?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Plankton, as an important part of the food chain in the river ecosystem, is sensitive to changes in the environment. To investigate the influence of plankton community structure on the aquatic ecosystem and further verify the applicability of biomanipulation, the midstream of the Jialing River was selected as the study area. A total of 149 species (varieties) from 7 phyla, 18 orders, 34 families, and 72 genera were identified for phytoplankton. A total of 48 species were found for zooplankton. The mean density of phytoplankton was 2.09 × 106 cells/L, and the mean density of zooplankton was 27.1 ind./L. Moreover, the mean values of the Shannon–Wiener diversity index (H′), Pielou homogeneity index (J′), and Margalef abundance index (D) for phytoplankton were 3.17, 0.76, and 4.47, respectively. The mean values of the Shannon–Wiener diversity index (H′), Pielou homogeneity index (J′), and Margalef abundance index (D) for zooplankton were 2.64, 0.92, and 5.07, respectively. Based on the diversity indices, the water quality was evaluated as oligo pollution to β-medium pollution level. Redundancy analysis (RDA) was used to explore the relationship between dominant plankton species and environmental factors. The RDA results show that the main environmental factors affecting the community structure of phytoplankton and zooplankton were water temperature (WT), electrical conductivity (EC), pH, total phosphorus (TP), and transparency (Secchi depth; SD). There were 17 functional groups in phytoplankton, which are X1, S1, Y, Y2, and so on. In zooplankton, there were six functional groups, which are Pmsl, Prsu, Pfif, Pmip, Lrsf and Lmic. Many articles have demonstrated the feasibility of using biological manipulation to control algae. The number of piscivorous fish in the midstream of the Jialing River had increased, and phytoplankton density in this survey was significantly decreased. The results show that traditional biomanipulation can be applied to this region, which can provide better scientific support for the future management of the aquatic environment in the Jialing River basin.
1. Introduction
Plankton usually refers to phytoplankton and zooplankton, of which the former is the primary producer and the latter is the primary consumer in aquatic ecosystems (Wang et al. Citation2015). Since plankton is more sensitive to the water environment, it is typically found in higher species diversity and abundance during the summer (Gao et al. Citation2023; Hu et al. Citation2023). The community structure of plankton can have a direct impact on the structure and function of the aquatic ecosystem while also serving as a better indicator of the condition of the aquatic environment, therefore, plankton is frequently used to assess the health of the aquatic ecosystem (Niu et al. Citation2021; Wang et al. Citation2022; Jiang et al. Citation2023). Zooplankton plays a significant role in the food webs and is influenced by both upward and downward mechanical processes in aquatic ecosystems (Zhou et al. Citation2020; Oparaku et al. Citation2021).
Phytoplankton can be controlled using biomanipulation through effective regulation of food webs in the aquatic ecosystems (Shi et al. Citation2022), and many practices have confirmed its effectiveness (Liu et al. Citation2018; Geletu Citation2023; Xiao et al. Citation2023). However, there are two major views of algal control based on biomanipulation (Chen et al. Citation2023). One is traditional biomanipulation, which involves introducing piscivorous fish to control smaller zooplanktivorous fish, thereby increasing the abundance of zooplankton to inhibit algae (Zhang et al. Citation2024). The other one is nontraditional biomanipulation, which utilizes filter-feeding fish such as bighead carp (Aristichthys nobilis) and silver carp (Hypophthalmichthys molitrix) to directly control plankton like cyanobacteria (Hu et al. Citation2017). These two viewpoints have been subject to intense debate in recent years. The results of Yi et al. (Citation2016) suggested that nontraditional biomanipulation is more applicable for controlling algae, while other investigations suggested that traditional biomanipulation is more suited for this purpose (Ekvall et al. Citation2014). However, the geographic location and climate of this area have a major impact on how well biomanipulation works (Geletu Citation2023).
With the greatest watershed area among the Yangtze River basin’s tributaries, the Jialing River is abundant in aquatic biological resources (Ren et al. Citation2011). Anthropogenic influences including overfishing and the construction of cascade hydropower stations once had an impact on the basin, resulting in a decrease in fish abundance and an increase in the abundance of planktonic algae (Tao et al. Citation2021). The provincial aquatic germplasm resource protection zone of Nanchong section of the Jialing River is located in the midstream of the Jialing River, between Fengyi hydropower station and Xiaolong hydropower station. Neither of the two hydropower stations has built a fish passage facility, thus creating a relatively closed channel. The major objectives of conservation in this reserve are Mystus macropterus, Siniperca chuatsi, and Silurus meridionalis, which are typical piscivorous fish (Zhang, Zeng, et al. Citation2022). With the strengthening of the management of the reserve and the continuous promotion of the ten-year ban on fishing in the Yangtze River basin (starting 1st January 2020), the protected objects of the reserve are better protected, and the resources are increasing continuously (currently, the percentages of the populations of the Mystus macropterus, Siniperca chuatsi, and Silurus meridionalis are 5%, 10%, and 0.3%, respectively (Unpublished data)) (Zeng and Zhou Citation2012). Because of this, the provincial aquatic germplasm resource protection zone of Nanchong section of the Jialing River makes for a great natural field experimental area, was selected to (i) investigate the effect of characteristics of community structure plankton on ecosystems in the aquatic environment; and (ii) verify the applicability of traditional biomanipulation in midstream of the Jialing River, which can provide better theoretical support for the future management of the aquatic environment in the Jialing River basin.
2. Materials and methods
2.1. Study area and sampling
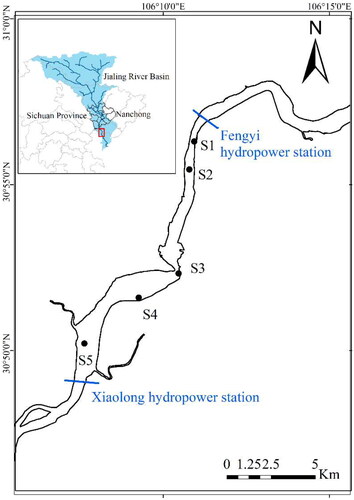
Located in Nanchong city, Sichuan province, the provincial aquatic germplasm resource protection zone of Nanchong section of the Jialing River spans a total of 24 km, between latitudes 30°46′–30°57′N and longitudes 106°07′–106°11′E. The reserve starts with Fengyi hydropower station and ends with Xiaolong hydropower station (). A field survey was conducted in the provincial aquatic germplasm resource protection zone of Nanchong section of the Jialing River in summer (August), 2022. Five sampling sites (S1–S5) were established, covering the entire protection zone, in accordance with the hydrological conditions of the protected zone and the actual circumstances of the river segment.
2.2. Sample collection and identification methods
The sampling of plankton was conducted in midstream of the Jialing River in the summer of 2022. Qualitative phytoplankton samples were collected with a 25# plankton net, samples were placed in 50 mL sampling bottles and fixed with neutral Lugol’s solution for examination. Quantitative samples were collected with a 1 L water sampler below the surface of the water and fixed with neutral Lugol’s solution, and then sent back to the laboratory. Samples were left to settle in the laboratory for 48h and then concentrated to 30 mL, 0.1 mL was placed in a phytoplankton counting frame and identified under a microscope. Phytoplankton species were identified to the lowest possible taxonomic level (usually genus or species) in the laboratory according to the relevant references (Zhou and Chen Citation2005; Hu and Wei Citation2006).
Qualitative zooplankton samples were collected with a 25# plankton net and fixed in formaldehyde solution for examination. Quantitative samples were collected below the surface of the water with a water sampler, concentrated by filtering through a 25# plankton net and fixed with formaldehyde solution, then returned to the laboratory. The samples were concentrated to 30 mL after 24h of settling in the laboratory and were identified under a microscope. The species identification of Zooplankton was based on the references (Editorial Committee of Fauna Sinica 1979; Jiang and Du Citation1979; Shen Citation1990; Wang Citation1961), and all individuals were identified to the lowest possible taxonomic level (genus or species).
2.3. Water quality determination
Water temperature (WT), pH, electrical conductivity (EC), and dissolved oxygen (DO) were measured at each sampling site using a multiparameter probe (YSI6600); transparency (Secchi depth; SD) was measured using the Secchi disk in situ (Li et al. Citation2022); A 1 L water sample was brought back to the laboratory for determination of other physicochemical indicators. Among them, total phosphorus (TP) was determined by ammonium molybdate spectrophotometric method (GB 11893-89); total nitrogen (TN) was obtained through alkaline potassium persulfate digestion UV spectrophotometric method (HJ 636-2012); nitrate nitrogen (N -N) was analyzed with ultraviolet spectrophotometry (HJ/T 346-2007); ammonia nitrogen (N
-N) was examined according to Nessler’s reagent spectrophotometry (HJ 535-2009); phosphate (P
) was quantified using Mo-Sb Anti spectrophotometric method (HJ 632-2011).
2.4. Functional group delineation
The functional group delineation of phytoplankton and zooplankton was carried out by the methods of Padisák et al. (Citation2009) and Fintelman-Oliveira et al. (Citation2023), respectively.
2.5. Statistical analysis
The Shannon–Wiener diversity index (H') (Shannon Citation1948), Pielou evenness index (J') (Pielou Citation1967), and Margalef richness index (D) (Margalef Citation1957) of plankton were computed by following equations:
where Pi=Ni/N, Ni is the number of the i species; N is the number of individuals of all species; S is the total number of species in the sample.
The dominant species of plankton was determined by the dominant degree (Y) (Li, Yu, et al. Citation2019) formula:
where fi is the proportion of the number of individuals of the i species to the total number of individuals; The dominant population was defined as Y ≥ 0.02.
The relationship between the dominant species density and environmental factors was examined using the Canoco 5.0 software. Firstly, there was a detrended correspondence analysis (DCA). Canonical correspondence analysis (CCA) is applied if the first axis’ length is larger than 4.0, however, redundancy analysis (RDA) is employed if the first axis’ length is less than 3.0. The species data were transformed into a normal distribution using the log(X + 1) method prior to analysis. All the environmental variables are also log(X + 1) transformed except for pH. Excel was applied to count species data of plankton. Figures were completed using Origin 2018 software. The sampling map was created by ArcGIS 10.7.
3. Results
3.1. Physical and chemical factors of water bodies
The physicochemical characteristics of each sampling site in the provincial aquatic germplasm resource protection zone of Nanchong section of the Jialing River in summer of 2022 were different. Values of pH were fluctuated between 7.0 and 7.5. The mean dissolved oxygen (DO) concentration at the five sites was 7.68 mg/L, with S4 having the highest value (9.34 mg/L) and S5 having the lowest value (6.96 mg/L). The transparency (Secchi depth; SD) of the sampling sites S1–S5 were 71, 65, 85, 51, and 69 cm, respectively. S1 recorded the lowest water temperature (28.6 °C), while the highest water temperature appeared at S4 (30.3 °C). Electrical conductivity (EC) reached a maximum value at S2 (2.9 μs/cm) and a minimum value at S5 (0.7 μs/cm). Total nitrogen (TN) was relatively stable with a mean value of 1.2 mg/L. The concentration of total phosphorus (TP) ranged from 0.026 mg/L (S5) to 0.063 mg/L (S4). The range of ammonia nitrogen (N -N) was 0.118–1.808 mg/L. The average values of phosphate (P
) and nitrate nitrogen (N
-N) were 0.036 and 0.750 mg/L, respectively. The lowest values of phosphate (P
) and nitrate nitrogen (N
-N) were observed at S4 ().
Table 1. Physicochemical indices at each sampling site in the provincial aquatic germplasm resource protection zone of Nanchong section of the Jialing River in summer of 2022.
3.2. Plankton community structure
3.2.1. Plankton species composition
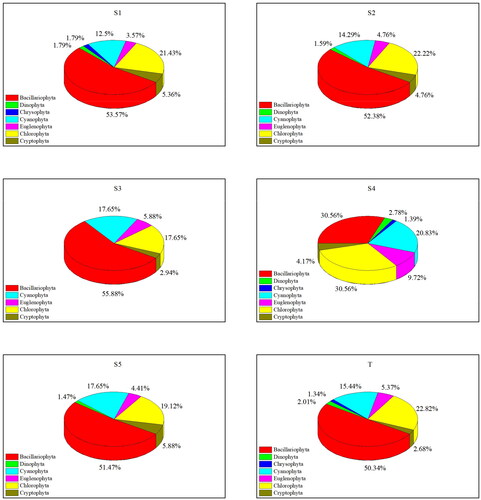
A total of 149 species (varieties) of phytoplankton from 7 phyla, 18 orders, 34 families, and 72 genera were collected in the provincial aquatic germplasm resource protection zone of Nanchong section of the Jialing River in summer of 2022, of which Bacillariophyta was the most abundant, with 75 species accounting for 50.34% of the total species. However, only two species were identified for Chrysophyta, which accounts for 1.34% of the total species. The species proportion of the other five phyla ranged from 2.01% to 22.82% ().
Figure 2. Proportion of phytoplankton species at each sampling site in the provincial aquatic germplasm resource protection zone of Nanchong section of the Jialing River in summer 2022. S1–S5 are the proportion of phytoplankton species at five sampling sites, respectively, and T is the proportion of total phytoplankton species.
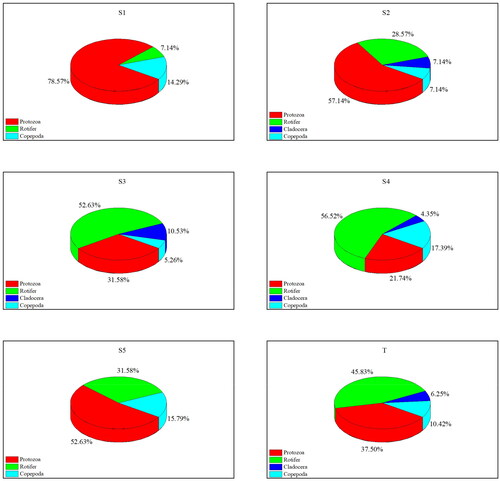
A total of 48 species of zooplankton were identified, of which rotifer was the most abundant, with 22 species (45.83%). However, only three species were identified for cladocera, which accounts for 6.25% of the total species. The species proportion of protozoa and copepoda was 37.50% and 10.42%, respectively ().
Figure 3. Proportion of zooplankton species at each sampling site in the provincial aquatic germplasm resource protection zone of Nanchong section of the Jialing River in summer 2022. S1–S5 are the proportion of zooplankton species at five sampling sites, respectively, and T is the proportion of total zooplankton species.
3.2.2. Plankton density
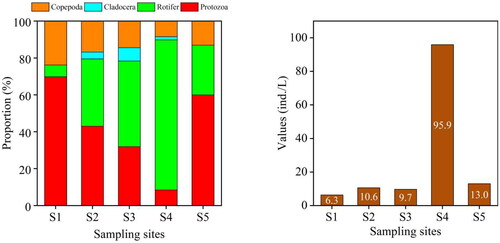
The mean density of phytoplankton was 2.09 × 106 cells/L, with the highest density of phytoplankton found at S4 (4.42 × 106 cells/L) (). The mean density of zooplankton was 27.1 ind./L. The result showed that the highest density of zooplankton appeared at S4 (95.9 ind./L) ().
Figure 4. The Density of phytoplankton at each sampling site in the provincial aquatic germplasm resource protection zone of Nanchong section of the Jialing River in summer of 2022. The figure on the left shows the proportion of phytoplankton density at five sampling sites. The figure on the right shows the values of phytoplankton density at five sampling sites.
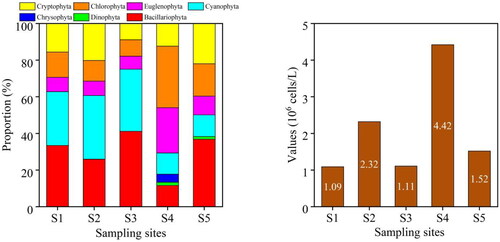
Figure 5. The Density of zooplankton at each sampling site in the provincial aquatic germplasm resource protection zone of Nanchong section of the Jialing River in summer of 2022. The figure on the left shows the proportion of zooplankton density at five sampling sites. The figure on the right shows the values of zooplankton density at five sampling sites.
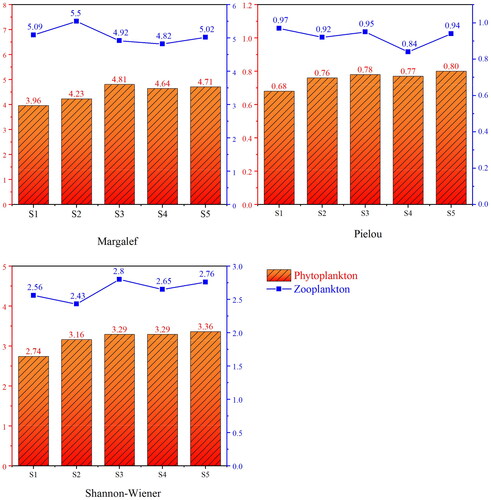
3.2.3. Plankton diversity index
The mean value of the Shannon–Wiener diversity index (H′) of phytoplankton was 3.17, with S5 having the highest value of 3.36, and S1 having the lowest value of 2.74. The mean value of the Pielou evenness index (J′) of phytoplankton was 0.76, with S5 having the maximum value of 0.80, and S1 having the lowest value of 0.68. The mean value of the Margalef richness index (D) of phytoplankton was 4.47, with S3 having the highest value of 4.81, and the lowest value occurring in S1 (3.96). The average value of the Shannon–Wiener diversity index (H′) of zooplankton was 2.64, S2 had the lowest H′ index (4.82) and the highest H′ index (2.80) in S3. The average value of the Pielou evenness index (J′) of zooplankton was 0.92, with a minimum value of 0.84 in S4 and a maximum value of 0.97 in S1 (). Similar to phytoplankton, the evenness of zooplankton is less variable. S2 had the highest value of 5.07 for the Margalef richness index (D), and the lowest value occurred in S4 (4.82). Based on the diversity indices, the water quality was evaluated as oligo pollution to β-medium pollution level.
3.2.4. Dominant plankton species
The survey showed that a total of eight phytoplankton species were dominant species (Y ≥ 0.02), with the dominant species originating from the Cyanophyta (three species), Euglenophyta (one species), Chlorophyta (two species), and Cryptophyta (two species). Of these, the dominant species of Cyanophyta accounted for the largest proportion (37.5%). A total of seven dominant species of zooplankton originated from protozoa (two species), rotifer (four species), and Copepoda (one species). The highest proportion of dominant species of rotifer was 57.14% ().
Table 2. Dominant species and dominant degree of plankton in the provincial aquatic germplasm resource protection zone of Nanchong section of the Jialing River in summer 2022.
3.2.5. Relationships between dominant plankton species and environmental factors
Detrended correspondence analysis (DCA) showed that the maximum length of the first axes of phytoplankton was 0.46, and the maximum length of the first axes of zooplankton was 1.07, which were both less than 3.0. Therefore, the correlation analysis between the dominant species of the plankton community and environmental factors was carried out by using redundancy analysis (RDA). Environmental factors involved in RDA include water temperature (WT), electrical conductivity (EC), transparency (Secchi depth; SD), pH, dissolved oxygen (DO), total nitrogen (TN), total phosphorus (TP), ammonia nitrogen (N -N), nitrate nitrogen (N
-N), and phosphate (P
-). After screening, the environmental factors affecting the phytoplankton community were total phosphorus (TP), pH, and transparency (Secchi depth; SD). The environmental factors affecting the zooplankton community were water temperature (WT), electrical conductivity (EC), and pH. The pseudo-canonical correlation between species-environment for both two sorting axes was 1.000 (), indicating that the results of sorting were reliable and better reflected the interrelationships between dominant plankton species and environmental factors. The results of RDA sorting showed that the density of dominant phytoplankton species was significantly influenced by TP, pH, and SD (). The density of dominant zooplankton species was significantly influenced by WT, EC, and pH ().
Figure 7. Redundancy analysis (RDA) of dominant phytoplankton species and environmental factors in the provincial aquatic germplasm resource protection zone of Nanchong section of the Jialing River in summer of 2022. Lim.sp.—Limnothrix sp.; C.ero.—Cryptomonas erosa; Pse.sp.—Pseudoanabaena sp.; C.ros.—Cryptomonas rostrate; D.var.—Dysmorphococcus variabilis; Tra.sp.—Trachelomonas sp.; C.vul.—Chlorella vulgaris; N N–N
–N; NO3–N–N
N; P
—P
.
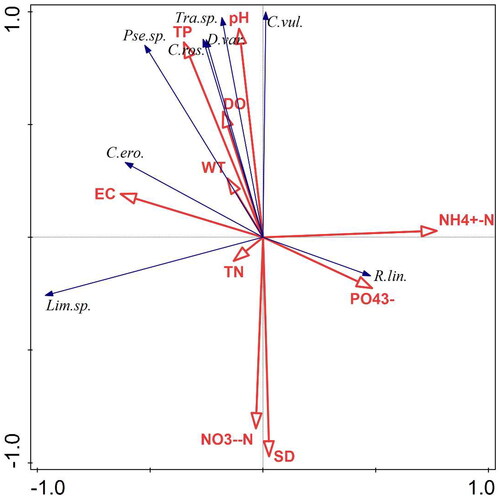
Figure 8. Redundancy analysis (RDA) of dominant zooplankton species and environmental factors in the provincial aquatic germplasm resource protection zone of Nanchong section of the Jialing River in summer of 2022. C.uni.—Conochilus unicornis; B.cal.—Brachionus calyciflorus; T.sim.—Trichocerca similis; K.coc.—Keratella cochlearis; D.glo.—Difflugia globulosa; C.arc.—Cyclopyxis arcelloides; N –N–N
–N; P
–P
.
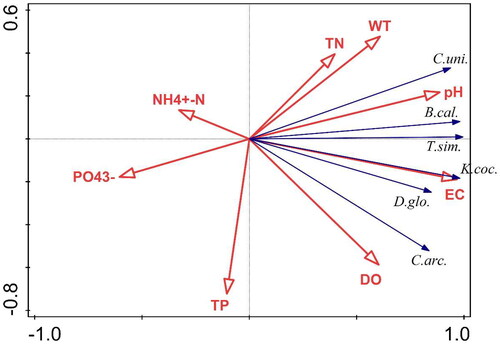
Table 3. Redundancy analysis (RDA) of dominant plankton species and environmental factors in the provincial aquatic germplasm resource protection zone of Nanchong section of the Jialing River in summer of 2022.
3.2.6. Plankton functional group
Phytoplankton in the provincial aquatic germplasm resource protection zone of Nanchong section of the Jialing River in summer of 2022 was classified into 17 functional groups based on sensitivity and tolerance. These groups included C, D, P, TB, S1, S2, X3, X2, X1, E, Y, G, J, L0, M, W1, and W2 ().
Table 4. Classification of phytoplankton functional groups in the provincial aquatic germplasm resource protection zone of Nanchong section of the Jialing River in summer 2022.
Based on size, habitat, feeding and predatory escape response, zooplankton was categorized into six functional groups: Pmsl, Prsu, Pfif, Pmip, Lrsf, and Lmic ().
Table 5. Classification of zooplankton functional groups in the provincial aquatic germplasm resource protection zone of Nanchong section of the Jialing River in summer of 2022.
4. Discussion
4.1. Characterization of the plankton community
A total of 149 species were identified for phytoplankton in the provincial aquatic germplasm resource protection zone of Nanchong section of the Jialing River in summer of 2022. The species composition was dominated by diatoms, cyanobacteria, and green algae, which accounted for 87.46% of the total species composition for phytoplankton. The results of this article differ from the findings of Min Yang, who found that diatoms were the dominant species of phytoplankton in the middle and lower reaches of the Jialing River, and there exists a dominance in spring, autumn, and winter. The reason for this may be that diatoms predominate in spring and winter (Rolland et al. Citation2009; Xiao et al. Citation2013), while green algae and cyanobacteria predominate in summer (Zhang et al. Citation2010). Research shows that diatoms prefer flowing water (Tao et al. Citation2021). The construction of the hydropower station resulted in lower flow rates in this protection zone, which may have inhibited diatom density and reduced the dominance of diatoms. Phytoplankton density at five sampling sites varied from 1.09 × 106 to 4.42 × 106 cells/L, with an average density of 2.09 × 106 cells/L. Phosphorus is the main limiting factor for phytoplankton in the Jialing River (Tao et al. Citation2021). S4 had the highest concentration of TP, and therefore, the phytoplankton density at S4 was higher than other sampling sites (S1, S2, S3, and S5). Compared to phytoplankton density in the midstream of the Jialing River in summer of 2019 (8.50 × 106 cells/L), phytoplankton density in this survey was decreased (Tao et al. Citation2021). It may be due to the enhanced management of the protected zone as well as the ten-year ban on fishing in the Yangtze River (starting 1st January 2020), which has increased the number of protected fishes (piscivorous fish) in the protected zone and the control of phytoplankton abundance through food webs (Chen et al. Citation2023). A total of 48 species were found for zooplankton in the provincial aquatic germplasm resource protection zone of Nanchong section of the Jialing River, and the composition of zooplankton species was mainly dominated by rotifer and protozoa, which accounted for 83.33% of the total species composition for zooplankton. In contrast, the number of species of copepoda and cladocera was relatively low, accounting for only 16.67%. The dominant species of zooplankton were dominated by rotifer and protozoa, and the results of the study differed from those reported in previous surveys (Ren and Ma Citation2012). On the one hand, this is due to the differences in the surveyed area and season; on the other hand, it is due to the influence of human activities, which led to a large change in the composition and diversity of zooplankton species. Rotifer is characterized by small size, short life-cycle, rapid development, and so on, and can quickly adapt to the changes in the physical and chemical environment in the water body, therefore, rotifer is often dominant in the aquatic environment (Wang et al. Citation2019). Zooplankton density varied between 6.3 and 95.9 ind./L at five sampling sites, with an average density of 27.1 ind./L. S4 is located in the widest part of the sampling area, where slower water velocity results in high predation success of zooplankton, which may be responsible for the high density of zooplankton at this site. There was a certain positive correlation between the overall zooplankton density and phytoplankton density in this study. Zooplankton shows the same trend by feeding on phytoplankton (Wei et al. Citation2022). It is possible that the aggregation of phytoplankton has an important effect on the distribution of zooplankton (Nielsen and Andersen Citation2002), which is consistent with the results of study on plankton in the Weihe River (Bai et al. Citation2022). It is generally recognized that zooplankton density is positively correlated with the trophic level of the water body (Qin et al. Citation2023). Therefore, the reason for the higher zooplankton density at sampling site S4 is related to the trophic status of the water body in which the sampling site is located (S4 had the highest concentration of total phosphorus).
4.2. Relationship between plankton community and environmental factors
Some differences in environmental factors affecting plankton community structure in different water bodies (Li, Feng, et al. Citation2019). Meanwhile, different groups of organisms respond differently to environmental factors (Bai et al. Citation2022). The primary environmental factors influencing the community structure of benthic fauna in the Jialing River were dissolved oxygen, total phosphorus, permanganate index, and flow velocity (Xu et al. Citation2022). Environmental factors that significantly influence community structure of fish in the watershed were temperature, flow velocity, water level, and transparency (Zhang, Zeng, et al. Citation2022). In this study, the redundancy analysis (RDA) of environmental factors with plankton community revealed that the environmental factors affecting the community structure of phytoplankton and zooplankton were mainly water temperature, electrical conductivity, pH, total phosphorus and transparency. Among them, water temperature is an extremely important environmental factor that affects the growth, reproduction and development, community composition, and abundance changes of zooplankton (Zhao et al. Citation2020). Many existent studies indicated that water temperature has a significant effect on the growth, reproductive rate, and feeding of rotifer (Oyoo-Okoth et al. Citation2011; Chalkia and Kehayias Citation2013). RDA analysis also showed that dominant species such as Conochilus unicornis, Brachionus calyciflorus, Keratella cochlearis, and Trichocerca similis were affected by water temperature, with which there was a close positive correlation. pH likewise has a close influence. Moreover, pH has an important effect on the spatial distribution of phytoplankton and is essential for the normal growth and development of plankton (Kim et al. Citation2013). The level of pH will have a great influence on the changes in the structure and distribution of the phytoplankton community, reflecting the status of algal growth in the water body and the changes in the water environment. Phytoplankton in alkaline water has better primary productivity due to the favorable photosynthesis of phytoplankton in an alkaline environment (Jakobsen et al. Citation2015). Different zooplankton have different pH levels at which they are suitable for growth, resulting in differences in species composition. The range of pH in the provincial aquatic germplasm resource protection zone of Nanchong section of the Jialing River is between 7.0 and 7.5, which is a weakly alkaline water body with a high density of rotifer. This may be due to the fact that rotifer shows a pattern of high density when distributed in alkaline water bodies (Yin and Niu Citation2008). As a result, the pH of water bodies varies with sites and plankton produce corresponding physiological responses, leading to spatial heterogeneity in the community structure of plankton. Higher electrical conductivity maintains higher species richness and biodiversity of zooplankton (Brysiewicz et al. Citation2017). It has been shown that among many environmental variables, electrical conductivity significantly affects the community structure of zooplankton (Sousa et al. Citation2008). Our study has confirmed this phenomenon, results of the RDA analysis showed that electrical conductivity was positively correlated with the density of all dominant zooplankton species. Total phosphorus is one of the major factors limiting the growth and development of phytoplankton (Lv et al. Citation2011). Some studies have reported that phosphorus is a preferential factor controlling the growth and development of phytoplankton (Howarth and Marino Citation2006). Phosphorus is regarded as a limiting factor by Redfield’s law when N/P is greater than 16:1 (Redfield Citation1960). The average N/P in this protection zone was roughly 24, suggesting that total phosphorus was a limiting factor of growth for phytoplankton in the provincial aquatic germplasm resource protection zone of Nanchong section of the Jialing River. This may also have contributed to the highest abundance of phytoplankton at S4 (S4 had the highest concentration of total phosphorus). The results of the RDA analysis show a positive correlation between all phytoplankton and total phosphorus, which also suggests that the environmental factor affecting the community structure of phytoplankton in the provincial aquatic germplasm resource protection zone of Nanchong section of the Jialing River is total phosphorus. Another significant environmental factor influencing the community structure of phytoplankton is transparency (Li et al. Citation2022). Low transparency increases the growth of surrounding plants, which affects the photosynthesis of phytoplankton, and therefore, limits the growth and development of phytoplankton (Zhang et al. Citation2015). And it has also been reported that reduced water clarity diminishes effective light for phytoplankton, thus affecting phytoplankton density (Zhang, He, et al. Citation2022).
4.3. Applicability of traditional biomanipulation in the provincial aquatic germplasm resource protection zone of Nanchong section of the Jialing River
Biomanipulation may promote a more balanced community structure of organisms (Polauke et al. Citation2024). Various biomanipulations have been attempted for ecosystem restoration, including the planting of submerged plants to improve water clarity and inhibit nutrient concentrations, the stocking of mussels to increase feeding pressure on phytoplankton, or the utilization of a variety of fish species (Liu et al. Citation2018; Geletu Citation2023; Xiao et al. Citation2023). Biomanipulation theory that uses fish to control phytoplankton has been proven to be effective (Ekvall et al. Citation2014; Bruggen et al. Citation2016). There are two major views of algal control based on biomanipulation: traditional biomanipulation and nontraditional biomanipulation (Chen et al. Citation2023). Although stocking filter-feeding fish into eutrophic freshwaters has demonstrated the potential for nontraditional biomanipulation to combat algal blooms (Chen et al. Citation2023). Fish that are safeguarded in the provincial aquatic germplasm resource protection zone of Nanchong section of the Jialing River are piscivorous fish (Zhang, Zeng, et al. Citation2022), which is more in accordance with traditional biomanipulation. Traditional biomanipulation involves introducing piscivorous fish to control smaller zooplanktivorous fish, thereby increasing the abundance of zooplankton to inhibit algae (Zhang et al. Citation2024). This manipulation promotes a trophic cascade of aquatic organisms and contributes to the growth of zooplankton, which are the main consumers of phytoplankton (Amorim and Moura Citation2020). The provincial aquatic germplasm resource protection zone of Nanchong section of the Jialing River is located in a subtropical monsoon climate zone, and fish communities in subtropics are characterized by small individuals, rapid growth, and short life spans (Brucet et al. Citation2010). Small fishes promote the growth of phytoplankton through grazing pressure on herbivorous zooplankton, thus the presence of small fishes can adversely affect the aquatic environment (He et al. Citation2018; Yu et al. Citation2021; Guo et al. Citation2023). Partial removal of zooplanktivorous fish can significantly improve the aquatic environment (Setubal and Riccardi Citation2020). Controlling the abundance of zooplanktivorous fish by stocking piscivorous fish and thus regulating the community structure of phytoplankton (Guo et al. Citation2022). Compared to phytoplankton density in the midstream of the Jialing River in summer of 2019, phytoplankton density in this survey was decreased (Tao et al. Citation2021). It may be due to the strengthened management of the reserve in recent years as well as the continued promotion of a ten-year ban on fishing in the Yangtze River (starting 1st January 2020), piscivorous fish have been better protected in this reserve, resulting in increasing abundance of piscivorous fish and limiting algal growth, proving that traditional biomanipulation has some applicability in this reserve. There may also be another reason that the aquatic environment in the protected area has been improved to some extent, and the reduction of nitrogen and phosphorus may also cause changes in algal density, but of course, this requires further research.
5. Conclusions
The provincial aquatic germplasm resource protection zone of Nanchong section of the Jialing River collected 149 species (varieties) from 7 phyla, 18 orders, 34 families, and 72 genera for phytoplankton, and 48 species of zooplankton. The mean density of phytoplankton was 2.09 × 106 cells/L. The mean density of zooplankton was 27.1 ind./L. Based on the diversity indices, the water quality was evaluated as oligo pollution to β-medium pollution level. Our results show that traditional biomanipulation has some applicability in this reserve. However, there may also be another reason that the aquatic environment in the protected area has been improved to some extent, and the reduction of nitrogen and phosphorus may also cause changes in algal density, consequently, this reason will be further explored in subsequent research.
Author contributions
Xiaopeng Tang: Conceptualization, Methodology, Software, Writing—original draft preparation, Writing—review and editing. Liang Liu: Investigation. Yongcheng Wang: Investigation. Jianghaoyue Xu: Software. Yiling Huang: Investigation. Qiang Qin: Investigation. Fei Xu: Investigation. Fubin Zhang: Methodology, Writing—review and editing, funding acquisition.
Acknowledgment
We thank Xin Liu and other colleagues for the assistance in field sampling and experiment.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
Data was contained within the article and will be available upon request.
Additional information
Funding
References
- Amorim CA, Moura AN. 2020. Effects of the manipulation of submerged macrophytes, large zooplankton, and nutrients on a cyanobacterial bloom: a mesocosm study in a tropical shallow reservoir. Environ Pollut. 265(Pt B):114997. doi: 10.1016/j.envpol.2020.114997.
- Bai HF, Wang YR, Song JX, Kong FH, Zhang XX, Li Q. 2022. Characteristics of Plankton Community Structure and Its Relation to Environmental Factors in Weihe River, China. Ecol Environ Sci. 31(1):117.
- Brucet S, Boix D, Quintana XD, Jensen E, Nathansen LW, Trochine C, Meerhoff M, Gascón S, Jeppesena E. 2010. Factors influencing zooplankton size structure at contrasting temperatures in coastal shallow lakes: implications for effects of climate change. Limnol Oceanogr. 55(4):1697–1711. doi: 10.4319/lo.2010.55.4.1697.
- Bruggen V, Lurling NCB, Waajen M, Pires GW, Dionisio LM, Lengkeek W. 2016. Biomanipulation with quagga mussels (Dreissena rostriformis bugensis) to control harmful algal blooms in eutrophic urban ponds. Ecol Eng. 90:141–150. doi: 10.1016/j.ecoleng.2016.01.036.
- Brysiewicz A, Sugocki U, Wesoowski P, Czerniawski R. 2017. Zooplankton community structure in small ponds in relation to fish community and environmental factors. Appl Ecol Env Res. 15(4):929–941. doi: 10.15666/aeer/1504_929941.
- Chalkia E, Kehayias G. 2013. Zooplankton and environmental factors of a recovering eutrophic lake (Lysimachia Lake, Western Greece). Biologia (Bratisl.). 68(3):459–469. doi: 10.2478/s11756-013-0171-9.
- Chen J, Liu JR, Han SP, Su HJ, Xia WL, Wang HJ, Liu Y, Zhang L, Ke ZX, Zhang X, et al. 2023. Nontraditional biomanipulation: a powerful ecotechnology to combat cyanobacterial blooms in eutrophic freshwaters. TIL. 1(3):100038. doi: 10.59717/j.xinn-life.2023.100038.
- Editorial Committee of Fauna Sinica, Chinese Academy of Sciences. 1979. Chinese fauna-freshwater copepod. Beijing: Science Press.
- Ekvall MK, Pablo UC, Lars-Anders H, Dam HG. 2014. Linking cascading effects of fish predation and zooplankton grazing to reduced cyanobacterial biomass and toxin levels following biomanipulation. PLoS One. 9(11):e112956. doi: 10.1371/journal.pone.0112956.
- Fintelman-Oliveira E, Kruk C, Lacerot G, Klippel G, Branco CWC. 2023. Zooplankton functional groups in tropical reservoirs: discriminating traits and environmental drivers. Hydrobiologia. 850(2):365–384. doi: 10.1007/s10750-022-05074-6.
- Gao WQ, Xiong FY, Lu Y, Qu X, Xin W, Chen YS. 2023. Development of a phytoplankton-based index of biotic integrity for ecological health assessment in the Yangtze River. Ecol Process. 12(1):41. doi: 10.1186/s13717-023-00456-7.
- Geletu TT. 2023. Lake eutrophication: control of phytoplankton overgrowth and invasive aquatic weeds. Lakes Reserv Res Manag. 28:e12425.
- Guo C, Li SQ, Ke J, Liao CS, Hansen AG, Jeppesen E, Zhang TL, Li W, Liu JS. 2023. The feeding habits of small-bodied fishes mediate the strength of top-down effects on plankton and water quality in shallow subtropical lakes. Water Res. 233:119705. doi: 10.1016/j.watres.2023.119705.
- Guo C, Li W, Li SQ, Mai Z, Zhang TL, Liu JS, Hansen AG, Li L, Cai XW, Hicks BJ. 2022. Manipulation of fish community structure effectively restores submerged aquatic vegetation in a shallow subtropical lake. Environ Pollut. 292(Pt B):118459. doi: 10.1016/j.envpol.2021.118459.
- He H, Jin H, Jeppesen E, Li KY, Liu ZW, Zhang YD. 2018. Fish-mediated plankton responses to increased temperature in subtropical aquatic mesocosm ecosystems: implications for lake management. Water Res. 144:304–311. doi: 10.1016/j.watres.2018.07.055.
- Howarth RW, Marino R. 2006. Nitrogen as the limiting nutrient for eutrophication in coastal marine ecosystems: evolving views over three decades. Limnol Oceanogr. 51(1part2):364–376. doi: 10.4319/lo.2006.51.1_part_2.0364.
- Hu HJ, Wei YX. 2006. The freshwater algae of China-systematic, taxonomy and ecology. Beijing: Science Press.
- Hu JW, Hua L, You AJ, Chen L, Xu ZQ, Wang ZM, Zhang W, Zhang CC, Yu GT, Tang WJ. 2023. Taxon-specific effects of seasonal variation and water connectivity on the diversity of phytoplankton, zooplankton and benthic organisms in urban wetland. J Freshw Ecol. 38(1):2253265.
- Hu L, Yang Z, Pan XJ, Zhao N, Peng JH, Wan CY. 2017. Use of fish species from different trophic levels to control algae and water quality: an enclosure experiment in eutrophic area of Xiaojiang River. PLoS One. 12(3):e0171953. doi: 10.1371/journal.pone.0171953.
- Jakobsen HH, Blanda E, Staehr PA, Højgård JK, Rayner TA, Pedersen MF, Jepsen PM, Hansen BW. 2015. Development of phytoplankton communities: implications of nutrient injections on phytoplankton composition, pH and ecosystem production. J Exp Mar Biol Ecol. 473:81–89. doi: 10.1016/j.jembe.2015.08.011.
- Jiang LL, Yao YP, Zhang SY, Wan LQ, Zhou ZZ. 2023. Effects of stream connectivity on phytoplankton diversity and community structure in sunken lakes: a case study from an August survey. Diversity. 15(2):291. doi: 10.3390/d15020291.
- Jiang YZ, Du NS. 1979. Chinese fauna-freshwater cladocera. Beijing: science Press.
- Kim H, Spivack AJ, Menden-Deuer S. 2013. pH alters the swimming behaviors of the raphidophyte Heterosigma akashiwo: implications for bloom formation in an acidified ocean. Harmful Algae. 26:1–11. doi: 10.1016/j.hal.2013.03.004.
- Li CC, Feng WY, Chen HY, Li XY, Song FH, Guo WJ, Giesy JP, Sun FH. 2019. Temporal variation in zooplankton and phytoplankton community species composition and the affecting factors in Lake Taihu-a large freshwater lake in China. Environ Pollut. 245:1050–1057. doi: 10.1016/j.envpol.2018.11.007.
- Li XY, Yu HX, Wang HB, Ma CX. 2019. Phytoplankton community structure in relation to environmental factors and ecological assessment of water quality in the upper reaches of the Genhe River in the Greater Hinggan Mountains. Environ Sci Pollut Res Int. 26(17):17512–17519. doi: 10.1007/s11356-019-05200-3.
- Li XY, Zhao YX, Chai FY, Yu HX, Sun X, Liu D. 2022. Phytoplankton community structure dynamics in relation to water environmental factors in Zhalong Wetland. Int J Environ Res Public Health. 19(22):14996. doi: 10.3390/ijerph192214996.
- Liu ZW, Hu JR, Zhong P, Zhang XF, Ning JJ, Larsen SE, Chen DY, Gao YM, He H, Jeppesen E. 2018. Successful restoration of a tropical shallow eutrophic lake: strong bottom-up but weak top-down effects recorded. Water Res. 146:88–97. doi: 10.1016/j.watres.2018.09.007.
- Lv J, Wu HJ, Chen MQ. 2011. Effects of nitrogen and phosphorus on phytoplankton composition and biomass in 15 subtropical, urban shallow lakes in Wuhan, China. Limnologica. 41(1):48–56. doi: 10.1016/j.limno.2010.03.003.
- Margalef R. 1957. Information theory in ecology. Soc Gen Syst Res. 31:36–71.
- Nielsen T, Andersen C. 2002. Plankton community structure and production along a freshwater-influenced Norwegian fjord system. Mar Biol. 141(4):707–724.
- Niu Y, Liu CL, Lu XL, Zhu LX, Sun QW, Wang SF. 2021. Phytoplankton blooms and its influencing environmental factors in the southern Yellow Sea. Reg Stud Mar Sci. 47:101916. doi: 10.1016/j.rsma.2021.101916.
- Oparaku NF, Andong FA, Nnachi I, Okwuonu E, Ezeukwu JC, Ndefo J. 2021. The effect of physicochemical parameters on the abundance of zooplankton of River Adada, Enugu, Nigeria. J Freshw Ecol. 37(1):33–56. doi: 10.1080/02705060.2021.2011793.
- Oyoo-Okoth E, Muchiri M, Ngugi CC, Njenga EW, Ngure V, Orina PS, Chemoiwa EC, Wanjohi BK. 2011. Zooplankton partitioning in a tropical alkaline–saline endorheic Lake Nakuru, Kenya: spatial and temporal trends in relation to the environment. Lakes Reserv. 16(1):35–47. doi: 10.1111/j.1440-1770.2011.00461.x.
- Padisák J, Crossetti LO, Naselli-Flores L. 2009. Use and misuse in the application of the phytoplankton functional classification: a critical review with updates. Hydrobiologia. 621(1):1–19. doi: 10.1007/s10750-008-9645-0.
- Pielou EC. 1967. The measurement of diversity in different types of biological collection. J Theor Biol. 15(1):177. doi: 10.1016/0022-5193(67)90048-3.
- Polauke E, Sø JS, Carl H, Møller PR, Reitzel K, Sand-Jensen K, Kragh T. 2024. Water quality in a shallow eutrophic lake is unaffected by extensive thinning of planktivorous and benthivorous fish species. J Environ Manage. 356:120570. doi: 10.1016/j.jenvman.2024.120570.
- Qin YX, Zhou W, Qiao YM, Chen R, Yang HY. 2023. Zooplankton community structure in Xinfengjiang reservoir. J Hydroecol. 44(4):35–43.
- Redfield AC. 1960. The biological control of chemical factors in the environment. Sci Prog. 11:150–170.
- Ren LP, Ma YH. 2012. The characteristics analysis of community structure of plankton and water quality assessment of cascade hydropower development on Jialing River in Sichuan. Nat Prod Res. 24(8):7.
- Ren LP, Zhang Z, Zeng X, Ma YH, Zeng Y, Zhou CQ. 2011. Community structure of zooplankton and water quality assessment of Jialing River in Nanchong. Procedia Environ Sci. 10(1):1321–1326. doi: 10.1016/j.proenv.2011.09.211.
- Rolland A, Bertrand F, Maumy M, Jacquet S. 2009. Assessing phytoplankton structure and spatio-temporal dynamics in a freshwater ecosystem using a powerful multiway statistical analysis. Water Res. 43(13):3155–3168. doi: 10.1016/j.watres.2009.03.049.
- Setubal RB, Riccardi N. 2020. Long-term effects of fish biomanipulation and macrophyte management on zooplankton functional diversity and production in a temperate shallow lake. Limnology. 21(3):305–317. doi: 10.1007/s10201-020-00617-z.
- Shannon CE. 1948. A Mathematical Theory of Communication. Bell Syst Tech J. 27(4):623–656. doi: 10.1002/j.1538-7305.1948.tb00917.x.
- Shen YF. 1990. New technology of micro-biological monitoring. Beijing: China Architecture and Building Press.
- Shi XL, Yang JS, Chen KN, Zhang M, Yang Z, Yu Y. 2022. Review on the control and mitigation strategies of lake cyanobacterial blooms. J Lake Sci. 34(2):349–375. doi: 10.18307/2022.0201.
- Sousa W, Attayde JL, Rocha EDS, Eskinazi-Sant’Anna EM. 2008. The response of zooplankton assemblages to variations in the water quality of four man-made lakes in semi-arid northeastern Brazil. J Plankton Res. 30(6):699–708. doi: 10.1093/plankt/fbn032.
- Tao M, Xiong Y, Li B, Wang ZJ, Huang J. 2021. Spatio-temporal distribution of phytoplankton and its environmental impact factors in Sichuan section of Jialing River. Resour Environ Yangtze Basin. 30(07):1680–1694.
- Wang HB, Huo TB, Du X, Wang L, Song D, Huang XL, Zhao C. 2022. Zooplankton community and its environmental driving factors in Ulungur Lake, China. J Freshw Ecol. 37(1):387–403. doi: 10.1080/02705060.2022.2093279.
- Wang HL, Liu YF, Ren YF, He YX, Wang SQ, Zhang HX, Wang XK, Li ZX. 2019. Analysis of river zooplankton community characteristics in autumn in Beijing. Environ Sci. 40(8):3568–3576.
- Wang JJ. 1961. Freshwater rotifers of China. Beijing: science Press.
- Wang L, Wang C, Deng DG, Zhao XX, Zhou ZZ. 2015. Temporal and spatial variations in phytoplankton: correlations with environmental factors in Shengjin Lake, China. Environ Sci Pollut Res Int. 22(18):14144–14156. doi: 10.1007/s11356-015-4640-2.
- Wei N, Yu LM, Du KK, Yang CS, Shen ZW, Ni ZH. 2022. Phytoplankton communities and correlations analysis of environmental factors in mainstream of Three Gorges Reservoir. Resour Environ Yangtze Basin. 31(3):615–623.
- Xiao R, Su SL, Ghadouani A, Wu JP. 2013. Spatial analysis of phytoplankton patterns in relation to environmental factors across the southern Taihu basin, China. Stoch Environ Res Risk Assess. 27(6):1347–1357. doi: 10.1007/s00477-012-0670-1.
- Xiao XZ, Sun HY, Ren HP, Xing MX, Huang J, Wang YC, Hu S, Zhang J, Tang JF. 2023. Biomanipulation of periphytic algae in the middle route of south–north water diversion project canal: an in situ study in the Lushan section. Water. 15(12):2144. doi: 10.3390/w15122144.
- Xu DD, Zhan XM, Tao M, Huang J, Wang ZJ, Li B. 2022. Macrozoobenthos community structure and water quality evaluation of Jialing River in Sichuan Province. Resour Environ Yangtze Basin. 31(3):602–614.
- Yi CL, Guo LG, Ni LY, Yin CJ, Wan J, Yuan CB. 2016. Biomanipulation in mesocosms using silver carp in two Chinese lakes with distinct trophic states. Aquaculture. 452:233–238. doi: 10.1016/j.aquaculture.2015.11.002.
- Yin XW, Niu CJ. 2008. Effect of pH on survival, reproduction, egg viability and growth rate of five closely related rotifer species. Aquat Ecol. 42(4):607–616. doi: 10.1007/s10452-007-9136-9.
- Yu J, Zhen W, Kong L, He H, Zhang Y, Yang X, Chen F, Zhang M, Liu Z, Jeppesen E. 2021. Changes in pelagic fish community composition, abundance, and biomass along a productivity gradient in subtropical lakes. Water. 13(6):858. doi: 10.3390/w13060858.
- Zeng Y, Zhou XY. 2012. An analysis of ichthyologic fauna of Jialing River. J Huazhong Agric Univ. 31(4):506–511.
- Zhang H, Chen R, Li FP, Chen L. 2015. Effect of flow rate on environmental variables and phytoplankton dynamics: results from field enclosures. Chin J Ocean Limnol. 33(2):430–438. doi: 10.1007/s00343-015-4063-4.
- Zhang Q, Zeng Y, Xiao J, Xiang LL, Bao JH, Zhang CS, Mi XY, Duan M. 2022. Functional diversities of fish community in Peng’an section of the middle reach of Jialing River. Acta Hydrobiol Sin. 46(5):630–642.
- Zhang SM, He PM, Liu W, Liu JL, Chen SW, Han Z, Tang CY, Tan M, Wu MQ. 2022. Seasonal variation of zooplankton and its relationship with environmental factors in urban rivers of Xuhui district, Shanghai. J Hydroecol. 43(5):42–48.
- Zhang YL, Feng LQ, Li JS, Luo LC, Yin Y, Liu ML, Li YL. 2010. Seasonal–spatial variation and remote sensing of phytoplankton absorption in Lake Taihu, a large eutrophic and shallow lake in China. J Plankton Res. 32(7):1023–1037. doi: 10.1093/plankt/fbq039.
- Zhang YT, Yang J, Lin XM, Tian B, Zhang TL, Ye SW. 2024. Phytoplankton community dynamics in ponds with diverse biomanipulation approaches. Diversity. 16(2):75. doi: 10.3390/d16020075.
- Zhao YF, Liu ST, Niu XL. 2020. Effect of water temperature on the dynamic behavior of phytoplankton–zooplankton model. Appl Math Comput. 378:125211. doi: 10.1016/j.amc.2020.125211.
- Zhou FX, Chen JH. 2005. Atlas of microbiology in freshwater. Beijing: Chemical Industry Press.
- Zhou J, Qin BQ, Zhu GW, Zhang YL, Gao G. 2020. Long-term variation of zooplankton communities in a large, heterogenous lake: implications for future environmental change scenarios. Environ Res. 187:109704. doi: 10.1016/j.envres.2020.109704.