Abstract
Round goby Neogobius melanostomus – a small, benthic fish native to Eurasia – was first introduced to North America in the 1980s through ballast water of cargo ships. In 1990, the Round goby was first discovered in the Laurentian Great Lakes Basin and rapidly spread through all Great Lakes from 1993 to 1998. The Round goby is an aggressive, prolific, and efficient egg predator that subsequently displaced native fishes from their preferred habitats and resources in the Great Lakes, where they are well established and abundant. From Lake Michigan, Round goby moved south into the Chicago Area Waterway System in 1993 and slowly progressed down the Illinois Waterway to the Mississippi River from 2004 to 2019, where less is known about their abundance, establishment, and impact. The goal of this study was to gain a comprehensive understanding of the first large river invasion by Round goby in the Mississippi River Basin by leveraging Round goby capture data from 2019 to 2022 on all pools of the Illinois Waterway. We describe their current distribution, relative abundance (i.e., catch-per-unit-effort), frequency of occurrence, and establishment (i.e., presence of young-of-year and adults) status throughout the Illinois Waterway. Results show that catch-per-unit-effort, frequency of occurrence, and the proportion of sites where multiple life stages are present are considerably higher in the upstream pools relative to more downstream pools of the Illinois Waterway. Our data support that the Round goby has established self-sustaining populations in the Chicago Area Waterway System, the upper Illinois River (i.e. Dresden Island Pool downstream through Starved Rock Pool), and a portion of the lower Illinois River (i.e. Peoria Pool downstream through Alton Pool) of the Illinois Waterway. In the lower Illinois River, Round gobies appear to only be established in Peoria Pool, with captures occurring infrequently in La Grange Pool, and no Round gobies being captured in Alton Pool.
Introduction
The Round goby (Neogobius melanostomus) is a small, benthic fish native to Europe’s Black and Caspian seas that is widely recognized as a globally significant invasive species and a model organism for invasion biology (Kornis et al. Citation2012). The Round goby is a highly adaptable, euryhaline species tolerant of oceanic salinities < 30 parts per thousand (Kornis et al. Citation2012) and reproduces successfully across freshwater, brackish, and marine systems (Charlebois et al. Citation1997; Kalamarz-Kubiak and Guellard Citation2019). The Round goby can reproduce multiple times a year, achieving hatch rates as high as 95% via male nest guarding (MacInnis and Corkum Citation2000). This species also has high phenotypic plasticity (Cerwenka et al. Citation2014; Kornis et al. Citation2017), consumes a broad diet of macroinvertebrates (mollusks, amphipods, chrionomids, cladocerans) and fish eggs (Pennuto et al. Citation2010; Brush et al. Citation2012; Kornis et al. Citation2012), survives within a wide temperature range (-1–30 °C), reaches comparatively high density (∼50 per m2, Charlebois et al. Citation1997; Vélez-Espino et al. Citation2010) and large body size (Charlebois et al. Citation1997; MacInnis and Corkum Citation2000) relative to native benthic fishes (Kornis et al. Citation2012), and aggressively competes for resources (Charlebois et al. Citation1997; Wickett and Corkum Citation1998; Bergstrom and Mensinger Citation2009). These traits make the Round goby a prolific invader, with invasive populations driving strong, negative impacts on native fish and invertebrate communities across their introduced range (Astorg et al. Citation2022, and references therein; Clark et al. Citation2022).
Both North America and Europe have many established, invasive populations of Round goby within lakes and rivers (Kornis et al. Citation2012). The Round goby was likely introduced to North America in the 1980s through ballast water of trans-Atlantic cargo ships. Round goby was first discovered in the Laurentian Great Lakes in 1990 (Jude et al. Citation1992) and rapidly spread through all Great Lakes from 1993 to 1998 (Corkum et al. Citation2004). From Lake Michigan, Round goby moved south into the Chicago Area Waterway System (CAWS) in 1993 (Charlebois et al. Citation1997), and since 2004, has slowly progressed downstream throughout the Illinois Waterway (IWW; Irons et al. Citation2006; Merry et al. Citation2018). In 2018, a single Round goby was detected near Grafton, Illinois, in pool 26 of the Mississippi River by the U.S. Fish and Wildlife Service, within 3 river kilometers of the confluence of the IWW and the Mississippi River (Freedman et al. Citation2023). Similarly, two more Round gobies were captured in 2019 near Grafton, Illinois and two additional Round gobies near Alton, Illinois – approximately 24 river kilometers downstream of Grafton, Illinois (Freedman et al. Citation2023).
Although the Round goby has reached the Mississippi River, the status of its invasion within the IWW is still largely unknown (Irons et al. Citation2006; Merry et al. Citation2018), and this population may serve as a springboard for their spread into other large rivers and their tributaries across the Mississippi River Basin. Our study seeks to build upon limited existing published Round goby capture data in the IWW (19 total individuals from 2004 to 2017, Irons et al. Citation2006; Merry et al. Citation2018) by leveraging 4 years of sampling data from a multi-gear, stratified random sampling program spanning all navigational pools of the IWW, which yielded 1,507 new Round goby capture records from 2019 to 2022. Objectives of this study were to 1) describe the current distribution, relative abundance, and frequency of occurrence of Round goby in the IWW, and 2) determine the establishment status of Round goby across the IWW.
Materials and methods
Study site
The IWW is comprised of the CAWS, portions of the Des Plaines River, and the entire Illinois River (), and the hydrology of the IWW has been heavily modified from its natural state to control flooding and facilitate vessel navigation (Lian et al. Citation2012). The CAWS is a collection of rivers, canals, channels, and port facilities throughout the Chicago metropolitan area. These waterways collectively flow into the Des Plaines River, which eventually joins the Illinois River. As a main tributary to the Mississippi River, the Illinois River forms the majority of the >500-kilometer-long IWW that connects Lake Michigan and the Great Lakes Basin to the Mississippi River and Mississippi River Basin (). Together, these three elements of the IWW contain nine navigation lock and dam structures that separate the system into eight pools.
Figure 1. Map of the Chicago Area Waterway System (T.J. O’Brien, Lockport, and Brandon Road pools) to Lake Michigan, upper Illinois River (Dresden Island, Marseilles, and Starved Rock pools), and lower Illinois River (Peoria, La Grange, and Alton pools) sections of the Illinois Waterway sampled during 2019–2022 under the Multi-Agency Monitoring program. Locks and dams on the Illinois Waterway are represented by square symbols separating the navigational pools.
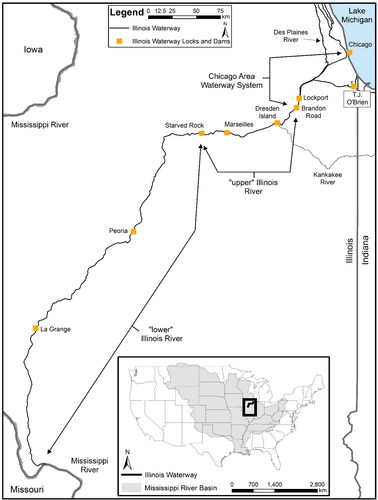
For the purpose of this study, we refer to the CAWS as only the Lockport and Brandon Road navigational pools, because no sampling occurred upstream in the T.J. O’Brien Pool/Calumet-Sag waterway complex (). The Illinois River comprises the six remaining navigational pools of the Illinois Waterway and flows into the Mississippi River near Grafton, Illinois (). The Illinois River is divided into two distinct geomorphic regions, the upper Illinois River and lower Illinois River (Delong Citation2005; McClelland et al. Citation2012). The upper Illinois River includes Dresden Island, Marseilles, and Starved Rock pools, and the lower Illinois River includes Peoria, La Grange, and Alton pools. The IWW shares two main connections to Lake Michigan, at the Chicago Lock & Dam and at the Calumet River upstream of T.J. O’Brien Lock & Dam (). These two connections are the likely source of the IWW Round goby invasion.
Data collection
Round gobies were collected from all pools of the IWW from 2019 to 2022 through the Multi-Agency Monitoring (MAM) program which used a stratified, random sampling design ( and ). River strata (hereafter referred to as main-channel, side-channel, and backwater [fully contiguous] habitat) in each navigational pool were designated according to Wilcox (Citation1993). Other than a few exceptions, all three habitat types were present in pools and were subsequently sampled by the MAM program (). The MAM program uses four gear types, including 15-minute, daytime boat-pulsed, direct-current electrofishing runs, 24-h sets of miniature (hereafter mini; 3-mm bar mesh) and regular fyke nets (18-mm bar mesh), and 48-h sets of paired large- (37-mm bar mesh) and small-hoop (18-mm bar mesh) nets each baited with 3 kg of soybean cake. The sampling design occurs over three, separate 6-week, sampling time periods that spanned June 15 – October 31. Sampling was modeled after the Upper Mississippi River Restoration Program’s Long Term Resource Monitoring element, and more detailed information on sampling and gear specifications can be found in their standard operating procedures manual (Ratcliff et al. Citation2014). All Round goby catch and length (mm) data presented in this study came from the MAM program, which was not designed to target Round goby but to monitor the entire fish community. Round goby >100-mm in length were measured to the nearest mm and all round goby <100-mm were grouped into 10-mm length bins.
Table 1. Target effort for each sampling gear, sampling time period, and habitat type (MCB = main-channel border, SCB = side-channel border, BWC = backwater (fully contiguous) for each Pool of the Illinois Waterway.
Statistical analysis
Distribution of Round goby and relative abundance in the IWW were determined using MAM daytime electrofishing runs and mini fyke net sets because these were the gears that successfully (only one Round goby was caught by a different sampling gear) captured Round goby from 2019 to 2022. Though daytime electrofishing runs and mini fyke net sets may have gear-specific selectivity attributes, data from these two gears were combined to determine presence/absence (i.e. capture/non-capture) and infer distribution, as we considered these two gears’ strengths and weaknesses for Round goby detection similar enough for collective comparison. However, relative abundance was estimated with mini fyke nets only, and catch per unit effort (CPUE) was calculated as one, 24-h net set representing one unit of effort.
A particular pool or section of the IWW was classified as established when it satisfied three criteria: 1) detection of multiple life stages in 1 year, 2) detection of a given life stage in consecutive years, and 3) consistent or increasing CPUE across years. We chose these criteria because it can be difficult to determine whether an invader has become established – defined as the persistence of an immigrant population through local reproduction and recruitment (Vermeij Citation1996) – because populations can appear to maintain themselves by being continually recharged with dispersers from nearby populations (Vermeij Citation1996). The life history of Round goby further complicates this scenario given larvae are known to passively disperse downstream via drift (Janáč et al. Citation2013), so presence of juveniles does not necessarily indicate reproduction.
We considered two distinct life stages: young-of-year (YOY) and adult, defined by a total length cutoff of 47-mm. This cutoff was selected by performing Gaussian mixture analysis using the R packages ‘diptest’ (Maechler Citation2021) and ‘mixtools’ (Benaglia et al. Citation2009), placing the threshold at the maximum distance between the means of the two-component length-frequency distributions identified by the analysis (see Supplemental Material section; Figure S1). Our use of ‘adult’ here does not necessarily describe an individual’s sexual maturity, but rather its likely status as a year 1+ fish and helps contextualize whether it arrived at a downstream site by passive larval drifting (see Janáč et al. Citation2013) or through bold pioneering behavior (see Brownscombe et al. Citation2012) that expands the invasion front.
Whether we captured only YOY at a site, only adults at a site, or both life stages at a given site may be relevant to inferring establishment status of Round goby at that site or in that pool. For example, if only YOY are captured at a site, we may know little about the establishment status of fish in that area, as YOY-only presence may indicate passive drifting of YOYs (Janáč et al. Citation2013) into this area, or it may indicate that successful reproduction has happened. Similarly, if only adults are captured at a site, this may indicate that a ‘bold’ large-bodied adult has colonized a new area (or was forced to expand its range by competition, Brownscombe et al. Citation2012), or it may indicate that the area is supporting reproductive-aged individuals that were hatched there and have overwintered. However, if both YOY and adults are captured in an area, it may be the best indicator that the area is supporting a multi-life-stage, reproducing, self-sufficient, and therefore established population of Round goby. Additionally, strong trends in the ratio of YOY-only captures to adult-only captures from upstream to downstream may give insight into which life stage is pushing the invasion front forward – passively drifting larvae or large-bodied adults.
Lastly, we visualized the spatial distribution of sampling effort and capture of life stages of Round goby in each pool as an additional way of supporting establishment outside of our formal criteria. This is useful in a scenario where the pool-specific establishment criteria were satisfied – but at the lowest level possible. If establishment data come from a single area within a pool (with high pool-wide spatial sampling coverage) then we considered this as secondary support that a population was established, just not broadly distributed. Statistical analyses and data visualization were performed using R Statistical Software (version 4.0.5; R Core Team Citation2022) and ArcGIS Desktop (version 10.8.1; ESRI Citation2020).
Results
In total, 4,485 sites (n = 2,701 boat electrofishing sites; n = 1,784 mini fyke net sites) were sampled across all IWW pools during 2019–2022, resulting in the capture of 1,507 Round gobies ( and ). Of the Round gobies captured, 12.4% (187 of 1,507) were captured using boat electrofishing and 87.6% (1,320 of 1,507) were captured in mini fyke nets. Round gobies were captured each year in all pools comprising the CAWS and upper Illinois River portions of the IWW ( and ). Round gobies were captured each year in the lower Illinois River, although Round gobies were only captured in 2 of 4 years (2019, 2021) in La Grange Pool, and no Round gobies were captured in Alton Pool ( and ).
Figure 2. Map of 2019–2022 Round goby captures from Lockport, Brandon Road, and Dresden Island pools of the Illinois Waterway. Note that RM stands for river mile.
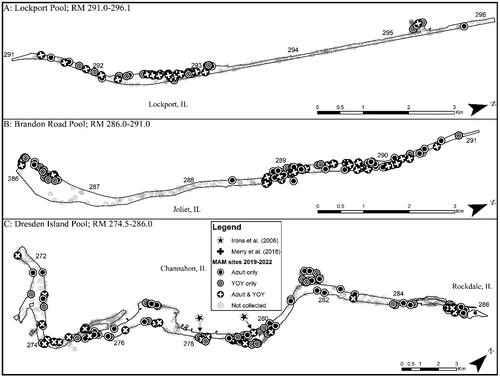
Figure 3. Map of 2019–2022 Round goby captures from Marseilles and Starved Rock pools of the Illinois Waterway. Note that RM stands for river mile.
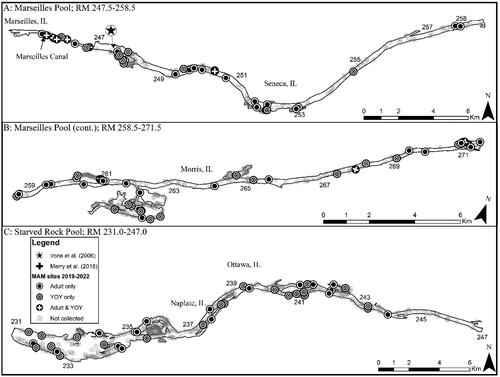
Figure 4. Map of 2019–2022 Round goby captures from Peoria Pool of the Illinois Waterway. Note that RM stands for river mile.
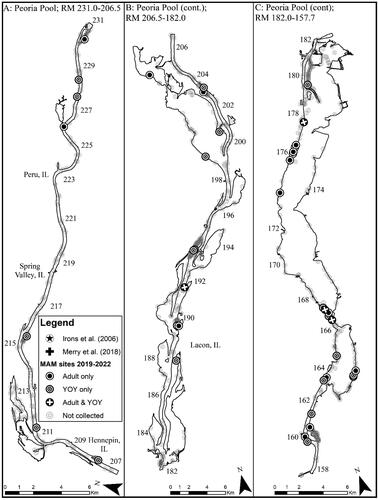
Figure 5. Map of 2019–2022 Round goby captures from La Grange Pool of the Illinois Waterway. Note that RM stands for river mile.
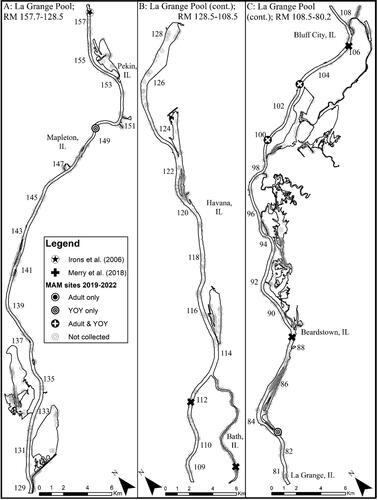
Figure 6. Map of 2019–2022 Round goby captures from Alton Pool of the Illinois Waterway. Note that RM stands for river mile.
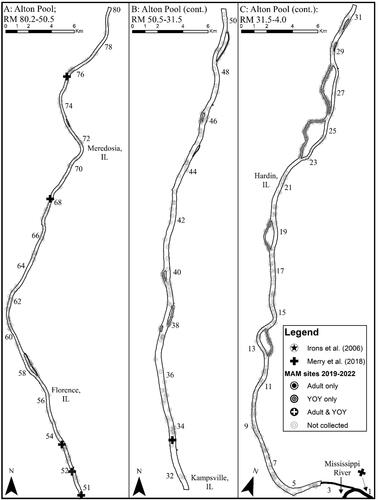
Table 2. Sampling effort (Total sites), total catch of Round goby (Total RDGY), frequency of occurrence (Occurrence [%]), and mean catch-per-unit-effort (Mean CPUE ± SE) for each Pool of the Illinois Waterway by year. Also included is the ratio of the number of sites at which we captured ‘adults’ only to the number of sites at which we captured ‘YOY’ only (adult-only:YOY-only); undefined ratios (i.e. with a zero denominator) are indicated by (und.).
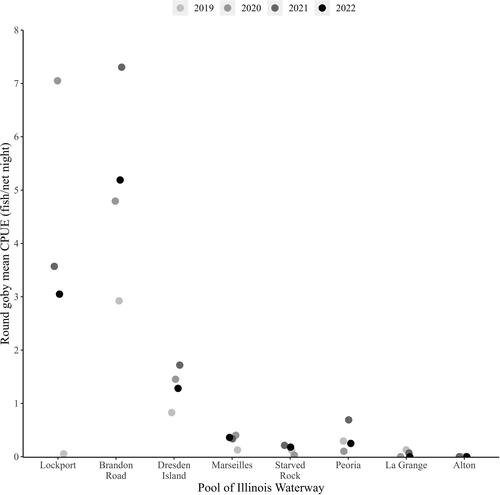
Mean CPUE (± SE) of Round goby across all years was highest in the CAWS (4.25 ± 0.72), followed by the upper Illinois River (0.59 ± 0.08) and the lower Illinois River (0.15 ± 0.04; , and ). Brandon Road Pool (5.07 ± 0.74) had the highest CPUE and La Grange Pool (0.05 ± 0.03) had the lowest (besides Alton where none were captured), and mean CPUE was generally highest upstream and followed a decreasing gradient from upstream to downstream in the IWW ( and ). There was variability in pool-specific mean CPUE across years, but there was a sustained increase in mean CPUE when comparing the first and last year of collection for all pools in the CAWS and upper Illinois River. As an example, if we compared mean CPUE in 2022 vs. 2019, there was a 61.0-fold increase in Lockport Pool, 1.8-fold increase in Brandon Road Pool, 1.5-fold increase in Dresden Island Pool, 2.8-fold increase in Marseilles Pool, and 1.5-fold increase in Starved Rock Pool ( and ).
Figure 7. Round goby catch-per-unit-effort (CPUE) across the eight navigational pools of the Illinois Waterway. The circles representing CPUE values are offset to reduce overlap.
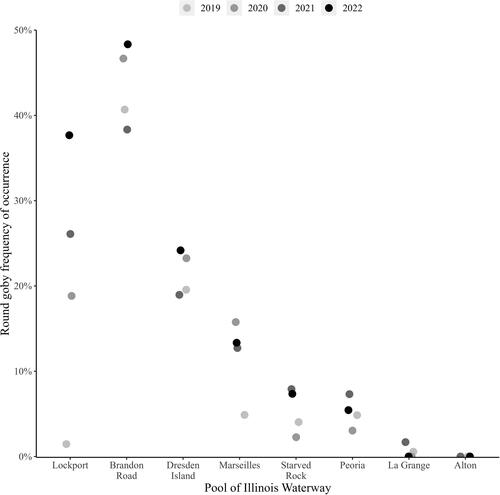
Frequency of occurrence (mean ± SE) generally followed a similar decreasing trend of mean CPUE from upstream to downstream (, and ). Mean frequency of occurrence across years was highest in the CAWS (31.43 ± 4.72), followed by the upper Illinois River (12.33 ± 1.23), and then the lower Illinois River (2.27 ± 0.48; and ). Pool-specific frequency of occurrence across years was highest upstream and decreased moving downstream in the IWW, although Brandon Road Pool frequency of occurrence was higher than Lockport Pool ( and ). Frequency of occurrence across years was highest in Brandon Road Pool (43.5 ± 2.38) and lowest in La Grange Pool (0.56 ± 0.40; and ). Lockport and Marseilles pools showed sustained increases in annual frequency of occurrence in 2020–2022 compared with 2019, similar to sustained increases in CPUE for these pools (, and ). Brandon Road and Dresden Island pools also showed sustained increases in frequency of occurrence but to a lesser extent (i.e., smaller increase and/or only in 2021–2022 and not 2020) than Lockport or Marseilles pools (). For example, if we compared annual frequency of occurrence in the first and last year of study in the CAWS and upper Illinois River, there was a 26.0-fold increase in Lockport Pool, 1.2-fold increase in Brandon Road Pool, 1.2-fold increase in Dresden Island Pool, 2.7-fold increase in Marseilles Pool, and 1.8-fold increase in Starved Rock Pool ().
Figure 8. Round goby frequency of occurrence (%) across the eight navigational pools of the Illinois Waterway. The circles representing frequency of occurrence values are offset to reduce overlap.
The CAWS had a relatively large and stable proportion of sites (12-25%) where both life stages were captured across all years (). One notable exception was Lockport Pool, the upstream-most pool in our study, which had a surprisingly low proportion of capture of both life stages (together and separately) in 2019 considering its closest proximity to the likely source of the invasion. However, Lockport Pool’s proportion of capture of all life stage categories quickly increased to rival the other CAWS pool, Brandon Road, by 2021. The proportion of sites where both life stages were captured was moderate in the upper Illinois River and relatively low in the lower Illinois River, which had pool-specific annual proportions <5% for captures of both life stages together as well as captures of ‘YOY only’ and ‘adult only’. Across all pools, proportions of captures of all life stage categories were variable year-to-year, but generally held a trend of decreasing proportions from upstream to downstream. There was no consistent upstream-to-downstream trend in the pool-specific ratio of the ‘adult only’ to ‘YOY only’ captures ( and ).
Figure 9. The proportion of sites where only adult (adult only), adult and young-of-year (adult and YOY), and only YOY (YOY only) Round goby were captured in all pools of the IWW from 2019 to 2022.
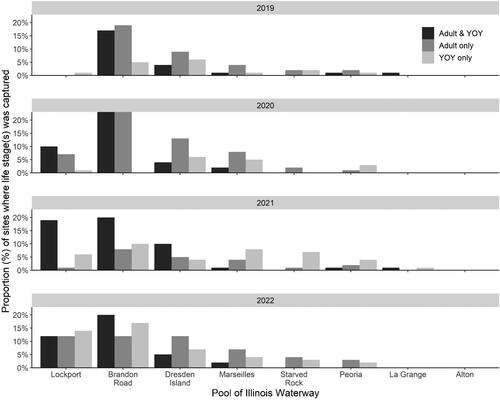
Based on our criteria of species establishment requiring that multiple life stages are detected in a single year, detection of a given life stage in consecutive years, and that CPUE is consistent or increasing across years, we concluded that there are established, self-sustaining Round goby populations in the CAWS, upper Illinois River, and one pool of the lower Illinois River of the IWW (). The Round goby has established a robust population in both the Lockport and Brandon Road pools of the CAWS relative to the other two sections of the IWW (). The established areas of the upper Illinois River vary considerably across pools, with larger relative abundances being found in the most upstream pool (i.e., Dresden Island Pool) and relative abundance decreasing downstream into Marseilles and Starved Rock pools (). Regardless of differences in relative abundance, the CAWS and upper Illinois River appear to have widespread Round goby establishment throughout all respective pools ( and ). For the lower Illinois River, Peoria Pool represents the only pool with an established population by our criteria; relative abundance was much lower than in the CAWS and upper Illinois River except for Starved Rock Pool (). Even though Peoria Pool has fewer Round goby than most upstream pools, capture locations indicate Round goby are widespread throughout Peoria Pool (). Unlike Alton Pool where no captures occurred, Round gobies were captured in the La Grange Pool, but captures were inconsistent among years (2 of 4 years) and a decreasing CPUE led us to conclude that the species has not become established in La Grange Pool (). The few captures of Round goby in La Grange Pool were not clustered in a single area, providing no secondary support that a small, localized establishment may exist ().
Discussion
Our findings provide a spatially comprehensive understanding of the distribution, relative abundance, and pool-specific establishment of Round goby in the IWW by leveraging 4 years of monitoring data from a stratified, random sampling program. We documented Round goby throughout the entire IWW, except in Alton pool – the farthest downstream navigational pool. Relative abundance of Round goby in the IWW was typically highest in the CAWS and then decreased downstream through the upper- and lower Illinois River. Large, sustained increases in mean CPUE and frequency of occurrence (when comparing 2020–2022 vs. 2019) suggest that Round gobies have recently become more abundant and widespread across the IWW. Based on our criteria, the Round goby is established and self-sustaining in all three major sections of the IWW. This includes establishment in all pools within the CAWS and upper Illinois River but was limited to only Peoria Pool in the lower Illinois River.
Over the 30 years of invasion history of Round goby in the IWW there have been published species records from the CAWS, Dresden Island, Marseilles, La Grange, and Alton pools (Charlebois et al. Citation1997; Irons et al. Citation2006; Merry et al. Citation2018; Happel and Gallagher Citation2021) and reports of captures throughout most of the IWW (Freedman et al. Citation2023). However, the use of differing sampling gears, protocols, and lack of capture and sampling effort data have excluded any comprehensive temporal or spatial comparisons of Round goby establishment status, distribution, relative abundance, and frequency of occurrence. Our comprehensive dataset showed that the Round goby is established in – and distributed throughout – most pools of the IWW other than the La Grange and Alton pools. No Round gobies were captured in the Alton Pool during this study, but six Round gobies were captured in the Alton Pool during 2014–2017 (Merry et al. Citation2018). Merry et al. (Citation2018) stated that it remained unknown whether the Round goby was established in the Alton Pool, with our results supporting that the Round goby has not yet become established in the Alton Pool.
Round goby CPUE and frequency of occurrence have increased considerably since 2019 for the CAWS and upper Illinois River but not the lower Illinois River. Regardless of recent increases, Round goby CPUE (Lockport = 3.05; Brandon Road = 5.19; Dresden Island = 1.28; Marseilles = 0.36) in these sections was generally towards the lower end of published literature values when comparing our most recent data (i.e., typically highest CPUE across study years) Round goby CPUE values among studies that used similar small-mesh fyke nets. For example, in Lake Michigan, mean CPUE (± SE) of Round goby in the nearshore zone was 4.8 ± 1.8 (Smith and Simpkins Citation2017), while CPUE in the Beaver Island Archipelago ranged from 13.2 ± 8.7 in open habitats to 48.5 ± 32.8 in vegetated habitats (Coulter et al. Citation2012). In Lake Huron, Round goby CPUE in Le Cheneaux Islands ranged from 4.4 ± 2.6 (open habitats) to 4.8 ± 2.1 (vegetated habitats), while it ranged from 0.04 ± 0.04 (open habitats) to 2.8 ± 1.3 (vegetated habitats) in Saginaw Bay (Coulter et al. Citation2012). Lastly, Round goby CPUE (30 ± 9.3) also was considerably higher in Oneida Lake, New York compared with the IWW (Brooking et al. Citation2022).
We identified three plausible reasons that may explain why Round gobies in the IWW are generally less abundant than invasive Round goby populations in other areas and why we found a decreasing trend in abundance and frequency of occurrence along an upstream to downstream gradient in the IWW. First, the lack of consistent hard substrate throughout the entire IWW (Delong Citation2005; Kornis et al. Citation2012, and references therein) may be limiting Round goby presence because they preferentially use hard substrate (most commonly abundant in rocky substrates) to reproduce, feed, and as refugia. Soft substrates have not been resistant to invasion, although soft substrates appear to be less hospitable to Round goby (Ray and Corkum Citation2001; Young et al. Citation2010; Kornis et al. Citation2012). For example, the proportion of sampled sites in this study that contained hard substrate in the CAWS and upper Illinois River was between 35-50% but decreased precipitously to <10% in Peoria Pool and <5% in La Grange and Alton pools (data not presented). Second, zebra mussels Dreissena polymorpha – a major food source for Round goby >50-mm total length (Janssen and Jude Citation2001; Kornis et al. Citation2012, and references therein) – only sporadically colonize and are patchily distributed downstream of the CAWS due to complex recruitment dynamics requiring that competent larvae from upstream source populations settle out in the presence of suitable habitat and environmental conditions (Stoeckel et al. Citation1997). A lack of this major food resource that is plentiful in much of the round gobies native and invaded range may account for higher abundances in the IWW being associated, at least in part, with habitats containing more zebra mussels. However, the diverse diet among Round goby populations is one attribute that has facilitated the species ability to invade new areas (Kornis et al. Citation2012, and references therein), and we have no data to suggest that non-mussel benthic resources are limiting to round goby populations in the IWW. Finally, Round goby in the lower Illinois River portion of the IWW (representing the most southern populations of Round goby in North America) experience mean summer surface water temperatures approaching 28 °C (mean of water temperature across sample sites, data not presented), which is near the upper limit of 28.9 °C for positive growth (Lee and Johnson Citation2005). Round goby CPUE and frequency of occurrence in this study was highest in the CAWS and Dresden Island Pool of the upper Illinois River, which coincides with habitats featuring a higher proportion of hard substrate, higher densities of zebra mussels (with a more consistent distribution), and 2-3 °C lower surface water temperature (relative to other portions of the IWW) that may be more favorable for Round goby populations.
There remains debate as to which sampling techniques (i.e., passive or active) and gears are most effective in capturing Round goby – especially when sampling large non-wadeable rivers (Kornis et al. Citation2012) such as the IWW. The long-term MAM program leveraged for this study used multiple gear types (including small-mesh, mini fyke nets, regular fyke nets, large- and small hoop nets, and standardized daytime electrofishing runs) to provide community-wide sampling coverage. Though not specifically designed to optimize Round goby capture, the MAM program’s intensive sampling effort spanned across multiple seasons and habitat strata, providing data that are directly comparable through space and time. Small-mesh fyke nets used for CPUE in our study have been commonly used to target Round goby (e.g. Cooper et al. Citation2007; Coulter et al. Citation2012; Brooking et al. Citation2022; Herlevi et al. Citation2023) and have been found to be more effective for capturing Round goby than beach seining and pulsed-direct-current-boat electrofishing in shallow nearshore areas in lakes (Breen and Ruetz Citation2006; Ruetz et al. Citation2007). Conversely, a study in non-wadeable habitats of Lake Michigan found that bottom trawling effectively captured Round goby (Clapp et al. Citation2001). Furthermore, the six Round gobies captured in the Alton Pool by Merry et al. (Citation2018) were captured using benthic trawls (i.e., bow trawls). Merry et al. (Citation2018) found that the bow trawl outperformed (i.e., only gear to capture Round goby) the push trawl, mini fyke net, and minnow trap in the Alton Pool, but CPUE for bow trawling was still low (0.76-2.58 fish/hr). Although we did not capture Round goby in the Alton Pool of the IWW, it is our opinion that the MAM program’s sampling approach performed well in describing the ecosystem-scale status of Round goby in the IWW despite its community-sampling design.
Our multi-faceted approach in determining whether Round goby was established in each IWW pool considered the presence/absence of different life stages (i.e., YOY and adult). These life stage assignments were based on total length cutoffs derived from a Gaussian mixture analysis of all Round goby captured in our study (Figure S1). We viewed using this analysis as an improvement over naively using length cutoffs determined for other populations, considering Round goby length-at-age varies greatly by site (Kornis et al. Citation2012), and the IWW is somewhat a novel ecosystem for Round goby. Our 47-mm length cutoff was similar to other freshwater populations of Round goby (range = 44.0-54.5 mm total length) that determined length at age-0 (i.e., YOY) by aging otoliths (Simonovic et al. Citation2001; Johnson et al. Citation2005; Huo et al. Citation2014). We recommend direct aging of otoliths when determining length cutoffs for a particular life stage or age when possible, though the Gaussian mixture analysis approach provided a lower-effort alternative.
The Round goby has invaded many river systems worldwide, but the invasion in the IWW represents the first invasion of a large river in the Mississippi River Basin, USA. This study focused on aspects of abundance and establishment in the IWW, but determining the best habitat (e.g. main channel, side channel, backwater), substrate type, and sampling time period for maximizing catch of Round goby would aid in early detection and monitoring efforts in other large rivers. Future research is needed to document Round goby age and growth in the IWW for comparison among populations given the variability of Round goby life history across its invaded range. Additionally, it would be beneficial to determine whether Round goby in the IWW – a very different habitat from Lake Michigan – differ genetically and/or morphologically from nearby Lake Michigan. Although the relative abundance and potential impact of Round goby on the IWW currently may be low, Round goby can have profound impacts on invaded ecosystems. It remains unknown whether Round goby will thrive in the Mississippi River and its tributaries and have undesirable ecosystem effects, but the highly adaptable Round goby will likely continue to spread, so understanding its continued expansion throughout North America and its large river systems will better inform future management in invaded ecosystems.
Supplemental Material
Download MS Word (127.9 KB)Acknowledgments
The authors thank all agencies and individuals that conduct sampling for or provide data to the Multi-Agency Monitoring program on the IWW. We also thank the reviewers and editorial staff at the Journal of Freshwater Ecology for greatly improving this manuscript. The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the funding source.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
These data that support the findings of this study are available from the corresponding author [BSH] upon request.
Additional information
Funding
References
- Astorg L, Charette C, Windle MJ, Derry AM. 2022. Two decades since first invasion: revisiting round goby impacts on nearshore aquatic communities in the Upper St. Lawrence River. J Great Lakes Res. 48(2):1–18. doi: 10.1016/j.jglr.2022.01.017.
- Benaglia T, Chauveau D, Hunter DR, Young D. 2009. mixtools: an R package for analyzing finite mixture models. J Stat Soft. 32(6):1–29. doi: 10.18637/jss.v032.i06.
- Bergstrom MA, Mensinger AF. 2009. Interspecific resource competition between the invasive round goby and three native species: logperch, slimy sculpin, and spoonhead sculpin. Trans Am Fish Soc. 138(5):1009–1017. doi: 10.1577/T08-095.1.
- Breen MJ, Ruetz CR.III 2006. Gear bias in fyke netting: evaluating soak time, fish density, and predators. N Am J Fish Manage. 26(1):32–41. doi: 10.1577/M05-013.1.
- Brooking TE, Rudstam LG, Jackson JR, VanDeValk AJ, Holeck KT, Hotaling CW, Cooper JE. 2022. Effects of round goby on the benthic invertebrate community and on diets and growth of yellow perch and white perch in Oneida Lake, New York. Trans Am Fish Soc. 151(5):641–654. doi: 10.1002/tafs.10377.
- Brownscombe JW, Fox MG, Marentette JR, Reddon AR, Groen M, Sopinka NM, Marsh-Rollo SE, Balshine S. 2012. Is there a role for aggression in round goby invasion fronts? Behaviour. 149(7):685–703. doi: 10.1163/1568539X-00002998.
- Brush JM, Fisk AT, Hussey NE, Johnson TB. 2012. Spatial and seasonal variability in the diet of round goby (Neogobius melanostomus): stable isotopes indicate that stomach contents overestimate the importance of dreissenids. Can J Fish Aquat Sci. 69(3):573–586. doi: 10.1139/f2012-001.
- Cerwenka AF, Alibert P, Brandner J, Geist J, Schliewen UK. 2014. Phenotypic differentiation of Ponto-Caspian gobies during a contemporary invasion of the upper Danube River. Hydrobiologia. 721(1):269–284. doi: 10.1007/s10750-013-1668-5.
- Charlebois PM, Marsden JE, Goettel RG, Wolfe RK, Jude DJ, Rudnika S. 1997. The Round Goby, Neogobius melanostomus (Pallas): a review of European and North American literature. Champaign (IL): Illinois-Indiana Sea Grant Program and Illinois Natural History Survey. INHS Special Publication 20. p. 76.
- Clapp DF, Schneeberger PJ, Jude DJ, Madison G, Pistis C. 2001. Monitoring round goby (Neogobius melanostomus) population expansion in eastern and northern Lake Michigan. J Great Lakes Res. 27(3):335–341. doi: 10.1016/S0380-1330(01)70649-1.
- Clark KH, Iwanowicz DD, Iwanowicz LR, Mueller SJ, Wisor JM, Bradshaw-Wilson C, Schill WB, Stauffer JR, Jr., Boyer EW. 2022. Freshwater unionid mussels threatened by predation of Round Goby (Neogobius melanostomus). Sci Rep. 12(1):12859. doi: 10.1038/s41598-022-16385-y.
- Cooper MJ, Ruetz IC, Uzarski DG, Burton TM. 2007. Distribution of round gobies in coastal areas of Lake Michigan: are wetlands resistant to invasion? J Great Lakes Res. 33(2):303–313. doi: 10.3394/0380-1330(2007)33[303:DORGIC]2.0.CO;2.
- Corkum LD, Sapota MR, Skora KE. 2004. The round goby, Neogobius melanostomus, a fish invader on both sides of the Atlantic Ocean. Biol Invasions. 6(2):173–181. doi: 10.1023/B:BINV.0000022136.43502.db.
- Coulter DP, Murry BA, Uzarski DG. 2012. Use of wetland versus open habitats by round gobies in lakes Michigan and Huron: patterns of CPUE, length, and maturity. J Great Lakes Res. 38(3):439–444. doi: 10.1016/j.jglr.2012.06.001.
- Delong MD. 2005. Upper Mississippi River Basin. In: Beke AC, Cushing CE, editors. Rivers of North America. Burlington (MA): Elsevier Academic Press; p. 327–374.
- ESRI. 2020. ArcGIS Desktop: release 10.8.1. Redlands (CA): Environmental Systems Research Institute.
- Freedman JA, Fuller P, Benson A, Maynard E, Neilson ME, Larson J, Fusaro A, Sturtevant R. 2023. Neogobius melanostomus (Pallas, 1814): U.S. Geological Survey. Gainesville (FL): Nonindigenous Aquatic Species Database. https://nas.er.usgs.gov/queries/factsheet.aspx?SpeciesID=713., Revision Date: 4/26/2022, Peer Review Date: 12/5/2019, Access Date: 2/10/2023
- Happel A, Gallagher D. 2021. Chicago’s fish assemblage over ∼30 years – more fish and more native species. Urban Ecosyst. 24(2):311–325. doi: 10.1007/s11252-020-01020-3.
- Herlevi H, Kihlberg IW, Aarnio E, Bonsdorff E, Florin A-B, Ljung W, Lundström K, Mattila J, Östman Ö. 2023. Environmental abundances of the non-native round goby Neogobius melanostomus influence feeding of native fish predators. J Fish Biol. 102(6):1340–1357. doi: 10.1111/jfb.15380.
- Huo B, Madenjian CP, Xie CX, Zhao Y, O’Brien TP, Czesny SJ. 2014. Age and growth of round gobies in Lake Michigan, with preliminary mortality estimation. J Great Lakes Res. 40(3):712–720. doi: 10.1016/j.jglr.2014.07.003.
- Irons KS, McClelland AM, Pegg MA. 2006. Expansion of round goby in the Illinois Waterway. Am Midl Nat. 156(1):198–200. doi: 10.1674/0003-0031(2006)156[198:EORGIT]2.0.CO;2.
- Janáč M, Šlapanský L, Valová Z, Jurajda P. 2013. Downstream drift of round goby (Neogobius melanostomus) and tubenose goby (Proterorhinus semilunaris) in their non-native area. Ecol Freshwater Fish. 22(3):430–438. doi: 10.1111/eff.12037.
- Janssen J, Jude DJ. 2001. Recruitment failure of mottled sculpin Cottus bairdi in Calumet Harbor, southern Lake Michigan, induced by the newly introduced round goby Neogobius melanostomus. J Great Lakes Res. 27(3):319–328. doi: 10.1016/S0380-1330(01)70647-8.
- Johnson TB, Bunnell DB, Knight CT. 2005. A potential new energy pathway in central Lake Erie: the round goby connection. J Great Lakes Res. 31:238–251. doi: 10.1016/S0380-1330(05)70317-8.
- Jude DJ, Reider RH, and Smith GR. 1992. Establishment of Gobiidae in the Great Lakes basin. Can J Fish Aquat Sci. 49(2):416–421. doi: 10.1139/f92-047.
- Kalamarz-Kubiak H, Guellard T. 2019. Oocyte hydration in round goby Neogobius melanostomus from the Gulf of Gdańsk: another invasive strategy? Oceanologia. 61(4):534–539. doi: 10.1016/j.oceano.2019.06.001.
- Kornis MS, Mercado-Silva N, Vander Zanden MJ. 2012. Twenty years of invasion: a review of round goby Neogobius melanostomus biology, spread, and ecological implications. J Fish Biol. 80(2):235–285. doi: 10.1111/j.1095-8649.2011.03157.x.
- Kornis MS, Weidel BC, Vander Zanden MJ. 2017. Divergent life histories of invasive round gobies (Neogobius melanostomus) in Lake Michigan and its tributaries. Ecol Freshwater Fish. 26(4):563–574. doi: 10.1111/eff.12300.
- Lee VA, Johnson TB. 2005. Development of a bioenergetics model for the round goby (Neogobius melanostomus). J Great Lakes Res. 31(2):125–134. doi: 10.1016/S0380-1330(05)70244-6.
- Lian Y, You J-Y, Sparks R, Demissie M. 2012. Impact of Human Activities to Hydrologic Alterations on the Illinois River. J Hydrol Eng. 17:537–546. doi: 10.1061/(ASCE)HE.1943-5584.0000465.
- MacInnis AJ, Corkum LD. 2000. Fecundity and reproductive season of the round goby Neogobius melanostomus in the upper Detroit River. Trans Am Fish Soc. 129(1):136–144. doi: 10.1577/1548-8659(2000)129<0136:FARSOT>2.0.CO;2.
- Maechler M. 2021. Diptest: Hartigan’s dip test statistic for unimodality – corrected. R package version 0.76-0. https://CRAN.R-project.org/package=diptest.
- McClelland MA, Sass GG, Cook TR, Irons KS, Michaels NN, O’Hara TM, Smith CS. 2012. The long-term Illinois River fish population monitoring program. Fisheries. 37(8):340–350. doi: 10.1080/03632415.2012.704815.
- Merry JL, Fritts MW, Bloomfield NC. 2018. Invasive Round Goby (Neogobius melanostomus) nearing the Mississippi River. Am Midl Nat. 180(2):p. 290–297.
- Pennuto CM, Krakowiak PJ, Janik CE. 2010. Seasonal abundance, diet, and energy consumption of round gobies (Neogobius melanostomus) in Lake Erie tributary streams. Ecol Freshw Fish. 19(2):206–215. doi: 10.1111/j.1600-0633.2010.00405.x.
- R Core Team. 2022. R: a language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing. https://www.R-project.org/.
- Ratcliff EN, Gittinger EJ, O’Hara TM, Ickes BS. 2014. Long term resource monitoring program procedures: fish monitoring. Reston (VA): U.S. Geological Survey.
- Ray WJ, Corkum LD. 2001. Habitat and site affinity of the round goby. J Great Lakes Res. 27(3):329–334. doi: 10.1016/S0380-1330(01)70648-X.
- Ruetz CR, IIIUzarski DG, Krueger DM, Rutherford ES. 2007. Sampling a littoral fish assemblage: comparison of small-mesh fyke netting and boat electrofishing. N Am J Fish Manage. 27(3):825–831. doi: 10.1577/M06-147.1.
- Simonovic P, Paunović M, Popović S. 2001. Morphology, feeding, and reproduction of the round goby, Neogobius melanostomus (Pallas), in the Danube River Basin, Yugoslavia. J Great Lakes Res. 27(3):281–289. doi: 10.1016/S0380-1330(01)70643-0.
- Smith BJ, Simpkins DG. 2017. A comparison of three paired modified fyke nets for characterizing fish assemblages in the nearshore zone of Lake Michigan. N Am J Fish Manage. 37(5):962–969. doi: 10.1080/02755947.2017.1336134.
- Stoeckel JA, Schneider DW, Soeken LA, Blodgett KD, Sparks RE. 1997. Larval dynamics of a riverine metapopulation: implications for zebra mussel recruitment, dispersal, and control in a large-river system. N Am Benthol Soc. 16(3):586–601. doi: 10.2307/1468146.
- Vélez-Espino LA, Koops MA, Balshine S. 2010. Invasion dynamics of round goby (Neogobius melanostomus) in Hamilton Harbour, Lake Ontario. Biol Invasions. 12(11):3861–3875. doi: 10.1007/s10530-010-9777-9.
- Vermeij GJ. 1996. An agenda for invasion biology. Biol Conserv. 78(1–2):3–9. doi: 10.1016/0006-3207(96)00013-4.
- Wickett RG, Corkum LD. 1998. OPINION-fisheries techniques essay–you have to get wet: a case study of the nonindigenous Great Lakes Fish, Round goby. Fisheries. 23(12):26–27.
- Wilcox DB. 1993 May. An aquatic habitat classification system for the Upper Mississippi River System. Onalaska (WI): U.S. Fish and Wildlife Service, Environmental Management Technical Center. EMTC 93-T003. 9 pp. + Appendix A. (NTIS PB93-208981).
- Young JA, Marentette JR, Gross C, McDonald JI, Verma A, Marsh-Rollo SE, Macdonald PD, Earn DJ, Balshine S. 2010. Demography and substrate affinity of the round goby (Neogobius melanostomus) in Hamilton Harbour. J Great Lakes Res. 36(1):115–122. doi: 10.1016/j.jglr.2009.11.001.
