?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Ceratopteris pteridoides is an endangered annual floating fern in China. This study tested the effect of cadmium on gametophyte growth and archegonia development of the aquatic fern C. pteridoides in culture. The results show that C. pteridoides spores germinated after being treated with different cadmium, but it was not significant compared with the reference group (45.63%). With the increase of cadmium, the rates of hermaphrodite gametophyte, the number of archegonia of C. pteridoides decreased, and archegonium differentiation was delayed gradually, indicating that cadmium caused adverse effects on sexual organ differentiation of C. pteridoides, further exacerbating the endangered status of this species in China. We suggest that it is necessary to pay more attention to protecting the primary habitats of C. pteridoides in China and reduce the impact of human activities from excessive aquaculture and industrial wastewater discharge on the water body in its living water body.
Key Policy Highlights
Ceratopteris pteridoides is an endangered aquatic fern, and listed in the second category of protected wild plants in China.
With the increase of the concentration of Cd2+, the proportion of hermaphroditic gametophyte and the number of archegonium decreased gradually, indicating that cadmium caused adverse effects on sexual organ differentiation of C. pteridoides.
We suggest that it is necessary to protect the primary habitat of the species, especially the protection of its living water body.
1. Introduction
Ceratopteris pteridoides (Hook.) Hieron. (Parkeriaceae), is an endangered, annual, floating, fern (Yu Citation1999), growing mainly in ponds, lakes, rivers and ditches in Central and South America, Southeastern Asia, Eastern India and China (Hickok et al. Citation1995). In China, the species is endangered, and was listed in the second category of protected wild plants in 1999 (Yu Citation1999) and 2021 (National Forestry and Grassland Administration, PRC Citation2021). Sexual reproduction by spores and subsequent gametophytes, and clonal reproduction are the main methods of reproduction of this species (Yu Citation1999; Hickok et al. Citation1995). Previous research showed that aquatic ferns could represent ideal species for studying the effects of water pollutants on the entire life cycle under laboratory conditions (Gupta et al. Citation1992). The growth and differentiation of fern gametophytes can be affected by several physical and chemical factors (Hickok and Kiriluk Citation1984). Dong et al. (Citation2012, Citation2022) reported that water chemistry with pH values and dissolved oxygen might have significant influences on the sexual reproduction of C. pteridoides, and could be closely correlated with the decline and extirpation of the species. Several earlier studies reported that the effect of auxins (such as IBA, IAA, NAA, a-naphthaleneacetic acid, 2,4,5-trichlorophenoxy acetic acid, 2,4-D, ABA, GA), antheridiogen, cadmium on sexual differentiation and gametophyte growth in the fern Ceratopteris richardii, Ceratopteris thalictroides (Banks et al. Citation1993; Hickok et al. Citation1995; Gregorich and Fisher Citation2006; Dong et al. Citation2012; Ganger and Sturey Citation2012). Herbicides Bensulfuron-methyl and quinclorac, and pH values affected sexual reproduction and gametophyte growth in aquatic fern C. pteridoides (Tao et al. Citation2008; Dong et al. Citation2022). Bora and Sarma (Citation2021) reported anatomical and ultrastructural alterations in C. pteridoides under cadmium stress. Monashree et al. (Citation2020) estimated the tolerance mechanism of translocation and subcellular distribution for cadmium in C. pteridoides. Wang et al. (Citation2021) and Ji (Citation2019) reported that some pteridophytes such as Phyllitis japonica, Sphaeropteris lepifera, Alsophila costularis and Alsophila gigantea suffered from chlorosis of gametophyte chloroplasts and damage to gametophyte development under cadmium (Cd2+) stress, thus affecting reproductive development. Cadmium is a widely present non-essential element in organisms in aquatic environments and is recognized as one of the main pollutants in the water environment (Adam et al. Citation2019; Long et al. Citation2022). Its concentration in polluted water bodies ranges from 6 to 360 μmol L−1, the highest concentration in wastewater can reach 2224 μmol L−1 (Deng et al. Citation2014). Cadmium in the water environment mainly comes from the development and utilization of metal mining, urban domestic sewage, agricultural production and cadmium-containing exhaust gases in the atmosphere (Hua et al. Citation2019). With the rapid development of industrialization, a large amount of cadmium-containing wastewater is discharged, and human production and household waste disposed of improperly, the problem of cadmium pollution in the water environment is becoming increasingly serious (Qu et al., Citation2016). Cadmium has a long half-life in living organisms and is not easily degraded. Even at lower concentrations, it has strong biological toxicity and hence poses a serious threat to plants, animals and microorganisms, and can subsequently harm human health through the food chain. However, studies on the effect of heavy metals on sexual reproduction and gametophyte growth of C. pteridoides have not been reported. The principal aims of this study were to investigate the effect of heavy metals cadmium (Cd) on gametophyte growth and archegonia development in the endangered floating fern C. pteridoides, and to provide information for the conservation of the species.
2. Materials and methods
2.1. Plant material
In this study, the mature spores of C. pteridoides come from Houguan Lake (N 30°30′, E 114°08′) in Wuhan, China. The spores are kept in airtight, dry bags at room temperature.
2.2. Growth condition
In this experiment, the concentration gradient of Cd2+ was set according to the maximum allowable discharge concentration of 0.1 mg L−1 Cd in China’s water pollutant discharge limit standard and surface water environmental quality standard (GB 3838-2002). Cd2+ concentrations of 0, 0.01, 0.1, 1, 5, 10 and 15 mg L−1 were made in Knop’s nutrient solution containing 1.5% agar (Gupta et al. Citation1992; Dong et al. Citation2022). A reference group (Cd2+ concentrations was 0 mg L−1) using Knop’s nutrient solution containing 1.5% agar was performed simultaneously with the experimental run. The main process is as follows: first, disinfect the spores, about 500–600 sterilized spores are inoculated in each Petri dish, and then culture the spores in SPRX-600B intelligent artificial climate box. A total of 8 days after spore sowing, the spore germination rate was recorded under a Jiangnan BM2000 digital microscope (Dong et al. Citation2022). Three horizons for observation in each culture plate of three replicate plates were selected randomly. A total of 14 days after spore sowing, gametophyte size and occupancy were recorded for the three culture plates representing each Cd2+ concentration. Two gametophytes were selected in each horizon and photographed under the digital microscope. Each gametophyte area was measured as pixels through digital photographs. A total of 16 days after spore sowing, the number of hermaphroditic gametophytes in each culture plate was recorded, and recorded one time per 2-day interval, for a total of three times (Dong et al. Citation2022).
2.2.1. Data collection and statistical analysis
All parameters were analyzed using SPSS 17.0 software. Level of significance was set at p < .05. Gametophyte areas of C. pteridoides were fitted using Log-Logistic Concentration Model (Seefeldt et al. Citation1995):
Here, U represents response of the gametophyte area to different concentrations of Cd2+, namely the size of the gametophyte area after fitting, C and D represent response of the upper and lower limits of the gametophyte area to different concentrations of Cd2+ respectively, EC50 represents the concentration of Cd2+ where the gametophyte area was inhibited and reached half effect. B represents the slope of the curve at EC50. In the present study, the responses of upper and lower limits of the gametophyte area to different concentrations of Cd2+ were set as the control value and 0, respectively.
3. Results
3.1. Effect of Cd2+ on spore germination
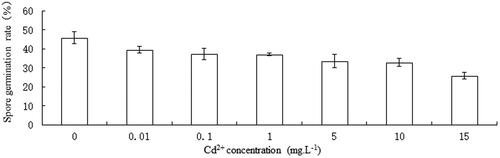
Spores of C. pteridoides germinated after being treated with different heavy metal Cd2+. With the increase of Cd2+concentration, spore germination rates decreased (). The spore germination rate of reference group was 45.63 ± 5.48%. In the maximum allowable discharge concentration of 0.1 mg L−1 Cd2+ in GB 3838-2002, spore germination rate was 37.27 ± 5.17%. At 15 mg L−1 Cd2+, the spore germination rate was 25.77 ± 3.30%, which was the lowest. However, an ANOVA analysis revealed that spore germination rates of different Cd2+ were no significant difference than that of the reference group (45.63%, ).
3.2. Effect of Cd2+ on gametophyte growth
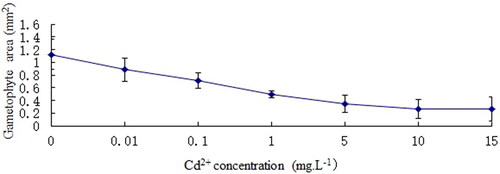
Gametophyte area (mm2) was greatest in the reference group, when Cd2+ concentration is 15 mg L−1, the gametophyte area is the lowest (). However, an ANOVA showed gametophyte areas of different Cd2+ treatments were no significant difference than that of the reference group. EC50 is equal to 0.5 mg L−1.
3.3. Effect of Cd2+ on hermaphrodite gametophytes
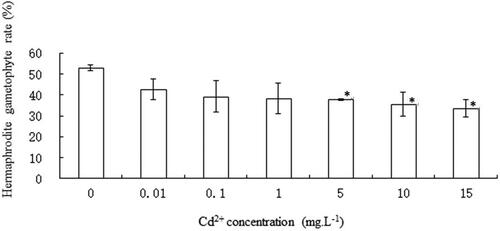
After 14 days of C. pteridoide spore culture, the control group gametophytes formed large, cordate hermaphroditic gametophytes and male gametophytes, indicating that sex differentiation had occurred (). Hermaphrodite gametophyte rate was 52.8 ± 2.45% in the reference group. The proportion of hermaphrodite gametophyte tended to decrease with the increase of Cd2+ concentration. At Cd2+concentration of 15 mg L−1, the hermaphrodite gametophyte rate was the lowest, which was 33.43 ± 7.19%. An ANOVA analysis exhibited that hermaphrodite gametophyte rates in 5, 10, and 15 mg L−1 Cd2+ were significantly lower (p ﹤.05) than that of the reference group ().
3.4. Effect of Cd2+ on time of archegonia emergence
Sixteen days after sowing C. pteridoides spores, archegonia were observed in the reference group. With the increase of Cd2+concentration, the number of archegonia decreased gradually. In the range of 0.1–15 mg L−1, the formation time of archegonia on the gametophyte was delayed with the increase of Cd2+ concentration. Compared to the reference group, time of archegonia emergence was delayed four days when Cd2+ concentration was 15 mg L−1, and the means number of archegonia was also reduced ().
Table 1. Effect of Cd2+ on time and number of archegonia emergence in C. pteridoides.
4. Discussion
Earlier studies revealed that dose-dependent effects of cadmium on the spore germination and sporophyte development in some ferns differed in degree and magnitude (Gupta and Devi Citation1991). Gupta et al. (Citation1992) reported that the germination rate of the second generation of C. thalictroides decreased gradually with the increase of cadmium concentration from 0.1 to 2.5 mg L−1, but did not affect the germination rate of the first-generation spores, indicating that the damage of cadmium concentration to spore germination showed a cumulative systemic effect. In this study, although spore germination rates of floating C. pteridoides (these spores are equivalent to the first-generation spores of C. thalictroides) decreased with the increase of Cd2+concentration from 0.01 to 15 mg L−1, it was not significant compared with the reference group (45.63%; ), indicating that different Cd treatments did not affect the germination rate of the first-generation spores of C. pteridoides. The results were similar to those reported by Gupta et al. (Citation1992) on the germination rate of the first-generation C. thalictroides spores treated with cadmium. However, in this study, the effect of cadmium on the germination of the second-generation spores of C. pteridoides was not investigated.
This study showed that the rates of hermaphrodite gametophyte of C. pteridoides reduced with the increase of cadmium concentration, and showed that those in 5, 10, and 15 mg L−1 Cd were significantly lower (p < .05) than that of the reference group (). Meanwhile, with the increase of Cd2+concentration, the number of archegonia reduced and archegonium differentiation was delayed gradually. These results indicated that cadmium concentration higher than 5 mg L−1 caused adverse effects on the sexual organ differentiation and reproduction of C. pteridoides. The results of affecting sexual reproduction under Cd-treated C. pteridoides in this study were similar to those reported in previous studies of Ceratopteris and some other ferns. For example, Gupta et al. (Citation1992) exhibited that times of antheridia and archegonia formation in C. thalictroides was delayed in concentrations up to 0. l mg L−1 Cd. Wang et al. (Citation2021) also reported that times of antheridia and archegonia formation in the endangered fern P. japonica were delayed, and the number of archegonia reduced with the increase of Cd2+. Cadmium absorption can cause cell damage, interfere with the normal physiological metabolic process of cells, and affect plant growth and development (Li et al. Citation2022). It is well known that cadmium particularly interferes with the function of cations such as calcium and zinc, thus affecting growth and differentiation in plants (Ernst Citation1980). The visible toxic effects on the aerial parts of sporophytes were chlorosis, reduction in the size of vascular bundles and damage to the radial walls of the endodermal cells (Gupta et al. Citation1992). C. pteridoides treated with cadmium showed that the stomata of leaves were closed, the diameter of xylem tracheids of stem and root was reduced, the chloroplast and mitochondrial membrane system were damaged, and the components of chloroplast were disordered (Ji Citation2019). Gupta et al. (Citation1992) showed that the chlorophyll and protein contents of C. thalictroides gametophytes treated with cadmium decreased gradually. In 1.0 mg L−1 cadmium, the total chlorophyll decreased by approximately 50%. Wang et al. (Citation2021) observed that chloroplast of gametophyte of P. japonica turned yellow and dissolution under 0.4 mg/kg Cd stress, and the gametophyte growth and sexual differentiation were affected by the increase of Cd concentration. Under the treatment of 0.8 mg/kg Cd, most of the antheridia could not be released sperm freely, accompanied by macular, and there was obvious necrosis of the archegonia, which might lead to the failure of fertilization, to affect sexual reproduction (Wang et al. Citation2021). These studies give insights into the metabolic changes in ferns, which lead to various phytotoxic symptoms caused by cadmium. The effect of cadmium concentration is more common during the formation of gametophytes which is possibly attributed to more cadmium concentration accumulated and increases toxicity, and consequently affecting the sexual reproduction.
Early studies have shown that the reduction and extinction of C. pteridoides in China are related to the destruction and loss of primary habitats, especially the increase in water pollution and the reduction of wetland areas (Dong et al. Citation2012, Citation2022). Based on the analysis of eight heavy metals contamination in surface sediments of 31 major lakes in China, Ding et al. (Citation2017) reported that the average pollution degree of cadmium was the highest, the average content was 0. 497 mg/kg, and the pollution class of Cd and Hg were moderately and heavily polluted respectively in many lakes, indicating that the potential ecological risks in the sediments of lakes in China were mainly caused by Cd and Hg. Heavy metal pollution in sediments of eastern and central lakes is more serious than that of western lakes in China (Ding et al. Citation2017). In China, C. pteridoides occur mainly in eastern and central lakes, ponds and rivers. C. pteridoides used in this study were collected from lakes in central China.
Thus, in the study, water environmental factors such as the changes in cadmium concentration might have significant influences on the sexual reproduction of C. pteridoides, further exacerbating the endangered degree of this species in China. Therefore, it is necessary to pay more attention to protecting more habitats of C. pteridoides in China and reduce the impact of human activities such as excessive aquaculture and industrial wastewater discharge on the water body in which C. pteridoides live.
Previous studies have shown that Ceratopteris species are sensitive to water pollutants (Gupta et al. Citation1992; Deng et al. Citation2014; Dong et al. Citation2012, Citation2022). Gupta et al. (Citation1992) reported that dose-dependent toxicity of Cd manifestations, including ultrastructural pathomorphological changes were detected in all stages of the entire life cycle (gametophyte, juvenile sporophyte, mature sporophytes, and spores) of the aquatic fern C. thalictroides, with gametophytes being the most sensitive. Deng et al. (Citation2014) showed that cadmium can lead to a decrease in the growth and photosynthesis of endangered aquatic fern C. pteridoides, thus enhancing its mortality risk. An efficient strategy to restore environmental quality for this endangered fern implies the reduction of Cd pollution in the environment. In this study, our results showed that cadmium had an adverse effect on the differentiation of sexual organs of C. pteridoides. Field investigations and water quality analysis also indicate that the survival of C. pteridoides is closely related to water quality changes (Dong et al. Citation2012, Citation2022). The study therefore recommends C. pteridoides to be used as an indicator species for water pollution.
In conclusion, with the increase of the cadmium concentration, the proportion of hermaphroditic gametophyte and the number of archegonium decreased gradually. Since the reproductive organ is a crucial part of the conservation of species, to conserve this endangered C. pteridoides species, it is necessary to protect the primary habitat of C. pteridoides, especially the protection of its living water body.
Acknowledgment
The authors would like to thank Jifei Zhang for his assistance.
Disclosure statement
The authors have no conflicts of interest to declare.
Data availability statement
The data presented in this study are available on request from the corresponding author.
Additional information
Funding
References
- Adam MA, Maftuch M, Kilawati Y, Risjani Y. 2019. The effect of cadmium exposure on the cytoskeleton and morphology of the gill chloride cells in juvenile mosquito fish (Gambusia affinis). Egypt J Aquat Res. 45(4):1–9. doi: 10.1016/j.ejar.2019.11.011.
- Banks JA, Hickok L, Webb MA. 1993. The programming of sexual phenotype in the homosporous fern Ceratopteris richardii. Int J Plant Sci. 154(4):522–534. doi: 10.1086/297135.
- Bora MS, Sarma KP. 2021. Anatomical and ultrastructural alterations in Ceratopteris pteridoides under cadmium stress: a mechanism of cadmium tolerance. Ecotoxicol Environ Saf. 218:112285. doi: 10.1016/j.ecoenv.2021.112285.
- Deng G, Li M, Li H, Yin L, Li W. 2014. Exposure to cadmium causes declines in growth and photosynthesis in the endangered aquatic fern (Ceratopteris pteridoides). Aquat Bot. 112:23–32. doi: 10.1016/j.aquabot.2013.07.003.
- Ding ZY, Pu J, Abuduwaili J. 2017. Heavy metal contamination characteristics and its assessment in surface sediments of major lakes in China. Environ Eng. 35(6):136–141.
- Dong YH, Wang QF, Robert GW. 2012. Effect of habitat modification on the distribution of the endangered aquatic fern Ceratopteris pteridoides (Parkeriaceae) in China, and conservation strategy. Amer Fern J. 102(2):136–146. doi: 10.1640/0002-8444-102.2.136.
- Dong Y, Liao K, Zeng C, Xu G, Dia X. 2022. Effects of pH on sex organ differentiation and sexual reproduction in the endangered aquatic fern Ceratopteris pteridoides. IOP Conf Ser Earth Environ Sci. 983(1):012081. doi: 10.1088/1755-1315/983/1/012081.
- Ernst WHO. 1980. Biochemical aspects of cadmium in plants. In: Nriagu JO, editor Cadmium in the environment. NY: John Wiley and Sons; p. 639–653.
- Ganger M, Sturey T. 2012. Antheridiogen concentration and spore size predict gametophyte size in Ceratopteris richardii. Botany. 90(3):175–179. doi: 10.1139/b11-097.
- Gregorich M, Fisher R. 2006. Auxin regulates lateral meristem activation in developing gametophytes of Ceratopteris richardii. Can J Bot. 84(10):1520–1530. doi: 10.1139/b06-113.
- Gupta M, Devi S. 1991. Effects of cadmium on spore germination and gametophyte development in some ferns. Bull Environ Contain Toxicol. 48:337–343.
- Gupta M, Devi S, Singh J. 1992. Effects of long-term low-dose exposure to Cadmium during the entire life cycle of Ceratopteris thalictroides, a water fern. Arch Environ Contam Toxicol. 23(2):184–189. doi: 10.1007/BF00212273.
- Hickok LG, Kiriluk RM. 1984. Effects of auxins on gametophyte development and sexual differentiation in the fern Ceratopteris thalictroides (L.) Brongn. Bot. Gaz. 145(1):37–42. doi: 10.1086/337423.
- Hickok LG, Warne TR, Fribourg RS. 1995. The biology of the fern Ceratopteris and its use as a model system. Int J Plant Sci. 156(3):332–345. doi: 10.1086/297255.
- Hua XY, Huang XM, Tian JQ, Dong DM, Liang DP, Guo ZY. 2019. Migration and distribution of cadmium in aquatic environment: the important role of natural biofilms. Sci Total Environ. 670:478–485. doi: 10.1016/j.scitotenv.2019.03.246.
- Ji SB. 2019. Effect of Cd2+, Pb2+ on development and rhizosphere soil microecology of three species of Chinese Cyatheaceae. Harbin: Harbin Normal University.
- Li HJ, Ming LL, Zhang WS. 2022. Uptake, transport and tolerance of cadmium in plants: a review. Asian J Ecotoxicol. 17(2):86–95.
- Long M, Wang ZH, Chen H, Hu YP, Zhu Y, Li GY. 2022. Research progress on the toxicity of cadmium to fish. J Hydroecol. 43(2):142–150.
- Monashree SB, Nirmali G, Kali PS. 2020. Tolerance mechanism of cadmium in Ceratopteris pteridoides: translocation and subcellular distribution. Ecotox Environ Safe. 197:110599.
- National Forestry and Grassland Administration, PRC. 2021. Announcement of the National Forestry and Grassland Administration, PRC and Ministry of Agriculture and Rural Affairs, PRC (No. 15 of 2021) (List of National Key Protected Wild Plants). http://www.forestry.gov.cn/2021-09-08.
- Qu R,Liu J,Wang L,Wang Z. 2016. The toxic effect and bioaccumulation in aquatic oligochaete Limnodrilus hoffmeisteri after combined exposure to cadmium and perfluorooctane sulfonate at different pH values. Chemosphere. 152:496–502. doi: 10.1016/j.chemosphere.2016.03.024.
- Seefeldt SS, Jensen JE, Fuerst E. 1995. Log-logistic analysis of herbicide dose-response relationships. Weed Technol. 9(2):218–227. doi: 10.1017/S0890037X00023253.
- Tao L, Yin LY, Li W. 2008. Effects of herbicide bensulfuron-methyl on gametophyte growth and sex organ differentiation in Ceratopteris pteridoides. J Plant Ecol. 32:408–412.
- Wang ZC, Yang S, Guan Y, Liu BD. 2021. Comparative study on gametophyte development of Phyllitis japonica under Cd2+ and Pb2+ stress. J Henan Agric Sci. 50(9):128–134.
- Yu YF. 1999. A milestone of wild plant conservation in China. Plants. 5:3–11.