Abstract
Understanding potential limiting factors affecting population growth of the endangered pallid sturgeon, Scaphirhynchus albus, is important in the upper (UMOR) and lower Missouri River (LMOR) basins. The UMOR is upstream of several reservoirs and generally has more natural habitat features, whereas the LMOR is downstream of these reservoirs and has been channelized to support navigation. In both sections, pallid sturgeon recruitment to age-1 is a concern, but hypothesized for different reasons. One hypothesis in the LMOR centers on food limitation for age-0 fish, which is not considered an issue in the UMOR, but evaluating this hypothesis is challenging given the rarity of age-0 pallid sturgeon. As a result, the related, more abundant shovelnose sturgeon (S. platorhynchus) has been considered as a potential surrogate to assess food-related hypotheses. Thus, the purpose of our study was to compare diets of age-0 sturgeon captured in 2016 from three reaches in the LMOR (Copeland, Langdon, and Lexington) with individuals captured from a reach in the UMOR (Williston) during the same year to provide additional context regarding potential food limitation in the LMOR. Consumption percentage (prey weight in the gut as a percentage of body weight) was similar among all reaches, but diet composition was different for the most downstream reach in the LMOR. Age-0 sturgeon in the UMOR reach as well as the two upstream LMOR reaches primarily consumed Diptera larvae along with Ephemeroptera nymphs. In contrast, age-0 sturgeon in the most downstream LMOR reach (Lexington) almost exclusively consumed Diptera larvae. These results may provide information on relative differences in prey availability between Lexington and the other upstream reaches but the similarity in consumption percentage values across all reaches provide further evidence that age-0 sturgeon are not food limited in the LMOR.
KEY POLICY HIGHLIGHTS
Habitat alteration is a factor affecting fishes, including Scaphirhynchus sturgeon, in the Missouri River.
The upper portion of this system is fragmented by several dams, yet contains relatively natural riverine habitat features between the reservoirs; the lower portion is heavily altered for barge navigation and bank stabilization.
Feeding by age-0 sturgeon in the upper portion has generally been considered adequate for survival, whereas lack of prey has been suggested as limiting survival in the lower portion.
We studied age-0 sturgeon prey use in the upper and lower portions of the Missouri River during 2016 and found minimal differences.
Introduction
Understanding potential factors limiting population growth of the endangered pallid sturgeon (Scaphirhynchus albus) in the United States is a high priority in both the upper (UMOR) and lower Missouri River (LMOR) basins. These basins are separated by a series of dams and reservoirs from Fort Peck Dam in Fort Peck, Montana to Gavins Point Dam in Yankton, South Dakota. Although major alterations have occurred in both systems, the UMOR is primarily affected by a series of six mainstem reservoirs downstream of free-flowing sections of the Missouri River and its tributaries whereas the LMOR has undergone extensive channelization and bank stabilization to maintain a channel for commercial navigation. As a result, the riverine areas above the reservoirs in the UMOR tend to have more natural habitat features (e.g. a wide range of depths and velocities with abundant islands, sandbars and secondary channels; Braaten et al. Citation2012a) compared to the highly engineered LMOR with relatively fast-flowing, deep water, and reduced channel complexity (NRC Citation2011). The reduction of food-producing and foraging habitats due to river alteration (e.g. channelization, bank stabilization) in the LMOR has been hypothesized as a factor limiting recruitment of pallid sturgeon to age 1 (Jacobson et al. Citation2016; Gemeinhardt et al. Citation2019). However, the rarity of wild age-0 pallid sturgeon has made it difficult to evaluate this hypothesis directly. For example, from 2014 to 2018, only seven wild age-0 pallid sturgeon were documented in the LMOR out of >10,000 age-0 sturgeons captured (Gosch et al. Citation2018, Citation2019). In contrast, the shovelnose sturgeon (Scaphirhynchus platorynchus) is an abundant and sympatric congener that has been considered a potential surrogate for certain aspects of pallid sturgeon early life history, including prey consumption (Gosch et al. Citation2016, Citation2021; Civiello et al. Citation2018; González et al. Citation2021).
Information to date indicates that pallid sturgeon and shovelnose sturgeon in the LMOR generally consume similar prey (Gosch et al. Citation2018, Citation2019) in similar amounts (Gosch et al. Citation2019), indicating that data on age-0 shovelnose sturgeon can provide useful information relative to foraging by pallid sturgeon. In the LMOR, much has been learned about age-0 sturgeon habitat use (Ridenour et al. Citation2011; Gosch et al. Citation2015, Citation2017), diet, and condition (Gosch et al. Citation2016, Citation2021; Civiello et al. Citation2018; González et al. Citation2021), indicating that food is not a limiting factor (Civiello et al. Citation2018). However, these studies have all been conducted in the LMOR without comparison to the UMOR where food limitation is not hypothesized as a concern. For example, Braaten et al. (Citation2007) suggested their UMOR study areas “had suitable food supplies” given the rarity of empty stomachs observed in age-0 shovelnose sturgeon. Stockings of pallid sturgeon free embryos have also demonstrated that the UMOR can support a successful transition from endogenous to exogenous feeding and subsequent recruitment to age-1 (Braaten et al. Citation2012b), further supporting that food is not limited in the UMOR. Thus, the purpose of this study is to compare the composition and quantity of prey items in the diet of age-0 sturgeon between fish captured in the UMOR and LMOR as a more direct test of the food limitation hypothesis. Because age-0 sturgeon in the UMOR are presumed to be able to obtain sufficient prey resources for survival and recruitment, similar prey-use between these two different basins of the Missouri River would infer that prey resources are not limiting in the LMOR. Given that inter-annual variation may be a potential factor affecting prey use and body condition of age-0 sturgeon (Civiello et al. Citation2018; González et al. Citation2021), we used data from a common year (2016) when age-0 individuals from both systems were available for analysis.
Materials and methods
Sampling
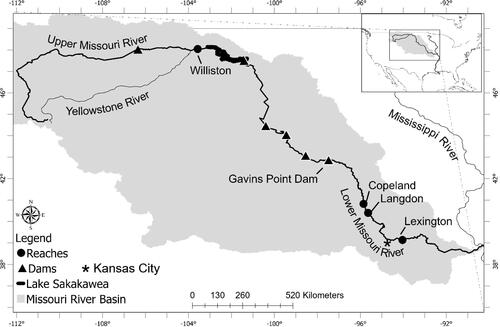
Sampling for age-0 (15–120 mm) sturgeon occurred from May to October 2016 with a target goal of approximately 20 individuals in each of 6 length categories (≤ 20 mm, 21-40 mm, 41-60 mm, 61-80 mm, 81-100 mm, and 101-120 mm). In the UMOR, sampling was conducted with a benthic beam trawl (3.2 mm mesh; 2.0 m wide; 0.5 m high; 5.5 m length; Braaten et al. Citation2007) in the Williston reach (). In the LMOR, sampling was conducted with a POT02 bow-mounted push trawl (4 mm mesh; 2.4 m wide with 0.76 × 0.38 m otter doors) for shallow-water (< 2 m depth) and a OT04 trawl (4 mm mesh; 4.9 m wide with 0.91 × 0.38 m otter doors) for deeper water (> 2 m depth) by the Nebraska Game and Parks Commission and the U.S. Army Corps of Engineers (USACE) at three reaches: Copeland, Langdon, and Lexington (). These gears are used for standard monitoring and research efforts of these life stages in their respective portions of the Missouri River reaches. Following capture, all Scaphirhynchus sturgeon were measured to the nearest millimeter for fork length (FL), or total length (TL) when the caudal fin did not have a well-defined fork, and preserved in ≥95% ethanol for analysis at Oklahoma State University. A fin clip was also taken from all specimens for genetic analysis and none of these individuals were genotyped as pallid sturgeon.
Figure 1. Map of the Missouri River basin. The upper Missouri River basin is above Lake Sakakawea. Williston reach, in the upper Missouri River, is located 2499-2563 river kilometers (rkm) from the Mississippi River confluence. The lower Missouri River basin is the area below Gavins Point Dam (rkm 1305) to the Mississippi River confluence. Copeland reach (rkm 901-925), Langdon reach (rkm 838-861), and Lexington reach (rkm 494-526) are located in the lower Missouri River.
Diet
In the laboratory, the entire stomach and the lower esophagus from each age-0 sturgeon was removed, blotted dry, and weighed to the nearest 0.1 mg (Civiello et al. Citation2018). The contents were then removed and the empty stomach was re-measured to estimate prey mass, including unidentifiable contents. Prey items were then identified to taxonomic order and life stage (larvae, pupae, where appropriate), and counted. If prey items were estimated to exceed 250 per stomach, non-larval Diptera prey (e.g. Ephemeroptera [EPH]) was counted separately. The remaining larval dipteran gut contents were sub-sampled by evenly spreading the gut contents on a plate gridded into 5 × 5-mm cells, and counting all items in three randomly selected cells. The number of prey items per cell were then averaged and multiplied by the total number of squares being occupied by the gut content sample (Hayslip Citation2007; Civiello et al. Citation2018).
Data analysis
To index the amount of prey eaten by different sizes of fish, we divided the total weight of prey in a stomach by the body weight and multiplied by 100 (hereafter called consumption percentage; Gemeinhardt et al. Citation2019) for each individual. The consumption percentage metric was compared among reaches (1 reach at UMOR, 3 reaches at LMOR) with a one-way ANOVA using the aov() function of base R statistical software (v. 3.6.4). Data distribution for the numeric quantity of prey items were not normally distributed and could not be corrected through data transformation so we analyzed these data with a generalized linear model (GLM). The relationship between numbers of prey eaten by type (Diptera larvae [DIPL], Diptera pupae [DIPP], and EPH) and reach was tested with likelihood-ratio tests using the glm.nb function in the MASS R Package (Venables and Ripley Citation2002) with a negative binomial distribution. Post-hoc, pairwise Tukey tests were conducted with the pairs() function of base R (Becker et al. Citation1988).
Results
A total of 284 wild age-0 sturgeon was captured for this study; 60 from the UMOR Williston reach and 224 from the LMOR reaches (53 from Copeland, 87 from Langdon, and 84 from Lexington; ). All length categories were represented, except for ≤ 20 mm at the UMOR Williston reach. All age-0 sturgeon contained prey except for one 20-mm TL individual at the Langdon reach of the LMOR, which was removed from further analysis. Another individual, from the UMOR Williston reach and measuring 40-mm, was removed from further analysis because the stomach was ruptured when it was received. Consumption percentage averaged 4.0% and did not differ statistically among reaches (F3,278 = 1.398; p = 0.24; ). Six prey taxa were found (Trichoptera, Plecoptera, Copepoda, Diptera pupae (DIPP), Diptera larvae (DIPL), and Ephemeroptera (EPH)), although DIPL was the most common (>62%; ). Prey composition at UMOR Williston, LMOR Copeland, and LMOR Langdon reaches was mostly DIPL (62.5-73.0%) followed by EPH (25.0-37.0%) (). However, DIPL accounted for a near total majority of prey items at the LMOR Lexington reach (97.8%; ).
Figure 2. Box plots of percent consumption for age-0 sturgeon among reaches in the upper Missouri River basin (UMOR; Williston reach) and lower Missouri River basin (LMOR; Copeland, Langdon, and Lexington reaches) in 2016. Box plots depict the minimum, first quartile, median, third quartile, and maximum, with outliers depicted as single points.
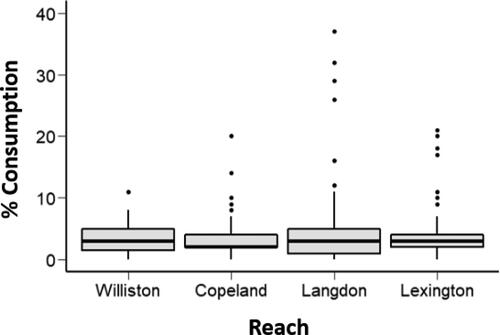
Figure 3. Percent composition of age-0 sturgeon diet among reaches in the upper Missouri River basin (UMOR; Williston reach) and lower Missouri River basin (LMOR; Copeland, Langdon, and Lexington reaches) in 2016.
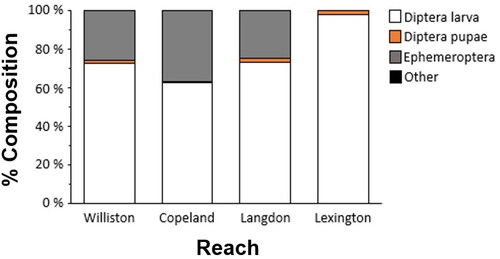
Table 1. Numbers of age-0 sturgeon in six length categories assessed for diet in select reaches of the upper (UMOR) and lower (LMOR) basins of the Missouri River in 2016.
Differences in mean number of prey consumed by type were significantly different among reaches (analysis of deviance; DDIPL(3) = 212.58, p < 0.001; DDIPP(3) = 73.95, p < 0.001; DEPH(3) = 307.21, p < 0.001). For DIPL and DIPP, the mean number of prey items consumed by age-0 sturgeon in every LMOR reach was as much or greater than in the UMOR reach with LMOR Lexington reach yielding a significantly greater mean by an order of magnitude compared to all other reaches (Tukey test; p < 0.05; ). However, consumption of EPH at UMOR was intermediate to the values observed at the LMOR reaches. The greatest consumption of EPH was observed at the LMOR Copeland and Langdon reaches, which were significantly higher than consumption of EPH at the UMOR Williston reach (Tukey test; p < 0.05). Significantly lower consumption of EPH was also observed at the UMOR Williston reach (Tukey test; p < 0.05), with consumption of EPH at the LMOR Lexington reach being significantly lower than all other sites (Tukey test; p < 0.05).
Table 2. Metrics for prey types found in the gut contents of age-0 sturgeon in the upper (UMOR) and lower (LMOR) basins of the Missouri River in 2016.
Discussion
The diet composition results indicate that sturgeon in the two upstream reaches of the LMOR (Langdon and Copeland) consumed prey similar to the UMOR reach, where prey is not considered a limiting factor. However, sturgeon prey use in the LMOR Lexington reach was the least similar to the upstream LMOR reaches. This is interesting because the UMOR reach is located much farther away (over four times the distance) from Langdon and Copeland reaches and is separated by multiple dams compared to the Lexington reach. Specifically, the use of EPH was limited at Lexington during 2016, which is consistent with the availability of this prey type (Poulton et al. Citation2003). However, González et al. (Citation2022) reported EPH to be commonly consumed in the Lexington reach during 2017 and 2018 for age-0 sturgeon < 50 mm long. Additionally, a diet study in 2018 of small (< 30 mm long) age-0 pallid sturgeon (n = 4) and shovelnose sturgeon (n = 18) captured in the Lexington reach found EPH were the most commonly consumed prey (Gosch et al. Citation2019). Both studies indicate EPH can occasionally be relatively abundant in the Lexington reach, though this may not have been the case in 2016. The differences observed at the Lexington reach may also be explained by differences in the timing of capture as most age-0 sturgeon from this reach were collected during August-September, whereas most individuals from Langdon and Copeland reaches were collected during June-July. Another possibility is that the availability of EPH in the Lexington reach was not low in 2016 compared to the other reaches, but instead the conditions may have been optimal for high DIPL production resulting in a relatively lower availability (or preference) of EPH. How these potential differences in prey availability might affect age-0 sturgeon vital rates, such as growth, condition, and survival, remains unclear.
Calculation of condition could have provided some additional insight to our foraging results, but this was not possible during our study. Lipids from the fish captured in the UMOR failed to extract, probably from issues with preservation, preventing lipid analysis. As a result, we also did not attempt to calculate a length-weight (L-W) index of condition, which can be affected by preservation (González et al. Citation2021). But, existing lipid and L-W condition assessments from the LMOR in 2016 both indicated that fish at Lexington were in better condition early in life, when smaller, compared to Langdon and Copeland. However, at larger sizes, fish at Langdon and Copeland were in similar or better condition than at Lexington by the time fish reached 120 mm (González et al. Citation2021; González and Long Citation2021), implying that conditions were more favorable for fish upstream of the Lexington reach. Although we did not measure prey availability to help explain these results, Angradi et al. (Citation2009) found that total benthic macroinvertebrate density throughout the entire Missouri River system peaked between 500 river kilometers (rkm) and 1,000 rkm above the mouth, which coincides with the Lexington reach and upstream to Gavins Point Dam in the LMOR. Moreover, Angradi et al. (Citation2009) found that Ephemeroptera was the dominant taxa throughout the LMOR. However, in contrast, Poulton et al. (Citation2003) showed that percent Ephemeroptera was reduced at Lexington with a corresponding increase in percent Chironomidae among their six LMOR study reaches. Relative to the other reaches, Lexington stood out due to extremely disproportionate consumption of Diptera larvae, such as Chironomidae, indicating that age-0 sturgeon may forage on prey items in relation to their abundance. However, consistent with studies that have assessed prey selection (e.g. Rapp Citation2014; Delauriers Citation2015), our data showed that age-0 sturgeon reliably consumed Diptera larvae as the dominant forage item. On an energy basis, given the mean dry mass and energy content of Diptera and Ephemeroptera prey in the LMOR (González et al. Citation2020), along with the mean number of age-0 sturgeon diet items, the lowest mean energy intake was observed in the UMOR at Williston (0.27 kJ), followed by LMOR sites at Lexington (0.31 kJ), Langdon (0.60 kJ), and Copeland (0.81 kJ). These energy basis measures indicate that, on average, fish in the LMOR are consuming greater amounts of energy than fish in the UMOR. Hall et al. (Citation2016) found that despite habitat differences and availability in UMOR and LMOR, age-0 sturgeon were collected from similar velocities in both the UMOR and LMOR, which may indicate similar energetic demands across both areas.
Accounting for temporal differences is also important given that most of the UMOR age-0 fish used in this study were captured in August compared to June-July for Copeland and Langdon reaches in the LMOR. In the UMOR, shovelnose sturgeon spawn during June - August based on collections of dispersing free embryos from tributaries or the mainstem river (Braaten and Fuller Citation2007; DeLonay et al. Citation2016a, free embryos from tributaries or the mainstem river (Braaten and Fuller Citation2007; DeLonay et al. Citation2016ab). Therefore, targeted sampling for age-0 fish in the UMOR typically occurs during late-July through August. In the LMOR, spawning is earlier and targeted sampling for age-0 sturgeon occurs in June. Gosch et al. (Citation2021) suggested that the best opportunity to catch age-0 sturgeon < 30 mm in the LMOR occurs before July and Hall et al. (Citation2016) reported that age-0 sturgeon <25 mm are available from June to July in the Copeland and Langdon reaches and late May to mid-September for the Lexington reach. This was generally the case in Copeland and Langdon reaches as the smaller age-0 sturgeon (≤ 40 mm) were captured in June and July, whereas sizes near Lexington were more protracted with the capture of many of the small individuals occurring in September. While differences in the timing of sample collections can be an issue in some circumstances, this was not the case for comparisons between the UMOR reach relative to the Copeland and Langdon reaches due to differences in the timing of spawning and age-0 sturgeon availability.
The spatial differences in prey use among reaches in the LMOR may have important implications regarding potential surrogacy between shovelnose sturgeon and pallid sturgeon due to the difference in larval drift distances. Drift simulations indicate larval shovelnose sturgeon may drift an average distance of 94 to 250 km and larval pallid sturgeon may drift an average distance of 245 to 530 km, depending on water velocity (Braaten et al. Citation2008). Contingent on the spawning location, shovelnose sturgeon could settle from the drift much sooner compared to pallid sturgeon, meaning that this endangered species may be more likely to settle below Kansas City than shovelnose sturgeon. This is consistent with LMOR field observations as the limited number (n = 7) of genetically confirmed exogenously feeding age-0 pallid sturgeon documented in the literature have all been captured at or downstream of the Lexington reach, which is located between rkm 494-526 (Gosch et al. Citation2018, Citation2019). If higher quality prey resources were available upstream of Lexington reach, this could have a disproportional effect on pallid sturgeon relative to shovelnose sturgeon. Even though fish at Lexington consumed much more DIPL than fish in the other reaches, the overall consumption percentage showed that the total amount of prey consumed was comparable with all the other reaches, including the UMOR. Additionally, recent studies in the UMOR that compared diets between age-0 pallid sturgeon and shovelnose sturgeon found 94% overlap in prey use identified to order (Holley et al. Citation2022), with the vast majority of prey belonging to Diptera for both species. Given that chironomids were preferred prey for age-0 pallid sturgeon in laboratory experiments (Rapp Citation2014; Delauriers Citation2015) and positively selected for by age-0 sturgeon in the middle Mississippi River (Sechler et al. Citation2012), it appears that prey resources for age-0 sturgeon in Lexington reach are sufficient, which supports previous research indicating the food is likely not a limiting factor for age-0 sturgeon in the LMOR (Civiello et al. Citation2018; Gosch et al. Citation2021). These results may have implications for pallid sturgeon recovery efforts if the surrogate relationship is validated.
Overall, this study provides valuable context regarding age-0 sturgeon prey consumption over a wide geographic range. We are not aware of any other studies incorporating diet data from both the UMOR and LMOR. Having additional years of data from the UMOR would provide further insight given that differences in prey consumption and condition among years have been previously noted in the LMOR (e.g. Civiello et al. Citation2018; González et al. Citation2021). Although shovelnose sturgeon in the UMOR and LMOR are considered self-sustaining, additional studies regarding other potential abiotic and biotic factors that may also be influencing recruitment and survival of age-0 sturgeon would also be valuable for future management efforts.
Acknowledgements
Edward Heist (Southern Illinois University) provided genetic analysis. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. The Oklahoma Cooperative Fish and Wildlife Research Unit is supported by the Oklahoma Department of Wildlife Conservation, Oklahoma State University, U.S. Geological Survey, the Wildlife Management Institute, and the U.S. Fish and Wildlife Service. We thank staff at the Nebraska Game and Parks Commission, Montana Fish, Wildlife and Parks, U.S. Geological Survey, Oklahoma State University, and USACE for field and laboratory assistance. Fish were handled as described in the U.S. Fish and Wildlife Service Biological Procedures and Protocols for Researchers and Managers Handling Pallid Sturgeon and the American Fisheries Society’s Guidelines for the Use of Fishes in Research.
Disclosure statement
The authors have no conflict of interest to declare.
Data availability statement
Data for this study are available on the USGS ScienceBase catalog, located at https://www.sciencebase.gov/catalog/item/64076ea8d34e76f5f75e387e and can be cited as:
Gonzalez, A., and Long, J. M., 2023, Stomach contents and consumption percentage from age-0 Scaphirhynchus sturgeon in the Missouri River, 2016: U.S. Geological Survey data release, https://doi.org/10.5066/P9KWJ8W7.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Angradi TR, Bolgrien DW, Jicha TM, Pearson MS, Taylor DL, Hill BH. 2009. Multispatial-scale variation in benthic and snag-surface macroinvertebrate assemblages in mid-continent US great rivers. J North Am Benthologic Soc. 28(1):1–11.
- Becker RA, Chambers JM, Wilks AR. 1988. The new S language. Monterey, California (USA): Wadsworth & Brooks/Cole.
- Braaten PJ, Fuller DB. 2007. Growth rates of young-of-year shovelnose sturgeon in the upper Missouri River. J Appl Ichthyol. 23(4):506–515.
- Braaten PJ, Fuller DB, Holte LD, Lott RD, Viste W, Brandt TF, Legare RG. 2008. Drift dynamics of larval pallid sturgeon and shovelnose sturgeon in a natural side channel of the upper Missouri River, Montana. North Am J Fish Manage. 28(3):808–826.
- Braaten PJ, Fuller DB, Lott RD, Haddix TM, Holte LD, Wilson RH, Bartron ML, Kalie JA, DeHaan PW, Ardren WR, et al. 2012b. Natural growth and diet of known-age pallid sturgeon (Scaphirhynchus albus) early life stages in the upper Missouri River basin, Montana and North Dakota. J Appl Ichthyol. 28(4):496–504.
- Braaten PJ, Fuller DB, Lott RD, Ruggles MP, Brandt TF, Legare RG, Holm RJ. 2012a. An experimental test and models of drift and dispersal processes of pallid sturgeon (Scaphirhynchus albus) free embryos in the Missouri River. Environ Biol Fish. 93(3):377–392.
- Braaten PJ, Fuller DB, McClenning ND. 2007. Diet composition of larval and young-of-year shovelnose sturgeon in the upper Missouri River. J Appl Ichthyol. 23(4):516–520.
- Civiello AP, Gosch NJC, Gemeinhardt TR, Miller ML, Bonneau JL, Chojnacki KA, DeLonay AJ, Long JM. 2018. Diet and condition of age-0 Scaphirhynchus sturgeon: implications for shallow-water habitat restoration. North Am J Fish Manage. 38(6):1324–1338.
- Delauriers D. 2015. Development and application of a spatially-explicit model for estimating growth of age-0 pallid sturgeon in the Missouri River [PhD dissertation]. Brookings, SD: South Dakota State University.
- DeLonay AJ, Chojnacki KA, Jacobson RB, Braaten PJ, Buhl KJ, Elliott CM, Erwin SO, Faulkner JDA, Candrl JS, Fuller DB, et al. 2016b. Ecological requirements for pallid sturgeon reproduction and recruitment in the Missouri River—annual report 2014. U.S. Geological Survey Open-File Report 2016-1013, p. 131.
- DeLonay AJ, Jacobson RB, Chojnacki KA, Braaten PJ, Buhl KJ, Eder BL, Elliott CM, Erwin SO, Fuller DB, Haddix TM, et al. 2016a. Ecological requirements for pallid sturgeon reproduction and recruitment in the Missouri River—annual report 2013. U.S. Geological Survey Open-File Report 2015-1197. p. 99.
- Gemeinhardt TR, Gosch NJC, Civiello AP, Chrisman NJ, Shaughnessy HH, Brown TL, Long JM, Bonneau JL. 2019. The influence of depth and velocity on age-0 Scaphirhynchus sturgeon prey consumption: implications for aquatic habitat restoration. River Res Appl. 35(3):205–215.
- González A, Barnes CL, Wilder SM, Long JM. 2020. Differences in macronutrient content of common aquatic macroinvertebrates available as prey for young-of-the-year Scaphirhynchus sturgeons in the lower Missouri River. J Freshwater Ecol. 35(1):191–202.
- González A, Long JM, Gosch NJC, Civiello AP, Gemeinhardt TR, Hall JR. 2021. Spatial and temporal variation in length-weight relationships of age-0 Scaphirhynchus sturgeon in the lower Missouri River. Am Midland Nat. 186(1):106–121.
- González A, Long JM, Gosch NJC, Civiello AP, Gemeinhardt TR. 2022. Factors affecting interannual variation in diet and body lipid content of age-0 Scaphirhynchus sturgeon in the lower Missouri River. River Res Appl. 38(6):1167–1178.
- González A, Long JM. 2021. Assessment of prey consumption and body condition of Missouri River age-0 Scaphirhynchus sturgeon. Final report W912HZ-16-2-0026 to U.S. Army Corps of Engineers, Kansas City, Missouri.
- Gosch NJC, Civiello AP, Gemeinhardt TR, Bonneau JL, Long JM. 2018. Are shovelnose sturgeon a valid surrogate for endangered pallid sturgeon during the first year of life? J Appl Ichthyol. 34(1):39–41.
- Gosch NJC, Gemeinhardt TR, Bouska WW, Miller ML, Brown TL, Bonneau JL. 2017. Age-0 sturgeon and shallow water: a local- and reach-scale assessment. River Res Appl. 33(9):1452–1462.
- Gosch NJC, Gemeinhardt TR, Civiello AP, Harrison AB, Bonneau JL. 2019. Dietary assessment of age-0 pallid sturgeon and shovelnose sturgeon: implications for surrogacy. Endang Species Res. 40:321–327.
- Gosch NJC, Hall JR, Civiello AP, Haas JD, Gemeinhardt TR, Bonneau JL. 2021. Floodplain connectivity and age-0 sturgeon prey consumption in the lower Missouri River. River Res Appl. 37(9):1243–1253.
- Gosch NJC, Miller ML, Gemeinhardt TR, Sampson SJ, Bonneau JL. 2015. Age-0 sturgeon accessibility to constructed and modified chutes in the lower Missouri River. North Am J Fish Manage. 35(1):75–85.
- Gosch NJC, Miller ML, Gemeinhardt TR, Starks TA, Civiello AP, Long JM, Bonneau JL. 2016. Age-0 shovelnose sturgeon prey consumption in the lower Missouri River. River Res Appl. 32(8):1819–1823.
- Hall J, Steffensen K, Mestl G, Eder B, Ames C, Winders K, Whiteman K. 2016. Characterizing best achievable habitat conditions for Missouri River side-channel habitat restoration projects. U.S. Army Corps of Engineers Report W9128F-15-D-0016 and W9128F-15-D-013 Task Order DH01. p. 55.
- Hayslip G. 2007. Methods for the collection and analysis of benthic macroinvertebrate assemblages in wadeable streams of the Pacific Northwest. Cook, Washington: Pacific Northwest Aquatic Monitoring Partnership.
- Holley C, Braaten P, Poulton B, Heist E, Umland L, Haddix T. 2022. Diet composition and overlap of larval pallid sturgeon and shovelnose sturgeon from the upper Missouri River, USA. Endang Species Res. 49:103–114. 10.3354/esr01205
- Jacobson RB, Annis ML, Colvin ME, James DA, Welker TL, Parsley MJ. 2016. Missouri River Scaphirhynchus albus (pallid sturgeon) effects analysis—integrative report 2016. U.S. Geological Survey Scientific Investigations Report 2016-5064.
- NRC (National Research Council). 2011. Missouri River planning: recognizing and incorporating sediment management. Washington, D.C.: National Academies Press.
- Poulton BC, Wildhaber ML, Charbonneau CS, Fairchild JF, Mueller BG, Schmitt CJ. 2003. A longitudinal assessment of the aquatic macroinvertebrate community in the channelized lower Missouri River. Environ Monit Assess. 85(1):23–53.
- Rapp T. 2014. Determinants of growth and survival of larval pallid sturgeon: a combined laboratory and field approach (PhD dissertation). Brookings, SD: South Dakota State University.
- Ridenour CJ, Doyle WJ, Hill TD. 2011. Habitats of age-0 sturgeon in the lower Missouri River. Trans Am Fish Soc. 140(5):1351–1358.
- Sechler DR, Phelps QE, Tripp SJ, Garvey JE, Herzog DP, Ostendorf DE, Ridings JW, Crites JW, Hrabik RA. 2012. Habitat for age-0 shovelnose sturgeon and pallid sturgeon in a large river: interactions among abiotic factors, food, and energy intake. North Am J Fish Manage. 32(1):24–31.
- Venables WN, Ripley BD. 2002. Modern applied statistics with S. 4th ed. New York: Springer.
