ABSTRACT
Purpose/Aims: This study assessed the efficacy and safety of brimonidine tartrate ophthalmic solution, 0.025% for treating ocular redness in adult subjects.
Materials and Methods: This was a single-center, double-masked, randomized, vehicle-controlled, parallel-group study in subjects ≥40 years, with ocular redness. Subjects were randomized 2:1 to brimonidine or vehicle, instilled QID for four weeks. Subjects completed four visits, the last occurring one week after treatment discontinuation. The investigator assessed ocular redness on a scale of 0–4 pre-instillation and 5–240 minutes post-instillation on Day 0, pre-instillation and 5 minutes post-instillation on Days 14 and 28, and on Day35; subjects assessed redness in diaries throughout the 28-day treatment period and following treatment discontinuation. Safety assessments included adverse events (AEs), rebound redness on treatment discontinuation, comprehensive ophthalmic exams, and vital signs. Drop comfort was assessed upon instillation, and 30 seconds and 1 minute post-instillation at Day 0.
Results: Fifty-seven subjects (brimonidine, n = 38; vehicle, n = 19) were randomized. Investigator-assessed ocular redness was significantly reduced with brimonidine across the entire post-instillation time period (overall treatment difference: −1.37; P < 0.0001) and at all individual time points (P < 0.0001). Subject-assessed ocular redness was also significantly lower with brimonidine (P ≤ 0.0005). No tachyphylaxis was evident. There were few ocular AEs, all mild to moderate in severity, and no redness rebound was observed upon brimonidine discontinuation. There were no effects on any safety measures, and both brimonidine and its vehicle were reported to be very comfortable.
Conclusions: Brimonidine 0.025% appeared safe, well tolerated, and reduced ocular redness for at least 4 hours. No tachyphylaxis or rebound redness upon treatment discontinuation was observed.
Introduction
Ocular redness, or hyperemia, is a common ophthalmic condition typically caused by inflammation of the conjunctiva due to allergy, exposure to environmental irritants, or as a reaction to infectious agents (e.g., bacteria, virus). Preferred treatment depends on the cause of redness.Citation1,Citation2 For instance, current agents used to treat allergic conjunctivitis include antihistamines and mast cell stabilizers. Topical vasoconstrictor agents (i.e. ocular decongestants) are commonly used to treat ocular redness, particularly non-allergic and non-infectious redness caused by minor eye irritations.Citation1,Citation2 Current over-the-counter (OTC) vasoconstrictors are α-adrenergic receptor (α-AR) agonists. These agents induce smooth muscle contraction and differ in their affinity for the α1- and α2- AR subtypes. Phenylephrine and tetrahydrozoline are considered selective α1-AR agonists,Citation3 while naphazoline and oxymetazoline are considered mixed α1/α2- AR agonists.Citation4
Current topical vasoconstrictors are highly efficacious in treating congestion in the eye (ocular redness); however, their use can be associated with tachyphylaxis (tolerance or loss of effectiveness) and redness rebound upon treatment discontinuation (worsening of condition as compared to baseline), pupil dilation, and systemic side effects (e.g., somnolence, dizziness), all of which may restrict long term use of these drugs.Citation5–Citation8 Research suggests that tachyphylaxis is caused by an internalization of the α1-ARs and subsequent downregulation of surface α1-ARs and that α2-AR vasoconstrictors may not be subject to this side-effect.Citation7,Citation9–Citation11 In addition, α1-ARs appear to be predominantly expressed in the arteries and therefore sustained use of α1-AR agonists may result in vasoconstrictor-induced tissue ischemia and release of vasodilators.Citation8,Citation10,Citation11 In contrast, α2-ARs appear to be predominantly expressed in veins and likely have a reduced potential for this associated risk.Citation10,Citation11 Hence, recent efforts have been aimed at developing α2-AR specific agonists for use in the reduction of non-allergic or non-infectious ocular redness.
Brimonidine is a third-generation selective α2-AR agonist with a relative binding affinity for the α2- and α1-AR of ~1000:1.Citation12–Citation14 First approved at a concentration of 0.2% in 1996 for the reduction of elevated intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension,Citation12–Citation14 brimonidine has been shown to be an effective vasoconstrictor in the skin, and a 0.33% brimonidine gel (Mirvaso®, Galderma Laboratories, Fort Worth, TX) was approved in 2013 as a topical dermal treatment for non-transient facial erythema secondary to rosacea in adults.Citation15,Citation16 Brimonidine has been shown to control bleeding and/or subconjunctival hemorrhaging during ocular surgery, attesting to its potent vasoconstriction function in ocular tissue.Citation17–Citation22 It has also been reported to prevent bleeding associated with intravitreal injections and to induce conjunctival blanching prior to surgery.Citation17,Citation23,Citation24 While allergic reactions are associated with α2-AR agonists and have been reported with brimonidine, the incidence appears dose-related and is expected to occur less frequently at lower doses.Citation25,Citation26
The aim of this study was to evaluate the safety and efficacy of a low dose formulation of brimonidine, namely brimonidine tartrate ophthalmic solution, 0.025%, compared to its vehicle for relief of ocular redness in a population of adult (defined as 40 years of age or older) subjects with redness of an undetermined nature representative of a real world population. Redness reduction was evaluated in-office through 4 hours following a single-dose. Thereafter treatment was instilled four times daily for one month to assess the potential for tachyphylaxis; in addition, redness was assessed following treatment discontinuation to evaluate the potential for rebound redness.
Materials and methods
Study design
This was a single-center, double-masked, randomized, vehicle-controlled, parallel-group, safety and efficacy study (ClinicalTrials.gov identifier: NCT01675609). The study was comprised of four visits and lasted approximately five weeks. The study was approved by an Institutional Review Board (Alpha IRB, San Clemente, CA), and was performed in compliance with the ethical principles of the Declaration of Helsinki and Good Clinical Practice (GCP). All subjects, or their legally acceptable representative, signed IRB-approved written consent and Health Insurance Portability and Accountability forms before study participation.
Subject eligibility criteria
Healthy adult (aged ≥40 years) subjects with stable ocular health, defined as no ocular conditions requiring therapy or surgical intervention, were enrolled in the study. Additionally, subjects were required to have a history of vasoconstrictor (redness relief drops) use or a desire to use vasoconstrictors for redness relief within the last six months and have ocular health otherwise within the normal limits, including a calculated best-corrected (if necessary) visual acuity of 0.3 logMar or better in each eye. Females of child-bearing potential had to agree to a pregnancy test at Day 0 (Visit 1) and at study exit. At Visit 1, the subject must have had a baseline redness score >1 on a scale of 0–4 (where 0 = no ocular redness, 4 = extremely severe ocular redness) in both eyes as scored by the investigator using the Investigator Ocular Redness Scale (Ora Calibra®, Ora, Inc., Andover, MA); this scale is based on photographic standards and has been used in a previous study.
Exclusion criteria included: known contraindications or sensitivities to study medication; ocular surgery within three months or refractive surgery within the past six months; any current or prior ocular or systemic condition that, in the opinion of the investigator, could have confounded study data, interfered with the subject’s study participation, or affected the subject’s safety or trial parameters; a known history of retinal detachment, diabetic retinopathy, or active retinal disease; the presence of an active ocular infection or positive history of an ocular herpetic infection; prior (within 5 days of beginning study treatment) or anticipated concurrent use of artificial tear products, eye whiteners (e.g., vasoconstrictors), decongestants, antihistamines (including OTC and herbal topical ophthalmic medications), any other topical ophthalmic agents, and contact lenses; prior (within 14 days of beginning study treatment) or anticipated concurrent use of systemic corticosteroids or cancer chemotherapy, and/or any other systemic medications which the investigator felt may have confounded study data, interfered with subject’s study participation, or affected the subject’s safety or trial parameters; prior (within 30 days of beginning study treatment) or anticipated concurrent use of an investigational drug or device; planned surgery (ocular or systemic) during the trial period or within 30 days after the study period; abnormal blood pressure; IOP <5 mm Hg or >22 mm Hg; or a normal IOP with a diagnosis of glaucoma at Day 0 (Visit 1).
Treatment and assessments
The concentration of brimonidine selected for this study was based on results of a prior dose-response study (ClinicalTrials.gov identifier: NCT01275105), which evaluated two low-dose concentrations of brimonidine tartrate (0.01%, 0.025%) in alleviating ocular redness and found the 0.025% dose to have a stronger effect. The investigational product, brimonidine tartrate ophthalmic solution, 0.025%, and its vehicle (formulation without brimonidine tartrate), were manufactured by Bio-Concept Laboratories (Salem, NH) and contained benzalkonium chloride 0.01% as the preservative. Treatments were supplied in identical 10-mL sterile bottles. For masking purposes, all kits of active treatment or vehicle were identical in appearance.
On Day 0 (Visit 1), subjects were randomized at a ratio of 2:1 (active: vehicle) to receive either brimonidine tartrate ophthalmic solution, 0.025% or vehicle. Subjects instilled one drop of study treatment bilaterally under the supervision of a study technician and assessed drop comfort on a 0–10 unit scale (0 = very comfortable, 10 = very uncomfortable) immediately upon instillation and 30 seconds and one minute post-instillation. The investigator assessed ocular redness on a scale of 0–4 (0 = none, 4 = extremely severe, with half-unit increments) at 5(+1), 15(+1), 30(+1), 60(+10), 90(+10), 120(+15), 180(+15), and 240(+15) minutes post study treatment instillation using the Investigator Ocular Redness Scale. Following these assessments, subjects were instructed to dose one drop bilaterally, four times daily (QID) through Day 28 + 2 days (Visit 3) with no less than a 3.5 hour separation between doses, beginning the next morning. Subjects were instructed to assess ocular redness for each eye (0–4 unit scale, with whole unit increments, based on the same photographic standards as those used in the Investigator Ocular Redness Scale) prior to dosing and then approximately 2 minutes after each dose for each of the four daily doses, and to record the time of dosing in their diaries. For the first dose of each day, the subjects were also asked to assess whether or not the drop made their eyes whiter. If the subject reported eye whitening, he/she was to record how long the whitening effect lasted.
Subjects returned for clinic visits on Day 14 ± 2 days (Visit 2), Day 28 + 2 (Visit 3), and Day 35 + 1 day (Visit 4), and were instructed not to dose within 3.5 hours of their scheduled clinic visits on Day 14 and Day 28. At these visits, study treatment was instilled by a study technician and ocular redness was assessed by the investigator prior to and five minutes post-instillation. Subjects did not instill any study treatment from Day 28 to Day 35, but continued to record their ocular redness QID. Finally, the investigator assessed ocular redness at the last visit, approximately 7 days following treatment discontinuation, to evaluate the presence of any rebound redness.
Subject compliance with dosing instructions was evaluated by in-office review of subject dosing diaries at Day 14 (Visit 2) and Day 28 (Visit 3) and was determined by dividing the number of doses taken (based on diary entries) by the number of doses expected, multiplied by 100. Subjects were considered non-compliant if they had dosing compliance <80%.
Efficacy and safety parameters
The primary efficacy endpoints included ocular redness evaluated by the investigator prior to study treatment instillation and at indicated time points post instillation at Day 0 (Visit 1), and ocular redness evaluated by the subject throughout the treatment period (between Day 0 [Visit 1] and Day 14 [Visit 2] and between Day 14 [Visit 2] and Day 28 [Visit 3]). Ocular redness evaluated by the investigator at Day 14 (Visit 2) and Day 28 (Visit 3) was considered a secondary efficacy endpoint. Duration of the whitening effect evaluated by the subject throughout the treatment period was an exploratory efficacy endpoint. Drop comfort was a tolerability endpoint.
Safety measures included vital signs, visual acuity (VA; best-corrected if necessary), slit lamp biomicroscopy, IOP, dilated ophthalmoscopy, alertness evaluation, and rebound redness examinations. All adverse events (AEs), regardless of relationship to the study drug, were monitored and reported throughout the study. A subject was considered to have ocular redness rebound if they had ocular redness evaluated by the investigator at Day 35 (Visit 4) greater than that at baseline prior to drug treatment and/or they had noted ocular redness in their dosing diary in the follow-up period, after dosing had ceased, greater than that observed at baseline. Investigators evaluated subject alertness by asking questions based on the previous week using a 6-point scale (0 = fully alert, 5 = coma).
Data analysis and statistical methods
For efficacy, tolerability, and non-ocular safety assessments, data were analyzed by subject and the average of both eyes was used for analysis of assessments. Ocular safety assessments were analyzed for each eye with the exception of ocular redness rebound, which used the average of both eyes. Categorical variables were summarized using frequencies and percentages and continuous variables were summarized using the descriptive statistics. All randomized subjects who received at least one dose of study treatment were included in the intent-to-treat (ITT) and safety population. All subjects who completed the study with no major protocol deviations were included in the per-protocol (PP) population.
A sample size of 40 subjects in the brimonidine group and 20 subjects in the vehicle group was determined to have approximately 95% power to detect a between treatment difference of 1.0 unit in mean ocular redness using a two-sample t-test, assuming a standard deviation (SD) of 1.0 unit.
For the primary efficacy parameter of ocular redness as assessed by the investigator at Day 0, brimonidine was compared to vehicle using an analysis of covariance (ANCOVA) model accounting for repeated measures and adjusting for treatment, time point, treatment by time point interaction, and baseline (pre-instillation) scores. In addition, the differences between treatments at each time point were analyzed using two-sample t-tests. The primary efficacy parameter of ocular redness, as recorded in subject diaries during the treatment period, was analyzed using a repeated measures analysis of variance (ANOVA) model. Treatment, day, and the treatment by day interaction were all included in the model and daily averages were used for each subject. The primary efficacy analyses were performed on the ITT population with the last observation carried forward (LOCF) or on observed data only, where indicated. Supportive efficacy analyses were performed on the PP population as observed.
Treatment differences in ocular redness as assessed by investigators at Days 14 and 28 (Visits 2 and 3) were analyzed using two-sample t-tests. Additionally, Fisher’s exact test was used to compare the percentage of subjects with total clearance (redness scores of 0) in the two treatment groups post-instillation at each of the on-treatment visits (Day 0, 14, and 28). Ocular whiteness assessments, as captured in subject diaries throughout the treatment period, were summarized descriptively.
AEs were coded by MedDRA (version 13.1) system organ class and preferred term. An AE was considered if it occurred or worsened after the first dose of study treatment. AEs were summarized with frequencies and percentages by treatment group and for all subjects. Drop comfort was summarized in the safety population using descriptive statistics and comparisons were made across treatment groups using two-sample t-tests. All other safety outcomes were summarized by treatment group using continuous summary statistics for continuous variables and percentages for discrete variables.
Results
Subject disposition and demographics
A total of 72 subjects were screened and 57 subjects were randomized (brimonidine, n = 38; vehicle, n = 19) at Day 0 (Visit 1) and included in the ITT population and safety population. Of the 57 subjects in the ITT population, 14 subjects did not complete the study (brimonidine, n = 11; vehicle, n = 3). Reasons for discontinuation included administrative reasons (no-show/loss to follow-up; brimonidine, n = 6; vehicle, n = 2), AEs (brimonidine, n = 3; vehicle, n = 1), and protocol violations (brimonidine, n = 2, both non-compliance). One additional brimonidine-treated subject was excluded due to a major protocol violation (non-compliance). Hence, the PP population contained 42 subjects. Subject demographics were balanced between the two treatment groups with regard to age, gender, race, and iris color (). Day 0 (Visit 1) mean (SD) ocular redness score before drug instillation was 1.82 (0.376) in the brimonidine group and 1.96 (0.346) in the vehicle group.
Table 1. Baseline subject demographics.
Overall, 47/57 (82%) of subjects were >80% compliant with dosing instruction (30/38 [79%] in the brimonidine group and 17/19 [89%] in the vehicle group). None of the 10 subjects that were <80% compliant completed the study.
In-office ocular redness assessment
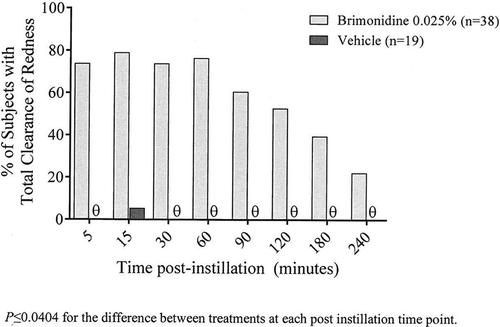
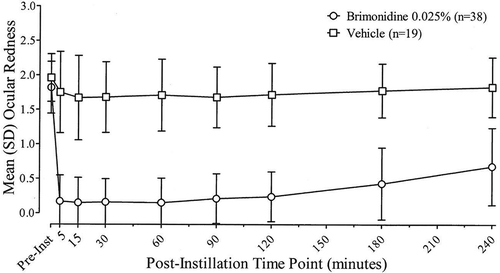
Day 0 (Visit 1) in-office investigator-assessed ocular redness scores prior to study treatment instillation through 4 hours post study treatment instillation are shown in . The mean post-dose redness for the entire post-instillation time period was statistically significantly different in favor of brimonidine 0.025% treatment with a least squares (LS) mean difference (95% CI) between brimonidine and vehicle treatment of −1.37 (−1.56, −1.18) for both mean redness and for mean change from baseline in redness (P < 0.0001 for both). Additionally, redness was significantly lower in brimonidine-treated eyes compared with vehicle-treated eyes at each individual post-instillation time point from 5 minutes to 240 minutes for both mean scores and for mean change from baseline (P < 0.0001 for all). Brimonidine had a rapid onset of action with a significant reduction in ocular redness seen at 5 minutes post-instillation, the first evaluation time point. Mean (SD) ocular redness scores were 0.17 (0.377) and 1.75 (0.589) in the brimonidine and vehicle group, respectively, at this time point. Peak efficacy was achieved at 15 minutes post-instillation, and brimonidine maintained statistically significant efficacy compared to vehicle through 4 hours post-instillation, the last evaluation time point. Analyses using the PP population with data as observed were consistent with findings with the ITT population.
Figure 1. Investigator-evaluated ocular redness scores (0–4 scale) before and after instillation of brimonidine tartrate ophthalmic solution, 0.025% or its vehicle at Day 0 (Visit 1). Data are the mean (SD) for the ITT population with last observation carried forward.
Day 14 and 28 (Visits 2 and 3) investigator-evaluated ocular redness prior to study treatment instillation and 5 minutes post instillation were comparable to those observed at Day 0 (Visit 1). Day 14 and 28 mean (SD) ocular redness scores were 1.46 (0.731) and 1.40 (0.529) pre-instillation decreasing to 0.63 (0.646) and 0.59 (0.524) post-instillation, respectively, in the brimonidine group. In contrast, Day 14 and 28 mean (SD) ocular redness scores were 1.69 (0.583) and 1.69 (0.520) pre-instillation and 1.50 (0.690) and 1.61 (0.532) post-instillation, respectively, in the vehicle group. The difference between treatments at these visits 5 minutes post study treatment instillation was significant whether comparing the mean redness or mean change from baseline in redness (P ≤ 0.0002 for all). These results were supported by the ocular redness analyses in the PP population with data as observed.
As expected based on results for mean ocular redness, the percentage of subjects with total clearance of ocular redness at Day 0 (Visit 1) was significantly higher in the brimonidine treatment group compared to the vehicle treatment group at all post-instillation assessment times (P ≤ 0.040; ). The proportion of subjects with total clearance peaked at the 15 minute time point with 78.9% of the subjects in the brimonidine group showing total clearance, and at 4 hours post-instillation, 22% of subjects still maintained total clearance. Brimonidine remained effective at reducing ocular redness throughout the study with 34.5% and 33.3% of brimonidine-treated subjects showing total clearance of redness at 5 minutes post-instillation on Days 14 and 28 (Visits 2 and 3), respectively, compared to none of the vehicle-treated subjects (P ≤ 0.0161). Similarly, in the PP population, a greater percentage of subjects in the brimonidine treatment group had total clearance of ocular redness compared to the vehicle treatment group at all time points throughout the study (P ≤ 0.014), with the exception of the 240 minute time point on Day 0 (P = 0.1374).
At-home subject diary ocular redness assessment
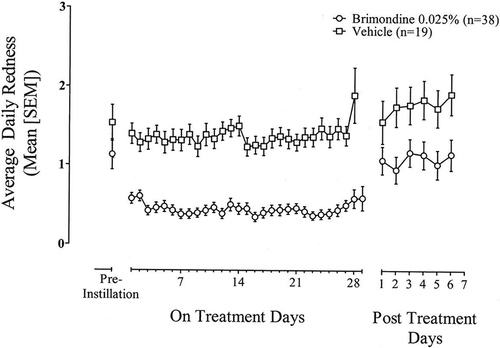
Evaluation of the subject diary data showed that subjects treated with brimonidine 0.025% had lower pre-dose and post-dose mean ocular redness compared to subjects in the vehicle group (). Mean (SD) post-dose redness scores were significantly lower in the brimonidine 0.025% group compared to vehicle group for post-instillation time points evaluated between Days 0 and 14 (Visits 1 and 2; P = 0.0005) and between Days 14 and 28 (Visits 2 and 3; P < 0.0001). These results were supported by analyses in the PP population as observed. presents average daily post-instillation ocular redness scores based on subject diaries during each on-treatment day of the study and redness scores reported for the 7 days following treatment discontinuation. Brimonidine-treated eyes had greater reductions in redness starting on Day 1 of the 4 week treatment period, and this treatment effect continued through Day 28. Thereafter, following discontinuation of treatment, ocular redness scores returned to baseline levels. Analysis of morning and last daily dose diary data showed that brimonidine-treated eyes had significantly less ocular redness than vehicle-treated eyes at each time point on treatment through Day 28 (P ≤ 0.0069 and P ≤ 0.0264, respectively).
Table 2. Ocular redness (Mean [SD]) – subject diaries – ITT population with last observation carried forward.
Figure 3. Average daily post-instillation ocular redness scores (0–4 scale) based on subject diary data during treatment (Days 0 to 28) and for seven days following treatment discontinuation (Days 29 to 35). Data are the mean (SEM) for the ITT population with observed data only.
Subjective assessment of ocular whitening was an exploratory endpoint. There were more subjects reporting that their eyes were whiter in the brimonidine treatment group compared with the vehicle treatment group following the first dose of each day throughout the treatment period (). However, less than half of subjects reporting whiter eyes reported the duration of whitening in their diaries. Analysis of the available data showed that there was a significant difference between treatments in duration of whiteness in favor of vehicle between Days 0 and 14 (Visits 1 and 2; P < 0.0001), but not between Days 14 and 28 (Visits 2 and 3).
Table 3. Ocular whitening – subject diaries – ITT population with observed data only.
Ocular redness rebound
To assess ocular redness rebound, study treatment was discontinued at Day 28 (Visit 3), and subjects continued to record their ocular redness QID for seven days until Day 35 (Visit 4) at which point the investigator assessed ocular redness. There was no evidence of redness rebound (i.e. worsening) after subjects discontinued dosing based on investigator-evaluated ocular redness at Day 35 (Visit 4) and based on subject diaries for assessments over the seven days between Days 28 and 35 (Visit 3 and 4) as shown in . Day 35 (Visit 4) mean in-office ocular redness score for the brimonidine and vehicle treatment groups were similar to mean in-office pre-instillation scores on Day 0, 14, and 28 (Visits 1, 2 and 3). Likewise, subject diary data showed that the ocular redness scores recorded between Days 28 and 35 (Visit 3 and 4) were similar to those recorded at pre-instillation time points evaluated between Days 0 and 14 (Visits 1 and 2) and between Days 14 and 28 (Visits 2 and 3) for each treatment group. Diary assessments of ocular redness following treatment discontinuation (diary days 28–35) showed that the return of ocular redness to baseline levels occurred within 24 hours of treatment discontinuation ().
Table 4. Rebound redness – safety population.
Safety evaluations
Three (7.8%) subjects in the brimonidine group and 1 (5.3%) subject in the vehicle group discontinued from the study due to AEs. The three brimonidine-treated subjects were withdrawn for AEs (2 ocular and one non-ocular) suspected of being related to study treatment (eye irritation, instillation site pain, and nasal discomfort), and the vehicle-treated subject was withdrawn for bacterial pneumonia that was considered unrelated to study treatment. The brimonidine-treated subject with eye irritation was recovering at the end of the study, and the other three subjects’ AEs had resolved by the end of the study.
There were no reports of serious AEs, and all AEs were considered mild to moderate in severity. In the brimonidine group, 10 (26.3%) subjects reported 11 ocular AEs and 8 (21.1%) subjects reported 10 non-ocular AEs. In the vehicle group, three (15.8%) subjects reported four ocular AEs and four (21.1%) subjects reported five non-ocular AEs. Ocular AEs are presented in . The most common ocular AEs with brimonidine were instillation site pain and dry eye, whereas the most common ocular AEs with vehicle were instillation site pain and eye irritation. Among ocular AEs, all but three events in brimonidine subjects (dry eye, conjunctival hemorrhage, visual acuity reduced) and two events in one vehicle-treated subject (eye irritation) were suspected of being treatment-related. Of the 15 non-ocular AEs, two events, both in brimonidine-treated subjects were suspected of being treatment-related and included rhinitis (2.6%) and nasal discomfort (2.6%).
Table 5. Ocular adverse events – safety population.
Brimonidine 0.025% did not appear to have any significant effects on the overall health or mean heart rate/blood pressure of subjects (data not shown). No clinically meaningful changes were noted in VA, ophthalmic examinations, or dilated fundoscopy. Mean (SD) change from baseline in IOP at Visit 3 was similar across treatment groups (brimonidine: OD = −0.1 [1.49], OS = −0.4 [1.31]; vehicle: OD = −0.1 [0.93], OS = 0.0 [0.82]). Of the brimonidine eyes that showed a decrease in IOP, the majority (67%) had a decrease of 1 mm Hg suggesting that brimonidine had no substantial impact on IOP following QID dosing for four weeks. There were no subjects with a decrease (or increase) in IOP >3 mm Hg.
Brimonidine 0.025% did not cause any somnolence when dosed QID for 28 days; all subjects were assessed as “fully alert” by the investigator throughout the study. Subjects assessed drop comfort immediately upon instillation, 30 seconds and 1 minute after drug instillation and reported the drug to be very comfortable. No significant differences were noted between the two treatment groups for drop comfort. Mean (SD) drop comfort scores in the brimonidine and vehicle groups, respectively, were 0.8 (1.48) and 0.5 (1.42) upon instillation (P = 0.4399), 0.7 (1.05) and 0.4 (1.38) 30 seconds post-instillation (P = 0.5637), and 0.6 (0.93) and 0.3 (0.93) 1 minute post-instillation (P = 0.2175)
Discussion
While treatment of ocular redness should be directed at the cause of redness, topical vasoconstrictors may be an option when there is no obvious underlying pathology. Ocular redness relief medications available today have a less than optimal safety and efficacy profile with side effects that include tachyphylaxis, rebound redness upon discontinuation, pupillary dilation, and systemic side effects. Therefore, there is a need to develop drugs that provide effective relief from ocular redness with minimal side effects. Brimonidine is a third-generation selective α2-AR agonist. This study evaluated the safety and efficacy of brimonidine tartrate ophthalmic solution, 0.025% compared to vehicle for treatment of ocular redness when dosed QID in adult subjects with redness of an undetermined nature. Subjects with redness from common conditions such as bacterial or viral infections or prior/current illness that could confound the study results were excluded from participation.
Results of investigator assessments showed that brimonidine 0.025% effectively reduced ocular redness as early as 5 minutes following drug instillation and maintained ocular redness reduction through 4 hours, the last post-instillation time point evaluated. The majority (79%) of brimonidine-treated subjects had total clearance of redness 15 minutes after drug administration and a significant number (22%) of subjects maintained total clearance through 4 hours. Consistent with research suggesting that the tachyphylaxis is caused by downregulation of α1-AR receptors,Citation7,Citation9–Citation11 brimonidine, a selective α2-AR agonist, remained effective in reducing ocular redness even after 4 weeks of QID dosing, with significant reductions in investigator-evaluated ocular redness 5 min post-instillation in brimonidine-treated eyes at each follow-up in-office visit compared to baseline. Likewise, subject post-dose diary data showed that initial reductions in redness in brimonidine-treated eyes were maintained through the 4 week treatment duration (). Of interest, diary data also showed that subjects in the brimonidine group had lower pre-dose and post-dose mean ocular redness scores compared to subjects in the vehicle group. The differences in the pre-dose averages between the two treatment groups were likely driven by the fact that the duration of action of redness reduction with brimonidine was longer than 3.5 hours, the minimum suggested dosing interval for at-home dosing. Finally, subjects’ assessment of “eye whitening”, an exploratory endpoint, showed that approximately twice as many brimonidine-treated subjects reported whiter eyes compared with vehicle-treated subjects.
Treatment with brimonidine 0.025% appeared safe and well tolerated. Among the safety outcomes examined in this study, investigators reported no significant ocular redness rebound following discontinuation of brimonidine 0.025%. Indeed, 1 week following treatment discontinuation, ocular redness scores for both treatment groups were lower than baseline scores. While there appeared to be a small difference in ocular redness at baseline between these groups, this difference was present and constant throughout the pre-dose assessments and was apparent again following treatment discontinuation on Day 28. Similarly, there was no evidence of redness rebound following brimonidine 0.025% discontinuation based on subject diaries.
There were no substantial effects of brimonidine 0.025% on IOP, and there were few AEs reported overall. Only two ocular AEs were reported more than once with each treatment (dry eye and instillation site pain with brimonidine, and eye irritation and instillation site pain with vehicle), and there were only two withdrawals due to an ocular AE with brimonidine (instillation site pain, eye irritation). Notably, there were no reports of mydriasis for any brimonidine-treated subject suggesting that brimonidine 0.025% does not dilate the pupil contrary to non-selective α1-AR agonists. Studies with high dose formulations of brimonidine (0.15–0.2%) used for lowering IOP in subjects with glaucoma or ocular hypertension have reported pupillary constriction, particularly under scotopic conditions; however, there were also no reports of miosis with low dose brimonidine in this study.Citation27–Citation29 High doses of brimonidine have also been associated with development of ocular surface allergic disease,Citation30,Citation31 yet no allergic reactions were reported during the course of our study involving one month of treatment, nor were there AEs commonly associated with ocular allergy such as conjunctival hyperemia or pruritus. In keeping with the reported observation that the 0.15% brimonidine dose has a lower rate of associated allergic disease than the 0.2% dose,Citation25,Citation26 the rate with the 0.025% brimonidine dose (6- to 8-fold lower than in currently marketed formulations) may be negligible. It follows that much larger studies and/or real-world experience may be required to determine the precise incidence of ocular allergy with low dose brimonidine; as well, studies with longer treatment duration would inform or rule out the potential for late-onset allergic reaction on long-term and/or repeated use. Finally, most patients rated drop comfort of both brimonidine and its vehicle as very comfortable with no difference between treatments.
Systemic α2-AR agonists have been exploited pharmacologically for their sedative properties (e.g. dexmedetomidine)Citation32 and cardiovascular effects.Citation24,Citation33 Previous studies following topical instillation of brimonidine at concentrations up to 0.2% found minimal to no cardiovascular effects, although fatigue and drowsiness were reported,Citation24,Citation34–Citation36 especially in pediatric patients.Citation37,Citation38 Results from the current study demonstrate brimonidine 0.025% did not cause any somnolence and had no significant effect on mean heart rate or blood pressure, suggesting that any sedative and cardiovascular effects following topical administration are dose-dependent and not expected in adults following low dose brimonidine, although further studies are warranted.
A limitation of the study was the small sample size. Of the subjects randomized, 14 (25%) failed to complete the study, including 8 (14%) who were lost to follow-up. Despite the small sample size, a significant treatment difference in redness of >1.0 unit was observed in the ITT population as well as the PP population, attesting to the efficacy of low dose brimonidine as a redness reducer. Another potential study limitation was the use of subject diaries to measure dosing compliance, as subject self-reported data may sometimes be unreliable.
In conclusion, the results from this trial suggest that brimonidine tartrate ophthalmic solution, 0.025% is safe and well tolerated and is effective in reducing ocular redness in adult subjects. Additionally, use of brimonidine 0.025% does not appear to be limited by side effects associated with currently marketed ocular redness relief medications.
Declaration of interest
P Gomes is the vice president of Allergy and an employee at Ora, Inc., an organization that consults and conducts research and clinical trials in the field of ocular allergy and other areas of ophthalmology and allergic diseases. CM Sanfilippo and HH DeCory are employees of Bausch & Lomb, Inc. G Torkildsen is a study investigator; she has received consultancy fees from Ora, Inc., reimbursement of meeting traveling expenses from Alcon Research Ltd., and research grants from Allergan. The authors report no other conflicts of interest in this work. The authors alone are responsible for the content and writing of the article.
Acknowledgments
Joseph B. Ciolino, MD, served as Medical Monitor for the study. Medical writing assistance was provided by Ora, Inc. This study was sponsored by Eye Therapies, Inc. The Sponsor and Ora, Inc. participated in the design of the studies and analysis of the data. Ora, Inc. supported the conduct of the trial. Gail L. Torkildsen was the study principal investigator at the single center of Andover Eye Associates. Following the completion of this study, brimonidine 0.025% ophthalmic solution was licensed to Bausch & Lomb, Inc. Bausch & Lomb, Inc. participated in the analysis of the data and approval of the manuscript.
References
- Cronau H, Kankanala RR, Mauger T. Diagnosis and management of red eye in primary care. Am Fam Physician. 2010;81(2):137–44.
- Galor A, Jeng BH. Red eye for the internist: when ot treat, when to refer. Cleve Clin J Med. 2008;75(2):137–44. doi:10.3949/ccjm.75.2.137.
- Haveles EB. Chapter 4: Autonomic drugs. In: Applied pharmacology for the dental hygienist. 7th ed. Maryland Heights, MO: Elsevier; 2016, p. 44.
- Shanbhag T. Pharmacology: Prep manual for undergraduates. India: Elsevier; 2008, p. 73.
- Spector SL, Raizman MB. Conjunctivitis medicamentosa. J Allergy Clin Immunol. 1994;94(1):134–36. doi:10.1016/0091-6749(94)90081-7.
- Tappeiner C, Sarra GM, Abegg M. Abuse of vasoconstrictive eyedrops mimicking an ocular pemphigoid. Eur J Ophthalmol. 2009;19(1):129–32.
- Vaidyanathan S, Williamson P, Clearie K, Khan F, Kipworth B. Fluticasone reverses oxymetazoline induced tachyphylaxis of response and rebound congestion. Am J Respir Crit Care Med. 2010;182(1):19–24. doi:10.1164/rccm.200911-1701OC.
- Soparkar CN, Wilhelmus KR, Koch DD, Wallace GW, Jones DB. Acute and chronic conjunctivitis due to over-the-counter ophthalmic decongestants. Arch Ophthalmol. 1997;115(1):34–38. doi:10.1001/archopht.1997.01100150036004.
- Fratelli M, De Blasi A. Agonist-induced alpha 1-adrenergic receptor changes. Evidence for receptor sequestration. FEBS Lett. 1987;212(1):149–53. doi:10.1016/0014-5793(87)81575-2.
- Corboz MR, Rivelli MA, Mingo GG, McLeod RL, Varty L, Jia Y, Hey JA. Mechanism of decongestant activity of alpha-2 agonists. Pulm Pharmacol Ther. 2008;21:449–54. doi:10.1016/j.pupt.2007.06.007.
- Corboz MR, Mutter JC, Rivelli MA, Mingo GG, McLeod RL, Varty L, Jia Y, Cartwright M, Hey JA. Alpha2-adrenoceptor agonists as nasal decongestants. Pulm Pharmacol Ther. 2007;20(2):149–56. doi:10.1016/j.pupt.2006.03.012.
- Adkins JC, Balfour JA. Brimonidine. A review of its pharmacological properties and clinical potential in the management of open-angle glaucoma and ocular hypertension. Drugs Aging. 1998;12(3):225–41. doi:10.2165/00002512-199812030-00005.
- Fudemberg SJ, Batiste C, Katz LJ. Efficacy, safety, and current applications of brimonidine. Expert Opin Drug Saf. 2008;7(6):795–99. doi:10.1517/17425250802457609.
- Rahman MQ, Ramaesh K, Montgomery DM. Brimonidine for glaucoma. Expert Opin Drug Saf. 2010;9(3):483–91. doi:10.1517/14740331003709736.
- Fowler J, Jr., Jackson JM, Moore A, Jarratt M, Jones T, Meadows K, Steinhoff M, Rudisill D, Leoni M. Efficacy and safety of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of two randomized, double-blind, and vehicle-controlled pivotal studies. J Drugs Dermatol. 2013;12(6):650–56.
- Tong LX, Moore AY. Brimonidine tartrate for the treatment of facial flushing and erythema in rosacea. Expert Rev Clin Pharmacol. 2014;7(5):567–77. doi:10.1586/17512433.2014.945910.
- Dahlmann-Noor AH, Cosgrave E, Lowe S, Bailly M, Vivian AJ. Brimonidine and apraclonidine as vasoconstrictors in adjustable strabismus surgery. J Aapos. 2009;13:123–26. doi:10.1016/j.jaapos.2008.12.001.
- Gupta A, Kekunnaya R, Sachdeva V, Rao HL. Strabismus surgery hemostasis. Ophthalmology. 2012;119(3):649–50. doi:10.1016/j.ophtha.2011.10.005.
- Hong S, Kim CY, Seong GJ, Han SH. Effect of prophylactic brimonidine instillation on bleeding during strabismus surgery in adults. Am J Ophthalmol. 2007;144(3):469–70. doi:10.1016/j.ajo.2007.04.038.
- Desco MC, Navea A, Ferrer E, Menezo JL. Effect of prophylactic brimonidine on bleeding complications after cataract surgery. Eur J Ophthalmol. 2005;15(2):228–32.
- Pasquali TA, Aufderheide A, Brinton JP, Avila MR, Stahl ED, Durrie DS. Dilute brimonidine to improve patient comfort and subconjunctival hemorrhage after LASIK. J Refract Surg. 2013;29:469–75. doi:10.3928/1081597X-20130617-05.
- Norden RA. Effect of prophylactic brimonidine on bleeding complications and flap adherence after laser in situ keratomileusis. J Refract Surg. 2002;18:468–71.
- Kim CS, Nam KY, Kim JY. Effect of prophylactic topical brimonidine (0.15%) administration on the development of subconjunctival hemorrhage after intravitreal injection. Retina. 2011;31(2):389–92. doi:10.1097/IAE.0b013e3181eef28e.
- Derick RJ, Robin AL, Walters TR, Barnebey HS, Chopin N, Schuman J, Kelley EP, Chen K, Stoecker JF. Brimonidine tartrate: A one month dose response study. Ophthalmology. 1997;104:131–36. doi:10.1016/S0161-6420(97)30349-2.
- Kim CY, Hong S, Seong GJ. Brimonidine 0.2% versus brimonidine Purite 0.15% in Asian ocular hypertension. J Ocul Pharmacol Ther. 2007;23(5):481–86. doi:10.1089/jop.2007.0042.
- Katz LJ. Twelve-month evaluation of brimonidine-purite versus brimonidine in patients with glaucoma or ocular hypertension. J Glaucoma. 2002;11:119–26. doi:10.1097/00061198-200204000-00007.
- McDonald JE, El-Moatassem Kotb AM, Decker BB. Effect of brimonidine tartrate ophthalmic solution 0.2% on pupil size in normal eyes under different luminance conditions. J Cataract Refract Surg. 2001;27:560–64. doi:10.1016/S0886-3350(01)00769-6.
- Thordsen JE, Bower KS, Warren BB, Stutzman R. Miotic effect of brimonidine tartrate 0.15% ophthalmic solution in normal eyes. J Cataract Refract Surg. 2004;27:1702–06. doi:10.1016/j.jcrs.2003.12.037.
- Kesler A, Shemesh G, Rothkoff L, Lazar M. Effect of brimonidine tartrate 0.2% ophthalmic solution on pupil size. J Cataract Refract Surg. 2004;30:1707–10. doi:10.1016/j.jcrs.2004.02.043.
- Katz LJ, Brimonidine Study Group. Brimonidine tartrate 0.2% twice daily vs timolol 0.5% twice daily: 1-year results in glaucoma patients. Am J Ophthalmol. 1999;127:20–26. doi:10.1016/S0002-9394(98)00286-4.
- LeBlanc RP. Twelve-month results of an ongoing randomized trial comparing brimonidine tartrate 0.2% and timolol 0.5% given twice daily in patients with glaucoma or ocular hypertension. Ophthalmology. 1998;105:1960–67. doi:10.1016/S0161-6420(98)91048-X.
- Carollo DS, Nossaman BD, Ramadhyani U. Dexmedetomidine: a review of clinical applications. Curr Opin Anaesthesiol. 2008;21(4):457–61. doi:10.1097/ACO.0b013e328305e3ef.
- Walters TR. Development and use of brimonidine in treating acute and chronic elevations of intraocular pressure: a review of safety, efficacy, dose response, and dosing studies. Surv Ophthalmol. 1996;41(Suppl 1):S19–26. doi:10.1016/S0039-6257(96)82028-5.
- Lee D, Gornbein J, Abrams C, Group ftGTS. The effectiveness and safety of brimonidine as mono-, combination, or replacement therapy for patients with primary open-angle glaucoma or ocular hypertension: a post hoc analysis of an open-label community trial. J Ocul Pharmacol Ther. 2000;16:3–18. doi:10.1089/jop.2000.16.3.
- Cantor LB. The evolving pharmacotherapeutic profile of brimonidine, an alpha 2-adrenergic agonist, after four years of continuous use. Expert Opin Pharmacother. 2000;1(4):815–34. doi:10.1517/14656566.1.4.815.
- Schuman JS. Effects of systemic beta-blocker therapy on the efficacy and safety of topical brimonidine and timolol. Brimonidine Study Groups 1 and 2. Ophthalmology. 2000;107(6):1171–77. doi:10.1016/S0161-6420(00)00081-6.
- Enyedi LB, Freedman SF. Safety and efficacy of brimonidine in children with glaucoma. J Am Assoc Pediatr Ophthalmol Strab. 2001;5(5):281–84. doi:10.1067/mpa.2001.117571.
- Bowman RJ, Cope J, Nischal KK. Ocular and systemic side effects of brimonidine 0.2% eye drops (Alphagan) in children. Eye (Lond). Jan 2004;18(1):24–26. doi:10.1038/sj.eye.6700520.