Abstract
This study aimed to examine the bacterial communities present in the macrophyte- (MDZs) and algae-dominated zones (ADZs) of the Chengdong Lake, by utilizing high-throughput sequencing techniques. The physicochemical characterization showed that there were obvious differences in the trophic statuses of the two lake regions, which were mainly due to the differences in pollutant concentration and hydrophyte coverage. The results obtained through the analysis of Shannon indices indicated that the biodiversity of water samples in the ADZs was greater compared to the MDZs. However, no statistically significant difference was observed in sediment samples across different regions of the lake. Our findings indicate that Cyanobacteria phylum abundance was highest in the ADZs, while the MDZs had Proteobacteria as the most abundant phylum, comprising over 30% of the bacterial community. The db-RDA analysis revealed that total nitrogen (TN) and total phosphorus (TP) in the water, as well as organic matter (OM) in the sediment, were the primary environmental factors influencing the variation in bacterial communities. This study contributes fundamental data towards comprehending the diversity variation of bacterial communities across different habitats within Chengdong Lake.
1. Introduction
Lake ecosystems, especially those that are shallow, exhibit two distinct stable states: a turbid state characterized by an abundance of phytoplankton and a clear state dominated by submersed macrophytes (Scheffer et al. Citation2001). The shift between alternative states is consistently linked to the increase in nutrient levels, particularly phosphorus and nitrogen, predominantly attributed to anthropogenic activities and global alterations (Scheffer et al. Citation1993). Microorganisms play a crucial role in lake ecosystems as they are key biological components responsible for driving nutrient cycling, including carbon, nitrogen, phosphorus and sulfur (Frossard et al. Citation2012).
Huaihe River is one of the seven major rivers in China, located in the east of China. The Chengdong Lake of Huoqiu City, Anhui Province, are located on the south bank of the middle section of the Huaihe River (Wu et al. Citation2022). As the major urban lake in the Huaihe River basin, Chengdong Lake holds national significance as an ecological protection area for flood control and water storage, and it serves as a crucial source of drinking water for local residents. This lake is shallow and characterized by the alternating presence of macrophytes and algae. The middle region of Chengdong Lake is predominantly occupied by macrophytes, with the water column surface being primarily covered by aquatic plants, particularly the dominant group of submerged plants known as Potamogeton crispus. A significant proportion of Chengdong Lake is typically characterized by an algae-dominated zone, which experiences frequent outbreaks of Cyanobacterial. Wu et al. Citation2022 reported the concentrations, sources, and potential ecological risks of heavy metals in the sediments of Chengdong Lake. In addition, numerous investigations have been conducted on hydrological alterations and engineering interventions in Chengdong Lake (Zhang Citation2021; Jin and Chen Citation2021). However, research on water quality and ecosystems has primarily focused on phytoplankton communities (Zhao et al. Citation2020), neglecting the examination of bacterial populations, including those residing in the sediments. Consequently, there is a pressing need to assess the ecological functions of bacteria in Chengdong Lake, to enhance comprehension and safeguard the well-being of this ecosystem in the forthcoming years.
In this study, we investigated the spatial distribution of bacterial communities in the water and sediment in different zones of Chengdong Lake with distinct trophic statuses and sought to determine how different habitats affect the bacterial community structure. Water and sediment samples were collected in 2 regions (a algae-dominated region and a macrophyte-dominated region). This study (1) examined the variation in the bacterial communities in water and sediments in 2 lake zones of Chengdong Lake and further studied the regulation of changes in bacterial communities and (2) aimed to understand the potential effects of habitats on the bacterial communities. The findings will offer novel perspectives and useful resources for understanding bacterial communities in different habitats. Additionally, we anticipate that the insights gained from this research will provide fundamental data for future water microbial monitoring endeavors aimed at safeguarding Chengdong Lake.
2. Materials and methods
2.1. Study site and sampling
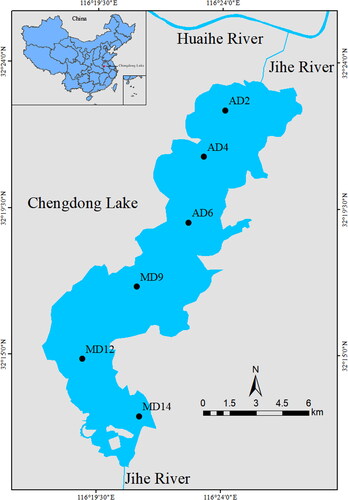
Chengdong Lake is situated in the southern region of the Huaihe River, The lake has an elongated shape with a mean length of 25 km and a mean width of 4 km, it covers a total area of 383.3 km2 and has a mean depth of 2 m. As a result of the prevalent occurrence of south and southwest winds during the summer season, the concentration of Cyanobacteria blooms primarily manifests in the northern vicinity of Chengdong Lake (). Consequently, the algae-dominated zones (ADZs) are mainly situated around the northern region. While the macrophyte-dominated zones (MDZs) are mainly distributed in the southern part of the lake, where the dominant aquatic macrophytes is Potamogeton crispus. In May 2021, six sampling sites were selected from contrasting zones within Chengdong lake, with 3 sites in ADZs (AD2, AD4, AD6) and 3 sites in MDZs (MD9, MD12, MD14) (). Water (30–50 cm below the water surface) and surface sediment (0–3 cm) samples were collected using a Plexiglas water collector and a Peterson mud collector, respectively. Three replicate samples were taken from each sampling site and they were stored on ice during transport back to the laboratory for further processing within 24 h.
2.2. Determination of environmental factors
The pH in the water samples were measured on location using a multiparameter water quality analyzer (Professional Plus, YSI, USA). Chemical analysis, including total nitrogen (TN), chlorophyll-a (Chl a), and total phosphorus (TP) measurements, in the water samples was conducted in the laboratory according to the standard procedures (APHA (American Public Health Association)) Citation2005). The pH, TN, TP and OM (Oganic Matter) in the sediment samples were conducted following the previously published methods (Bai et al. Citation2012, Tang et al. Citation2009).
2.3. Sample processing, DNA extraction and sequencing
For microbiological analyses, the water samples were filtered using a 0.22-μm polycarbonate membrane. The filtered membranes were stored at −80 °C for DNA extraction. The fresh sediment samples were centrifuged at a speed of 10,000 rpm for 1 min and then retained at −80 °C. The E.Z.N.A. soil DNA isolation kit (OMEGA) was used to extract the genome DNA of the samples following the manufacturer’s instructions. We used the extracted DNA as a template to amplify the V3-V4 hypervariable region of the 16S rRNA gene using the 338 F and 806 R primers (Peng et al. Citation2019). The EXtaq enzyme from TaKaRa was used to ensure the efficiency and accuracy of the amplification. Then, the amplified target fragment was enriched, and a specific index sequence was added. Sequencing was conducted at Majorbio BioPharm Technology Co.Ltd. (Shanghai, China) using the Illumina MiSeq platform. The raw data generated in this study were submitted into the NCBI database under the BioProject accession numbers PRJNA1019119 (water samples) and PRJNA1019121 (sediment samples).
2.4. Data processing and statistical analysis
Raw sequences were subjected to a quality control procedure using QIIME (Caporaso et al. Citation2010). USEARCH was employed to filter chimeras and the high-quality sequences were clustered into operational taxonomic units (OTUs) at a similarity level of 97% (Fan et al. Citation2018). For each representative sequence, Ribosomal Database Project (RDP, https://rdp.cme.msu.edu/) by Bayesian classifier was used for the taxonomic analysis based on the Silva Database (https://www.arb-silva.de) at 70% threshold. All statistical analyses were analyzed on the online platform of Majorbio Cloud Platform (https://cloud.majorbio.com/) developed by Majorbio BioPharm Technology Co. Ltd. Mothur software was used to analyze α diversity, including community richness parameters (Chao 1, OTU), community diversity parameters (Shannon), and community coverage index (Good’s coverage) (Schloss et al. Citation2009). At the OTU level, the similarity between samples was visualized using the principal coordinate analysis (PCoA) based on the Bray-Curtis dissimilarity matrix. Analysis of similarities (ANOSIM) was then used to test whether the differences between different groups were significant (Clarke and Warwick Citation1994). A variance inflation factor (VIF) assessed multicollinearity of physicochemical variables. If VIF ≥ 10, variable(s) were removed. Redundancy analysis (RDA) was performed on the Majorbio Cloud Platform to assess the effects of physicochemical factors on the bacterial communities. Spearman’s rank correlation was calculated to estimate the relationship between the environmental factors and relative abundances of phyla.
3. Results
3.1. Water and sediment chemical properties
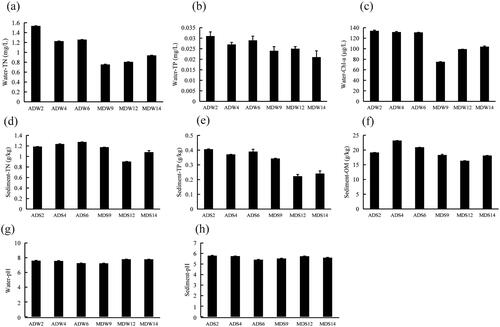
The values of the environmental factors in the lake water and sediment are presented in . Based on the environmental parameters, Chengdong lake was determined to be slightly alkaline (pH: 7.24-7.8). The average concentrations of TN, TP, and Chl a in the overlying water of the ADZs were found to be 1.34 mg/L, 0.029 mg/L, and 132.2 μg/L, respectively. In the MDZs, the mean concentrations of TN, TP and Chl a were observed to be 0.834 mg/L, 0.023 mg/L and 92.75 μg/L, respectively. The concentrations of TN, TP, and Chl a in the overlying water of the ADZs zones (AD2, AD4, AD6) were determined to be higher than those in the MDZs zones (MD9, MD12, MD14), as indicated in . Similarly, the concentrations of TN, TP, and OM in the sediment of the ADZs were also observed to be higher than those in the MDZs.
Figure 2. Physicochemical properties of the water and sediment in different regions of Chengdong Lake. (a) Total nitrogen in the water, (b) total phosphorous in the water, (c) Chl a in the water, (d) total nitrogen in the sediment, (e) total phosphorous in the sediment, (f) total organic matter in the sediment, (g) pH in the water, and (h) pH in the sediment. Abbreviations: ADW, lake water from the algae-dominated zones; ADS, sediment from the algae-dominated zones; MDW, lake water from the macrophyte-dominated zones; MDS, sediment from the macrophyte-dominated zones.
3.2. Diversity and spatial distribution of bacterial communities
A total of 872,136 valid bacterial 16S rRNA sequences were obtained from both water and sediment for all 12 samples. Using a 97% sequence similarity cutoff, these sequences resulted in a bacterial operational taxonomic unit (OTU) count ranging from 505 to 933 in water samples and 4,689 to 5,476 in sediment samples (). The coverage estimator for water and sediment samples was determined to be 99.3% and 94.2%, respectively. This finding supports the notion that the existing bacterial profiles accurately represent the primary microbial communities. further presents the α-diversity indices for the two lake regions, encompassing measures of community diversity (Shannon index) and richness (Chao 1 index). The Shannon indices demonstrated that the biodiversity of water in the ADZs surpasses that of the MDZs. Little difference in bacterial diversity in sediments from two different regions. Additionally, the analysis also revealed that bacterial community diversity is higher in lake sediment samples compared to water samples.
Table 1. Bacterial richness and diversity of the bacterial community in water and sediments of different regions.
3.3. Characteristics and comparative of the bacterial communities in different lake regions
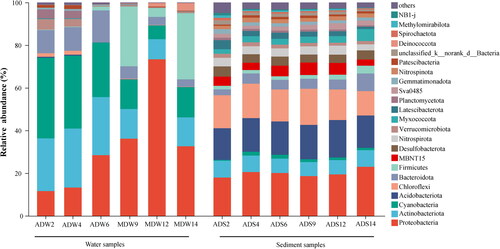
The analysis of bacterial phylogenetic assignments based on the sequencing results revealed the presence of 44 phyla in the water samples and 65 phyla in the sediment samples. The relative abundances of all detected bacterial phyla (relative abundance > 0.1%) in the water and sediment samples are shown in . Notably, the dominant phyla differed substantially between the two sample types (). Among all the lake regions, Proteobacteria represented the most abundant phylum in water (average 32.58%), followed by Cyanobacteria (average 22%), Actinobacteriota (average 19.41%), Firmicutes (average 10.96%), Bacteroidota (average 8.33%). In contrast to water samples, the dominant bacterial phyla in the sediment were Proteobacteria (19.95%), Acidobacteriota (15.88%), Chloroflexi (14.84%), Actinobacteriota (7.2%) and Bacteroidota (4.95%). The ADZs exhibited a notably higher relative abundance of Cyanobacteria compared to the MDZs. Additionally, the sediments displayed significantly higher relative abundances of Acidobacteriota and Chloroflexi in comparison to the water, while the relative abundance of Cyanobacteria in the sediments was significantly lower than that in the water.
Figure 3. Analysis of the relative abundance at the phylum level of the bacterial community in the water bodies and sediment of Chengdong Lake.
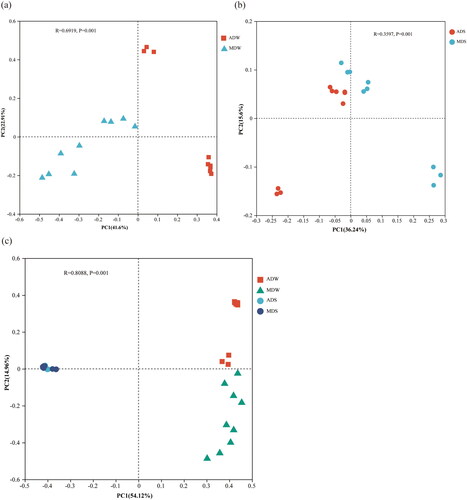
The difference of microbiota composition among samples was analyzed by β diversity analysis and is presented herein using principal coordinates analysis (PCoA) based on Bray-Curtis distances at the OTU level (). Generally, the proximity of two samples on the plot indicates a higher similarity in their compositions. The principal coordinates analysis yielded two components that accounted for 69.08% of the total variation in the bacterial community structure. Furthermore, the water and sediment samples were categorized into two distinct groups, indicating significant disparities in the bacterial community composition between the water bodies and sediments of Chengdong Lake (; PC1 of 54.12%). revealed that water samples were separated by two different ecological states (; PC1 of 41.6%), the ANOSIM results were also confirmed that there were significant variations in bacterial community structure of the water between the ADZs and MDZs (R = 0.6919, p = 0.001), the sediment samples clustered together suggesting high similarity in microbial structure ().
3.4. Relationship between bacterial community composition and environmental factors
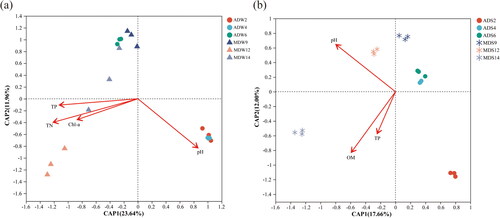
Distance-based redundancy analysis (db-RDA) was employed to analyze the impact of environmental factors on the diversity of bacterial communities in lake water and sediments at the OTU level (). In order to address the issue of collinearity among specific variables, only physicochemical variables with lower collinearity, as determined through variance inflation factor analysis, were incorporated into the model. As shown in , TN (r2 = 0.7338, p = 0.001) emerged as the most influential factor in shaping the bacterial communities in lake water, as indicated by the length of the arrow-lines. Additionally, OM played a significant role in structuring the bacterial community composition in the sediment of Chengdong Lake (r2 = 0.9563, p = 0.001).
Figure 5. db-RDA ordination diagram of bacterial communities associated with environmental variables in the (a) water and (b) sediments of Chengdong Lake. Chl-a chlorophyll-a, TP total phosphorus, TN total nitrogen, pH in the water; OM organic matter, TP total phosphorus, pH in the sediment.
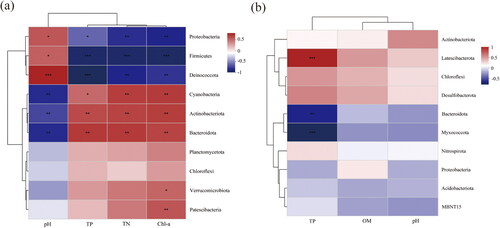
The Spearman rank correlation test was utilized to investigate the relationship between the 10 most prevalent phyla and environmental factors (). In the case of water samples, the relative abundances of Cyanobacteria, Actinobacteriota, and Bacteroidota exhibited a positive correlation with nitrogen and phosphorus nutrients (p < 0.05), whereas Proteobacteria, Deinococcota, and Firmicutes displayed a negative correlation with these nutrients (p < 0.05). In sediment samples, the relative abundances of Myxococcota and Bacteroidetes were found to be negatively correlated with TP (p < 0.01), while Latescibacterota exhibited a positive correlation with TP (p < 0.001).
4. Discussion
The objective of this study was to examine the bacterial community composition in the water and sediment of Chengdong Lake across two distinct trophic states, and to explore the potential factors influencing their distribution and diversity. Disparities in trophic status and lake habitats can result in variations in water quality and sediment geochemical properties (Fan and Xing Citation2016). In line with prior research, the trophic statuses of the water bodies exhibited significant dissimilarities in the ADZs and MDZs, primarily attributed to disparities in pollutant concentration and hydrophyte coverage (Chang et al. Citation2020). The mean concentrations of TN in the ADZs surpassed the China national surface water quality criteria of category IV (i.e. industrial waters) (MEP Citation2002). The environmental variables in the MDZs complied with the China national surface water quality criteria of category III (i.e. fishery and swimming waters) (MEP Citation2002). Numerous prior studies have explored the disparities in microbial diversity between sediment and water samples (Chang et al. Citation2020; Shang et al. Citation2020). For instance, Shang et al. (Citation2020) showed that in the Hulun Lake, the Shannon diversity index values revealed significantly greater bacterial diversity in sediment samples compared to water samples. The findings of our study align with prior research indicating that sediments exhibit a greater Shannon’s diversity in comparison to water samples, potentially attributable to the intricate physicochemical characteristics inherent in sediments (Chang et al. Citation2020). The findings of our study demonstrated that notable disparities in the composition of bacterial communities in the water column between states dominated by macrophytes and algae (), potentially attributable to variations in the trophic conditions of the water. Within the MDZs, Proteobacteria were the most prevalent phylum in the water (average 47.46%), whereas in the ADZs, the phylum with the largest relative abundance was Cyanobacteria (32.52%) (). Prior research has suggested that the presence of hydrophytes may have a suppressive impact on Cyanobacteria in regions dominated by hydrophytes (Wu et al. Citation2011; Chang et al. Citation2020). Numerous studies have provided evidence indicating the significant involvement of Proteobacteria phylum in nitrogen removal, biological phosphorus removal, and organic degradation (Nguyen et al. Citation2011). Zhang et al. (Citation2014) have reported the prevalence of Proteobacteria as the dominant bacterial group in a low-nutrient lake.
The results of db-RDA analysis demonstrated that TN significantly influenced the bacterial community structure in water samples (), in agreement with previous studies. Mao et al. (Citation2019) have additionally identified TN as the most influential factor driving the dynamics of planktonic bacterial communities in the Yulin River. This study indicated that OM exerts a more significant influence on bacterial community composition in sediments compared to other measured parameters, which was supported by Shao et al. (Citation2013). They reported that OM was also identified as a driving force of vertical changes of bacterial community composition in the sediment of Lake Taihu (Shao et al. Citation2013). Zeng et al. (Citation2009) also found that OM is responsible for causing changes in the bacterial community in sediments of Lake Xuanwu, China. A Spearman correlation analysis was conducted to examine the relationship between the abundance of microbiota and various water quality parameters. The results revealed that significant associations between certain phyla and the concentrations of total phosphorus and total nitrogen in the water, suggesting that their potential contribution to nitrogen and phosphorus cycling processes.
5. Conclusions
The findings of this study demonstrate significant differences in the trophic statuses of water bodies between the ADZs and MDZs. High-throughput sequencing indicated that the water from different regions in Chengdong Lake contained distinct bacterial communities. The Shannon indices indicated higher biodiversity in water samples from the ADZs compared to the MDZs. The db-RDA analysis showed that TN, TP and OM were important environmental factors affecting the composition and distribution of bacterial communities in Chengdong Lake. Our research provides basic data for microbial monitoring and protections of Chengdong Lake.
Authors’ contributions
Yangyang Liang wrote the manuscript with help from Ting Fang. Na Gao carried out the MiSeq sequencing process and data analysis. Wenxuan Lu conceived the presented idea.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data generated or analyzed during this study are included in this published article. The 16S rRNA gene sequences obtained in this study have been submitted to the NCBI SRA database under accession numbers PRJNA1019119 (water samples) and PRJNA1019121 (sediment samples).
Additional information
Funding
References
- APHA (American Public Health Association). 2005. Standard methods for the examination of water and wastewater. Washington, DC: American Public Health Association.
- Bai YH, Shi Q, Wen DH, Li ZX, Jefferson WA, Feng CP, Tang XY. 2012. Bacterial communities in the sediments of Dianchi Lake, a partitioned eutrophic Waterbody in China. PLoS One. 7(5):e37796. doi: 10.1371/journal.pone.0037796.
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, et al. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 7(5):335–336. doi: 10.1038/nmeth.f.303.
- Chang W, Sun J, Pang Y, Zhang S, Gong L, Lu J, Feng B, Xu R. 2020. Effects of different habitats on the bacterial community composition in the water and sediments of Lake Taihu, China. Environ Sci Pollut Res Int. 27(36):44983–44994. doi: 10.1007/s11356-020-10376-0.
- Clarke KR, Warwick RM. 1994. Similarity-based testing for community pattern: the two-way layout with no replication. Mar Biol. 118(1):167–176. doi: 10.1007/BF00699231.
- Fan X, Xing P. 2016. Differences in the composition of archaeal communities in sediments from contrasting zones of Lake Taihu. Front Microbiol. 7:1510. doi: 10.3389/fmicb.2016.01510.
- Fan XY, Gao JF, Pan KL, Li DC, Dai HH, Li X. 2018. Functional genera, potential pathogens and predicted antibiotic resistance genes in 16 full-scale wastewater treatment plants treating different types of wastewater. Bioresour Technol. 268:97–106. doi: 10.1016/j.biortech.2018.07.118.
- Frossard A, Gerull L, Mutz M, Gessner MO. 2012. Disconnect of microbial structure and function: enzyme activities and bacterial communities in nascent stream corridors. ISME J. 6(3):680–691. doi: 10.1038/ismej.2011.134.
- Jin YQ, Chen Y. 2021. The key issues of the reinforcement project of the East Lake Gate in Huoqi County. Engin Construct (In Chinese with English Abstract). 35:785–786.
- Mao Y, Liu Y, Li H, He Q, Ai H, Gu W, Yang G. 2019. Distinct responses of planktonic and sedimentary bacterial communities to anthropogenic activities: case study of a tributary of the Three Gorges Reservoir, China. Sci Total Environ. 682:324–332. doi: 10.1016/j.scitotenv.2019.05.172.
- MEP. 2002. Environmental quality standards for surface water (GB3838-2002). China Environmental Science, Beijing.
- Nguyen HT, Le VQ, Hansen AA, Nielsen JL, Nielsen PH. 2011. High diversity and abundance of putative polyphosphate-accumulating Tetrasphaera-related bacteria in activated sludge systems. FEMS Microbiol Ecol. 76(2):256–267. doi: 10.1111/j.1574-6941.2011.01049.x.
- Peng JX, Zhou SM, Xiao K, Zeng JY, Yao CH, Lu SL, Zhang W, Fu YZ, Yang YX, Bi XH, et al. 2019. Diversity of bacteria in cloud water collected at a national atmospheric monitoring station in Southern China. Atmos Res. 218:176–182. doi: 10.1016/j.atmosres.2018.12.004.
- Scheffer M, Carpenter S, Foley JA, Folke C, Walker B. 2001. Catastrophic shifts in ecosystems. Nature. 413(6856):591–596. doi: 10.1038/35098000.
- Scheffer M, Hosper SH, Meijer ML, Moss B, Jeppesen E. 1993. Alternative equilibria in shallow lakes. Trends Ecol Evol. 8(8):275–279. doi: 10.1016/0169-5347(93)90254-M.
- Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, et al. 2009. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 75(23):7537–7541. doi: 10.1128/AEM.01541-09.
- Shang Y, Wu X, Wei Q, Dou H, Wang X, Chen J, Zhang H, Ma S, Zhang H. 2020. Total Arsenic, pH, and sulfate are the main environmental factors affecting the microbial ecology of the water and sediments in Hulun Lake, China. Front Microbiol. 11:548607. doi: 10.3389/fmicb.2020.548607.
- Shao K, Gao G, Wang Y, Tang X, Qin B. 2013. Vertical diversity of sediment bacterial communities in two different trophic states of the eutrophic Lake Taihu, China. J Environ Sci (China). 25(6):1186–1194. doi: 10.1016/s1001-0742(12)60122-3.
- Tang XM, Gao G, Qin BQ, Zhu LP, Chao JY, Wang JJ, Yang GJ. 2009. Characterization of bacterial communities associated with organic aggregates in a large, shallow, eutrophic freshwater Lake (Lake Taihu, China). Microb Ecol. 58(2):307–322. doi: 10.1007/s00248-008-9482-8.
- Wu D, Liu H, Wu J, Gao X. 2022. Spatial distribution, ecological risk assessment and source analysis of heavy metals pollution in urban lake sediments of Huaihe River Basin. Int J Environ Res Public Health. 19(22):14653. doi: 10.3390/ijerph192214653.
- Wu Y, Liu J, Yang L, Chen H, Zhang S, Zhao H, Zhang N. 2011. Allelopathic control of cyanobacterial blooms by periphyton biofilms. Environ Microbiol. 13(3):604–615. doi: 10.1111/j.1462-2920.2010.02363.x.
- Zeng J, Yang L, Li J, Liang Y, Xiao L, Jiang L, Zhao D. 2009. Vertical distribution of bacterial community structure in the sediments of two eutrophic lakes revealed by denaturing gradient gel electrophoresis (DGGE) and multivariate analysis techniques. World J Microbiol Biotechnol. 25(2):225–233. doi: 10.1007/s11274-008-9883-3.
- Zhang JY. 2021. Application of small and medium-sized steel bridge in the reinforcement project of Chengdong Lake Lock. Harnessing the Huaihe River; p. 56–57. (in Chinese with English abstract).
- Zhang X, Yan QY, Yu YH, Dai LL. 2014. Spatiotemporal pattern of bacterioplankton in Donghu Lake. Chin J Ocean Limnol. 32(3):554–564. (in Chinese with English abstract). doi: 10.1007/s00343-014-3037-2.
- Zhao XX, Lu WX, Li J, Liang YY, Fang T, Yang K. 2020. Community structure of phytoplankton and bio-assessment of water quality in Chengdong Lake in Anhui, China. Ecol Sci. 39:187–196. (in Chinese with English abstract).