a principal component analysis (PCA) to determine if larval fish occupy a subset of the microhabitat available to the larval fish. The PCA was run using a combined dataset of available microhabitat data from the grid design and occupied microhabitat data. To evaluate associations between larval fish assemblage structural and microhabitat environmental variables, we used a partial distance-based redundancy analysis (pRDA) based on Bray–Curtis dissimilarities on log(x)+1 transformed larval fish count within each microhabitat using the occupied microhabitat data. A partial RDA explores the linear effects of the microhabitat variables on the assemblage composition while accounting for the effects of a covariable. We used the number of days since the first sampling event as the covariable to account for temporal autocorrelation. The pRDA was performed using the function ‘rda’ in the VEGAN package (Oksanen et al. Citation2019) in R version 4.0.0 (R Core Team Citation2020). We tested the statistical significance of the environmental variables on assemblage structure using a permutated analysis of variance of 999 permutations (Legendre et al. Citation2011).
Results
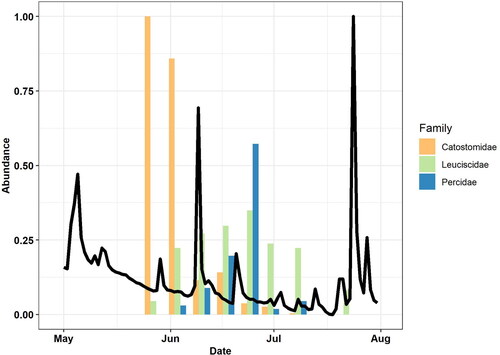
We collected 893 Catostomidae, 784 Leuciscidae and 393 Percidae individuals across all seven sampling events for a total of 2,077 individuals. The highest number of Leuciscidae and Percidae larvae collected occurred in late June, whereas the highest counts of Catostomidae were found in late May to early June (). The number of Percidae and Catostomidae larvae peaked and decreased rapidly, while Leuciscidae counts gradually increased and decreased (). Catostomidae larval occupied microhabitats with higher water velocities than Leuciscidae (). Percidae larvae tended to be collected in greater water depth, whereas Leuciscidae larvae tended to be found in microhabitats with the lowest depth, velocity, and greatest distance from shore ().
Figure 2. Bar graph of larval fish abundance by family in Twelvemile Creek, South Carolina for each sampling event scaled between 0 and 1. Line shows daily mean discharge scaled between 0 and 1.
Microhabitat patterns
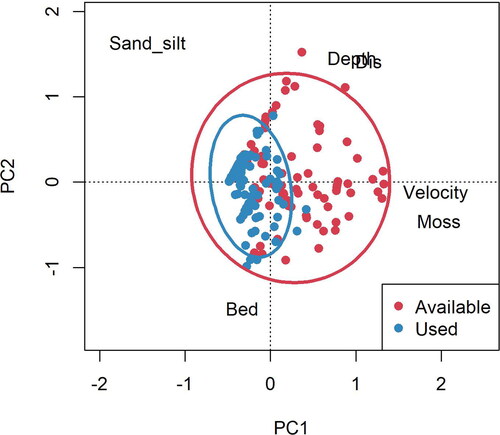
The PCA visualizing microhabitats occupied by larval fishes and overall available microhabitat indicated that larval fishes tended to occupy a subset of the available habitat space (). The first two PC axes accounted for 37% and 28% of the variance in the microhabitat data, respectively. Water velocity and Podostemum had high positive loadings on PC1; PC2 was negatively associated with bedrock substrate and positively related to sandy substrate, depth, and distance from shore (). We found used habitat space to be characterized by low water velocity, non-Podostemum, shallower habitats, and shorter distance to shore ().
Figure 3. Principal component analysis of all microhabitat variables for larval fish in Twelvemile Creek, South Carolina colored by used habitat and available habitat with 95% confidence ellipses. Microhabitat variables: distance from shore (Dis), water depth (Depth), % bedrock substrate (Bed), % Podostemum (Moss), % sand-silt substrate (Sand-silt), and water velocity (Velocity).
The first two axes of the distance-based RDA explained 87% of the variation in larval fish assemblage structure. Water velocity, depth, sand-silt and distance were positively correlated with RDA1. Depth, sand-silt, and Podostemum had positive loadings on RDA2 (; ). We found negative loading scores for Leuciscidae on RDA1, indicating counts of this family were negatively related to depth, distance to shore, and velocity (; ). The RDA2 axis was negatively associated with Catostomidae, suggesting Catostomidae counts were positively related to distance to shore. The microhabitat variables explained a significant proportion of the larvae count variation based on the full RDA model at the microhabitat scale (Adjusted R2 = 0.12, p = 0.05). Tests of the individual microhabitat variables showed a significant effect of water velocity on larval fish assemblage structure (F = 2.760, df= 1, p = 0.033).
Figure 4. Distance-based redundancy analysis (RDA) for larval fish abundance in Twelvemile Creek, South Carolina (log +1) using Bray–Curtis dissimilarity and constrained by microhabitat variables: distance from shore (Dis), water depth (Depth), % bedrock substrate (Bed), % Podostemum (Moss), % sand-silt substrate (Sand-silt), and water velocity (Velocity).
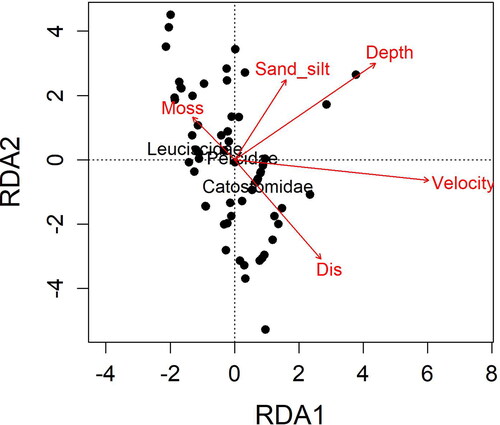
Table 2. The loading scores, eigenvalues, and variance explained for the first two axes of the distance-based redundancy analysis (RDA) of larval fish assemblage structural and microhabitat environmental variables in Twelvemile Creek, South Carolina. Variables are given as: distance from shore (Dis), water depth (Depth), % Podostemum (Moss), % sand-silt substrate (Sand-silt), water velocity (Velocity), proportion of variance explained (Proportion), and cumulative variance explained.
Discussion
Our study suggests distinct habitat use by metalarvae of three freshwater fish families, and provides insights on microhabitat use in a riffle-pool sequence of a southeastern USA Piedmont stream. Larval fishes in our study tended to occupy a subset of the available habitat characterized. These microhabitats were characterized by lower water velocity, absence of Podostemum sp. vegetation and, to a lesser extent, shallower habitats closer to shore. Depth and water velocity played a role in determining larval fish assemblage structure at the microhabitat scale, indicating these variables helped determine the habitats larval fish can occupy. We also observed temporal changes in the number of larvae at a weekly time step for all three families, with peaks of larvae counts in late May for Catostomidae and in late June for Leuciscidae and Percidae.
Temporal changes in larval fish counts varied among families in our study. For example, counts of Leuciscidae and Percidae larvae counts peaked in late June, whereas the number of Catostomidae larvae was highest in late May and early June. However, the abundance peak of Catostomidae may have occurred earlier before we started sampling, as the catostomid species in this assemblage spawn earlier than the percids and leuciscids. Larvae abundance changed at a weekly time step, suggesting sampling larval fish at a monthly time step may miss substantial variation in abundance patterns. The abundance of leuciscid larvae increased and decreased gradually across our study period, whereas the larval abundance of Percidae peaked and decreased rapidly. This rapid peak of percid abundance suggests a single large spawning event in line with an equilibrium life history strategy rather than the serial spawning of an opportunistic species (Winemiller and Rose Citation1992). Turner et al. (Citation1994) also noted an abrupt reduction in percid abundance after abundance peak. Abundance peaks observed in this study generally correspond with spawning times for species found in Twelvemile Creek after accounting for hatching time. Percina nigrofasciata and Etheostoma inscriptum, the dominate darter species in Twelvemile Creek, spawning is reported from March to June and May to June, respectively (Mathur Citation1973; Rohde et al. Citation2009). For two species in the genus Etheostoma (E. bellum and E. camurum), incubation time was reported to be 7–9 days. Our results generally match the larvae count peak seen in Twelvemile Creek after accounting for hatching time. Spawning of the dominant leuciscid species, Hybopsis rubrifrons, Nocomis leptocephalus, Notropis hudsonius and Notropis lutipinnis (Marion Citation2014), occurs from April to June (Rohde et al. Citation2009; Kim and Kanno Citation2020). Hatching of leuciscid eggs, such as Nocomis micropogon, is reported to occur 5–6 days after fertilization and measured 5.7–6.1 mm after hatching (Cooper Citation1980). We found leuciscid individuals within this size range in all our samples from early June to early July, indicating multiple spawning events of a single species or multiple spawning events of multiple species during this time period. The temporal patterns observed for leuciscid and percid larvae match Spawning of the most prevalent sucker species in Twelvemile Creek (Marion Citation2014), H. nigricans (Catostomidae), occurs primarily in April and May (Jenkins and Burkhead Citation1994). Eggs take about 10 days to hatch and H. nigricans larvae require about 8 days to become free-swimming (Buynak and Mohr Citation1978). Other catostomid species found in Twelvemile Creek, such as Minytrema melanops, Moxostoma collapsum, and Moxostoma rupiscartes (Marion Citation2014), are reported to spawn from March and May (Jenkins and Burkhead Citation1994). Given the delay in hatching and growth, the peak larvae counts found in our study appear to match reported spawning times of H. nigricans and most of the catostomid species.
Based on the PCA of the combined microhabitat datasets, larval fish families occupied a subset of the available microhabitat space in our study area. The larvae selected shallower habitats with lower water velocity and nearer to stream margins than was available. Similar to our results, Pusey et al. (Citation2002) reported a strong preference for microhabitats associated with low water velocity, shallow to moderate depth, and cover. However, we observed no large macrophytes or woody debris within our study site. Water velocity is thought to be one of the main environmental filters for larval fishes (Webb Citation1975; Turner et al. Citation1994; Scheidegger and Bain Citation1995), restricting individuals from occupying areas with high velocities. A majority of our specimens were collected along the margins of stream or near a bedrock outcropping, generally less than 2 m from any given stream bank or outcrop. Other studies also found greater larval fish densities closer to the margins of streams, where larvae abundance was up to seven times greater near the margins than in the main channel (Raborn et al. Citation2001). This may be a result of larval fish avoiding high water velocities that occurred at high frequency away from stream margins in our riffle-pool sequence. In our study, greater distances from shore were generally associated with runs or riffles with high water velocities where larval fish could not persist (Webb Citation1975). Larval fish tended to occupy shallower microhabitats than were available in our study. In streams and rivers, larval fish are often associated with moderate to shallow depths (Scheidegger and Bain Citation1995; Pusey et al. Citation2002). Interestingly, we did occasionally collect high numbers of larval fish in deep (>1 m), non-flowing pools as well as deep areas between two runs or riffles where the water swirled or formed pools. The riffle-pool sequence site in our study was made up of a series of small pools, riffles, runs divided by large bedrock outcroppings. These habitats provide high densities of shallow, low velocity microhabitats with greater marginal habitat area, suggesting riffle-pool sequence habitats may be well suited as nurseries for Percidae, Catostomidae and Leuciscidae metalarvae. However, further study is needed to determine the use of riffle-pool sequence as a nursery habitat by larval fish compared to other sections of a river. These riffle-pool sequences may also shelter larval fish from predators. Predation is known to influence larval fish microhabitat use (Schlosser Citation1987; Harvey Citation1991a; Harvey Citation1991b). Our study did not account for the presence of predators that could potentially alter larval fish habitat use.
The distance-based pRDA indicated that microhabitat variables influenced larval fish assemblage structure in our study site. Water velocity was the most important microhabitat variable affecting the assemblage structure of larval fish families in our study. Water velocity can strongly influence larval fishes’ ability to persist in a given habitat. For example, Percidae larvae were reported as being vulnerable to transport downstream at water velocities greater than 3 cm/sec (Houde Citation1969). Larvae mortality may result from increased stress, abrasion, predation on disoriented larvae, and inability to feed in higher flow habitats (Raborn et al. Citation2001). We also observed Leuciscidae larvae associated with lower water velocities. Scheidegger and Bain (Citation1995) reported similar velocity preferences for leuciscid and catostomid larvae (mean = 2 to 2.6 cm/sec and 1.6 to 3.2, respectively). Catostomidae larvae tended to occupy microhabitats with higher water velocity than previously reported by Scheidegger and Bain (Citation1995). However, a previous study showed an average prolonged swimming speed of 10.6 cm/sec for 16 mm catostomid larvae (Moxostoma robustum), which is much greater than the mean velocity found for Catostomidae in our study (Ruetz and Jennings Citation2000).
Many species of larval fishes are reported to select areas of moderate to shallow depths (Scheidegger and Bain Citation1995; Raborn et al. Citation2001; Pusey et al. Citation2002). We found that Leuciscidae and Catostomidae tended to prefer shallower microhabitats, similar to the findings of Scheidegger and Bain (Citation1995). However, these trends were not significant. Scheidegger and Bain (Citation1995) observed leuciscid larvae occupying deeper microhabitats than found in our study. This difference in depth preference may be due to our study occurring within riffle-pool sequences where only a few deep, low water velocity habitats occur. We observed Percidae larvae occupying deeper microhabitats relative to Leuciscidae or Catostomidae. Additionally, Percidae larvae tended to be found in deeper areas compared to the other two families. Similar results were found for Percidae larvae in the Tallapoosa and Cahaba Rivers, where larvae tended to select deeper microhabitats than other families (Scheidegger and Bain Citation1995).
It is important to acknowledge several limitations of this study. We collected larval fishes only within riffle-pool sequences at a single stream reach, preventing conclusions about habitat use and temporal patterns of larval count across different streams or habitat types within a stream. Habitat preferences of larval fish can vary across sites (Scheidegger and Bain Citation1995; Pusey et al. Citation2002), yet little is known about how much habitat use varies among streams for different taxa or regions, particularly for the species in our study. Additional studies are needed to explore the relative role of intraspecific variation in habitat preference across and within sites. Ontogenetic shifts in microhabitat use are also common among freshwater fishes (Pusey et al. Citation2002), adding to the need for additional studies on microhabitat use. Additionally, our samples were only collected during the day, potentially missing important diel patterns. The diversity and number of larvae captured have been shown to increase at night as drift occurs more often after dark (Reeves and Galat Citation2010). Our study highlights the need for future studies to explore intraspecific variation in habitat use across streams, habitat types within a stream, and regions to determine the intraspecific variability in habitat use. Our study started in late Spring, missing the full spawning season of many species in Twelvemile Creek. For example, our results suggest that peak spawning of Catostomidae likely occurred earlier in the season. Additionally, sampling for a single season may have missed potential temporal variation in spawning times and habitat use. To fully capture the temporal patterns of larval fish abundance and habitat use, a study would need to be conducted across the entire spawning season of all fishes in the creek and across multiple years.
The use of higher taxonomic levels restricts our ability to draw specific conclusions about larval fish ecology, preventing collections of more precise data needed for robust conservation and management efforts. This coarse taxonomic resolution likely masks patterns of interspecific variation in spawning phenology and larval habitat use. The lack of comprehensive keys for larval stream fish prevents the identification of specimens to the species level and restricts research on early life stages of stream fishes. The management and conservation of stream fishes would greatly benefit from the creation of comprehensive larval fish keys. Until then, species-level information on larval stream fishes can only be attained through molecular approaches such as DNA metabarcoding (Buckwalter et al. Citation2019); these tools have proven to be highly effective, but can be costly, especially for small-scale natural history studies. Furthermore, we did not separate specimens into development stage due to limited numbers of protolarvae for some families, likely missing potential habitat use differences by among larval development stages. For example, protolarvae of many species may occupy interstitial spaces of coarse substrate before developing to swim to favorable habitats, while other species drift as ichthyoplankton for days or weeks before gaining the ability to select for favorable habitats (Snyder Citation1990; Hoagstrom and Turner Citation2015). Accordingly, the results presented in this study cannot be applied across all larval fish stages.
Our results provide insights on the microhabitat preference for three families of larval fish and the temporal changes in their counts within a riffle-pool sequence of a southeastern USA Piedmont stream. Evidence from this and other studies show that larval fish occupy microhabitats with specific environmental characteristics (Scheidegger and Bain Citation1995; Childs et al. Citation1998; Falke et al. Citation2010), such as shallow habitats with low water velocity close to stream margins, and microhabitat use of larval fish can differ among taxa (Scheidegger and Bain Citation1995; Childs et al. Citation1998). The high proportion of marginal microhabitats with low water velocity and shallow depths in riffle-pool sequences suggests that these habitats may function as nursery areas for Percidae, Catostomidae, and Leuciscidae larvae. However, additional studies are needed to determine the suitability of riffle-pool sequences as nursery habitats relative to other habitats within a river. Anthropogenic alterations of the flow regime, such as dams, can increase discharge and hydropeaking, resulting in reductions of suitable nursery habitat and reduced larval fish density (Scheidegger and Bain Citation1995; Erickson et al. Citation2021). These impacts are likely greater when fish larvae are small and their swimming ability is poor (Ruetz and Jennings Citation2000). For example, flooding events were found to be fatal to larval fish under 10 mm (Harvey Citation1987). However, future studies are needed to determine the impact of hydrologic alteration on larval fish habitat and assemblage structure. The statistical association between microhabitat variables and larval fish counts in ours and previous studies indicate active orientation of the larvae to suitable microhabitats rather than pure random drift of larvae (Scheidegger and Bain Citation1995; Falke et al. Citation2010), particularly riffle-current species such as those in Percidae and Leuciscidae (Floyd et al. Citation1984). Our study suggests conservation and management efforts can benefit from focusing on low water velocity habitat close to stream margins.
Supplemental Material
Download MS Excel (13.2 KB)Acknowledgements
The authors thank Andrew Peel and Colby Denison for field work assistance, Ty’Celia Young, Daniel St. Amand, Anna Pereda, Charles Jackson, and Brianna Taylor for help identification and measurement of specimens. This work represents technical contribution number 7100 of the Clemson experiment station. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. This study was performed under the auspices of Clemson University protocol # 2020-006.
Data availability statement
The data that support the findings of this study will be openly available as a supplemental document once paper is accepted.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Luke M. Bower
Luke M. Bower is a freshwater ecologist broadly interested in community assembly and the drivers of biodiversity spatial patterns. His research focuses on understanding how changes in environmental gradients influence assemblage and functional trait structure of aquatic systems across multiple scales, and applying community, evolutionary, and functional ecology concepts to inform the management and conservation of freshwater fishes. Another research focus is to understand the roles of stream flow in driving the ecology and evolution of fishes across landscape gradients to better inform state flow standards.
Brandon K. Peoples
Brandon K. Peoples’ research is broadly focused on cross-scale approaches to freshwater ecology and conservation. His research ranges from applied issues in fisheries ecology and conservation to using fishes as a model system for examining general models of ecology. He uses observational field studies, experiments, and modeling of large datasets to investigate key questions regarding reproductive ecology, invasive species, population characteristics, and movement.
References
- Auer NA. 1982. Identification of larval fishes of the Great Lakes basin with emphasis on the Lake Michigan drainage. Great Lakes Fishery Commission.
- Balon EK. 1981. Saltatory processes and altricial to precocial forms in the ontogeny of fishes. Am Zool. 21(2):573–596.
- Balon EK. 1984. Reflections on some decisive events in the early life of fishes. Trans Am Fish Soc [Internet]. 113(2):178–185.
- Buckwalter J, Angermeier PL, Argentina J, Wolf S, Floyd S, Hallerman EM. 2019. Drift of larval darters (Family Percidae) in the upper Roanoke River basin, USA, characterized using phenotypic and DNA barcoding markers. Fishes. 4(4):59.
- Buynak GL, Mohr HW. 1978. Larval development of the Northern Hog Sucker (Hypentelium nigricans), from the Susquehanna River. Trans Am Fish Soc. 107(4):595–599.
- Childs MR, Clarkson RW, Robinson AT. 1998. Resource use by larval and early juvenile native fishes in the Little Colorado River, Grand Canyon, Arizona. Trans Am Fish Soc. 127(4):620–629.
- Cooper JE. 1980. Egg, larval and juvenile development of longnose dace, Rhinichthys cataractae, and river chub, Nocomis micropogon, with notes on their hybridization. Copeia. 1980(3):469.
- Dewey MR, Jennings CA. 1992. Habitat use by larval fishes in a backwater lake of the upper Mississippi River. J Freshw Ecol. 7(4):363–372.
- Erickson KA, Sakaris PC, Conner H, Irwin ER. 2021. Hydrologic effects on growth and hatching success of age-0 Channel Catfish in the Tallapoosa River Basin: implications for management in regulated systems. North Am J Fish Manag. 41(S1):S118–S132.
- Falke JA, Fausch KD, Bestgen KR, Bailey LL. 2010. Spawning phenology and habitat use in a Great Plains, USA, stream fish assemblage: an occupancy estimation approach. In: Kraft C, editor. Can J Fish Aquat Sci. 67(12):1942–1956.
- Floyd KB, Hoyt RD, Timbrook S. 1984. Chronology of appearance and habitat partitioning by stream larval fishes. Trans Am Fish Soc. 113(2):217–223.
- Hanks RD, Hartman KJ. 2019. Evaluation of the influences of dam release types, land use, and habitat affecting abundance, richness, diversity, and community structure of larval and juvenile fish. Can J Fish Aquat Sci. 76(8):1388–1397.
- Harvey BC. 1987. Susceptibility of young-of-the-year fishes to downstream displacement by flooding. Trans Am Fish Soc. 116(6):851–855.
- Harvey BC. 1991a. Interactions among stream fishes: predator-induced habitat shifts and larval survival. Oecologia [Internet]. 87(1):29–36.
- Harvey BC. 1991b. Interaction of abiotic and biotic factors influences larval fish survival in an Oklahoma Stream. Can J Fish Aquat Sci. 48(8):1476–1480.
- Helfman GS. 2007. Fish conservation: a guide to understanding and restoring global aquatic biodiversity and fishery resources. Island Press.
- Hoagstrom CW, Turner TF. 2015. Recruitment ecology of pelagic-broadcast spawning minnows: paradigms from the ocean advance science and conservation of an imperilled freshwater fauna. Fish Fish. 16(2):282–299.
- Hogue JJ, Wallus R, Kay LK. 1976. Preliminary guide to the identification of larval fishes in the Tennessee River. Tennessee Valley Authority, Division of Forestry, Fisheries, and Wildlife∼….
- Holland-Bartels L. E, Littlejohn SK, Huston ML. 1990. A guide to the larval fishes of the upper Mississippi River. Book. University of Minnesota: U.S. Fish and Wildlife Service National Fisheries Research Center.
- Houde ED. 1969. Sustained swimming ability of larvae of walleye (Stizostedion vitreum vitreum) and yellow perch (Perca flavescens). J Fish Res Bd Can. 26(6):1647–1659.
- Houde ED. 1987. Fish early life dynamics and recruitment variability. Am Fish Soc Symp. 2:17–29
- Jenkins RE, Burkhead NM. 1994. Freshwater fishes of Virginia. Bethesda, Md.: Am Fish Soc. 1079.
- Kim S, Kanno Y. 2020. Spawning periodicity and synchrony of bluehead chub (Nocomis leptocephalus) and a nest associate, yellowfin shiner (Notropis lutipinnis), across local streams. Ecol Freshw Fish. 29(2):299–310.
- Kupren K, Rams I, Żarski D, Kucharczyk D. 2016. Early development and allometric growth patterns of rheophilic cyprinid common dace Leuciscus leuciscus (Cyprinidae: Leuciscinae). Ichthyol Res. 63(3):382–390.
- Legendre P, Oksanen J, ter Braak CJF. 2011. Testing the significance of canonical axes in redundancy analysis. Methods Ecol Evol. 2(3):269–277.
- Marchetti MP, Moyle PB. 2000. Spatial and temporal ecology of native and introduced fish larvae in lower Putah Creek, California. Environ Biol Fishes. 58(1):75–87.
- Marion CA. 2014. South Atlantic stream fish assemblages: multi-scale structuring factors, trait associations and channelization, and responses to dam removal. Clemson University.
- Mathur D. 1973. The University of Notre Dame Some Aspects of Life History of the Blackbanded Darter, Percina nigrofasciata (Agassiz), in Halawakee Creek, Alabama Author (s): Dilip Mathur Source: The American Midland Naturalist, Vol. 89, No. 2 (Apr., 1973). Am Midl Nat. 89(2):381–393.
- Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H. 2019. vegan: community ecology package. R Package version.
- Omernik JM. 1987. Ecoregions of the conterminous United States. Ann Assoc Am Geogr. 77(1):118–125.
- Peoples BK, Cooper P, Frimpong EA, Hallerman EM. 2017. DNA barcoding elucidates cyprinid reproductive interactions in a Southwest Virginia Stream. Trans Am Fish Soc [Internet]. 146(1):84–91.
- Pusey BJ, Arthington AH, Close PG, Bird JR. 2002. Larval fishes in rainforest streams: recruitment and microhabitat use. Proc R Soc Queensl. 110:27–46. (February 2017)
- R Core Team. 2020. R: a Language and Environment for Statistical Computing. https://www.r-project.org/.
- Raborn SW, Will T, Miranda LE. 2001. An assessment of larval fish density and assemblage structure within mid-channel and backwater habitats in a mississippi stream. J Freshw Ecol. 16(3):395–401.
- Reeves KS, Galat DL. 2010. Do larval fishes exhibit diel drift patterns in a large, turbid river? J Appl Ichthyol. 26(4):571–577.
- Rodger AW, Mayes KB, Winemiller KO. 2016. Larval fish abundance in relation to environmental variables in two Texas Gulf Coast rivers. J Freshw Ecol. 31(4):625–640.
- Rohde FC, Arndt RG, Foltz JW, Quattro JM. 2009. Freshwater fishes of South Carolina. Columbia: University of South Carolina Press.
- Ruetz CR, Jennings CA. 2000. Swimming performance of larval robust redhorse Moxostoma robustum and low-velocity habitat modeling in the Oconee River, Georgia. Trans Am Fish Soc. 129(2):398–407.
- Scheidegger KJ, Bain MB. 1995. Larval fish distribution and microhabitat use in free-flowing and regulated rivers. Copeia [Internet]. 1995(1):125.
- Schlosser IJ. 1985. Flow regime, juvenile abundance, and the assemblage structure of stream fishes. Ecology [Internet]. 66(5):1484–1490.
- Schlosser IJ. 1987. The role of predation in age- and size-related habitat use by stream fishes. Ecology. 68(3):651–659.
- Schlosser IJ. 1991. Stream fish ecology: a landscape perspective. Bioscience. 41(10):704–712.
- Simon TP, Wallus R. 2003. Reproductive Biology and Early Life History of Fishes in the Ohio River Drainage: Ictaluridae-Catfish and Madtoms, Vol. 3. CRC Press.
- Snyder DE. 1990. Fish larvae - Ecologically distinct organisms. United States Fish Wildl Serv Biol Rep. 90(5):20–23.
- Sogard SM. 1997. Size-selective mortality in the juvenile stage of teleost fishes: a review. Bull Mar Sci. 60(3):1129–1157.
- Turner TF, Trexler JC, Miller GL, Toyer KE. 1994. Temporal and spatial dynamics of larval and juvenile fish abundance in a temperate floodplain river. Copeia [Internet]. 1994(1):174.
- Webb PW. 1975. Hydrodynamics and energetics of fish propulsion. Bull Fish Res Board Can. 190:1–159.
- Winemiller KO, Rose KA. 1992. Patterns of life-history diversification in North American Fishes: implications for population regulation. Can J Fish Aquat Sci. 49(10):2196–2218.
