ABSTRACT
Purpose: This study investigated the clinical usefulness of a multiple tear cytokine/chemokine test by simultaneously determining tear levels of CC chemokine ligand 17 (CCL17)/thymus and activation-regulated chemokine (TARC), CCL24/eotaxin-2, and interleukin-16 (IL-16) for assessing acute and chronic allergic inflammation in allergic conjunctival disorders (ACDs).
Subjects and Methods: This study included 37 patients with ACD and 11 healthy adults (controls). Patients with ACDs were divided into the following three groups; patients with allergic conjunctivitis (AC group, n = 17), patients with atopic keratoconjunctivitis (AKC group, n = 6), and patients with vernal keratoconjunctivitis (VKC group, n = 14). Tear samples were collected using the Schirmer I method with a filter paper. Tear levels of CCL17/TARC, CCL24/eotaxin-2, and IL-16 were determined by performing a magnetic bead assay (tear cytokine/chemokine test). Tear levels of eosinophil cationic protein (ECP) were determined by performing enzyme immunoassay. In patients with AC, clinical scores of objective findings and results of the tear cytokine/chemokine test at baseline were compared with those at 7 days after treatment with the histamine H1 receptor antagonist (epinastine) ophthalmic solution.
Results: Tear positive rates of CCL17/TARC, CCL24/eotaxin-2, and IL-16 were higher in patients with AC, AKC, and VKC compared with controls. Tear levels of CCL17/TARC, CCL24/eotaxin-2, and IL-16 in patients with AKC and VKC were significantly higher than those in patients with AC. Moreover, tear levels of IL-16 in patients with AC that showed improvement of their clinical score by treatment with epinastine ophthalmic solution decreased significantly after 7 days of the treatment compared with those at baseline. In patients with AKC and VKC, a significant correlation was observed between the tear levels of CCL24/eotaxin-2 and ECP.
Conclusion: Simultaneous measurement of the tear levels of CCL17/TARC, CCL24/eotaxin-2, and IL-16 may be a useful test for assessing acute and chronic allergic inflammation in ACDs.
Introduction
Allergic conjunctival disorders (ACDs) are inflammatory disorders characterized by an immediate hypersensitivity response in the ocular surface. ACDs are classified into three clinical forms, namely, allergic conjunctivitis (AC), atopic keratoconjunctivitis (AKC), and vernal keratoconjunctivitis (VKC).Citation1 The immediate hypersensitivity response includes two affinity reactions, namely, an early-phase reaction (EPR) and a late-phase reaction (LPR).Citation2 The EPR is characterized by the release of chemical mediators in the conjunctiva due to the degranulation of mast cells.Citation3 Representative chemical mediators released from mast cells include histamine, leukotriene, prostaglandin, thromboxane A2, and platelet-activating factor. Therefore, therapeutic ophthalmic solutions such as mast cell stabilizers and histamine H1 receptor antagonists are used for treating EPR. The LPR is an inflammatory reaction characterized by the infiltration of immediate hypersensitivity-specific inflammatory cells such as eosinophils and type 2 helper T cells (Th2 cells).Citation3,Citation4 Corticosteroids and immunosuppressive agents are used for treating LPR. Therefore, determining the pathophysiology of allergic inflammation in the ocular surface of patients with ACDs is important to ensure the proper use of the therapeutic drugs for these patients.
Several researchers have used a tear test to assess the levels of suitable biomarkers of allergic inflammation in the ocular surface. Aside from eosinophil cationic protein (ECP), which is a useful biomarker of eosinophilic inflammation,Citation5–Citation7 cytokines and chemokines such as CCL11/eotaxin-1 and CCL24/eotaxin-2 associated with eosinophils;Citation8–Citation10 interleukin-4 (IL-4), CCL17/thymus and activation-regulated chemokine (TARC), and CCL22/macrophage-derived chemokine associated with Th2 cellsCitation11,Citation12; and macrophage inflammatory protein-3 alpha (MIP-3α)/CCL20 associated with Th17 cellsCitation13 are useful biomarkers of allergic inflammation. Tear levels of eotaxin-2 are known to increase in patients with VKC,Citation10 therefore this chemokine is thought to be associated with eosinophilic inflammation of conjunctival tissues. TARC is associated with the migration of Th2 cells, and serum levels of TARC are used for determining the severity of atopic dermatitis.Citation14,Citation15 IL-16 is associated with the migration of CD4-positive cells and with the pathophysiology of immediate hypersensitivity reaction.Citation16 However, the significance of IL-16 as a biomarker in patients with ACDs is not completely understood.
It is difficult to establish a single cytokine/chemokine as a biomarker for ACD because many are associated with conjunctival inflammation. Cytokine/chemokine profiles obtained by simultaneously measuring the levels of multiple cytokines by using an antibody array or multiple bead assay are efficient at determining major factors involved in conjunctival allergic inflammation.Citation17,Citation18 However, the combination of several biomarkers is more helpful in diagnosis and makes it easier to understand the pathophysiology compared to a cytokine/chemokine profile analysis in the clinical setting of allergic inflammation in ACD. Therefore, we conducted a preliminary experiment whereby we selected the cytokines and chemokines for use in this study by applying a cytokine/chemokine profile method. The cytokines and chemokines were detected in the tears of the ACD patients abundantly and were detected in the tears of the normal control only in small quantities. In this study, we simultaneously measured the levels of IL-16, CCL17/TARC, and CCL24/eotaxin-2 in tear samples of patients with ACDs, which were obtained using the Schirmer I method. Furthermore, we investigated the utility of this tear test for evaluating allergic inflammation associated with ACDs.
Materials and methods
Subjects
This study was approved by the institutional review board of the Nihon University School of Medicine and adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all subjects enrolled in this study.
This study included 37 consecutive patients (37 eyes) with ACDs who were treated at the Department of Ophthalmology, Nihon University Itabashi Hospital, Tokyo, Japan, from January 2013 to September 2015 and 11 healthy volunteers who did not have atopic diathesis and or a history of wearing contact lenses as controls. Demographic data of the subjects are shown in . Only non-treated patients or patients treated with anti-allergic ophthalmic solutions alone, such as mast cell stabilizers, histamine H1 receptor antagonists, corticosteroids, and immunosuppressive agents were included in the study. Patients who used oral medicines or injections for treating allergic diseases and those who received immunotherapy were excluded from the study. In addition, patients with ocular surface diseases other than ACD, including lagophthalmos, blepharospasm, conjunctival chalasis, dry eye, infectious conjunctivitis, infectious keratitis, Stevens–Johnson syndrome, and ocular pemphigoid, and patients who could not provide a sufficient amount of tear sample were excluded from the study. The treatment regimen of ophthalmic solutions for each patient is shown in .
Table 1. Subjects and their demographic data.
The patients were divided into the following 3 groups: AC group, 17 patients (17 eyes) with AC; AKC group, 6 patients (6 eyes) with AKC; and VKC group, 14 patients (14 eyes) with VKC. ACDs were diagnosed using the guidelines of the Japanese Ophthalmological Society.Citation1 AC, AKC, and VKC were diagnosed by performing a clinical examination with a slit-lamp microscope, according to the Japanese guidelines for ACDs. The patients with ACDs had at least one positive antigen-specific IgE antibody in the serum, such as house-dust mite- or Japanese cedar pollen-specific IgE antibody, which was detected using ImmunoCAP® (Thermo Fisher Scientific, Tokyo, Japan).
Utility of tear cytokine/chemokine test
We performed the simultaneous measurement of allergic inflammation-associated cytokines (IL-16) and chemokines (CCL17/TARC and CCL24/eotaxin-2) in the tears of patients with ACDs using a magnetic bead assay.
Tear sampling
Tears were sampled from the affected eye in unilateral cases or from the more severely affected eye in bilateral cases. The tear samples from the ACD patients were collected under the following conditions; the seasonal ACD patients were performed In-season, and the perennial ACD patients were performed during the active stage. If clinical observations of the activity stage of ACD including conjunctival hyperemia, edema, and swelling were obtained in the perennial ACD patients with treatment of ophthalmic solutions, the tear samples were collected without a wash-out period. The right eyes of 11 healthy volunteers were used as controls.
Tears were sampled using the Schirmer I method with filter paper (Schirmer Tear Production Measuring Strips; Showa Yakuhin Kako, Tokyo, Japan),Citation7 and the Schirmer strips were stored at −20°C until further use. The Schirmer strips were thawed and eluted overnight at room temperature using 0.5 M NaCl and 0.5% Tween 20 containing 0.05 M phosphate-buffered solution (pH 7.2). The amount of tears obtained was calculated by considering 1 mm of a wet Schirmer strip to contain 1 μl of tears. Thus, the end concentration of the eluted solution corresponded to a 20-fold dilution of the original tear sample.
Magnetic bead assay
Tear levels of IL-16, CCL17/TARC, and CCL24/eotaxin-2 were simultaneously determined by performing a magnetic bead assay (Bio-Plex Pro™ human chemokine assay; Bio-Rad Laboratories, Hercules, CA, USA), according to the manufacturer’s instructions. The lower detection limit indicated by the manufacturer was 2.1 pg/mL for IL-16, 1.7 pg/mL for CCL17/TARC, and 6.2 pg/mL for CCL24/eotaxin-2.
Enzyme immunoassay
Tear ECP levels were determined by performing a chemiluminescent enzyme immunoassay (Immuryze®; LSI Medience, Tokyo, Japan). The lower detection limit for ECP, as indicated by the manufacturer, was 0.2 ng/mL.
Assessment of the measurement outcomes
The utility of the tear cytokine/chemokine test for diagnosing ACDs was determined by assessing its sensitivity and specificity in each clinical form of ACD and by comparing cytokine/chemokine levels associated with these clinical forms of ACD. We also determined the utility of the multiple tear cytokine/chemokine tests by comparing the tear levels of ECP, a biomarker of allergic inflammation, with the results of the test.
Statistical analysis
Differences in the positive ratios of cytokines and chemokines associated with each clinical form of ACD were evaluated using Fisher’s exact test. A Steel–Dwass test was used to compare tear levels of cytokines/chemokines. Partial correlations were performed to assess the relationship of tear levels between ECP and cytokines/chemokines. A Spearman rank correlation coefficient was used to determine the association between tear levels of ECP and eotaxin-2. A p value of <0.05 was considered statistically significant. All statistical analyses were performed using MAC Toukei-Kaiseki Ver. 2 (Esumi, Tokyo, Japan).
Therapeutic monitoring of patients with AC using the tear cytokine/chemokine test
Patients
This study included patients with AC (n = 17) who were treated with a histamine H1 receptor antagonist (epinastine) ophthalmic solution.
All patients with AC underwent the following clinical tests at the initiation of instillation treatment (baseline) and 7 days after the instillation treatment (day 7): determination of objective findings and tear cytokine/chemokine test. Objective findings were recorded at baseline and 7 days after the treatment by determining clinical scores with a 5-5-5 exacerbation grading scale for ACDs.Citation19
After baseline examination, all patients with AC received 1 drop of 0.05% epinastine hydrochloride ophthalmic solution (ALESION® Ophthalmic Solution 0.05%, Santen Pharmaceutical Co. Ltd, Osaka, Japan) in each eye 4 times daily for 7 days. The same clinical tests were repeated after 7 days.
Assessment of the measurement outcomes
The results of the cytokine/chemokine test performed at baseline and 7 days after the treatment were compared to determine whether CCL17/TARC, CCL24/eotaxin-2, or IL-16 were useful as biomarkers for monitoring patients with AC treated with epinastine ophthalmic solution.
Statistical analysis
Clinical scores and tear cytokine/chemokine levels of patients with AC at baseline were compared with those on day 7 using a Wilcoxon signed rank test. A p value of <0.05 was considered statistically significant. Statistical analysis was performed using MAC Toukei-Kaiseki Ver. 2.
Case presentation: monitoring patients with VKC using the tear cytokine/chemokine test
Two patients with VKC who were untreated at initial diagnosis in our hospital were treated with a combination of mast cell stabilizer and cyclosporine ophthalmic solutions. The effect of this treatment on VKC was monitored by performing the tear cytokine/chemokine test.
Results
Utility of the tear cytokine/chemokine test
The positive rates, sensitivity, and specificity of the tear cytokine/chemokine test for determining tear levels of IL-16, CCL17/TARC, and CCL24/eotaxin-2 are shown in . The positive rates of each cytokine/chemokine in patients with each clinical form of ACD were significantly higher than that in controls. The sensitivity of the test in determining the tear level of IL-16 was >90% for all the clinical forms of ACD. Moreover, the specificity of the test for determining the levels of all cytokines/chemokines was >90% for the 3 clinical forms of ACD.
Table 2. Detection ratio of each chemokine in tears by performing beaded assay.
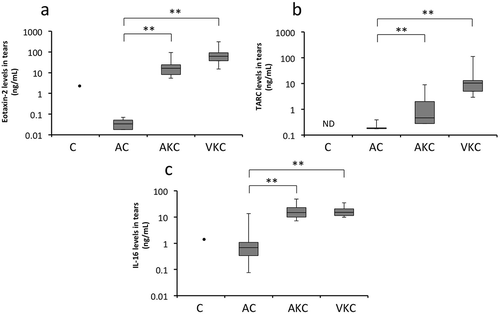
Tear levels of IL-16, CCL17/TARC, and CCL24/eotaxin-2 according to the clinical forms of ACD are shown in . Tear levels of IL-16 (median [range]) in patients with AC, AKC, and VKC were 7.7 (4.0–31), 13 (5.1–49), and 9.2 (3.9–35) ng/mL, respectively; levels of CCL17/TARC were 0.01 (0.01–0.39), 1.7 (0.48–8.9), and 9.2 (3.9–35) ng/mL, respectively; and levels of CCL24/eotaxin-2 were 0.034 (0.018–0.35), 16 (5.0–70), and 55 (8.2–2.2 × 102) ng/mL, respectively. Tear levels of IL-16, CCL17/TARC, and CCL24/eotaxin-2 levels in patients with AKC and VKC were significantly higher than those in patients with AC ().
Figure 1. Determination of chemokine levels in tears by using the magnetic bead assay. Tear levels of CCL17/TARC (A), CCL24/eotaxin-2 (B), and IL-16 (C) in patients with AKC and VKC are significantly higher than those in patients with AC; *p < 0.05, **p < 0.01. AC: allergic conjunctivitis, AKC: atopic keratoconjunctivitis, VKC: vernal keratoconjunctivitis, TARC: thymus and activation-regulated chemokine, IL-16: interleukin-16, ND: not detected.
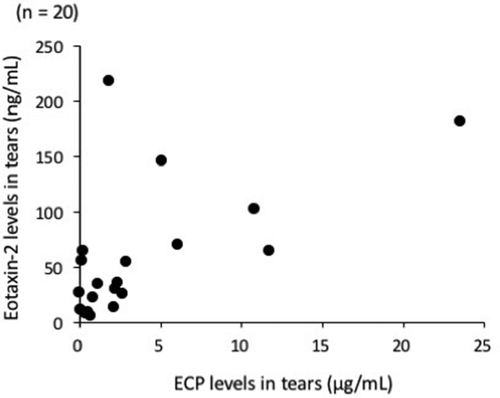
There was a significant correlation between tear levels of ECP and eotaxin-2 in patients with AKC and VKC (), with a correlation coefficient of 0.68 (Spearman rank correlation coefficient, p = 0.0032; ).
Table 3. Partial correlation coefficient of patients with AKC and VKC.
Figure 2. Correlation between eosinophil cationic protein (ECP) and eotaxin-2 levels in the tears of patients with atopic keratoconjunctivitis (AKC) and vernal keratoconjunctivitis (VKC). Tear levels of ECP were determined by chemiluminescent enzyme immunoassay and those of eotaxin-2 were determined by magnetic bead assay. Significant correlation was observed between ECP and eotaxin-2 levels in the tears of patients with AKC (n = 6) and VKC (n = 14) (Spearman rank correlation coefficient, ρ = 0.68, p < 0.005).
Therapeutic monitoring of patients with AC using the tear cytokine/chemokine test
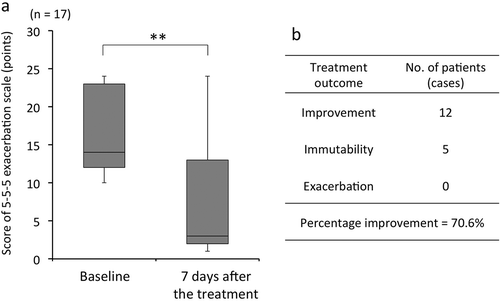
In patients with AC, clinical scores (median [range]) at baseline and on day 7 after the treatment were 14 (2–24) and 3 (1–24) points, respectively. Clinical scores of patients with AC treated with epinastine ophthalmic solution significantly decreased after 7 days compared to the baseline scores (p < 0.001, Wilcoxon signed rank test; ). Patients with AC were divided into the following 3 groups according to their outcomes after treatment with the epinastine ophthalmic solution: patients showing improvement (n = 12) whose clinical scores decreased after 7 days of treatment, patients showing immutability (n = 5) who had the same clinical scores at baseline and 7 days after the treatment, and patients showing exacerbation (n = 0) whose clinical scores increased after 7 days of treatment (). It was judged with effectiveness in the cases that clinical scores showed improvement. In contrast, there was a degree of invalidity in the cases with clinical scores that were immutable or exacerbated. As a result, the response rate of this treatment was found to be 70.6%.
Figure 3. Clinical scores of patients with AC. Clinical scores of patients with AC were determined using the 5-5-5 exacerbation scale.
a. Clinical scores of patients with AC treated with epinastine ophthalmic solution significantly decreased after 7 days compared with those at baseline (p < 0.001, Wilcoxon signed rank test).b. Patients with AC were divided into 3 groups based on their treatment outcomes: patients showing improvement (n = 12), patients showing immutability (n = 5), and patients showing exacerbation (n = 0).
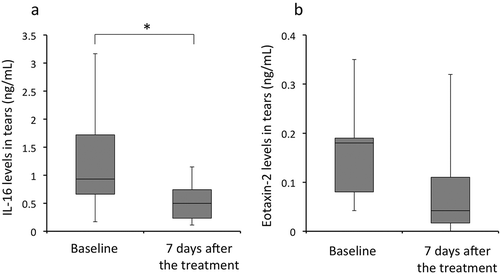
In 12 patients with AC that showed improvement, tear levels of IL-16 on day 7 were significantly lower than those at baseline (p < 0.05, Wilcoxon signed rank test; ). Furthermore, tear levels of CCR24/eotaxin-2 in these patients were lower on day 7 than at baseline (). However, statistical analysis could not be performed because only 5 patients with AC who showed improvement yielded positive results for CCR24/eotaxin-2 in the tear cytokine/chemokine test. Furthermore, no significant differences were observed in the tear levels of CCL17/TARC at baseline and 7 days after the treatment.
Figure 4. Tear levels of IL-16 and eotaxin-2 in patients with AC. In patients with AC showing improvement (n = 12), tear levels of IL-16 decreased significantly 7 days after the treatment compared with those at baseline (p < 0.05, Wilcoxon signed rank test). Similarly, tear levels of eotaxin-2 decreased at 7 days after the treatment compared with those at baseline. However, statistical significance could not be calculated due to a small sample size.
Case presentation
Patient 1: An 8-year-old girl presented with a chronic history of severe itching, hyperemia, and ropy discharge in both eyes. In addition, she had a history of atopic dermatitis. Her best corrected visual acuity score was 20/20 for each eye. Slit-lamp microscopy showed several giant papillae in the upper tarsal conjunctiva and diffuse thickening of the limbal tissue, with Trantas’ dots in both eyes. The patient was treated with topical 2% sodium cromoglycate (Intal® Eye Drops UD2%, Sanofi, Tokyo, Japan) 4 times daily and 0.1% cyclosporine (PAPILOCK® Mini Ophthalmic Solution 0.1%; Santen Pharmaceutical Co. Ltd, Osaka, Japan) 3 times daily. Topical instillation treatment markedly decreased the size of the giant papillae, loss of limbal diffuse thickening, and Trantas’ dots 3 weeks following treatment. The results of the tear cytokine/chemokine test showed that levels of CCL17/TARC, CCL24/eotaxin-2, and IL-16 gradually decreased after the treatment ().
Figure 5. Clinical features of 2 patients with VKC, and the results of the tear cytokine/chemokine test in these patients.
a.An 8-year-old girl with VKC. Slit-lamp photographs showing several giant papillae in the upper tarsal conjunctiva and diffuse thickening of the limbal tissue, with trantas spots in both eyes at baseline. These clinical observations improved and tear levels of CCL17/TARC and CCL24/eotaxin-2 decreased after treatment with 2% sodium cromoglycate and 0.1% cyclosporine ophthalmic solution.b.An 8-year-old boy with VKC. Slit-lamp photographs showing several giant papillae in the upper tarsal conjunctiva in both eyes. These clinical observations improved and tear levels of CCL17/TARC and CCL24/eotaxin-2 decreased after treatment with 0.5% tranilast and 0.1% cyclosporine ophthalmic solution. ND: not detected.
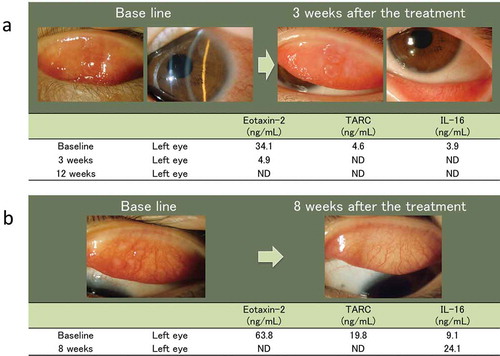
Patient 2: An 8-year-old boy presented with a 6-month history of severe itching, hyperemia, and ropy discharge in both eyes. His symptoms increased from spring to summer. His best corrected visual acuity score was 20/20 in each eye. Slit-lamp microscopy showed several giant papillae in the upper tarsal conjunctiva of both eyes. The patient was treated using topical 0.5% tranilast (Rizaben® Eye Drops 0.5%; Kissei Pharmaceutical Co. Ltd, Nagano, Japan) 4 times daily and 0.1% cyclosporine (PAPILOCK® Mini Ophthalmic Solution 0.1%) 3 times daily. Topical instillation treatment markedly decreased the size of the giant papillae at 8 weeks following treatment. The results of the tear cytokine/chemokine test showed a decrease in the levels of CCL17/TARC and CCL24/eotaxin-2 but an increase in the level of IL -16 after treatment ().
Discussion
The tear test is a useful tool that can be used to determine the local pathophysiology of the ocular surface in patients with ACDs. In this study, we investigated the utility of the tear cytokine/chemokine test not only for diagnosing ACDs but also for determining the therapeutic effect of treatment with different ophthalmic solutions. As there are pathological differences in the different clinical forms of ACD, no optimal biomarker is available for diagnosing and assessing the therapeutic effects of different treatments on these clinical forms. Although ECP is a useful biomarker for determining therapeutic effects in patients with VKC,Citation20 no biomarker is available for assessing therapeutic effects in patients with AC. In this study, we investigated the clinical utility of the tear cytokine/chemokine test by simultaneously determining the tear levels of IL-16, CCL17/TARC, and CCL24/eotaxin-2, which are associated with the migration of inflammatory cells, in patients with different clinical forms of ACDs.
Positive rate and levels of IL-16, CCL17/TARC, and CCL24/eotaxin-2 were significantly higher in the tears of patients with AKC and VKC than in controls and patients with AC. Furthermore, a significant correlation was observed between tear levels of eotaxin-2 and ECP. These findings suggest that patients with AKC and VKC have severe allergic inflammation due to the infiltration of eosinophils and Th2 cells and that eotaxin-2 is an optimal biomarker for assessing the severity of allergic inflammation in these patients. Eotaxin promotes eosinophil infiltration through CC chemokine receptor 3 (CCR3).Citation21 CCR3 is expressed by both eosinophils and Th2 cells and may be associated with their migration into conjunctival tissues. Eotaxin has 3 types: CCL11/eotaxin-1, CCL24/eotaxin-2, and CCL26/eotaxin-3. The results obtained from the tear cytokine/chemokine test showed that CCL24/eotaxin-2 had the highest detection rate and level among the 3 types of eotaxinsCitation10 and that eotaxin-2 was a useful biomarker compared with eotaxin-1 and -3 for determining eosinophilic inflammation. This study showed that the eotaxin-2 levels in tears were significantly correlated with ECP levels in tears. Furthermore, the ECP levels in tears had a wide range from <20 to 20 000 ng/mL and were not an appropriate parameter for the clinical tear test. Therefore, eotaxin-2 may be a suitable substitute for ECP as a marker for eosinophilic inflammation in ACD. The results obtained from the tear cytokine/chemokine test in the 2 patients with VKC showed that the tear levels of CCL24/eotaxin-2 and CCL17/TARC decreased after 7 days of cyclosporine instillation. However, tear levels of IL-16 increased in one patient but decreased in the other patient after 7 days of treatment with topical cyclosporine. Therefore, CCL24/eotaxin-2 and CCL17/TARC are suggested to be useful biomarkers of cyclosporine instillation. However, IL-16, which has a different property to eotaxin-2, may be a useful biomarker for evaluating the pathophysiology of the immediate hypersensitivity reaction.
In patients with AC, IL-16 was the most useful biomarker, with a positive rate of >90%. Furthermore, positive rates of allergic inflammation-associated chemokines CCL17/TARC and CCL24/eotaxin-2 in these patients were intermediate compared with controls. These results suggest that patients with AC had a mild allergic inflammation and that eotaxin-2 and TARC were not appropriate biomarkers for assessing the therapeutic effects of the ophthalmic solution. The positive rate of IL-16 was high in patients with AC, and tear levels of IL-16 in these patients decreased after epinastine instillation.
IL-16 acts as a chemoattractant for various CD4-positive immune cellsCitation22,Citation23 and as an immunomodulatory cytokine in allergic diseases.Citation24,Citation25 IL-16 is produced by epithelial cells and immune cells such as eosinophils, mast cells, lymphocytes, macrophages, and dendritic cells. Increased secretion of IL-16 is observed in patients with allergen-induced rhinitis and those with allergic asthma.Citation26,Citation27 Moreover, serum IL-16 levels are significantly elevated in patients with allergic rhinitis compared with controls.Citation28 IL-16 expression in the nasal mucosa of patients with allergic rhinitis is upregulated during the grass pollen season.Citation29 In our study, a high positive rate of IL-16 in tears was determined irrespective of the clinical form of ACD. The results of previous studies and our study suggest that local and systemic levels of IL-16 are a suitable biomarker for allergic diseases. Tear levels of IL-16 in patients with AC decreased at 7 days following treatment with epinastine ophthalmic solution. In an ovalbumin-sensitized mouse model of allergic rhinitis, fexofenadine, and ramatroban treatment significantly inhibited IL-16 expression in the nasal tissue.Citation30 Therefore, local expression of IL-16 might be useful as a biomarker for assessing the therapeutic effects of histamine H1 receptor antagonists but not of immunosuppressive drugs such as cyclosporine. However, it is unclear whether treatment with epinastine ophthalmic solution directly or indirectly reduced IL-16 levels in the tears of patients with AC. Furthermore, in the two case studies of VKC, the difference in the anti-allergic ophthalmic solutions (Intal® vs. Rizaben®) and the effect on the results of simultaneous measurement of cytokine/chemokine including IL-16 was a critical problem; however, we were unable to clarify this problem in this case study. IL-16 exerts immunomodulatory effects, including suppression of regulatory T cell migrationCitation24 and inhibition of eosinophilia and Th2 cytokine production in lymphocytes.Citation31 Therefore, further clinical studies should be performed to investigate the clinical and pathophysiological significance of altered IL-16 levels in the tears of patients with ACDs.
The limitations of this study are as follows. First, this tear test showed high sensitivity and specificity in the comparison between ACD patients and normal controls. Therefore, we have shown the usefulness of the clinical test to assist in the diagnosis of ACD. However, other diseases of the ocular surface were not included in this study. Therefore, further studies are necessary to investigate true sensitivity and specificity for ACD diagnosis. Second, in this study, we compared the tear test result before and after the treatment in the untreated cases, and this tear test showed usefulness as a possibility for treatment assessment. However, the efficacy of this tear test for the treatment assessment was not evaluated thoroughly. A large-scale investigation for the untreated ACD patients is necessary to determine the efficacy of this tear test for treatment assessment.
In conclusion, the use of a tear cytokine/chemokine test for simultaneously measuring tear levels of IL-16, CCL17/TARC, and CCL24/eotaxin-2 improves diagnostic accuracy and helps in quantifying the therapeutic effect of different treatments.
Declaration of interest
The authors wish to make the following disclosure: Jun Shoji has received an honorarium from Santen Pharmaceutical Co., Ltd, Osaka, Japan. Hiroshi Aso has declared no conflict of interest.
Acknowledgments
The authors thank Professor Mitsuko Yuzawa (Chairman, Division of Ophthalmology, Department of Visual Sciences, Nihon University School of Medicine) for editing this manuscript. We wish to thank Mrs. Akiko Tomioka (Department of Visual Sciences, Division of Ophthalmology, Nihon University School of Medicine) for her expert technical assistance. This study was performed under a collaborative research agreement between the Nihon University School of Medicine and Santen Pharmaceutical Co., Ltd.
Funding
Noriko Inada has received research funding from Santen Pharmaceutical Co., Ltd.
Additional information
Funding
Notes on contributors
Noriko Inada
Designing and conducting the study: J.S.; analyzing and interpreting data: J.S., N.I., and H.A.; writing the manuscript: J.S. and N.I.; critically revising the manuscript: J.S. and N.I.; providing final approval for the manuscript: J.S., N.I., and H.A.; collecting data: J.S., N.I., and H.A.; providing statistical expertise: J.S.; searching literature: J.S. and N.I.; and providing administrative, technical, or logistic support: J.S., N.I., and H.A.
References
- Takamura E, Uchio E, Ebihara N, Ohno S, Ohashi Y, Okamoto S, et al. Japanese guideline for allergic conjunctival diseases. Allergol Int 2011;60:191–203.
- Leonardi A. Allergy and allergic mediators in tears. Exp Eye Res 2013;117:106–117.
- Bacon AS, Ahluwalia P, Irani AM, Schwartz LB, Holgate ST, Church MK, et al. Tear and conjunctival changes during the allergen-induced early- and late-phase responses. J Allergy Clin Immunol 2000;106:948–954.
- Reyes NJ, Saban DR. T helper subsets in allergic eye disease. Curr Opin Allergy Clin Immunol 2014;14:477–484.
- Montan PG, van Hage-Hamsten M. Eosinophil cationic protein in tears in allergic conjunctivitis. Br J Ophthalmol 1996;80:556–560.
- Montan PG, van Hage-Hamsten M, Zetterström O. Sustained eosinophil cationic protein release into tears after a single high-dose conjunctival allergen challenge. Clin Exp Allergy 1996;26:1125–1130.
- Shoji J, Kitazawa M, Inada N, Sawa M, Ono T, Kawamura M, et al. Efficacy of tear eosinophil cationic protein level measurement using filter paper for diagnosing allergic conjunctival disorders. Jpn J Ophthalmol 2003;47:64–68.
- Leonardi A, Jose PJ, Zhan H, Calder VL. Tear and mucus eotaxin-1 and eotaxin-2 in allergic keratoconjunctivitis. Ophthalmology 2003;110:487–492.
- Fukagawa K, Nakajima T, Tsubota K, Shimmura S, Saito H, Hirai K. Presence of eotaxin in tears of patients with atopic keratoconjunctivitis with severe corneal damage. J Allergy Clin Immunol 1999;103:1220–1221.
- Shoji J, Inada N, Sawa M. Evaluation of eotaxin-1, -2, and -3 protein production and messenger RNA expression in patients with vernal keratoconjunctivitis. Jpn J Ophthalmol 2009;53:92–99.
- Uchio E, Ono SY, Ikezawa Z, Ohno S. Tear levels of interferon-gamma, interleukin (IL)-2, IL-4 and IL-5 in patients with vernal keratoconjunctivitis, atopic keratoconjunctivitis and allergic conjunctivitis. Clin Exp Allergy 2000;30:103–109.
- Fujishima H, Saito I, Takeuchi T, Tsubota K. Immunological characteristics of patients with vernal keratoconjunctivitis. Jpn J Ophthalmol 2002;46:244–248.
- Inada N, Ishimori A, Shoji J. CCL20/MIP-3 alpha mRNA expression in the conjunctival epithelium of normal individuals and patients with vernal keratoconjunctivitis. Graefes Arch Clin Exp Ophthalmol 2014;252:1977–1984.
- Hijnen D, De Bruin-Weller M, Oosting B, Lebre C, De Jong E, Bruijnzeel-Koomen C, et al. Serum thymus and activation-regulated chemokine (TARC) and cutaneous T cell-attracting chemokine (CTACK) levels in allergic disease: TARC and CTACK are disease-specific markers for atopic dermatitis. J Allergy Clin Immunol 2004;113:334–340.
- Jahnz-Rozyk K, Targowski T, Paluchowska E, Owczarek W, Kucharczyk A. Serum thymus and activation-regulated chemokine, macrophage-derived chemokine and eotaxin as markers of severity of atopic dermatitis. Allergy 2005;60:685–688.
- Laberge S, Ghaffar O, Boguniewicz M, Center DM, Leung DY, Hamid Q. Association of increased CD4+ T-cell infiltration with increased IL-16 gene expression in atopic dermatitis. J Allergy Clin Immunol 1998;102:645–650.
- Shoji J, Inada N, Sawa M. antibody array-generated cytokine profiles of tears of patients with vernal keratoconjunctivitis or giant papillary conjunctivitis. Jpn J Ophthalmol 2006;50:195–204.
- Leonardi A, Curnow SJ, Zhan H, Calder VL. Multiple cytokines in human tear specimens in seasonal and chronic allergic eye disease and in conjunctival fibroblast cultures. Clin Exp Allergy 2006;36:777–784.
- Shoji J, Inada N, Sawa M. Evaluation of novel scoring system named 5-5-5 exacerbation grading scale for allergic conjunctivitis disease. Allergol Int 2009;58:591–597.
- Leonardi A, Borghesan F, Faggian D, Secchi A, Plebani M. Eosinophil cationic protein in tears of normal subjects and patients affected by vernal keratoconjunctivitis. Allergy 1995;50:610–613.
- Kitaura M, Nakajima T, Imai T, Harada S, Combadiere C, Tiffany HL, et al. Molecular cloning of human eotaxin, an eosinophil-selective CC chemokine, and identification of a specific eosinophil eotaxin receptor, CC chemokine receptor 3. J Biol Chem 1996;29:7725–7730.
- Center DM, Kornfeld H, Cruikshank WW. Interleukin 16 and its function as a CD4 ligand. Immunol Today 1996;17:476–481.
- Cruikshank WW, Center DM, Nisar N, Wu M, Natke B, Theodore AC, et al. Molecular and functional analysis of a lymphocyte chemoattractant factor: association of biologic function with CD4 expression. Proc Natl Acad Sci USA 1994;91:5109–5113.
- McFadden C, Morgan R, Rahangdale S, Green D, Yamasaki H, Center D, et al. Preferential migration of T regulatory cells induced by IL-16. J Immunol 2007;179:6439–6445.
- Trudelle A, El Bassam S, Pinsonneault S, Mazer B, Laberge S. Interleukin-16 inhibits immunoglobulin e production by B lymphocytes. Int Arch Allergy Immunol 2007;143:109–118.
- Baumann R, Rabaszowski M, Stenin I, Tilgner L, Gaertner-Akerboom M, Scheckenbach K, et al. Nasal levels of soluble IL-33R ST2 and IL-16 in allergic rhinitis: inverse correlation trends with disease severity. Clin Exp Allergy 2013;43:1134–1143.
- Cruikshank WW, Long A, Tarpy RE, Kornfeld H, Carroll MP, Teran L, et al. Early identification of interleukin-16 (lymphocyte chemoattractant factor) and macrophage inflammatory protein 1 alpha (MIP 1 alpha) in bronchoalveolar lavage fluid of antigen-challenged asthmatics. Am J Respir Cell Mol Biol 1995;13:738–747.
- Karaki M, Dobashi H, Kobayashi R, Tokuda M, Ishida M, Mori N. Expression of interleukin-16 in allergic rhinitis. Int Arch Allergy Immunol 2005;138:67–72.
- Pullerits T, Lindén A, Malmhäll C, Lötvall J. Effect of seasonal allergen exposure on mucosal IL-16 and CD4+ cells in patients with allergic rhinitis. Allergy 2001;56:871–877.
- Akiyama K, Karaki M, Kobayashi R, Dobashi H, Ishida T, Mori N. IL-16 variability and modulation by antiallergic drugs in a murine experimental allergic rhinitis model. Int Arch Allergy Immunol 2009;149:315–322.
- De Bie JJ, Jonker EH, Henricks PA, Hoevenaars J, Little FF, Cruikshank WW, et al. Exogenous interleukin-16 inhibits antigen-induced airway hyper-reactivity, eosinophilia, and Th2-type cytokine production in mice. Allergy 2002;32:1651–1658.
