Abstract
The health of the Dayang River ecosystem, critical freshwater resource for Dandong City, is intertwined with local water management and coastal development. In June 2023, we conducted a biodiversity survey in the Dayang River basin using a combined morphological and environmental DNA (eDNA) approach. We assessed both species and functional diversity of summer macroinvertebrates. A total of 81 macroinvertebrates species were identified, with some overlap in species detection between the methods. The trunk stream exhibited lower species diversity with uneven distribution across sites, whereas the tributaries displayed a more stable species composition. Functional diversity analysis mirrored this pattern, revealing lower functional diversity in the trunk stream, indicative of intense competition and niche occupation. Conversely, the tributaries displayed more uniform and complete functional diversity. This study highlights the differential influence of hydrological environments on macroinvertebrate communities during the summer season, with the trunk stream favoring certain species with specific habitat preferences and adaptations. Furthermore, it underscores the value of combining traditional morphological surveys and eDNA techniques for comprehensive biodiversity assessments.
1. Introduction
Freshwater ecosystems, crucial for ecological services such as climate regulation and biodiversity, are profoundly influenced by human activities. These interactions affect both the ecosystem’s internal dynamics and biodiversity levels (Irfan and Alatawi Citation2019; Damseth et al. Citation2024). Macroinvertebrates, recognized as keystone species within these ecosystems, are highly sensitive ecological indicators due to their limited migration and responsiveness to environmental changes (Tampo et al. Citation2021). Not only are they integral components of aquatic food webs, but their life activities, such as shredding, grazing, and bioturbation, also significantly influence material cycling and energy flow within freshwater ecosystems. This contributes directly to maintaining regional water quality, biodiversity, and ecological equilibrium (Allan et al. Citation2021).
While traditional morphology-based methods, such as the kick net method and Surber method, have played a crucial role in collecting samples of macroinvertebrates, these approaches have inherent limitations, including dependence on species visibility, life cycle stages, and the level of morphological identification (Orozco González Citation2023; Vourka et al. Citation2023). In contrast, Environmental DNA (eDNA) technology has emerged as a powerful complement in ecological monitoring, offering enhanced non-invasiveness, efficiency, and precision. eDNA detection relies on analyzing trace DNA released by organisms into the environment, overcoming several challenges posed by traditional methods (Gao et al. Citation2020). Nevertheless, the application of eDNA technology faces hurdles, such as the need for standardization, a lack of universal primers, and potential false-positive or false-negative results (Liu et al. Citation2023). Hence, integrating traditional morphology with eDNA techniques in ecosystem studies promises a more comprehensive and precise framework for ecological monitoring.
Dayang River, located in Liaoning Province in southeast China, is an important freshwater ecological corridor (Che Citation2014). Its biodiversity and ecological health are critical to maintaining regional water quality and ecosystem services. However, rapid economic development and urbanization have intensified the pressure on the Dayang River aquatic ecosystem, including population growth and related stressors (Sun et al. Citation2012; Zhao and Zhao Citation2015). In addition, due to the upcoming water conservancy project, the Dayang River is about to undergo important changes. This human intervention, combined with urbanization and facility construction, is expected to have a profound impact on river ecosystems, leading to changes in water quality, flow patterns and habitat, which in turn affect macroinvertebrate biodiversity and community structure. Therefore, an in-depth understanding of the major aquatic taxa of the Dayang River is essential for developing effective conservation strategies (Shi Citation1985). In order to meet this demand, this study comprehensively evaluated the biodiversity of summer macroinvertebrates in Dayang River Basin from three aspects: species, function and water environmental factors, and combined the traditional morphological investigation method with eDNA technology to provide important scientific data for protecting freshwater ecosystems and promoting regional sustainable development.
2. Materials and methods
2.1. Study sites
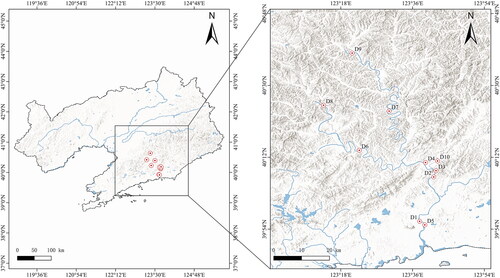
The Dayang River, located in the southeastern of the Liaoning Peninsula (122°51′ − 124°7′E, 39°49′ − 40°49′N), flows into the sea. It has a total length of 230 km and a watershed area of 1968.4 km2 (Wang Citation1993). The river provides crucial freshwater source for Anshan and Dandong Cities. Its topography slopes northward, and its basin features dense vegetation and minimal human disturbance. The habitat varies along the river’s course, with upper-reach mountain streams, mid-reach hilly curved channels, and lower-reach plains leading to the sea. Rainfall is concentrated primarily in the summer (Zheng and Wang Citation2006). In June 2023, this study established 10 sampling stations along the Dayang River six (D1-D6) along the trunk stream and four (D7-D10) on tributaries ().
2.2. Sample collection
For morphological sample collection, a D-net was used for qualitative sampling, while a Surber net (0.0625 m2 area, 420 μm mesh) was employed for quantitative sampling (Lin et al. Citation2024). Three parallel samples were collected per site. In the field, samples were mixed and purified using a 500 μm sieve. Visible macroinvertebrates were sorted immediately and preserved in a 75% alcohol (Wang et al. Citation2023). Water environmental factors were measured with a portable water quality analyzer (Hengxin® AZ-86031 CHN). In the laboratory, samples were identified to the species level under a dissecting microscope (Phenix® SMZ180-LB CHN) using specialized taxonomic references (Liu Citation1979; You Citation1995; Editorial Committee of Fauna Sinica CAOS Citation1996; Liu et al. Citation1999; Wang Citation2011; Chou Citation2014).
To collect Environmental DNA sample, 50 ml sterile sampling vials (ERTAREE® CHN) were used to collect mud samples at established Sampling sites. Two parallel samples were collected from each point, and stored in a 0-4 °C vehicle refrigerator (Philips® TB7101 CHN) (Wu et al. Citation2023). Within 6 h, samples were transferred to a −20 °C freezer for storage (ZhongkeXileng® DW-45L50 CHN) (Zhang et al. Citation2022).
2.3. eDNA technology and sequencing
eDNA experiments including extraction of eDNA and PCR of technology were conducted at a sequencing facility (Shanghai Shenggong Bioengineering Co., Ltd). This study employed universal primers designed for freshwater macroinvertebrates (Wang Citation2022; Yin Citation2023). The primers (Forward:5′-GGWACWGGWTGAACWGTWTAYCCYCC-3′);(Reverse:5′-TAAACTTCAGGGTGACCAAARAAYCA-3′) target variable regions of the mitochondrial cytochrome oxidase (CO1) gene, amplifying fragments of approximately 300-310 base pairs. Following high-throughput sequencing, DNA barcodes were uploaded to the National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov) for matching against reference databases and species identification. The resulting species information was cross-referenced with morphology data to confirm species-level taxonomy and generate the final OTU (Uchida et al. Citation2020).
2.4. Data processing and analysis
This study calculated the relative abundance of macroinvertebrate species within the Dayang River basin using data for both methodological surveys and eDNA analyses. Abundance calculations were performed at the genus level. For the morphological surveys, relative abundance represented the proportion of individuals of a given species within a genus to the total individuals in that genus. For eDNA, relative abundance reflected the proportion of sequences reads for a given species within a genus (Ji et al. Citation2022).
Species α diversity (within-community) and species β diversity (between-community) were computed for Dayang River macroinvertebrate communities. Alpha diversity indices included Shannon (H’), Simpson (D), observed species, Chao1, ACE, and Pielou index (Hou et al. Citation2024). Beta diversity was assessed using Jaccard similarity and Bray-Curtis distance, followed by permutation multifactor analysis of variance (PERMANOVA) to compare communities position between trunk stream and tributaries (Callisto et al. Citation2021). Principal Coordinate Analysis (PCoA) and (RDA) are conducted using the ‘vegan’ package in R (version 4.3.2).
Informed by relevant studies (Park et al. Citation2006; Poff et al. Citation2006; Zhang Citation2023) and the "A Database of Lotic Invertebrate Traits for North America" (https://pubs.usgs.gov/ds/ds187), 26 functional traits were categorized into five groups to assess the functional diversity of Dayang River macroinvertebrates. Indices included Functional Richness (FRic), Functional Evenness (FEve), Functional Divergence (FDiv), Functional Dispersion (FDis), and Rao’s Quadratic Entropy (RaoQ). Data visualization used ‘ggplot2’ and ‘gghalves’ packages in R (version 4.3.2), and ANOVA for multiple comparisons of alpha and functional diversity indices ().
Table 1. Groups and categories of functional traits.
3. Results
3.1. Water environmental factors
The pH values of the trunk stream samples ranged from 7.48 to 7.99, indicating relatively neutral water quality. However, the pH value of the tributary sample points is generally high, especially the pH value of the D9 sample point reaches 8.77, which indicates that the water quality is alkaline. In terms of total dissolved solids (TDS), there is a large difference between sampling points. In the trunk stream, D5 has the highest TDS value (127 ppm), while D6 has the lowest TDS value (56.7 ppm). In tributaries, D7 and D8 had relatively high TDS values of 153 ppm and 148 ppm respectively. For the dissolved oxygen (DO) levels, the DO level of D5 in the trunk stream was the highest (17.2 mg/L), while the DO level of D10 in the tributary was the lowest (6.21 mg/L). In general, the temperature of the trunk stream and the tributary was similar, mostly between 17.3 °C and 21.6 °C ().
Table 2. Water chemistry data of summer Dayang river.
3.2. Macroinvertebrate community composition
A combination of eDNA and morphological surveys revealed a total of 81 macroinvertebrate species across the the Dayang River Trunk stream and tributaries. These species are classified into 8 classes, 19 orders, 40 families, and 62 genera. The morphological survey identified 45 species (5 classes, 12 orders, 26 families, and 36 genera), while the eDNA survey detected 58 species (8 classes, 12 orders, 29 families, and 46 genera). Notably, 22 species were identified through both methods.
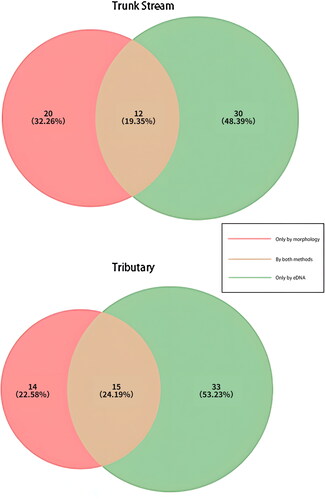
The two survey methods—morphological and environmental DNA (eDNA) analysis—exhibited a degree of overlap in the macroinvertebrate species they identified. This Venn diagram visually represents the synergistic nature of our approach, where the combination of both methods led to a more comprehensive assessment of biodiversity in the Dayang River Basin. Specifically, in the trunk stream, we detected a total of 62 macroinvertebrate species: 32 were identified through morphological analysis, 42 through eDNA analysis, and 12 species were detected by both methods, highlighting the additive value of integrating these techniques. A similar pattern was observed in the tributaries, where 62 species were identified in total, with 29 detected via morphology, 48 via eDNA, and 15 species identified by both methods. These findings underscore the importance of utilizing a multi-faceted survey strategy to capture the full extent of biodiversity present within an ecosystem ().
Figure 2. The distribution of macroscopic invertebrate species identified by (morphological) and environmental DNA (eDNA) surveys in the main channel and tributaries of the Dayang River.
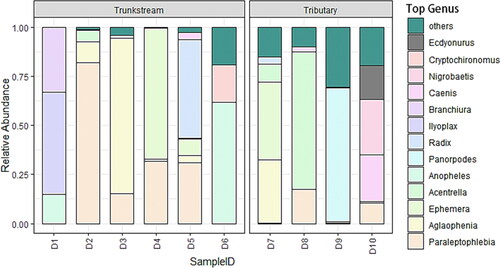
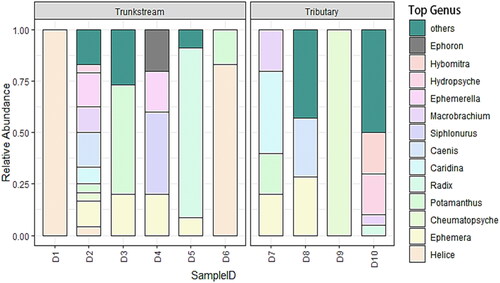
Depicted the relative abundance of macroinvertebrate genera based on the morphological survey. In the Dayang River Trunk stream, Radix was the most abundant genus (25%), followed by Potamanthus (13%), Ephemera (12%), and Helice (10%). The tributaries showed a different pattern, with Hybomitra and Hydropsyche both dominant (12% each) and Ephemera also relatively abundant (9%) ().
Figure 3. Morphological surveys on the genus level species composition of macroinvertebrates at various locations in the Dayang River.
Shows the relative abundance of macroinvertebrate genera in the Dayang River based on the eDNA survey. In the Trunk stream, Paraleptophlebia was the most abundant genus (47%), followed by Ephemera (25%), Radix (14.7%), and Aglaophenia (7.8%). The tributaries showed a different pattern, with Panorpodes most abundant (19%) and Acentrella at (12%) ().
3.3. Macroinvertebrate community diversity and structure
3.3.1. Alpha (α) diversity
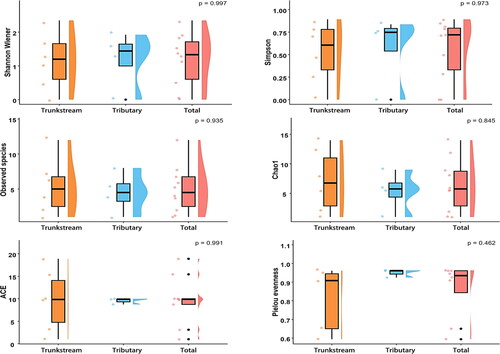
Alpha (α) diversity indices revealed no significant difference in species richness (p > 0.05) between the trunk stream and tributaries macroinvertebrate communities based on either morphology and eDNA surveys. However, morphology surveys indicated lower evenness in the trunk stream, with Shannon (H’), Simpson (D), and Pielou indices all lower compared to tributaries. eDNA surveys showed a similar trend of lower evenness in the trunk stream, reflected by the lower Shannon, Simpson, and Pielou indices ( and ).
3.3.2. Beta (β) diversity
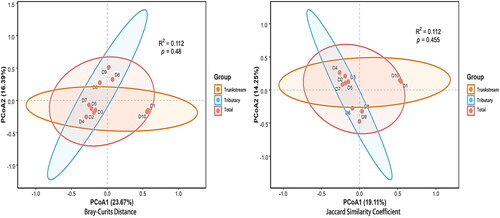
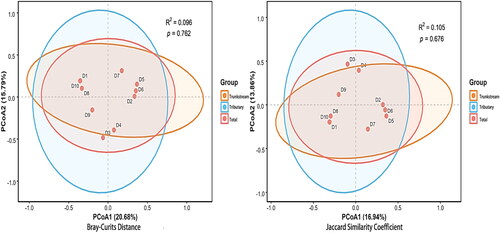
Comparison of macroinvertebrate communities between the Dayang River trunk stream and tributaries revealed differences in composition (beta diversity) using both morphology and eDNA surveys. Based on the morphology data set, PCoA with Bray-Curtis Distance and Jaccard Similarity indices explained 23.67% (p = 0.48, R2=0.122) and 19.11% (p = 0.455, R2=0.112) of the community variation, respectively. The eDNA survey revealed similar patterns: PCoA with Bray-Curtis Distance and Jaccard Similarity indices explained 20.68% (p = 0.762, R2=0.096) and 16.94% (p = 0.676, R2=0.105) of community variation, respectively ( and ).
3.3.3. Functional diversity
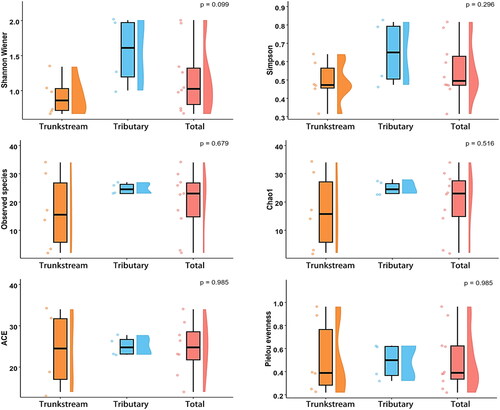
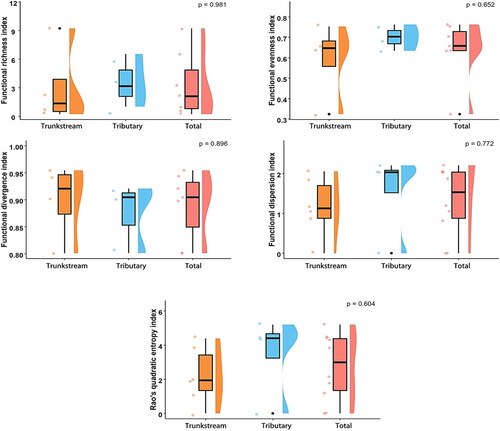
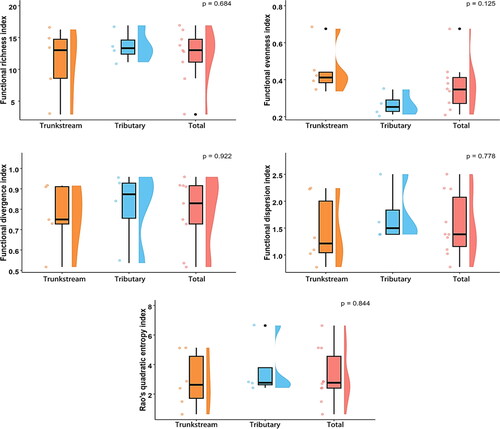
Morphological and environmental DNA (eDNA) surveys revealed no significant difference in overall functional diversity between the trunk stream and tributaries macroinvertebrate communities (p > 0.05). However, the trunk stream exhibited significantly higher functional divergence (FDiv) (0.90 ± 0.07) than the tributaries, indicating a greater diversity of resource utilization strategies in the trunk stream. Compared to the tributaries, the trunk stream had lower functional richness (FRic), functional evenness (FEve), functional dispersion (FDis), and quadratic entropy (RaoQ), suggesting lower functional trait diversity and evenness in the trunk stream ( and ).
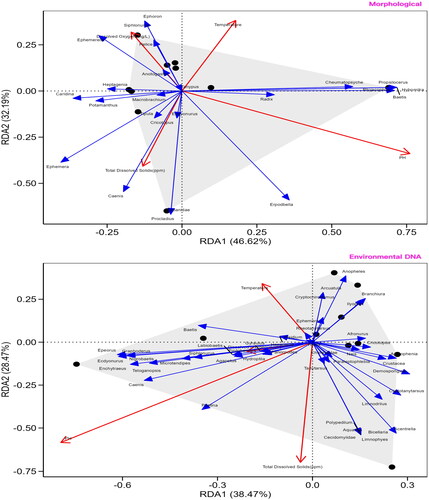
3.4. RDA analysis
In the data of morphological investigation, RDA1 axis explained 46.62% variation, while RDA2 axis explained 32.19% variation. Together, the two axes can explain the variation between species and environmental factors of about 78.81%. In the data investigated by environmental DNA technology, RDA1 axis explained 38.47% of the variation, while RDA2 axis explained 28.47% of the variation. This means that these two principal axes can explain about 66.94% of the total variation, and the RDA analysis charts of the two investigation methods have high interpretation rate ().
4. Discussion
This study combined morphological and environmental DNA (eDNA) surveys in the Dayang River basin, identifying 81 macroinvertebrate species. While both methods yielded unique insights, eDNA surveys detected more species, particularly crustaceans (e.g. Gastropoda, Bivalvia), which is consistent with previous findings (Kamil et al. Citation2021). Conversely, morphology excelled at identifying larger aquatic insect larvae (Hemiptera, Dragonflies, Troglodytes), aligning with the observations of Ntislidou et al. (Ntislidou et al. Citation2023). These findings highlight the complementary strengths of the two methods: eDNA surveys offer broader species detection, while morphology provides valuable information on individual size, age, and developmental status (Wang et al. Citation2021; Ji et al. Citation2022). The integration of both methods provides a more robust and comprehensive understanding of macroinvertebrate communities in aquatic environments.
In addition to species richness and functional diversity, water chemical parameters such as temperature, dissolved oxygen, total dissolved solids, and pH are also critical to the formation of macroinvertebrate communities. Through RDA analysis, that PH value had a certain impact on the overall macrobenthos in the DaYang River basin, but it was found that water temperature still had a strong regulatory ability on the representative species of the main stream and tributaries. These findings highlight the complex interaction between environmental factors and biological responses in aquatic ecosystems. Both morphology and eDNA surveys revealed Radix as the dominant macroinvertebrate in the trunk stream, with relative abundances of 25% and 14.7%, respectively. This dominance likely reflects its adaptation to warm summer temperatures (19.2 °C) (Huang et al. Citation2002). Radix species are known r-strategists with high reproductive rates and rapid growth under optimal conditions (Zhang et al. Citation2022). These traits likely contributed to their proliferation during the warm, rainy survey period. Conversely, tributaries were dominated by Cheumatopsyche, a genus typically associated with clean, well-oxygenated water bodies (Prommi et al. Citation2014). The lower water temperatures (18.3 °C) in tributaries likely provided suitable conditions for Cheumatopsyche larvae development and survival (Mochizuki Citation2008). Furthermore, Cheumatopsyche serves as an indicator of good water quality (Ge et al. Citation2022), suggesting healthier water conditions in tributaries, potentially due to their role as benthic predators utilizing abundant organic matter (Kobayashi et al. Citation2017; Hamid et al. Citation2021). These findings highlight how temperature, habitat quality, and life-history strategies interact to shape macroinvertebrate community composition within the Dayang River basin.
Despite higher observed species richness in the trunk stream, lower Shannon (H’), Simpson (D), and Pielou (J’) indices indicate uneven species distribution and potential dominance by a few taxa. Beta diversity analysis revealed significant variation in species composition along the trunk stream, compared to the more stable tributaries communities. This likely reflects the greater habitat heterogeneity within the trunk stream, encompassing mountainous headwaters, wider midsections, and brackish conditions near the sea (Dala-Corte et al. Citation2020). Summer rainfall further amplifies this environmental variability. Conversely, Dayang River tributaries exhibit more uniform habitat conditions, contributing to their relatively stable species composition (Kędzior et al. Citation2022; Szałkiewicz et al. Citation2022).
Functional diversity indices (FEve, FRic, FDis, RaoQ) were lower in the trunk stream, suggesting lower functional evenness and richness compared to tributaries. This indicates a potential for niche crowding and underutilized functional space in the trunk stream (Mason et al. Citation2005; Lam-Gordillo et al. Citation2021). Conversely, higher functional divergence (FDiv) in both habitats suggests a broader range of ecological roles. Seasonal precipitation likely intensifies selection for specific traits in the trunk stream, impacting functional diversity (Mesa Citation2012). The larger size and greater environmental heterogeneity of the trunk stream compared to tributaries (Ao et al. Citation2022) might further contribute to this pattern by limiting the development of certain functional groups. These findings suggest that habitat stability and environmental predictability may be crucial for maintaining functional diversity in aquatic ecosystems.
Despite the valuable insights gained, the limited overlap (28%) between eDNA and morphology highlights challenges associated with eDNA-based biodiversity assessments. These limitations likely stem from incomplete reference databases, OTU clustering biases, and environmental DNA degradation (Leray and Knowlton Citation2015; Rice et al. Citation2018). In addition, The focus on the Dayang River Basin during the summer season and the selection of specific water chemistry parameters may not fully capture the breadth of anthropogenic impacts. Addressing these issues will be crucial for refining eDNA methodologies and maximizing their effectiveness in watershed ecosystem monitoring. This study underscores the value of combining eDNA and morphology surveys for a more comprehensive understanding of macroinvertebrate communities. On the one hand, future studies should incorporate a broader range of environmental variables and extend the studies to different seasons to gain a more comprehensive understanding of the ecological effects of urbanization and river engineering. On the other hand, emphasis should be placed on improving eDNA reference databases, improving OTU clustering techniques, and exploring integrated methods to utilize the advantages of both methods while combining environmental variables to achieve more accurate and robust aquatic ecosystem monitoring.
Supplemental Material
Download MS Excel (15 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Allan JD, Castillo Uzcanga M, Capps K. 2021. Energy flow and nutrient cycling in aquatic communities. In: Stream ecology. Berlin, Germany: Springer; p. 357–381.
- Ao S, Ye L, Liu X, Cai Q, He F. 2022. Elevational patterns of trait composition and functional diversity of stream macroinvertebrates in the Hengduan Mountains region, Southwest China. Ecol Indic. 144:109558. doi: 10.1016/j.ecolind.2022.109558.
- Callisto M, Linares MS, Kiffer WP, Hughes RM, Moretti MS, Macedo DR, Solar R. 2021. Beta diversity of aquatic macroinvertebrate assemblages associated with leaf patches in neotropical montane streams. Ecol Evol. 11(6):2551–2560. doi: 10.1002/ece3.7215.
- Che YL. 2014. Analysis of hydrological characteristic of Dayang River Basin. Mod Agric Sci Technol. 7:264–265.
- Chou WG. 2014. Illustrated guide to benthic macroinvertebrates monitoring in the Liao River Basin. Beijing, China: China Environmental Science Publishing House.
- Dala-Corte RB, Melo AS, Siqueira T, Bini LM, Martins RT, Cunico AM, Pes AM, Magalhães ALB, Godoy BS, Leal CG, et al. 2020. Thresholds of freshwater biodiversity in response to riparian vegetation loss in the Neotropical region. J Appl Ecol. 57(7):1391–1402. doi: 10.1111/1365-2664.13657.
- Damseth S, Thakur K, Kumar R, Kumar S, Mahajan D, Kumari H, Sharma D, Sharma AK. 2024. Assessing the impacts of river bed mining on aquatic ecosystems: a critical review of effects on water quality and biodiversity. HydroRes. 7:122–130. doi: 10.1016/j.hydres.2024.01.004.
- Editorial Committee of Fauna Sinica CAOS. 1996. Fauna sinica: phylum Annelida, class Hirudinea. Beijing, China: China Science Publishing & Media.
- Gao X, Yang JH, Zhang XW. 2020. Study on the selection of marker genes in zooplankton DNA metabarcoding monitoring. Asian J Ecotoxicol. 15:61–70.
- Ge X, Jin J, Peng L, Zang H, Wang B, Sun C. 2022. The first chromosome-level genome assembly of Cheumatopsyche charites Malicky and Chantaramongkol, 1997 (Trichoptera: Hydropsychidae) reveals how it responds to pollution. Genome Biol Evol. 14(10):c136. doi: 10.1093/gbe/evac136.
- Hamid A, Ullah S, Jehangir A, Bhat S. 2021. Assessment of ecological characteristics of macroinvertebrate communities and their relationship with environmental factors in a stream ecosystem. J Chem Ecol. 37:746–766. doi: 10.1080/02757540.2021.1987419.
- Hou Y, Pan B, Yang H, Zhu P, Huang Z, Zhao G, Du D. 2024. Responses of multi-faceted benthic macroinvertebrates alpha and beta diversity to flooding in a highland floodplain. Environ Res. 250:118475. doi: 10.1016/j.envres.2024.118475.
- Huang YY, Xue XW, Ouyang S. 2002. Primary studies on biology of Radix plicatula (Gastropoda: Lymnaeidae). J Nanchang Univ Nat Sci. 26:96–99.
- Irfan S, Alatawi A. 2019. Aquatic ecosystem and biodiversity: a review. Open J Ecol. 9(1):1–13. doi: 10.4236/oje.2019.91001.
- Ji F, Han D, Yan L, Yan S, Zha J, Shen J. 2022. Assessment of benthic invertebrate diversity and river ecological status along an urbanized gradient using environmental DNA metabarcoding and a traditional surveymethod. Sci Total Environ. 806(Pt 2):150587. doi: 10.1016/j.scitotenv.2021.150587.
- Ji FF. 2022. Establishment of eDNA technology and application in diversity assessment of three kinds of aquatic organisms in typical lakes and reservoirs [Doctor dissertation]. Wuhan: Huazhong Agricultural University.
- Kamil H, Saskia S, Till-Hendrik M, Martina W, Florian L. 2021. Fresh insights into Mediterranean biodiversity: environmental DNA reveals spatio-temporal patterns of stream invertebrate communities on Sicily. Hydrobiologia. 849:155–173. doi: 10.1007/s10750-021-04718-3.
- Kędzior R, Kłonowska-Olejnik M, Dumnicka E, Woś A, Wyrębek M, Książek L, Grela J, Madej P, Skalski T. 2022. Macroinvertebrate habitat requirements in rivers: overestimation of environmental flow calculations in incised rivers. Hydrol Earth Syst Sci. 26(15):4109–4124. doi: 10.5194/hess-26-4109-2022.
- Kobayashi S, Nozaki T, Takemon Y. 2017. Caddisfly community in the Seta-Uji River, the outlet of Lake Biwa. Jpn J Ecol. 67:13–29.
- Lam-Gordillo O, Baring R, Dittmann S. 2021. Taxonomic and functional patterns of benthic communities in southern temperate tidal flats. Front Mar Sci. 8:1–19. doi: 10.3389/fmars.2021.723749.
- Leray M, Knowlton N. 2015. DNA barcoding and metabarcoding of standardized samples reveal patterns of marine benthic diversity. Proc Natl Acad Sci U S A. 112(7):2076–2081. doi: 10.1073/pnas.1424997112.
- Lin Z, Liu G, Guo K, Wang K, Wijewardene L, Wu N. 2024. Scales matter: regional environment factors affect α diversity but local factors affect β diversity of macroinvertebrates in Thousand Islands Lake catchment area. Ecol Indic. 158:111561. doi: 10.1016/j.ecolind.2024.111561.
- Liu KJ, Lai XX, Xiang J, Gui YT, Ge LR. 2023. Progress in the application of environmental DNA technology in aquatic environment study. J Aquacult. 44(16-21):27.
- Liu YY, Zhang WZ, Wang YX. 1999. Medical conchology. Beijing, China: China Ocean Press.
- Liu YY. 1979. Fauna of sinica: freshwater Mollusks. Beijing: China Science Publishing & Media.
- Mason NWH, Mouillot D, Lee WG, Wilson JB. 2005. Functional richness, functional evenness and functional divergence: the primary components of functional diversity. Oikos. 111(1):112–118. doi: 10.1111/j.0030-1299.2005.13886.x.
- Mesa LM. 2012. Interannual and seasonal variability of macroinvertebrates in monsoonal climate streams. Braz Arch Biol Technol. 55(3):403–410. doi: 10.1590/S1516-89132012000300011.
- Mochizuki S. 2008. Effect of water temperature on larval development of the caddis larvae, Cheumatopsyche brevilimeata (Iwata). Nat Insects. 43:12–15.
- Ntislidou C, Latinopoulos D, Skotida A, Giannoulis T, Moutou K, Kagalou I. 2023. Assessment of hydrological barriers effect in river benthic fauna coupled with eDNA metabarcoding monitoring. Ecohydrol Hydrobiol. 23(3):389–399. doi: 10.1016/j.ecohyd.2023.04.007.
- Orozco González CE. 2023. A comparative analysis of sampling methods for aquatic macroinvertebrates. Basel, Switzerland: Preprints.
- Park Y-J, Cho Y-H, Han Y-G, Oh H-S, Kwon O-S, Nam S-H. 2006. Fauna of macroinvertebrates and composition of functional feeding groups about the aquatic insects to microhabitats from the Geum River. Korea. J Ecol Environ. 29(5):415–424. doi: 10.5141/JEFB.2006.29.5.415.
- Poff N, Olden J, Vieira N, Finn D, Simmons M, Kondratieff B. 2006. Functional trait niches of north American lotic insects: traits-based ecological applications in light of phylogenetic relationships. J N Am Benthol Soc. 25(4):730–755. doi: 10.1899/0887-3593(2006)025[0730:FTNONA]2.0.CO;2.
- Prommi T, Laudee P, Chareonviriyaphap T. 2014. Biodiversity of adult Trichoptera and water quality variables in Streams, Northern Thailand. APCBEE Proc. 10:292–298. doi: 10.1016/j.apcbee.2014.10.055.
- Rice CJ, Larson ER, Taylor CA. 2018. Environmental DNA detects a rare large river crayfish but with little relation to local abundance. Freshw Biol. 63(5):443–455. doi: 10.1111/fwb.13081.
- Shi WL. 1985. The fish fauna characteristics of the Dayang River and its adjacent rivers. Fish Sci. 4:53–57.
- Sun YG, Zhao DZ, Wu T, Wei BQ, Gao SG, Li Y, Cao FF. 2012. Temporal and spatial dynamic changes and landscape pattern response of Hemeroby in Dayang estuary of Liaoning Province. China. Acta Ecol Sin. 12:3645–3655.
- Szałkiewicz E, Kałuża T, Grygoruk M. 2022. Detailed analysis of habitat suitability curves for macroinvertebrates and functional feeding groups. Sci Rep. 12(1):10757. doi: 10.1038/s41598-022-15096-8.
- Tampo L, Kaboré I, Alhassan EH, Ouéda A, Bawa LM, Djaneye-Boundjou G. 2021. Benthic macroinvertebrates as ecological indicators: their sensitivity to the water quality and human disturbances in a tropical river. Front Water. 3:1–17. doi: 10.3389/frwa.2021.662765.
- Uchida N, Kubota K, Aita S, Kazama S. 2020. Aquatic insect community structure revealed by eDNA metabarcoding derives indices for environmental assessment. PeerJ. 8:e9176. doi: 10.7717/peerj.9176.
- Vourka A, Karaouzas I, Parmakelis A. 2023. River benthic macroinvertebrates and environmental DNA metabarcoding: a scoping review of eDNA sampling, extraction, amplification and sequencing methods. Biodivers Conserv. 32(13):4221–4238. doi: 10.1007/s10531-023-02710-y.
- Wang GY. 2022. Analysis of benthic biodiversity in the Yalu River Estuary using environmental DNA [Master dissertation]. Dalian: Dalian Ocean University.
- Wang JC. 2011. Chironomidae larvae in Northern China. Beijing, China: China Yan Shi Press.
- Wang L, Li J, Tan L, Han B. 2023. Seasonal patterns of functional alpha and beta redundancies of macroinvertebrates in a disturbed (sub)tropical river. Ecol Indic. 146:109777. doi: 10.1016/j.ecolind.2022.109777.
- Wang M, Jin XW, Lin XL, Du L, Cui YD, Wu XP, Sun HY, Xie ZC, Wang XH, Wang BX. 2021. Advances in the macrozoobenthos biodiversity monitoring and ecosystem assessment using environmental DNA metabarcoding. Acta Ecol Sin. 41:7440–7453.
- Wang ZX. 1993. Xiuyan yearbook - 1985-1990. Shenyang, China: Shenyang Publishing House.
- Wu F, Zou Y, Qin S, Li F, Zhang Y. 2023. eDNA biomonitoring of macroinvertebrate communities for the bioassessment of a River’s ecological status. Water. 15(2):308. doi: 10.3390/w15020308.
- Yin X. 2023. Environmental DNA metabarcoding technology to monitor the diversity of aquatic insects in the Dongjiang River Basin [Master dissertation]. Dalian: Dalian Ocean University.
- You DS. 1995. Fauna sinica: insecta, volume 48 - order Trichoptera. Beijing, China: China Science Publishing & Media.
- Zhang DP. 2023. Patterns of taxonomic and functional diversity and associated community assembly mechanisms of macroinvertebrate across the mainstream of the Xijiang River [Master dissertation]. Dalian: Dalian Ocean University.
- Zhang L, Yang J, Zhang Y, Shi J, Yu H, Zhang X. 2022. eDNA biomonitoring revealed the ecological effects of water diversion projects between Yangtze River and Tai Lake. Water Res. 210:117994. doi: 10.1016/j.watres.2021.117994.
- Zhao XY, Zhao J. 2015. Study on influence of the different land used mode on runoff in the Dayang River Basin. Groundwater. 37:123–125.
- Zheng JP, Wang F. 2006. Research on ecological water requirement of Dayang River. J Hohai Univ Nat Sci. 34:502–504.