ABSTRACT
Purpose: To investigate plasma lutein (L) and zeaxanthin (Z) concentrations with grading-confirmed and self-reported prevalence of age-related macular degeneration (AMD).
Material and methods: Data collected from a nationally representative prospective cohort study of community-dwelling adults aged 50 years and over in the Republic of Ireland. Participants underwent a computer-assisted personal interview and a center-based health assessment. Plasma concentrations of L and total Z (Z and meso-zeaxanthin [MZ]) were measured by high performance liquid chromatography, and retinal photographs were graded using a version of the AMD International Classification and Grading System. Consumption of supplements containing L and/or Z and/or MZ was recorded as supplement use. Four groups were identified: Group 1 (n = 24): AMD-afflicted and correctly aware; Group 2 (n = 264): AMD-afflicted but unaware; Group 3 (n = 41): AMD-free and incorrectly believed that they were afflicted with the condition; Group 4 (n = 4094): AMD-free and correctly self-reported absence of AMD.
Results: Of 4,423 participants with plasma concentrations of L and Z and gradable retinal photographs, 288 (6.5%) were afflicted with AMD, and 65 (1.5%) self-reported AMD. Controlling for family history and age, the relationship between grading-confirmed AMD and plasma L was positive and significant (p < 0.001). Mean plasma concentrations of L in Group 2 (mean = 0.2162 ± 0.132 µmol) and Group 4 (mean = 0.2040 ± 0.121 µmol/L) were significantly lower than Group 1 (mean = 0.4691 ± 0.0.372 µmol/L) and Group 3 (mean = 0.3176 ± 0.0.235 µmol/L). Supplement use was reported by 41.7% and 17.1% of participants in Groups 1 and 3, respectively, but only 2.7% and 1.9% of participants in Groups 2 and 4, respectively.
Conclusion: A belief that one suffers from AMD, whether justified or not, is associated with supplement use and with higher plasma concentrations of L.
Introduction
The macula, a specialized area of the retina, mediates central and color vision.Citation1 Age-related macular degeneration (AMD) is a disease of the macula that, in its advanced stage, results in the loss of central vision if untreated or if untreatable.Citation2 Early (non-advanced) AMD is characterized by drusen and/or pigmentary abnormalities; whereas, the late (advanced) form of AMD is visually consequential and can be classified as atrophic (geographic atrophy or dry) or neovascular (choroidal neovascularization or wet).Citation3 AMD is the leading cause of irreversible blindness in the older population, especially in developed countries.Citation4 We have recently shown that the overall prevalence of any form of AMD (i.e. early or advanced) in adults aged 50 years or older in the Republic of Ireland (ROI) is 7.2% (census-weighted).Citation5 The incidence and prevalence of AMD will continue to rise because of increasing longevity and because of the growing world population.Citation6 The global projection of people with AMD is estimated at 196 million by 2020, further increasing to 288 million by 2040.Citation7
The loss of central vision in patients afflicted with AMD has a dramatic and adverse impact on their quality of life.Citation8 For example, the impact of vision loss associated with AMD may result in an inability to drive, to read, to recognize faces, or to watch television, with a consequential loss of social independence and increasing need for family support,Citation9 which is a major concern in the context of an advancing population. The financial burden of vision loss and/or impairment may be classed as direct or indirect.Citation10 The indirect costs include the loss of the patient’s income, the cost of care-givers, nursing homes and other costs (e.g. transport, etc.).Citation10 Direct costs include hospital care, outpatient and office visits, optometry costs, drugs and other direct expenses.Citation10 Currently, there is no effective treatment for atrophic AMD, whereas neovascular AMD is treated by intravitreal injections of anti-vascular endothelial growth factor (anti-VEGF) therapy, which has been shown to dramatically reduce the risk of vision loss,Citation11 but at an average annual cost per eye per year of $24,000.Citation12
Established risk factors for AMD include increasing age, family history of disease (genetic background), and tobacco use; whereas, exposure to short-wavelength (blue) light, obesity, cardiovascular disease and diet (antioxidant status) are described as putative risk factors for this condition.Citation13 Although the etiopathogenesis of AMD remains elusive, we now know that oxidative stress is a key factor in the development of this disease.Citation14,Citation15 Indeed, and over the last few decades, there has been a growing body of research investigating the protective role of carotenoids for AMD,Citation16 which culminated in the publication of the Age-Related Eye Disease Study (AREDS) 2 in 2013.Citation17,Citation18 Specifically, it has been demonstrated that supplementation with at least two of the three macular carotenoids (lutein [L], zeaxanthin [Z]) in association with co-antioxidants (vitamin C, vitamin E, zinc, and copper) reduces the risk of progression from intermediate AMD to advanced AMD.Citation17,Citation18 Moreover, a recent double-blind, randomized clinical trial reported that, in patients with early AMD, supplementation with all three of macular pigment’s constituent carotenoids in a meso-zeaxanthin [MZ]:L:Z (mg) ratio of 10:10:2 enhances visual performance and is non-inferior (in terms of macular pigment augmentation) to the AREDS 2 formulation.Citation19 Interestingly, carotenoids are entirely of dietary origin and, therefore, their concentration in plasma and consequential bioavailability for uptake by the retina is dependent on consumption of foods containing these nutrients (such as leafy greens and colored fruits and vegetables) or supplements.
The association between plasma concentrations of L and Z with diet, age, ethnicity, and AMD status has been previously reported.Citation20–Citation23 However, the current investigation, conducted as part of the Irish Longitudinal Study on Ageing (TILDA) study, see below, is the first population-based study to report on the relationship between plasma concentrations of L and Z and grading-confirmed AMD while investigating the impact of self-reporting of AMD, supplement use and plasma concentrations of the relevant carotenoids.
Materials and methods
Study design
TILDA is a nationally representative, longitudinal study of the health, economic and social status of 8,175 adults aged 50 years and over in the ROI. The design and methodology of TILDA are described in detail elsewhere.Citation24 In brief, a nationally representative sample of community-dwelling adults was drawn from the Irish Geodirectory, a comprehensive record of all residential addresses in the ROI. Addresses were selected by means of RANSAM (a random sampling design for the ROI) using a three stage process where all household residents aged 50 years or older were eligible to participate.Citation24
Participants
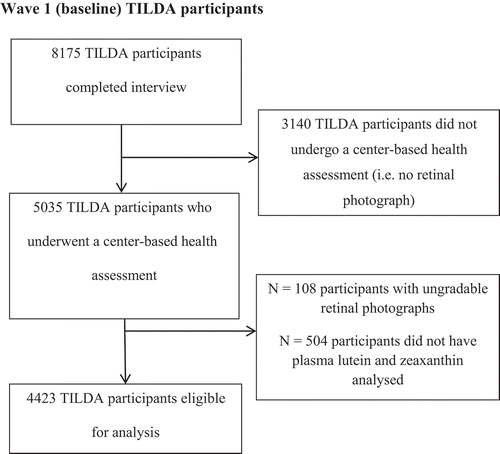
Wave 1 consisted of three separate components: (1) a face-to-face interview using a computer assisted personal interview (CAPI); (2) a self-completion questionnaire (SCQ); 3) a health assessment carried out in either a dedicated health center (based in Dublin and Cork) or, alternatively, a modified assessment was carried out in the participant’s own home if he/she was unable/unwilling to travel to the health center. Retinal photographs were not obtained from participants who opted not to attend a health center assessment (n = 3,140). Wave 1 health assessment had an overall response rate of 62% (i.e. 62% of 8,175 = 5035). illustrates TILDA participants included in this investigation.
Figure 1. The Irish Longitudinal Study on Ageing (TILDA) participants included in this investigation. Photographs were judged as ungradable based on a priori criteria of photographic quality. The phenomenon of participants without plasma concentration of lutein and zeaxanthin was attributable to one of the following: participant did not consent to give a blood sample, a failure to perform a venepuncture at the time of the health assessment, insufficient sample for carotenoid analysis or sample lost during carotenoid extraction/analysis.
This study was approved by the Faculty of Health Sciences Research Ethics Committee of Trinity College Dublin and the local Ethics Committee at the Waterford Institute of Technology. All participants provided signed informed consent prior to enrolment in the study. The study was conducted in accordance with the tenets of the Declaration of Helsinki regarding research into human volunteers.
Interview
Participants completed a CAPI, which was carried out by a trained social interviewer.Citation24 Participants were asked whether, to their knowledge, a doctor’s diagnosis of AMD had been made in their individual case and, whether, to their knowledge, there was a family history of AMD. A list of medications, including food supplements consumed on a daily basis, was recorded for each participant. The exact wording of the question was as follows: “MD001: Now I would like to record all medications that you take on a regular basis, like every day or every week. This will include prescription and non-prescription medications, over-the-counter medicines, vitamins, and herbal and alternative medicines.” For the purpose of the current analysis, information on supplements that contained at least one of the three constituent carotenoids of macular pigments (i.e. either L and/or Z and/or MZ) was also recorded, and this was then coded as yes or no (and henceforth referred to as supplement use).
Retinal photographs
All retinal photography was performed by TILDA nurses, who were trained and certified by experts from the Ocular Epidemiology Reading Centre at the University of Wisconsin, Madison, USA. One 45° monoscopic color photograph, centered on the macula (EDTRS standard field 2) was obtained for each eye using the NIDEK AFCE-210 non-mydriatic auto-fundus camera through a non-dilated pupil. Pupil dilation was not feasible for this large study, given that participants had to undergo many other tests, some of which would have been adversely influenced by pupil dilation (e.g. gait assessment).
AMD grading
Retinal photographs were graded using a modified version of the International Classification and Grading System for AMD.Citation5 Early (non-advanced) AMD was defined as the presence of more than 10 hard drusen (< 63 µm) and/or the presence of soft drusen (>125 µm). Late (advanced) AMD was defined as the presence of atrophic AMD and/or neovascular AMD.
Plasma L and Z assessment
The TILDA protocol for non-fasting venous blood sample collection, processing and storage has been described previously.Citation25 In summary, 1 ml of plasma wrapped in tinfoil was stored at −80°C and dedicated to carotenoid assessment. L and total Z (zeaxanthin and meso-zeaxanthin) was analyzed using a reversed phase high performance liquid chromatography (HPLC) method. Details of the extraction procedures and HPLC analysis that we used have been previously described.Citation26
Statistical analysis
The statistical package IBM SPSS Statistics for Windows Version 22.0 was used for analysis. In an earlier report of this TILDA cohort,Citation5 we found that AMD prevalence was linked to age and family history of AMD. In the current analysis, we investigated the respective relationships between self-reported AMD (justified and unjustified), use of supplements containing at least one of the three macular carotenoids and mean plasma concentrations of L and Z. The statistical methods employed included contingency table analysis and post hoc analysis of variance. Accordingly, and along with plasma concentrations of L and Z, we incorporated these variables into our logistic regression models for this current study. However, we did not incorporate other variables, such as sex, education, BMI, and so on, as these were not found to be significantly related to AMD in the earlier report of this cohort.Citation5 The 5% level of significance was used throughout, without adjustment for multiple testing.
Results
Demographic, health and lifestyle characteristics for the 4,423 TILDA participants reported herein are presented in . The participants’ mean age was 61 ± 8 years; and 231 participants (5.2%) reported a family history of AMD. 288 participants (6.5%) exhibited signs of early or late AMD (early AMD: n = 273; 6.2%; late AMD: n = 15; 0.34%;). Because of the small numbers of cases of late AMD, cases of early (non-advanced) and late AMD were combined in subsequent analyses (and henceforth referred to as AMD). As previously reported in this sample,Citation5 increasing age and a positive family history of AMD were strongly associated with prevalence of AMD.
Table 1. Demographics, health and lifestyle characteristics of participants in this investigation.
Using logistic regression, the association between grading-confirmed AMD and plasma concentrations of L and Z, in each case controlling for age and family history of AMD, were investigated. The relationship between AMD and plasma L was positive and highly significant (p < 0.001), demonstrating that AMD is associated with higher plasma concentrations of L in this cohort. There was no significant relationship between plasma concentration of Z and AMD (p > 0.05).
This unexpected association between AMD and plasma concentrations of L prompted us to explore whether self-reported family history of AMD and/or self-report of AMD (whether justified or not) were associated with use of supplements containing at least one of the three macular carotenoids, which in turn might have contributed to our observations. In our study cohort, 231 participants (5.2%) reported a family history of AMD and 102 participants (2.3%) reported use of supplements containing at least one of the three macular carotenoids (supplement use). A small number of participants (n = 65; 1.5%) believed that they were afflicted with AMD on the basis of a doctor’s diagnosis, whereas 4,358 participants (98.5%) reported that they were not afflicted with this condition. Subsequent AMD grading, however, revealed that not all of these self-reports were accurate, and that we had, in fact, four distinct subject groups in our study: Group 1 (n = 24): grading-confirmed AMD in association with justified self-report of AMD (i.e. grading positive/self-report positive); Group 2 (n = 264): grading-confirmed AMD in participants who were unaware that they suffer from AMD (i.e. grading positive/self-report negative); Group 3 (n = 41): grading-confirmed absence of AMD in participants who (incorrectly) self-reported AMD (i.e. grading negative/self-report positive); Group 4 (n = 4,094): grading-confirmed absence of AMD in participants who justifiably reported that they were not afflicted with AMD (i.e. grading negative/self-report negative).
We used post hoc analysis of variance (Tukey HSD) to investigate plasma concentrations of L and Z in these four groups. Plasma concentrations of L in Group 2 (grading positive/self-report negative; mean = 0.2162 ± 0.132 µmol/L;) and Group 4 (grading negative/self-report negative; mean = 0.2040 ± 0.121 µmol/L;) were found to be significantly lower than in Group 1 (grading positive/self-report positive; mean = 0.4691 ± 0.0.372 µmol/L; p < 0.001 and p < 0.001, respectively) and Group 3 (grading negative/self-report positive; mean = 0.3176 ± 0.0.235 µmol/L; p < 0.001and p < 0.001, respectively) as shown in .
Figure 2. Mean plasma concentrations of lutein and total zeaxanthin of subgroups in this investigation. Plasma lutein and zeaxanthin concentrations measured by high performance liquid chromatography. Group 1 (n = 24): grading-confirmed AMD in association with self-reported AMD; Group 2 (n = 264): grading-confirmed AMD in the absence of self-reported AMD; Group 3 (n = 41): grading-confirmed absence of AMD in association with self-reported AMD; Group 4 (n = 4094): grading-confirmed absence of AMD in association with self-reported absence of AMD. Grading-confirmed AMD, retinal photographs were graded by a certified grader using a modified version of the International Classification and Grading System for AMD; Self-reported AMD, participants were asked whether a doctor had diagnosed them with AMD.
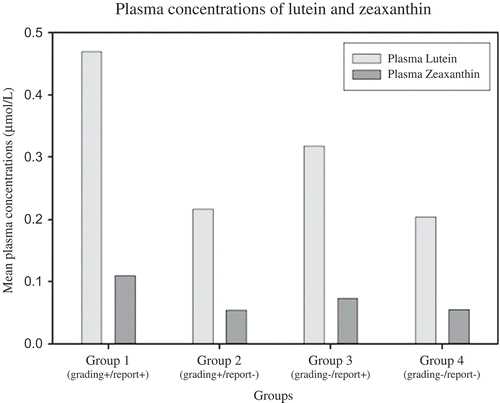
Plasma concentrations of Z in Group 1 (grading positive/self-report positive; mean = 0.1101 ± 0.105 µmol/L;) were significantly greater than in Group 2 (grading positive/self-report negative; mean = 0.0551 ± 0.041 µmol/L; p < 0.05), Group 3 (grading negative/self-report positive; mean = 0.0735 ± 0.069 µmol/L; p < 0.001) and Group 4 (grading negative/self-report negative; mean = 0.0557 ± 0.046 µmol/L; p < 0.001) ().
The role of other variables that might have influenced the observed relationships between a grading-confirmed diagnosis of AMD and plasma concentrations of L were also investigated, including use of supplements containing at least one of the three macular carotenoids (supplement use) and factors that might have prompted the use of such supplements (i.e. family history of AMD and/or self-report of AMD). Supplement use was found to be significantly higher in participants who self-reported AMD (whether justified or unjustified); 41.7% and 17.1% of participants in Groups 1 and 3, respectively, were using a supplement containing at least one of the three macular carotenoids, and this compares with only 2.7% and 1.9% of Groups 2 and 4, respectively (p < 0.001, Pearson Chi-Square). Similarly, 9.1% of participants who self-reported a family history of AMD reported use of a supplement containing at least one of the three macular carotenoids, compared with just 1.9% who did not report a family history of AMD (p < 0.001, Pearson Chi-Square).
Discussion
The principal and novel finding in this population-based study was that participants who believed that they suffer from AMD (and irrespective of whether this belief is founded or unfounded) exhibit higher plasma concentrations of L than participants who do not believe they suffer from this condition (again, irrespective of whether this latter belief is justified or unjustified). Our findings have profound implications for epidemiologic studies investigating the prevalence of, and risk factors for, AMD; moreover, our findings also inform the debate regarding the appropriateness of introducing a screening program for non-advanced AMD.
In this report, plasma concentrations of L in Group 1 (grading positive/self-report positive) were 2.2 times greater than among participants in Group 2 (grading positive/self-report negative) and 2.3 times greater than participants in Group 4 (grading negative/self-report negative). Also, plasma concentrations of L in Group 3 (grading negative/self-report positive) were 1.5 times greater than among participants in Group 2 (grading positive/self-report negative) and 1.6 times greater than participants in Group 4 (grading negative/self-report negative). Further, our results also strongly suggest that these findings are attributable to greater use of a supplement containing at least one of the three macular carotenoids among those who believe (correctly or incorrectly) that they suffer from AMD. With respect to plasma Z concentrations, only Group 1 (grading positive/self-report positive) had significantly higher concentrations of Z when compared with the other groups. One possible explanation for this observation could rest on the fact that this group consisted solely of participants correctly reporting doctor-diagnosed AMD, and therefore more likely (perhaps) to secure a recommendation to consume a supplement that included at least two of the three macular carotenoids (including Z) and not merely a L-containing formulation. Importantly, the data reported herein were recorded as part of Wave 1 of TILDA (between 2009 and 2011), a period when supplementation with macular pigment’s constituent carotenoids was already in widespread use for the purpose of managing AMD, in spite of the fact that the findings of AREDS 2 were not published until 2013.Citation27
Of the 288 participants with grading-confirmed AMD, 264 participants (92%) were unaware that they were afflicted with the condition (Group 2), an unsurprising finding given that patients with non-advanced AMD are typically unaware of their condition because vision is only profoundly affected if and when the disease progresses to the advanced stageCitation28 and given that 273 participants (95%) with grading-confirmed AMD in this study suffered from the early (non-advanced) form of the condition.Citation5
With respect to epidemiologic studies reporting on serum concentrations of L and/or Z, it would appear that some cross-sectional studies may now need to be re-interpreted in light of our novel findings. For example, reference values for plasma caroteniods published after 1999 may now need to be revisited.Citation29 One could exclude participants with AMD, participants who believe that they suffer from AMD, participants with a family history of AMD and also participants who use a supplement containing at least one of the three macular carotenoids, for the purposes of generating reference values, but this measure would necessarily render the sample non-representative of the population at large and would exclude a sub-population that is of particular interest. Another concern arising from our findings rests on the interpretation of cross-sectional epidemiologic studies attempting to investigate a possible association between macular pigment’s constituent carotenoids and the prevalence of AMD. Such studies are, in any case, inherently problematic, not least because macular pigment’s constituent carotenoids are intracellular compounds and AMD (whether non-advanced or advanced) results in loss of photoreceptors and their axons.Citation30,Citation31 In other words, the principal shortcoming of cross-sectional studies in this respect rests not only on the impossibility of determining causality, but also because it is very likely that AMD causes loss of “housing” to accommodate macular pigment (and, therefore, a lack of macular pigment in association with the disease is probably the result [and not the cause] of the disease)Citation32; accordingly, cross-sectional studies investigating possible relationships between AMD and macular pigment are subject to greater confounding than those investigating possible relationships between AMD and serum concentrations of macular pigment’s constituent carotenoids. Nevertheless, and with full appreciation of the limitations of associative studies, there have been no less than 12 cross-sectional reports attempting to investigate the relationship between serum concentrations of macular pigment’s constituent carotenoids and the risk for AMD (see ).Citation20–Citation23,Citation33–Citation40 Further, and notwithstanding the fact that many of these cross-sectional studies were performed in the pre-AREDS 2 era, it should be appreciated that lutein-containing supplements were commercially available since 1999,Citation41 and since that date their use grew substantially as a result of widespread dissemination of their putative benefits.Citation42–Citation48 Meaningful comment on any such relationship should be predicated, therefore, on population-based studies where data were recorded pre-1999 and to subsequent population-based studies where the use of carotenoid-containing supplements was recorded and appropriately factored into analyses. ThreeCitation20-Citation22 of fourCitation20-Citation22,Citation35 (75%) population-based studies using data recorded pre-1999 found an inverse relationship between AMD and serum concentrations of L and/or Z, and this compares with none of oneCitation38 (0%) population-based studies utilizing data recorded after 1999 where supplement use was recorded and factored into analyses (see ).
Table 2. Summary of cross-sectional studies designed to investigate a possible relationship between age-related macular degeneration and serum concentrations of macular pigment’s constituent carotenoids.
The findings reported herein also have implications for the debate regarding the appropriateness of introducing a screening program for non-advanced AMD.Citation49 A screening program can only be justified when there is a proven intervention for subjects with pre-disease or asymptomatic disease, and who are identified by use of a test that is sensitive (i.e. few false negatives) and specific (i.e. few false positives).Citation50 Accordingly, non-advanced AMD would appear to be an ideal candidate for a screening program, especially given our findings that awareness of the condition is associated with supplement use and consequentially increased plasma concentrations of L. Indeed, a screening program for non-advanced AMD could avail of existing infrastructures (community-based cameras, centralized reading center, etc.), which had been shown to be efficacious and cost-effective for the purposes of screening for diabetic retinopathyCitation51 (although it would need to be extended to the entire population aged 50 years and older, which represents a substantially greater number of participants than the diabetic population).
Further, and beyond risk-reduction for disease progression and visual loss, it is important to emphasize that antioxidant supplements also confer visual benefits in patients with non-advanced AMD in the short- and medium-term, and are therefore not solely aimed at risk reduction of ultimate disease progression.Citation19 Indeed, in the recent study by Akuffo et al, antioxidant supplementation in patients with non-advanced AMD over a 24-month period was shown to enhance vision in non-advanced AMD patients (a condition traditionally associated with progressive visual loss), reflected in statistically significant improvements in contrast sensitivity, glare disability, photostress recovery, and reading speed.Citation19 These improvements are not trivial, and improve quality of life in patients with non-advanced AMD, as well as reducing the risk of adverse and vision-related insults to health (e.g. falls and hip fracture).Citation52
Accordingly, establishment of a screening program would facilitate appropriate disease-retardingCitation17, sight-savingCitation17 and vision-optimizingCitation19 nutritional interventions to be offered to subjects who would otherwise be unaware that they are afflicted with the non-advanced form of AMD, and would likely be justified by the financial savings accruing from early detection of disease.
The principal strengths of this study rest on its large population-based sample size (n = 4,423). Also, and somewhat uniquely, this cohort represents a racially homogeneous sample (99% were white and Irish born). However, this study also has limitations, including the fact that the TILDA study sample excluded individuals who were institutionalized (e.g. living in nursing homes) and, also, individuals aged 75 years and older were underrepresented in the sample.Citation24 Although diurnal variation of serum concentrations of L and Z has been reported to be negligible, there are seasonal effects for serum concentrations of L and Z, which differs between individuals.Citation53 Moreover, dietary data would have enriched the analysis and interpretation of our data, given that we were studying compounds, which are entirely of dietary origin.
In conclusion, we report that a belief that one suffers from AMD (irrespective of whether that belief is founded or unfounded) is associated with supplement use and consequentially higher plasma concentrations of L. This finding represents a hitherto unappreciated confounding variable for interpretation of cross-sectional epidemiologic studies investigating relationship between AMD and macular pigment’s constituent carotenoids.
Declaration of interest
S Beatty and JM Nolan are Directors of Nutrasight Consultancy Ltd, where they do consultancy work for companies with an interest in supplements for eye care. R Moran, J Stack, AM O’Halloran, J Feeney, KO Akuffo, T Peto and RA Kenny report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgements
We would like thank the TILDA participants, research team, field researchers and research nurses who conducted tests in TILDA.
Funding
This work was supported by Bayer, Ireland and Waterford Institute of Technology Presidential Scholarship. TILDA is funded by An Roinn Sláinte (Irish Department of Health), The Atlantic Philanthropies, and Irish Life plc. The funders had no role in the study design or in the collection, analysis and interpretation of the data or in the writing of the report or in the decision to submit the article for publication. JMN and KOA were funded by the European Research Council (ERC). JF and AOH were funded by the Centre for Ageing Development and Research in Ireland (CARDI). TP was funded by the NIHR BMRC at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology, London, UK.
References
- Hirsch J, Curcio CA. The spatial resolution capacity of human foveal retina. Vision Res. 1989;29(9):1095–101.
- Chew EY, Clemons TE, Agron E, Sperduto RD, Sangiovanni JP, Davis MD, Ferris FL. Ten-year follow-up of age-related macular degeneration in the age-related eye disease study: AREDS report no. 36. JAMA Ophthalmol. 2014;132(3):272–77.
- Bird A, Bressler N, Bressler S, Chisholm I, Coscas G, Davis M, de Jong P, Klaver C, Klein B, Klein R. An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group. Surv Ophthalmol. 1995;39(5):367–74.
- Resnikoff S, Pascolini D, Etya’ale D, Kocur I, Pararajasegaram R, Pokharel GP, Mariotti SP. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82(11):844–51.
- Akuffo KO, Nolan J, Stack J, Moran R, Feeney J, Kenny RA, Peto T, Dooley C, O’Halloran AM, Cronin H, et al. Prevalence of age-related macular degeneration in the Republic of Ireland. Br J Ophthalmol. 2015;99(8):1037–44.
- Kelliher C, Kenny D, O’Brien C. Trends in blind registration in the adult population of the Republic of Ireland 1996-2003. Br J Ophthalmol. 2006;90(3):367–71.
- Wong WL, Su X, Li X, Cheung CMG, Klein R, Cheng C-Y, Wong TY. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106–e116.
- Bonastre J, Le Pen C, Anderson P, Ganz A, Berto P, Berdeaux G. The epidemiology, economics and quality of life burden of age-related macular degeneration in France, Germany, Italy and the United Kingdom. Eur J Health Econ. 2002;3(2):94–102.
- Treas J. Family support systems for the aged some social and demographic considerations. Gerontologist. 1977;17(6):486–91.
- Köberlein J, Beifus K, Schaffert C, Finger RP. The economic burden of visual impairment and blindness: a systematic review. BMJ Open. 2013;3(11):e003471.
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY, Group MS. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–31.
- Schmier JK, Covert DW, Lau EC. Patterns and costs associated with progression of age-related macular degeneration. Am J Ophthal. 2012;154(4):675–81.
- Connell PP, Keane PA, O’Neill EC, Altaie RW, Loane E, Neelam K, Nolan JM, Beatty S. Risk factors for age-related maculopathy. J Ophthalmol. 2009;2009:360764.
- Beatty S, Koh H, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45(2):115–34.
- Hollyfield JG, Bonilha VL, Rayborn ME, Yang X, Shadrach KG, Lu L, Ufret RL, Salomon RG, Perez VL. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat Med. 2008;14(2):194–98.
- Bernstein PS, Li B, Vachali PP, Gorusupudi A, Shyam R, Henriksen BS, Nolan JM. Lutein, zeaxanthin, and meso-zeaxanthin: the basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog Retin Eye Res. 2016;50:34–66.
- Age-Related Eye Disease Study 2 Research G. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA Ophthalmol. 2013;309(19):2005–15.
- Age-Related Eye Disease Study 2 Research G, Chew EY, Clemons TE, Sangiovanni JP, Danis RP, Ferris FL, 3rd, Elman MJ, Antoszyk AN, Ruby AJ, Orth D, et al. Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report No. 3. JAMA Ophthalmol. 2014;132(2):142–49.
- Akuffo KO, Beatty S, Peto T, Stack J, Stringham J, Kelly D, Leung I, Corcoran L, Nolan JM. The Impact of Supplemental Antioxidants on Visual Function in Nonadvanced Age-Related Macular Degeneration: A Head-to-Head Randomized Clinical Trial. Invest Ophthalmol Vis Sci. 2017;58(12):5347–60. (6):2461–5.
- Delcourt C, Carriere I, Delage M, Barberger-Gateau P, Schalch W. Plasma lutein and zeaxanthin and other carotenoids as modifiable risk factors for age-related maculopathy and cataract: the POLA Study. Invest Ophthalmol Vis Sci. 2006;47(6):2329–35.
- Mares-Perlman JA, Fisher AI, Klein R, Palta M, Block G, Millen AE Wright JD. Lutein and zeaxanthin in the diet and serum and their relation to age-related maculopathy in the third national health and nutrition examination survey. Am J Epidemiol. 2001;153(5):424–32.
- Gale CR, Hall NF, Phillips DI, Martyn CN. Lutein and zeaxanthin status and risk of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2003;44(6):2461–65.
- Antioxidant status and neovascular age-related macular degeneration. Eye disease case-control study group. Arch Ophthalmol. 1993;111(1):104–09.
- Kearney PM, Cronin H, O’Regan C, Kamiya Y, Savva GM, Whelan B, Kenny R. Cohort profile: the Irish longitudinal study on ageing. Int J Epidemiol. 2011;40(4):877–84.
- Kenny R, Whelan B, Cronin H, Kamiya Y, Kearney P, O’Regan C, Ziegel M. The design of the Irish longitudinal study on ageing. Dublin: Trinity College Dublin; 2010.
- Moran R, Nolan JM, Stack J, O’Halloran AM, Feeney J, Akuffo KO, Kenny RA, Beatty S. Non-dietary correlates and determinants of plasma Lutein and Zeaxanthin concentrations in the Irish population. J Nutr Health Aging. 2017;21(3):254–61.
- Loughman J, Nolan J, Stack J, Beatty S. Online AMD research study for optometrists: current practice in the Republic of Ireland and UK. Opt in Prac. 2011;12(4):135–44.
- Neelam K, Nolan J, Chakravarthy U, Beatty S. Psychophysical function in age-related maculopathy. Surv Ophthalmol. 2009;54(2):167–210.
- Gorsek WF, inventor; Vitacost Inc., assignee. Ocular orally ingested composition for prevention and treatment of individuals. US patent US 6103756 A. 2000.
- Zeimer M, Dietzel M, Hense HW, Heimes B, Austermann U, Pauleikhoff D. Profiles of macular pigment optical density and their changes following supplemental lutein and zeaxanthin: new results from the LUNA study. Invest Ophthalmol Vis Sci. 2012;53(8):4852–59.
- Curcio CA, Medeiros NE, Millican CL. Photoreceptor loss in age-related macular degeneration. Invest Ophthalmol Vis Sci. 1996;37(7):1236–49.
- Nolan J, Beatty S. Profiles of macular pigment optical density and their changes following supplemental Lutein and Zeaxannthin letters. Invest Ophthalmol Vis Sci. 2012;53(10):6303–6303.
- Mares-Perlman JA, Brady WE, Klein R, Klein BE, Bowen P, Stacewicz-Sapuntzakis M, Palta M. Serum antioxidants and age-related macular degeneration in a population-based case-control study. Arch Ophthalmol. 1995;113(12):1518–23.
- Sanders T, Haines AP, Wormald R, Wright LA, Obeid O. Essential fatty acids, plasma cholesterol, and fat-soluble vitamins in subjects with age-related maculopathy and matched control subjects. Am J Clin Nut. 1993;57(3):428–33.
- Moeller SM, Parekh N, Tinker L, Ritenbaugh C, Blodi B, Wallace RB, Mares JA. Associations between intermediate age-related macular degeneration and lutein and zeaxanthin in the Carotenoids in Age-related Eye Disease Study (CAREDS): ancillary study of the Women’s Health Initiative. Arch Ophthalmol. 2006;124(8):1151–62.
- Dasch B, Fuhs A, Schmidt J, Behrens T, Meister A, Wellmann J, Fobker M, Pauleikhoff D, Hense HW. Serum levels of macular carotenoids in relation to age-related maculopathy: the Muenster Aging and Retina Study (MARS). Graefes Arch Clin Exp Ophthalmol. 2005;243(10):1028–35.
- Simonelli F, Zarrilli F, Mazzeo S, Verde V, Romano N, Savoia M, Testa F, Vitale DF, Rinaldi M, Sacchetti L. Serum oxidative and antioxidant parameters in a group of Italian patients with age-related maculopathy. Clin Chim Acta. 2002;320(1–2):111–15.
- Michikawa T, Ishida S, Nishiwaki Y, Kikuchi Y, Tsuboi T, Hosoda K, Ishigami A, Iwasawa S, Nakano M, Takebayashi T. Serum antioxidants and age-related macular degeneration among older Japanese. Asia Pac J Clin Nutr. 2009;18(1):1–7.
- Cardinault N, Abalain JH, Sairafi B, Coudray C, Grolier P, Rambeau M, Carre JL, Mazur A, Rock E. Lycopene but not lutein nor zeaxanthin decreases in serum and lipoproteins in age-related macular degeneration patients. Clin Chim Acta. 2005;357(1):34–42.
- Zhou H, Zhao X, Johnson EJ, Lim A, Sun E, Yu J, Zhang Y, Liu X, Snellingen T, Shang F, et al. Serum carotenoids and risk of age-related macular degeneration in a chinese population sample. Invest Ophthalmol Vis Sci. 2011;52(7):4338–44.
- Hadden WL, Watkins RH, Levy LW, Regalado E, Rivadeneira DM, Van Breemen RB, Schwartz SJ. Carotenoid composition of marigold (Tagetes erecta) flower extract used as nutritional supplement. J Agric Food Chem. 1999;47(10):4189–94.
- Bone RA, Landrum JT, Guerra LH, Ruiz CA. Lutein and zeaxanthin dietary supplements raise macular pigment density and serum concentrations of these carotenoids in humans. J Nutrition. 2003;133(4):992–98.
- Thomson LR, Toyoda Y, Langner A, Delori FC, Garnett KM, Craft N, Nichols CR, Cheng KM, Dorey CK. Elevated retinal zeaxanthin and prevention of light-induced photoreceptor cell death in quail. Invest Ophthalmol Vis Sci. 2002;43(11):3538–49.
- Johnson EJ, Neuringer M, Russell RM, Schalch W, Snodderly DM. Nutritional manipulation of primate retinas, III: effects of lutein or zeaxanthin supplementation on adipose tissue and retina of xanthophyll-free monkeys. Invest Ophthalmol Vis Sci. 2005;46(2):692–702.
- Leung IY-F, Sandstrom MM, Zucker CL, Neuringer M, Snodderly DM. Nutritional manipulation of primate retinas, II: effects of age, n–3 fatty acids, lutein, and zeaxanthin on retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2004;45(9):3244–56.
- Neuringer M, Sandstrom MM, Johnson EJ, Snodderly DM. Nutritional manipulation of primate retinas, I: effects of lutein or zeaxanthin supplements on serum and macular pigment in xanthophyll-free rhesus monkeys. Invest Ophthalmol Vis Sci. 2004;45(9):3234–43.
- Loane E, Kelliher C, Beatty S, Nolan JM. The rationale and evidence base for a protective role of macular pigment in age-related maculopathy. Br J Ophthalmol. 2008;92(9):1163–68.
- Nolan JM, Stack J, OD O, Loane E, Beatty S. Risk factors for age-related maculopathy are associated with a relative lack of macular pigment. Exp Eye Res. 2007;84(1):61–74.
- Chew EY, Schachat AP. Should we add screening of age-related macular degeneration to current screening programs for diabetic retinopathy? Am Acad Ophthal. 2015;122(11):2155–56.
- Spix C, Blettner M. Screening: part 19 of a Series on Evaluation of Scientific Publications. Dtsch Arztebl Int. 2012;109(21):385–90.
- Conlin PR, Fisch BM, Orcutt JC, Hetrick BJ, Darkins AW. Framework for a national teleretinal imaging program to screen for diabetic retinopathy in Veterans Health Administration patients. J of Rehab Res Dev. 2006;43(6):741.
- Klein BE, Klein R, Lee KE, Cruickshanks KJ. Performance-based and self-assessed measures of visual function as related to history of falls, hip fractures, and measured gait time. The Beaver Dam Eye Study. Ophthalmology. 1998;105(1):160–64.
- Nolan JM, Stack J, Mellerio J, Godhinio M, O’Donovan O, Neelam K, Beatty S. Monthly consistency of macular pigment optical density and serum concentrations of lutein and zeaxanthin. Curr Eye Res. 2006;31(2):199–213.
