ABSTRACT
Statement: The current article has not been published elsewhere and has not been submitted simultaneously for publication elsewhere.
Purpose: To investigate the preliminary use of three-dimensional (3D) heads-up display (HUD) viewing system for vitreoretinal surgery under various status.
Materials and Methods: Nonrandomized case–control study. Consecutive cases to have vitreoretinal surgery under various status were prospectively recruited. Twenty-five-gauge vitrectomy platform and 3D viewing system were used. Main outcomes included: luminous emittance (lux) of endoillumination pipe, surgical duration, the surgeon and residents’ preference and ergonomics. Consecutive patients to have vitreoretinal surgery with the conventional viewing system were recruited as control group following the same inclusion and exclusion criteria and underwent surgeries by the same surgeon with the same microscope and vitrectomy platform.
Results: Thirty-one patients (31 eyes; Group Study) and twenty-eight patients (28 eyes; Group Control) were included; without significantly statistical difference in terms of age, gender, main diagnosis, surgical duration, and difficulty rating between both groups (all P > 0.05). Lower endoillumination intensity was needed in Group Study than that in Group Control (10% vs. 35%; 598.7 ± 5.4 vs. 1913.0 ± 12.9 lux, P < 0.001). The surgeon and residents expressed overwhelming preference with the 3D system in both groups. Improved ergonomic was rated in Group Study (4.4 ± 0.8 vs. 3.2 ± 1.0, P < 0.001). Some intraoperative difficulties and discomforts appeared to the surgeon and assistants when using the 3D viewing system.
Conclusion: Vitreoretinal surgery under various status can be well finished with the HUD platform by novice at the system. Main benefits included lower endoillumination intensity, enhanced users’ preference, and improved ergonomics. Some further refinements of the system are expected.
Introduction
Heads-up display (HUD) viewing systems were firstly introduced for pilots to look straight ahead during flight. Surgical application of HUD has become increasingly popular in some medical specialties, including ophthalmology.Citation1–Citation7 A three-dimensional (3D) camera mounted on the microscope transmitted image from the surgical field to the platform, and the 3D image is terminally demonstrated on a large flat panel display. The surgeon has microsurgical performance by looking at the display with eyeglasses, rather than through the microscope eyepiece.
A study by Eckardt and Paulo found that the heads-up method could cover routine vitreoretinal procedures, with greater comfort during prolonged work.Citation1 A more recent study by Adam et al.Citation2 demonstrated that HUD vitreoretinal surgery could be well finished under less endoillumination intensity, which might have an intraoperative protecting effect on the retina. The current study aims to evaluate whether vitreoretinal surgery under various status can be accomplished with the heads-up method, with low endoillumination intensity by the novice at the 3D surgical platform.
Materials and methods
The study was approved by the Institutional Review Board of Zhongshan Ophthalmic Center (ZOC) affiliated to Sun Yat-sen University (Guangzhou, China) and performed in accordance with the World Medical Association’s Declaration of Helsinki. Written informed consent was obtained from each case in the study group and control group. All the patients were informed of the vitreoretinal surgery as routine. Additionally, all the patients were clearly informed of the kinds of viewing systems (3D or conventional microscope eyepiece) we would use during their vitreoretinal surgeries before they signed the consent. Consecutive patients who scheduled to have vitreoretinal surgery under various status in ZOC were prospectively included. Vitreoretinal surgeries under various status were defined as the following inclusion criteria: concomitant with phacoemulsification with/without intraocular lens implantation (because of visually significant cataract); recurrent retinal detachment (RD) with/without silicone oil tamponade (SOT); tractional retinal detachment (TRD) with/without vitreous hemorrhage (VH) secondary to severe proliferative diabetic retinopathy (PDR); RD with proliferative vitreoretinopathy (PVR) (≥Grade C1); RD with obvious choroidal detachment (CD) (confirmed with B ultrasound); and those with other conditions defined to be complex after group discussion. Exclusion criteria included: surgery solely for rhegmatogenous RD, epimacular membrane (EMM), idiopathic macular hole, and VH; and other conditions finally decided by group discussion to be not under complex status. For better justification of the study’s conclusions, a control group was additionally recruited at the same period as the study group. The main outcomes included: emittances of the endoillumination pipe in both groups and that of the 3D display in the study group; surgical duration; the surgeon and residents’ preference with the two systems (3D vs. conventional); difficulty rating; ergonomics rating; intraoperative and postoperative complications; and the surgeon and assistants’ using experiences. Best-corrected visual acuity (BCVA) and anatomic outcomes were considered secondary outcomes because of the short follow-up duration.
Study group
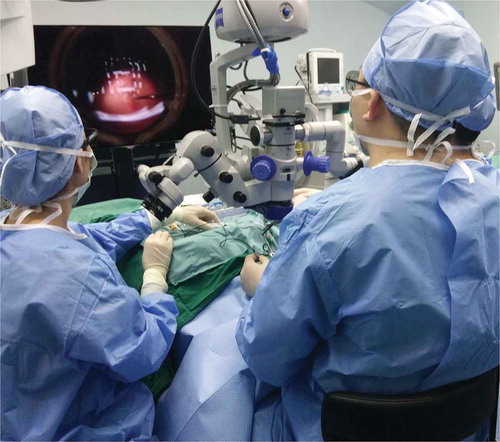
All surgeries were performed by one experienced surgeon of novice at the 3D surgical platform (S.Z.) with retrobulbar anesthesia (by the surgeon). Twenty-five-gauge pars plana vitrectomy (PPV) platform (Constellation Vitrectomy System, Alcon Laboratories, Fort Worth, TX, USA) was used for all the vitreoretinal surgery, including phacoemulsification and silicone oil removal. A wide-angle viewing system (Carl Zeiss AG, Jena, Germany) was used for all the vitreoretinal surgeries. The NGENUITY® 3D visualization system (Alcon Laboratories, Fort Worth, TX, USA) was used as a platform for the imaging processing. A 3D surgical camera set at 80% iris aperture (ICM5 A; Alcon Laboratories, Fort Worth, TX, USA) was connected with the surgical microscope (OPMI VISU 200 plus; Carl Zeiss AG, Jena, Germany). The surgeon and assistants wore passive 3D polarized glasses and were positioned 1.8 m from the heads-up 3D 4K Ultra HD flat panel display (; the display was a little side for the surgeon) (screen side 54.5 inches; resolution 3840 × 2160 pixels; mode number: 55EF9500-UA; LG Ltd., Seoul, Korea). The surgeon and assistants were examined not to have strabismus, amblyopia, or anisometropia, which have been reported being related with increased ocular and systemic discomforts when looking at 3D display.Citation8
Figure 1. The surgeon and assistants wore passive three-dimensional polarized glasses and were positioned 1.8 m from the heads-up display. The display was a little side for the surgeon. All the surgical performances of the surgeon and assistants were finished by looking at the display.
As in a previous study by Adam et al.Citation2, we set the endoillumination intensity to 10% for all surgeries. Before surgery, we measured the luminous emittance (lux) of the endoillumination light pipe three times. The light-pipe (set to 10%) was located right above 5 mm from a luxmeter (MS6612; Peak Meter Co., Shenzhen, China) with operating room lights off. After phacoemulsification and core vitrectomy (if required; for transparency of the visual axis), the light-pipe was positioned in the middle part of the vitreous cavity (about 10 mm from the optic disc, with the light cone being centered on it). Corresponding lux of the display was measured three times at the center of the monitor with the luxmeter positioned 5 cm from the display. After surgery, the surgeon rated the general difficulty of the whole surgery from 1 to 5 (1 = not difficult, 5 = absolutely difficult). Afterwards, all the surgical assistants were asked by the surgeon whether they preferred looking at the 3D display or looking through the eyepiece to have scleral indentation and other manipulations during the surgical procedure. The assistants were also required to offer the main reasons for their choices.
Control group
Additionally, to have better justification of the conclusions from the study, another group of consecutive patients were prospectively included as control. All the patients met the inclusion criteria mentioned earlier. The same surgeon (S.Z.) performed all the surgical manipulation using the same vitrectomy platform (Constellation Vitrectomy System, Alcon Laboratories, Fort Worth, TX, USA) and surgical microscope (OPMI VISU 200 plus; Carl Zeiss AG, Jena, Germany). The only difference was that the surgeon performed all the surgeries by looking through the eyepiece, rather than looking at the 3D display. The incoming images from the viewing system were split into two sections, one for the surgeon and the other for assistant; the ratio was 80% (surgeon) versus 20% (assistant). The main parameters to compare between the study group and control group included: the endoillumination intensity to see clearly for all the vitreoretinal surgeries, the surgeon and residents’ preferences with the two systems, and ergonomics. As the endoillumination intensity of the study group was set 10%, we only evaluated the minimum endoillumination intensity required for all the vitreoretinal surgeries when using the conventional viewing system (eyepiece of the surgical microscope). In each conventional surgery, different endoillumination intensities (stepwise increasing from 5%, 10%, 15%, 20%, 25%, 30%, 35%, 40% to 45%) were evaluated whether it was clear enough for the surgeon to well finish all the manipulations. The surgeon and residents (the same individuals in the study group) were required to answer their preferences after each surgery. In both the study group and control group, the surgeon rated the general ergonomics of each surgery from 1–5 (1 = very uncomfortable; 5 = very comfortable). General difficulty of each surgery was rated as mentioned earlier.
All Snellen visual acuity values were converted to the logarithm of the minimum angle of resolution (logMAR) for statistical analysis. Visual acuity of light perception was assigned as 2.9, hand movements as 2.6, and counting fingers as 2.3.Citation9 Unpaired t test, unpaired Mann–Whitney test, and χ2 analysis were used as appropriate. All data were analyzed using the SPSS 19.0 statistical software (SPSS Inc., Chicago, IL, USA). All the continuous data were expressed as mean ± standard deviation (SD). A P value less than 0.05 was considered statistically significant.
Results
Study group
In the study group, there were 31 eyes of 31 patients (18 men and 13 women) included, with a mean age of 49.2 ± 15.3 years (range, 19 years-70 years). The mean logMAR BCVA was 2.087 ± 0.591 (mean Snellen equivalent 20/2,444) (range, 0.699–2.600; Snellen equivalent, hand movements-20/100). The main diagnosis included: recurrent RD with/without SOT (9 eyes), SOT with significant cataract (1 eye), severe PDR with/without TRD (8 eyes), idiopathic EMM with significant cataract (1 eye), and primary RD with various complex conditions (12 eyes). The concomitant various status of the patients mainly included: significant cataract (4 eyes; EMM (1 eye), recurrent RD (1 eye), primary RD (1 eye) and SOT (1 eye)), recurrent RD with SOT (5 eyes; recurrent RD (4 eyes), PDR (1 eye)); TRD secondary to severe PDR (6 eyes), RD with obvious CD (10 eyes; recurrent RD (3 eyes), primary RD (7 eyes)), subretinal membrane with/without epiretinal membrane (4 eyes; recurrent RD (1 eyes), primary RD (2 eyes), PDR (1eye)), high myopia (7 eyes; recurrent RD (3 eyes), primary RD (4 eyes)), and uveitis with posterior iris synechiae (primary RD (1 eye)).
The main surgical procedure mainly included: PPV (30 eyes), silicone oil removal (6 eyes), phacoemulsification with/without intraocular lens implantation (3 eyes), membrane peeling (14 eyes), retinotomy (2 eyes), and silicone oil infusion (26 eyes). The mean duration of surgical procedure was 45.0 ± 12.6 min (range, 25–78 min). The mean emittance of endoillumination pipe (at 10% intensity) was 598.7 ± 5.4 lux (range, 587.3–610.0 lux). The mean emittance of the display was 62.4 ± 3.9 lux (range, 54.4–72.0 lux). The correlation between mean emittance of the endoillumination and that of the display was not statistically significant (P = 0.375).
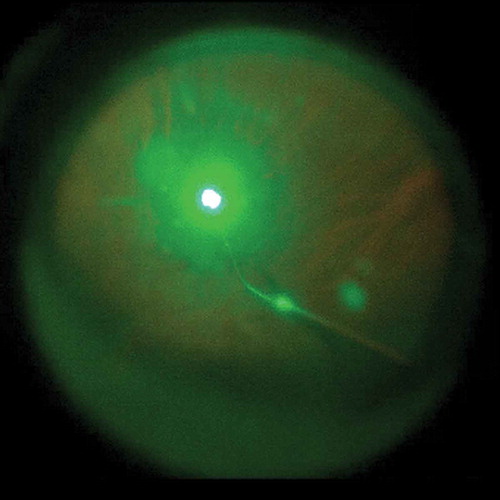
The mean general difficulty rating as graded by the surgeon was 1.6 ± 0.8 (between not difficult and slightly difficult) (range, 1–4). The surgeon was mainly satisfied with the improved comforts of the body and reported reduced fatigue during surgery. Two other impressing advantages were reduced eye fatigue when looking at the display and improved depth perception when having complicated manipulations. The main difficulties observed during the surgical procedure with the 3D display included: movement of patients’ head during scleral indentation () (Cases 5, 10, 11, 22, and 27), opacity of the anterior and/or posterior lens capsule () (Cases 1, 18, and 31), and nausea and dizziness experienced when performing prolonged laser photocoagulation () (Cases 5, 10, 14, 26, and 30; the surgeon’s discomforts disappeared after a pause in the manipulation, and there was no need to switch to the microscope eyepiece).
Figure 2. The peripheral vitreous could be shaved completely and safely with the help of scleral indentation by the assistant who used the heads-up method. However, under local anesthesia, inevitable movement of the patient’s eye and head made scleral indentation more difficult than when with the traditional method.
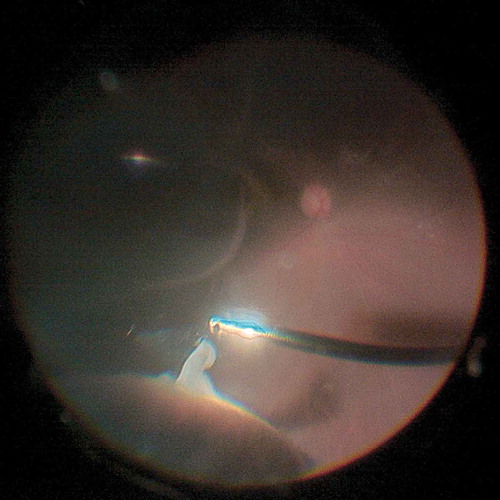
Figure 3. Diffuse small opacity of the anterior capsular membrane created obvious disturbance to the surgeon when using the three-dimensional platform. (A) Anterior segment photograph of Case 1; (B) Fundoscopy screenshot of Case 1 from the three-dimensional platform during the surgical procedure.
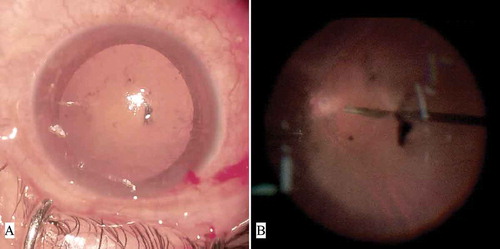
Figure 4. During laser photocoagulation, the surgeon sometimes experienced nausea and dizziness when looking at the display. The flash-like images also made some of the assistants uncomfortable.
The surgeon and all the surgical assistants (10 individuals) preferred the 3D platform to the traditional one. The main reasons for their preference included: comforts of the neck and back (3 people), stereoscopic sensation (2 people), and enhanced teaching ability to the assistants (5 people). However, three assistants complained of discomforts during having scleral indentation and when the surgeon was performing laser photocoagulation. The general characteristics of all the patients in the study group were listed in .
Table 1. General characteristics of the patients in the study group (n = 31) undergone three-dimensional vitreoretinal surgery under complex status.
Control group
There were totally 28 eyes of 28 patients (16 men and 12 women) included. The general characteristics of the patients in the control group were summarized into the table (supplementary data). There was no statistically significant difference in terms of age, gender, BCVA, main diagnosis, surgical duration and difficulty rating between the study group and control group (all P > 0.05). Finally, the minimum endoillumination intensity for the surgeon to see clearly all through the procedure was judged 35%. Overwhelming preference with the HUD surgical system were observed in the control group (the surgeon and all the 10 assistants expressed their preference with HUD at the conclusion of each conventional surgery). Improved ergonomics were rated by the surgeon when using the HUD system (P < 0.001). The main characteristics of both groups were listed in .
Table 2. Main characteristics of patients in the study group (n = 31) and control group (n = 28).
For both groups, there were no serious intraoperative or postoperative (with follow-ups ranging 2–5 weeks) complications observed. Temporary intraocular pressure elevation after the surgery was controlled well to the normal range with topical administration of eye drops.
Discussion
Two decades ago, HUDs were proposed as an ideal alternative to surgery performed using a microscope eyepiece for visualization.Citation6,Citation7 The 3D platform has been used in several medical specialties, including laparoscopy, neurosurgery, and ophthalmology.Citation1–Citation5,Citation10–Citation12 The application of 3D HUD surgical platform in vitreoretinal surgery was firstly introduced last year by Eckardt et al.,Citation1 and followed by a pilot study by Adam et al.Citation2 Their studies suggested that routine vitreoretinal surgery with/without cataract surgery could be completed with the 3D HUD viewing system. The advantages of the 3D platform were summarized as: reduces the potential risk of retinal phototoxicity during vitreoretinal surgery; improves ergonomics during prolonged vitreoretinal surgery; the high dynamic range provides increased color contrast with comparable depth of field; increased depth-of-field (due to single chip camera and small aperture); and color gain adjustment for different manipulatoins.Citation1,Citation2 Consistent with the previous ones, we also found that vitreoretinal surgery could be effectively and safely finished by the 3D HUD viewing system with a lower endoillumination intensity.
A recent study by Rizzo et al.Citation13 had a thorough and convincing evaluation of the whole surgical team’s perception on 3D surgical viewing system. The study suggested that the system was very comfortable for the primary surgeon. However, the second assistant and anesthetist experienced problems and discomforts during the surgery. Consistently, our study also evaluated the surgeon and assistants’ perception, revealing the surgeon and assistants’ positive perception, as well as the discomforts and difficulties on the system. However, our current article did not include the surgical nurses and anesthetists, neither had evaluation of the 3D HUD viewing system under various surgical platforms. In the current study, we considered that the various status might increase the difficulty of the surgical procedures, either with the 3D method or the traditional one. Additionally, the surgeon had not undergone any 3D surgical practice prior to the study. However, the complexity of the surgeries created minimal obstacle for the surgeon in completing all the procedures. Therefore, our study suggested that the 3D HUD viewing system could cover a wide range of vitreoretinal surgeries, even under various complex conditions. Another concern of the 3D surgical system might be the learning curve.Citation1,Citation10,Citation12 The surgeon had not undergone any noncomplicated vitreoretinal surgery with the 3D system before the current study, However, the current study revealed that an experienced surgeon was able to master heads-up vitreoretinal surgery without any obvious obstacles, even without presurgery 3D practice.
Reduced phototoxicity is one potential benefit of the 3D HUD system.Citation1,Citation2 Retinal phototoxicity would not be a critical point for the skilled vitreoretinal surgeon with the rapid advancement of modern vitrectomy. However, there are still continuous concern with it from the vitreoretinal surgeons and researchers.Citation1,Citation2,Citation14–Citation16 Light exposure time and illumination intensity are two main factors related to the effects of macular phototoxicity.Citation14–Citation16 The current study had direct measurement of the emittance of the endoillumination pipe with a luxmeter, which might supplement to the one by Adam et al.Citation2 Consistent with the study by Adam et al.,Citation2 the surgeon also felt comfortable to finish all the surgeries with the endoillumination set to 10% (Alcon’s Constellation). The emittance of display we measured was 62.4 ± 3.9 lux, which was much higher than that measured by Adam et al. (14.3 ± 9.5 lux).Citation2 Several factors might have contributed to this difference: the distance of the luxmeter from the display (5 vs. 40 cm); the display’s brightness settings; the service time of the endoillumination lamp; and the distance of the intravitreous endoillumination pipe to the retina and optic disc.
We also found that there was no statistically significant correlation between the emittance of the endoillumination pipe and that of the 3D display (P = 0.375). We could deduce that the emittance of the display might be affected by various factors. As we know, the macula is exposed to the light beam by the endoillumination pipe at a close distance, without any natural filtering effect from either the cornea or the lens. Therefore, the emittance of the display might not be an ideal factor to reflect the true intensity of the endoillumination during vitreoretinal surgery. Direct measurement of the emittance of the light pipe would serve better to reflect the true light intensity to which the macula is exposed.
Three possible factors might contribute to the lower requirement of endoillumination intensity of the 3D vitreoretinal surgery: digital amplification and processing of the camera signals; high resolution of the HUD; and improved depth perception.Citation1,Citation2,Citation10,Citation12 The service time of the endoillumination lamp in the Constellation® vitrectomy platform was about 40 h when we started the study. In the control group, minimum endoillumination intensity of 35% was required when looking through the eyepiece of the microscope. Therefore, it could be concluded that the 3D platform enabled us to finish complex vitreoretinal surgery with a much lower endoillumination intensity than that in the conventional viewing system (598.7 ± 5.4 vs. 1913.0 ± 12.9 lux; P < 0.001). Another factor related to retinal toxicity is the exposure time. The current study demonstrated that complex vitreoretinal surgery could be completed in a reasonable time range. There was no significantly statistical significance in surgical duration between the study group and control group (45.0 ± 12.6 vs. 47.1 ± 12.8, P = 0.407).
Even though vitreoretinal surgery could be performed effectively using the 3D platform, the surgeon and assistants’ experiences with its use is another major issue that should be evaluated in depth. The surgeon rated improved ergonomics in the study group than that in the control group (4.4 ± 0.8 vs. 3.2 ± 1.0, P < 0.001). The surgeon was satisfied with the experience of improved ergonomics: first, the surgeon was able to perform the surgeries with his back resting on the back of the surgical chair; and second, the surgeon could freely move his head and back to an acceptable extent so as to alleviate intraoperative fatigue. The surgeon also reported improved eye comfort when looking at the display, and ideal depth perception when performing complicated manipulations, such as membrane peeling and peripheral vitreous shaving. In the current study, the mean emittance of the 3D display was 62.4 ± 3.9 lux. We hypothesize that the surgeon’s improved eye comfort might be attributed to three factors: the emittance of light entering the surgeon’s eyes was lower than when the surgeon looks through the eyepiece; the surgeon could slightly move his eyes to alleviate fatigue during manipulations; and finally, the increased image size and improved depth perception on the display helped the surgeon to see more clearly and perform more accurately with less effort.
The surgical assistants’ experiences were consistent with previous reports.Citation1,Citation4,Citation11 The ten assistants overwhelmingly preferred the 3D platform. The main reasons were improved neck and back comfort, stereoscopic sensation, and an enhanced teaching/learning experience. The improved comfort experienced by the assistants might be attributed to the factors mentioned earlier for the surgeon. Interestingly, the assistants felt better about learning with the 3D platform as they shared the same stereoscopic images with the surgeon, which was a significant improvement and an opportunity that only the 3D surgical platform could offer to all in attendance. Therefore, we expect that the 3D surgical platform will play an important role in teaching institutions.Citation4,Citation10,Citation11
However, we could not discount the discomfort experienced by the surgeon and assistants when using the 3D surgical platform. This discomfort mainly included headache, nausea, and visual disturbances, which have also been reported in previous studies.Citation8,Citation11 The current study was the first one to report these “side effects” induced by the 3D surgical platform during vitreoretinal surgery. Movement of the patient’s head during scleral indentation might be resolved by pre- and intraoperative communication or by general anesthesia. It should be noted that the opacity of the optical media (such as the cornea and the lens capsule) greatly disturbed the surgeon, because the opacity-signals were also amplified by the 3D system. Additionally, unclear images can be caused not only by eye condition but also by adjustment of 3D imaging system. This problem is expected to be resolved in the future.
Moreover, the surgeon experienced nausea and dizziness when performed prolonged laser photocoagulation, even though various color profiles had been tried. Lund-Andersen et al.Citation17 demonstrated that flickering light may cause dysbalance/unstability. However, the specific mechanism of this process is still unclear. There are several possible links between vision and dizziness.Citation18 First, balance control (or postural stability) is achieved when the visual, vestibular, and proprioceptive systems are effectively coordinated. Second, vision may be associated with dizziness via changes to the vestibulo-ocular reflex. Third and finally, some patients are diagnosed with visual vertigo typically due to unilateral vestibular problems in patients suffering from anxiety. In our case, we suppose that constant flickering green light may be a disruptive visual stimulation that disturbs the vestibular and proprioceptive systems. We all know that in traditional methods, the surgeons are protected from potential laser-induced hazard by wearing goggles specifically matched to the laser’s wavelength.Citation19 In this process, not only the hazardous laser is filtered, the disturbing flickering light is also eliminated. Since the goggles are neither needed nor available in the new method, we speculate that better customized color profile might filtrate or soften the flickering light which disturbs the surgeon.
Therefore, we suggest that in the future, the 3D surgical platform should have a better customized color profile for laser photocoagulation. Another issue that should be mentioned is that the assistants complained of difficulty and fatigue when performing scleral indentation when the eyes were looking at the display. The underlying causes might include the learning curve and the fact that the assistants were forced to turn their heads nearly 90° in order to see the image on the display. Therefore, we suggest that the 3D surgical platform provides one more display (with a concomitant 2D or 3D image) at the front-upside position of the assistant’s body, in order to help the assistants see clearly during the surgical procedure without having to rotate their heads. The patients might benefit better from the 3D surgical platform in terms of reduced endoillumination intensity, and possibly better outcomes, because of the surgeon’s improved ergonomics. However, for patients as well as surgeons, one of the main problems for the 3D surgical system to be more popular would be the cost.
Our study presents several limitations that need to be recognized. This was a case series study with a small sample size, short follow-up duration. Even with a control group, it did not follow randomization. Furthermore, we did not use the same setting of endoillumination for HUD group to judge in a balanced manner. We could not provide in-depth analysis and comparison of the potential retinal phototoxicity of the endoillumination with different set intensities. There was a considerable variability in the complexity between the cases. Therefore, unless the study is designed as a randomized trial, it was very difficult to compare ergonomics rating between 3D and conventional surgery for complicated cases. Moreover, ergonomics rating was a subjective measure and could be biased.
In conclusion, our study demonstrated that vitreoretinal surgery under various status with a 3D HUD viewing system can be accomplished by an experienced vitreoretinal surgeon who is a novice at 3D surgery, with lower endoillumination intensity and improved ergonomics. Refinement to such systems is expected to provide better user experiences for surgeons and assistants in the future. Further randomized case–control studies with larger sample sizes and more objective parameters should be undertaken.
Supplemental Material
Download PDF (148.5 KB)Disclosure Statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Supplemental data for this article can be accessed on the publisher’s website.
References
- Eckardt C, Paulo EB. Heads-up surgery for vitreoretinal procedures: an experimental and clinical study. Retina. 2016;36(1):137–47. doi:10.1097/IAE.0000000000000689.
- Adam MK, Thornton S, Regillo CD, Park C, Ho AC, Hsu J. Minimal endoillumination levels and display luminous emittance during three-dimensional heads-up vitreoretinal surgery. Retina. 2017;37(9):1746–49. doi:10.1097/IAE.0000000000001420.
- Zhou J, Xu HJ, Liang CZ, Zhang L, Hao ZY, Feng LX. A comparative study of distinct ocular symptoms after performing laparoscopic surgical tasks using a three-dimensional surgical imaging system and a conventional two-dimensional surgical imaging system. J Endourol. 2015;29(7):816–20. doi:10.1089/end.2014.0759.
- Wong AK, Davis GB, Nguyen TJ, Hui KJ, Hwang BH, Chan LS, Zhou Z, Schooler WG, Chandrasekhar BS, Urata MM. Assessment of three-dimensional high-definition visualization technology to perform microvascular anastomosis. J Plast Reconstr Aesthet Surg. 2014;67(7):967–72. doi:10.1016/j.bjps.2014.04.001.
- Bhadri PR, Rowley AP, Khurana RN, Deboer CM, Kerns RM, Chong LP, Humayun MS. Evaluation of a stereoscopic camera-based three-dimensional viewing workstation for ophthalmic surgery. Am J Ophthalmol. 2007;143(5):891–92. doi:10.1016/j.ajo.2006.12.032.
- Taffinder N, Smith SG, Huber J, Russell RC, Darzi A. The effect of a second-generation 3D endoscope on the laparoscopic precision of novices and experienced surgeons. Surg Endosc. 1999;13:1087–92.
- Franken RJ, Gupta SC, Banis JJ, Thomas SV, Derr JW, Klein SA, Kon M, Barker JH. Microsurgery without a microscope: laboratory evaluation of a three-dimensional on-screen microsurgery system. Microsurg. 1995;16(11):746–51. doi:10.1002/micr.1920161109.
- Kim SH, Suh YW, Song JS, Park JH, Kim YY, Huh K, Son J, Kham K, Jeong T, Pyo KS. Clinical research on the ophthalmic factors affecting 3D asthenopia. J Pediatr Ophthalmol Strabismus. 2012;49(4):248–53. doi:10.3928/01913913-20120207-03.
- Holladay JT. Visual acuity measurements. J Cataract Refract Surg. 2004;30(2):287–90. doi:10.1016/j.jcrs.2004.01.014.
- Williams GA. Surgical viewing; do you see what I see? Retina. 2017;37(7):1219. doi:10.1097/IAE.0000000000001721.
- Guana R, Ferrero L, Garofalo S, Cerrina A, Cussa D, Arezzo A, Schleef J. Skills comparison in pediatric residents using a 2-dimensional versus a 3-dimensional high-definition camera in a pediatric laparoscopic simulator. J Surg Educ. 2017;74(4):644–49. doi:10.1016/j.jsurg.2016.12.002.
- Read SP, Fortun JA. Visualization of the retina and vitreous during vitreoretinal surgery: new technologies. Curr Opin Ophthalmol. 2017;28(3):238–41. doi:10.1097/ICU.0000000000000368.
- Rizzo S, Abbruzzese G, Savastano A, Giansanti F, Caporossi T, Barca F, Faraldi F, Virgili G. 3D surgical viewing system in ophthalmology: perceptions of the surgical team. Retina. 2018;38(4):857–61. doi:10.1097/IAE.0000000000002018.
- Charles S. Illumination and phototoxicity issues in vitreoretinal surgery. Retina. 2008;28(1):1–4. doi:10.1097/IAE.0b013e318156e015.
- Yanagi Y, Iriyama A, Jang WD, Kadonosono K. Evaluation of the safety of xenon/bandpass light in vitrectomy using the A2E-laden RPE model. Graefes Arch Clin Exp Ophthalmol. 2007;245(5):677–81. doi:10.1007/s00417-006-0428-x.
- van den Biesen PR, Berenschot T, Verdaasdonk RM, van Weelden H, van Norren D. Endoillumination during vitrectomy and phototoxicity thresholds. Br J Ophthalmol. 2000;84:1372–75.
- Lund-Andersen H. [Dizziness related to ophthalmological pathology]. Ugeskr Laeger. 2013;175:2703–05.
- Armstrong D, Charlesworth E, Alderson AJ, Elliott DB. Is there a link between dizziness and vision? A systematic review. Ophthalmic Physiol Opt. 2016;36(4):477–86. doi:10.1111/opo.12299.
- Hardy KJ, Lipton JR, Foster DH, Scarpello JH. Laser safety and ophthalmologists. Lancet. 1991;338(8778):1338. doi:10.1016/0140-6736(91)92643-G.
