ABSTRACT
Purpose/Aim: Inflammation is recognized as playing an etiological role in dry eye disease. This study aimed to assess the efficacy of various topical cyclosporine A (CsA) formulations on cornea inflammatory markers in a mouse model of dry eye.
Material and Methods: Six- to 7-week-old mice treated with scopolamine were housed in a controlled environment room to induce dry eye. Following dry eye confirmation by corneal fluorescein staining (CFS), the mice were treated three times a day with: 0.05%CsA (Restasis, Allergan), 0.1%CsA (Ikervis, Santen), 1%CsA oil solution, and 0.5% loteprednol etabonate (LE, Lotemax, Baush+Lomb), or left untreated. Aqueous tear production and CFS scores were assessed during the treatment period, and corneas were collected to measure the expression profile of a selection of inflammatory genes.
Results: After 7 days of treatment, the CFS scores were reduced by 21%, 31%, and 44% with 0.05%CsA, 0.1%CsA, and 1%CsA eye drops, respectively. By contrast, 0.5% LE did not decrease corneal fluorescein staining at day 10. A statistically significant dose-dependent CFS reduction was observed only between the 0.05% and 1%CsA formulations. The gene expression profiles indicated that 12, 18, 17 genes were downregulated by 0.05%CsA, 0.1%CsA, 1%CsA, respectively. Among them, the genes significantly downregulated were: IL1A, IL1R1, and TLR4 with 0.05%CsA; H2-Eb1, IL1A, IL1B, IL1RN, IL6, TGFB2, TGFB3, TLR2, TLR3, and TLR4 with 0.1%CsA; IL1B, IL6, TGFB3, and TLR4 with 1%CsA. TGFB1 and TGFBR1 were the only genes upregulated in all groups, but only TGFB1 upregulation reached significance. IL6RA was significantly upregulated by 0.05%CsA.
Conclusions: This study indicates that the three CsA formulations effectively modulated TLR4, TGFβ1, IL1, and IL6 pathways to reduce corneal epithelium lesions in a mouse model of severe dry eye. The study also suggests that the different anti-inflammatory eye drops modulated inflammatory genes in a slightly different manner.
Introduction
Inflammation is a process by which the body protects itself from environmental harmful stresses (e.g., pathogens or irritants such as pollutants) by eliminating the initial cause of cell injury, as well as injured and damaged cells and tissues. Controlled inflammation is beneficial, while chronic inflammation is detrimental and can lead to disease. Inflammation has been recognized as an important component of the physiopathology of dry eye disease (DED).Citation1–Citation4 This concept has been included in the definition of DED provided by the Dry Eye Workshop (DEWS) in 2007 and 2017, with inflammation recognized to play an etiological role.Citation5 Hence, the modulation of inflammation to manage DED is critical. Untreated (chronic) inflammation in DED patients can result in alterations of the cornea, ranging from punctate epitheliopathy to corneal ulcer and vision loss in the most severe cases.
Treatments currently available to control inflammation in DED and prevent severe cornea complications include corticosteroids,Citation6,Citation7 cyclosporine A (CsA),Citation8–Citation10 and lifitegrast (a novel integrin antagonist),Citation11,Citation12 even though the latter is intended for the treatment of mild-to-moderate DED in patients who are more prone to respond well to artificial tears.Citation13 Corticosteroids are very effective at controlling inflammation. They act by decreasing the expression of various pro-inflammatory cytokines and chemokines through genomic and nongenomic actions.Citation14,Citation15 Unfortunately, their long-term use is associated with serious side effects such as glaucoma and cataract.Citation16,Citation17 As a consequence, only soft steroids (e.g., fluorometholone or loteprednol etabonate) with an improved safety profile are suggested as treatments of DED, but still not for chronic disease.Citation6,Citation7,Citation18 Nonsteroid anti-inflammatory compounds are therefore preferred for the long-term treatment of DED patients, and CsA has demonstrated positive results in an animal model of DED and in clinical trials.Citation8–Citation10,Citation19–Citation21 Various CsA ophthalmic preparations are currently available for use: 0.05% CsA anionic emulsion (Restasis®, Allergan, Irvine, CA, USA), 0.1% CsA cationic emulsion (Ikervis®, Santen, Evry, France), and hospital compound preparations of CsA (at concentrations up to 2% CsA).Citation22 CsA exerts its anti-inflammatory action via its binding to cyclophilin A and the subsequent inhibition of calcineurin. As a consequence, the nuclear factor of activated T cell (NFAT)-dependent expression of pro-inflammatory interleukins (ILs) (IL2, etc.) is inhibited, and activation of lymphocyte T cells is blocked.Citation23 The efficacy of CsA at modulating calcineurin-dependent pathways (NFAT, etc.)Citation24 is related to the amount of calcineurin present in a specific cell type and consequently to the CsA dose that reaches the target tissues.Citation25
Recent studies have examined the physiopathology of DED and the effect of various anti-inflammatory treatments by studying inflammatory markers in the tear film (TF) and ocular surface tissues. Various disease biomarkers of DED were detected at the proteomic level in the TF and from conjunctival imprints (at transcriptomic and proteomic levels) in DED patients. Very scarce to no information on corneal cells-derived biomarkers is available for patients, and very few from animal models. Markers from the cytokine, chemokine, growth factor, extracellular matrix enzyme, cell adhesion, and chemoattractant family are among the most frequently described in the literatureCitation26–Citation32 (see ). However, the exact role these ocular surface inflammatory markers have in the pathophysiology of DED is not clearly established. In addition, how the inflammatory markers present in the cornea are modulated upon disease and treatment is not well known.
Table 1. List of the inflammatory genes followed in this study.
The goal of this study was to characterize the efficacy of the anti-inflammatory eye drop formulations in a mouse model of severe DEDCitation33,Citation34 and to explore for the first time their effects on the modulation of cornea inflammatory markers.
Materials and methods
Animals
Sixty pigmented C57BL/6N female mice aged 6–7 weeks (Charles River Laboratories, Saint-Germain-Nuelles, France) were used in this study. All animals were treated according to the Directive 2010/63/UE European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific PurposesCitation35 and the Association for Research in Vision and Ophthalmology (ARVO) statement for the Use of Animals in Ophthalmic and Vision Research. The study protocol was approved by the Iris Pharma Internal Ethics Committee.
Controlled environment room and dry eye model experimental procedure
Mice were placed in a controlled environmental room for 10 days (temperature: 20–22°C; relative humidity: 25%; airflow: 15 L/min) and received scopolamine on day 1, 3, 5, 7, and 9 via transdermal patches (0.5 mg/72 h, Scopoderm® TTS, Novartis, Reuil-Malmaison, France) adapted on the basal part of the mouse tails as previously described by Barabino and collaborators.Citation34 The mice were then randomly assigned to five groups (n = 10 per group), including an untreated group (DED control group) in which the mice received no treatment, and four treatment groups: 0.5% loteprednol etabonate (LE) ophthalmic suspension (Lotemax®, Bausch & Lomb, Rochester, NY, USA), 0.05% CsA anionic ophthalmic emulsion (Restasis®, Allergan, Irvine, CA, USA), 0.1% CsA cationic ophthalmic emulsion (Ikervis®, Santen, Evry, France), and a 1% CsA oil solution with medium-chain triglycerides as the oily vehicle. Treated animals received 3 μL of the test item three times a day in both eyes from days 3 to 10. The DED control group did not receive any eye drop treatment. The treatments were randomized, and the group allocation was masked to the technician administering the treatment and to the researcher assessing the outcome of the experiment. Group identification was unmasked at the end of the analysis.
Measurement of tear volume and cornea alterations
Tear volume was measured with the phenol red thread (PRT) test (Zone-Quick, Lacrimedics, Eastsound, WA, USA), as described previously.Citation36 Corneal fluorescein staining (CFS, evaluated using the National Eye Institute (NEI) scheme) was performed before dry eye induction (day 0) and during the experiment at days 3, 6, and 10 as described by Barabino and collaborators.Citation36 Briefly, 0.5 µL of a 0.5% fluorescein sodium solution (Fluoresceine Faure, 0.4 mL unit-dose vials, Novartis Pharma SAS, France) was instilled into the inferior conjunctival sac using a micropipette. The cornea was examined through a biomicroscope by light passing through a cobalt blue filter. The stained area was assessed and graded using the grading system from the NEI/Industry Workshop guideline.Citation37 The system provided a stepwise categorization of the cornea, by dividing it into five sectors, with each one scored on a 0–3 scale, for a total maximum score of 15.
Healthy control animals
Aged-matched (at the end of the 10-day DED experiment; aged 9–10 weeks) healthy pigmented C57BL/6N female mice (n = 10) were used as healthy controls to set the healthy baseline value for the gene expression analysis in the cornea. Tear volume (PRT test) and CFS scores were determined as for the DED mice.
Animal euthanasia and cornea sampling
The healthy untreated control mice and DED mice were euthanized at the end of the experiment by a systemic injection of overdosed pentobarbital, as recommended for euthanasia by the European authorities (French decree no. 2013–118).Citation35 Immediately after euthanasia, the corneas (n = 5 for each DED treatment group, n = 10 for the DED untreated group, and n = 10 for the healthy untreated control group) were collected from right eyes to isolate total RNA.
RNA preparation and quantification
Total RNA were extracted from corneas according to the manufacturer’s protocol using an RNA-XS kit from Macherey-Nagel (Macherey-Nagel, Hoerdt, France). Total RNA yield and integrity were assessed with an Agilent 2100 bioanalyzer (Agilent Technologies, Wilmington, DE, USA). RNAs with RNA integrity number (RIN) greater than 7 were used for analysis.
mRNA quantification of the inflammation-related genes () was performed with the NanoString nCounter® analysis system (NanoString Technologies, Seattle, WA, USA). This technology provided a direct and rapid quantification of the expressed genes (via direct digital counting of the copy numbers of the said mRNA without an amplification step and a wide dynamic range from 1 to >54,000 mRNA copies). This technology provides a sensitive and highly reproducible method for gene expression detection. High-quality total RNA (100 ng) (RIN >7) were used for the quantitative analysis of the genes of interest (n = 34; ) with the nCounter® mouse inflammatory codeset and analysis system from NanoString. mRNA copy numbers were normalized against six housekeeping genes (CLTC, GAPDH, GUSB, HPRT, PGK1, and TUBB5), and the mean copy number per group was determined and used for group comparison. The genes of interest were chosen among those previously identifiedCitation26 as being relevant to DED pathology and are listed in .
Statistical analysis
The statistical analysis (on CFS) was performed using GraphPad Prism 6.0b. The treatment effect was assessed on CFS, which was considered the primary outcome. The treated groups were compared with the DED untreated group. Tukey’s multiple comparisons test was used and the statistical significance was set at a p < 0.05. Results are presented as mean ± SD. The relative standard deviation (RSD) was used as a measure of the dispersion of the data around the mean.
Gene expression data were log-transformed and an unpaired t-test with unequal variance was then used to assess the significance (<0.05) in the fold changes between the DED-untreated and DED-treated mice, and a Spearman correlation test was used to identify the relationships between gene expression and CFS scores.
Results
Effect of anti-inflammatory eye drops on CFS scores
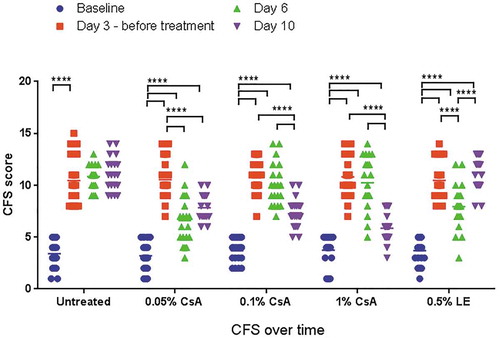
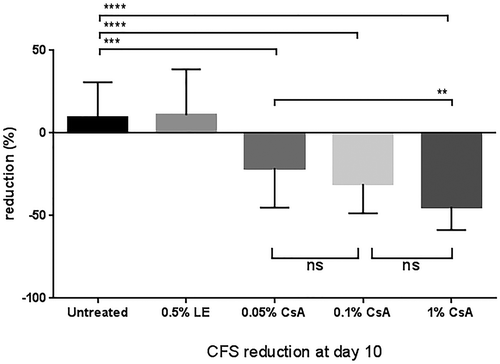
At day 3 after placement of the mice in the dry environment, the CFS score increased markedly from baseline (normal healthy state), ranging between 10.4 ± 2.2 and 10.8 ± 1.7 for the different assigned treatment groups and the untreated control (). Tear production, as measured by PRT, was reduced to a mean value per group lower than 2.3 ± 0.5 mm in all groups. After initiation of the treatment, the PRT values remained low (as a consequence of the scopolamine patches) throughout the experiment until the end of the experiment at day 10 (data not shown). The CFS score remained elevated in the untreated DED group animals, with a slight worsening from day 3 (DED baseline) to day 10: 10.4 ± 2.2 to 11.1 ± 1.6. By contrast, the different CsA eye drop treatments improved the CFS score (p < 0.0001), reaching 7.8 ± 1.3, 7.3 ± 1.4, and 5.9 ± 1.3 at day 10 for 0.05% CsA, 0.1% CsA, and 1% CsA oil solution, respectively (). Surprisingly, while the 0.5% LE treatment reduced the CFS score at day 6 (p < 0.0001), this improvement was not observed at day 10. Compared to the CFS score of the untreated animals at day 10 (), the CFS scores were reduced (from day 3, DED baseline) by −21.1 ± 24.0% (p < 0.001), −30.7 ± 17.8% (p < 0.0001), and −44.6 ± 14.1% (p < 0.0001) with 0.05% CsA, 0.1% CsA, and the 1% CsA oil solution, respectively. A statistically significant difference (p < 0.01) in CFS scores at day 10 was observed between the 0.05% CsA and the 1% CsA formulations. By contrast, the CSF scores of the untreated animals and those treated with 0.5% LE worsened by +9.4±21.4% and +10.9 ± 27.7%, respectively, between day 3 and day 10.
Differential modulation of inflammation-related genes in the cornea of DED animals receiving anti-inflammatory treatments
Among the 34 genes followed (), 11 were not detected in both healthy and DED mouse corneas after 10 days of induction (CCL3, CCL4, CXCL9, H2-Ea-ps, IFNA1, IFNB1, IFNG, IL17A, MMP9, TLR6, and TLR9). For the remaining 23 genes, the expression levels (i.e., the mRNA copy numbers) as means ± SD and the RSD (expressed in %) are presented in . To follow the effects of the treatments on the DED state, the fold changes vs. untreated DED with the t-test values are presented in . These 23 genes are classified in three groups according to their level of expression in the healthy animals’ corneas (low (n = 8), medium (n = 12), and high (n = 3)).
Table 2. Mean (±SD) expression levels of the 23 genes detected among the 34 genes followed in this study in the healthy and treated DED mice corneas.
Table 3. Fold changes and t-test values (vs. DED untreated) for the 23 genes detected among the 34 genes followed in this study in the DED (± treatment) mice corneas. Fold changes calculated with real expression values. The t-test was performed on log10-transformed values of the raw data (not parametric). Note, to estimate a fold change for the gene not detected (ND, counts = 0), a copy number was arbitrarily set to one (1) for calculation purpose.
Genes with low expression levels in the cornea
The low expression levels of these genes rendered the interpretation of the data difficult, as these levels were close to the background counts for the nCounter® assay kits. The RSD showed that the dispersion and the level of expression variability within a group was quite high; however, it can be noted that placing the mice in the DED environment tended to increase the expression of CCL20, CXCL10, IL6, IL1B, and IL1RN. Interestingly, CsA treatments seemed to reverse this upregulation in a dose–response manner. To estimate a fold change for the genes that are not detected (copy number = 0; ND in ), the copy numbers for the ND genes were arbitrarily set to one (1) for fold change calculation. Even if the fold changes (vs. untreated DED) upon treatment were quite substantial, only a few reached significance in the 0.1% CsA and 1% CsA groups, and none in the 0.05% CsA group: IL6 (p = 0.026), IL1B (p = 0.005), and IL1RN (p < 0.001) in the 0.1% CsA group and IL6 (p = 0.026) and IL1B (p = 0.009) in the 1%CsA group. The soft steroid (0.5% LE) only reduced the expression of CCL20, CXCL10, IL6, and IL1B but not significantly ().
Genes with medium and high expression levels in the cornea
A common trend in each group () was that with higher expression levels the variability in expression tend to be lower, with RSDs, as low as 8% for TGFB1 (0.1% CsA group), IL1RAP (1% CsA group) and IL6RA (0.5% LE group), 9% for TGFBR1 (untreated DED group) and 10% for TLR4 (untreated DED group), IL6RA, and TLR4 (0.05% CsA group), and TGFB1 (1% CsA group).
Among the 15 genes with medium-to-high expression levels, TNF, IL1R1, and IL1A were upregulated by placing the mice in the DED environment. With treatments, not only were these genes downregulated to return to the healthy level, but most of the others were also downregulated (vs. DED untreated) (). In the 0.05% CsA group, three genes were significantly downregulated: IL1R1 (p = 0.001), IL1A (p = 0.019), and TLR4 (p = 0.013). Only IL6RA (p = 0.001) and TGFB1 (p = 0.005) were upregulated. In the 0.1% CsA group, 7 out of the 13 downregulated genes significantly decreased expression levels: TLR3 (p = 0.026), TLR2 (p < 0.001), TLR4 (p = 0.009), H2-Eb1 (p < 0.001), IL1A (p = 0.049), TGFB3 (p = 0.010), and TGFB2 (p < 0.001). By contrast, TGFB1 (p < 0.001) was the only gene to be significantly upregulated. In the 1% CsA group, among the 10 downregulated genes, TGFB3 (p = 0.008) and TLR4 (p = 0.014) were significantly downregulated. TGFB1 was the only gene to be significantly upregulated (p = 0.038). In the 0.5% LE group, only H2-Eb1 (p = 0.024) was significantly downregulated among the eight genes downregulated by this treatment. TGFB1 (p < 0.001) and IL6RA (p < 0.001) were the only genes to be significantly upregulated by 0.5% LE treatment.
Correlation of gene expression levels with CFS scores
Spearman’s rank correlation coefficient (rho, ρ) was used to assess the relationship between CFS scores and gene expression levels. The untreated DED and DED with treatment groups (n = 30 animals) were used in this analysis. Seven genes were identified to have low-to-high positive correlations ranging from ρ = 0.267 to ρ = 0.547 (): TGFB2, IL6, TLR5, IL1R1, TGFB3, IL1RAP, and TLR4. A medium-to-high correlation was observed for TGFB3 (ρ = 0.406), IL1RAP (ρ = 0.440), and TLR4 (ρ = 0.547). Only five low-to-very low negative correlations were observed; the strongest negative correlation was for TLR8 (ρ = −0.225).
Table 4. Spearman correlation between CFS scores and gene expression levels for the untreated DED and DED with treatment groups for a total of 30 animals.
Discussion
We used a mouse model of severe DEDCitation34 to explore the relations between inflammatory markers within the cornea and CFS scores in DED animals and how the treatment with anti-inflammatory eye drop medicinal products impacts both CFS and the level of expression in the cornea of a set of inflammatory markers () generally found to be present on DED patients’ ocular surface.Citation26–Citation32 The different anti-inflammatory treatments were able to decrease CFS scores at day 6 and day 10 for the three CsA-containing eye drops. Interestingly, at day 10, 0.5% LE did not maintain the reduction observed at day 6. The reason for this lack of CFS reduction between days 6 and 10 is not clear. However, using the same model 1% methylprednisolone treatment decreased CFS by 6.2% at day 10; and no improvement in CFS decrease was observed from day 6 to day 10 either.Citation19 It was, therefore, interesting to explore the effect of these treatments on the modulation of the inflammatory markers in the cornea (). Inflammatory markers (n = 34) were chosen to be followed in this study, based on literature data and their relevance in DED pathology. For this purpose, we used NanoString’s technology that directly counts the number of mRNA copies of a target gene without needing an amplification step. This technology uses color-coded probes highly specific to a specified mRNA sequence that can directly count the number of target mRNA copies present in the total mRNA extract purified from the tissue. Whole corneas, with the different cell layers (epithelium, stroma, and endothelium), were used for total mRNA preparation. Hence, genes expressed by both corneal cells and immune cells infiltrating the different layers of the cornea can be detected. Since the dynamic range of detection for this technique is very large, it is possible to detect mRNA with very low-to-very high copy numbers. Among the 34 genes of interest (), 23 were readily detected in both healthy and DED mice cornea treated with the anti-inflammatory eye drops. Interestingly, the Th17-specific gene IL17A was not detected in the cornea of healthy and DED animals, even though IL17 has been implicated in DED physiopathology,Citation31,Citation38–Citation40 suggesting that the number of Th17 cells infiltrating the cornea is very low, or that IL17 is mainly produced by Th17 cells present in other ocular tissues, such as the conjunctiva.Citation41,Citation42 It is also possible that increased expression of IL17A takes place very early in the inflammatory cascade of DED development in the mouse, and was not detectable anymore at day 10.Citation43 Surprisingly, MMP9 was not detected in DED mouse cornea, even though cornea alterations were clearly visible in these mice.Citation44,Citation45 This needs to be further confirmed by qRT-PCR, even though a good correlation was demonstrated between NanoString and qRT-PCR gene expression data.Citation46 MMP9 protein was detected in the tears of DED patients. It is possible that the matrix metalloproteinase responsible for the corneal epithelial defects originates mainly from other ocular surface tissues. This needs to be explored further. Among the genes detected, 3, 10, and 4 were significantly downregulated by 0.05% CsA, 0.1% CsA, and 1% CsA eye drops, respectively (). While no statistically significant difference was observed for CFS score reduction between the 0.05% CsA and 0.1% CsA formulations and between the 0.1% CsA and 1% CsA formulations at day 10 (), it seems that there was a difference in the number of inflammatory genes downregulated upon CsA treatments. This difference may be the result of both the difference in CsA concentration found between the three formulations, and the difference in their vehicle composition. Indeed, the 0.1% CsA formulation also contains excipients, which by themselves can have a direct effect on the gene modulated by this formulation, hence explaining the difference in gene patterns among the three CsA formulations.Citation47–Citation49 Only two genes were simultaneously significantly modulated by the three CsA treatments: TLR4 was downregulated, whereas TGFB1 was upregulated. It is possible that the other genes modulated by the different CsA formulation results from the interactions of the excipients, such as the surfactants present in both emulsions, with corneal cells. TGFB1 was the only gene to be significantly upregulated in all treatment groups. Interestingly, TGFB1 was also similarly significantly modulated by 0.5% LE, even though the CFS reduction was not observed at day 10 in this treatment group. TLR4 was the only gene to be significantly downregulated in all groups with a CFS improvement at day 10 (i.e., in the CsA treatment groups), suggesting an important role for TLR4 in the management of DED and cornea alterations in the mouse.
Indeed, the exploration of the relationship (Spearman’s rank correlation) between CFS scores and gene expression in the cornea indicated that TLR4 was the gene with the highest positive correlation (ρ = 0.547) among the 23 genes followed; only seven genes had a positive correlation (ρ > 0.250) with CFS: TGFB2, IL6, TLR5, IL1R1, TGFB3, IL1RAP, and TLR4 ().
This positive ρ correlation suggests that TLR4 can be implicated in corneal epithelium alteration severityCitation50 (S100A8 and S100A9, identified endogenous TLR4 protein ligands, were demonstrated to be associated with DED severityCitation27), inflammation,Citation51 and the healing process.Citation52 In the present study, we found that DED did not significantly increase TLR4 gene expression in the cornea, but treatments that improved CFS resulted in downregulation of the TLR4 gene. The TLR4 protein is primarily expressed intracellularly in normal cornea, and DED increased its cell surface localization through its translocation. Interestingly, CsA treatment decreased the amount of available TLR4.
TGFB3 and IL1RAP also had ρ-values higher than 0.4, suggesting a link between CFS and their modulation. The TGFB3 gene correlated quite well with CFS (ρ = 0.406), while surprisingly TGFB1 did not, even though it was significantly modulated by the different treatments. TGF-β1, -β2, and -β3 are the three protein isoforms that have been identified in mammals. All three protein isoforms display overlapping and distinct spatial and temporal patterns of expression, and each isoform plays a distinct role. The TGF-β1protein was described as an anti-inflammatory factor,Citation26,Citation53 and TGFB1 gene overexpression upon anti-inflammatory treatments might explain the improvement in DED mice’s CFS condition. However, the TGF-β1 protein has also been implicated in T-helper cell differentiationCitation54 and the production of pro-inflammatory IL17 interleukin, complicating the identification of its role in the CFS reduction observed. The TGF-β3 protein was described to play a pro-inflammatory role,Citation55 but, like the TGF-β1 protein, the TGFB3 gene product was also identified as a bi-functional modulator of the immune system. Thus, the concomitant reduction in TGFB3 and increase in TGFB1 gene expression might support the improvement seen in CFS scores upon treatment. However, these two factors also need to be evaluated at the protein level, as the mRNA levels do not necessarily correlate with the protein distribution.
Other genes than TLR4 and the TGFB family were significantly downregulated in the 0.1% CsA group when compared to the 0.05% CsA group (IL1B, IL1RN, TLR2, TLR3, H2-Eb1; ), but the benefit of their modulation did not appear to have a significant effect on CFS improvement at day 10. The IL1 protein pathway seems to be downregulated in both the 0.05% and 0.1% CsA formulation (with the downregulation of IL1R1, IL1RAP, and IL1A in both groups), but only IL1R1 and IL1RAP had a medium correlation with CFS (), suggesting that the modulation of this pathway in the cornea, even though IL1B gene expression remained low in the DED condition (), was implicated in managing CFS. The IL1β protein has been described to be released from stressed ocular surface epithelium and cause corneal epithelium damage.Citation26 IL6 gene expression also appeared to correlate moderately with CFS, but its low level of expression (in the cornea at day 10) might not suggest a pivotal role of IL6 overexpression in the worsening of CFS, although the IL6 gene was described to be implicated in corneal inflammationCitation56 and corneal erosion.Citation57 The levels of expression of IL1B, IL1RN, TLR1, TLR2, and TLR3 were quite low in untreated DED mice cornea, suggesting that their impact on corneal epithelium damage might be limited. The TNF gene, which had higher expression in DED mice cornea () and was downregulated (although not significantly) in the 0.1% and 1% CsA treatment groups as well as in the 0.5% LE group, did not significantly correlate with the CFS score. This lack of correlation was unexpected, as the TNF protein is more likely to have a causative role in CFS worsening.Citation58
While all CsA treatments showed efficacy on CFS, the expression profiles of the inflammatory genes followed in this study tend to indicate that there were some differences in how the CsA-based formulations modulate these inflammatory genes. These differences might result from the difference in CsA concentration found in the three eye drops and from the difference in their vehicle composition too. This might also suggest that the actors within a pathway can be modulated differently by different CsA eye drop treatments. Different pathways could also be modulated in the different treatment groups to reach the same clinical efficacy at the CFS level.
This study suggests that the modulation of TLR4, TGF-β1, IL1, and IL6 pathways in the cornea might be implicated in the clinical improvement seen for CFS in this mouse model. However, it cannot be excluded that other genes, with lower levels of expression or with modulations that did not reach significance in this study, also play a role in improving the corneal epithelium. One limitation of this study was that only a small number of animals per group were analyzed, with sometimes important variation in the level of expression; hence, particularly for the genes with the lowest levels of expression, this number of animals may be too low. On the other hand, the NanoString gene expression data will have to be confirmed by qRT-PCR and by a proteomic analysis. Some additional studies are needed to better understand the role of the modulation of the inflammatory genes followed in the present study and assess the direct contribution of the different genes in such a complex multifactorial disease as DED.
Disclosure statement
The authors wish to acknowledge the following financial interests:
P.D.: Employee – Santen SAS
S.B.: Consultant – Santen Pharmaceutical Co., Ltd;
L.F.: Employee – Iris Pharma
C.B.: Consultant – Santen Pharmaceutical Co., Ltd;
J-S.G., Employee – Santen SAS
K.K., M.D., S.M.P.: none
Additional information
Funding
References
- Wei Y, Asbell PA. The core mechanism of dry eye disease is inflammation. Eye & Contact Lens. 2014;40(4):248–56. doi:10.1097/ICL.0000000000000042.
- Baudouin C, Irkec M, Messmer EM, Benitez-Del-Castillo JM, Bonini S, Figueiredo FC, Geerling G, Labetoulle M, Lemp M, Rolando M, et al. Clinical impact of inflammation in dry eye disease: proceedings of the Odyssey group meeting. Acta Ophthalmol. 2018;96(2):111–19. doi:10.1111/aos.13436.
- Ambroziak AM, Szaflik J, Szaflik JP, Ambroziak M, Witkiewicz J, Skopinski P. Immunomodulation on the ocular surface: a review. Cent Eur J Immunol. 2016;41(2):195–208. doi:10.5114/ceji.2016.60995.
- Calonge M, Enriquez-de-Salamanca A, Diebold Y, Gonzalez-Garcia MJ, Reinoso R, Herreras JM, Corell A. Dry eye disease as an inflammatory disorder. Ocul Immunol Inflamm. 2010;18(4):244–53. doi:10.3109/09273941003721926.
- Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo CK, Liu Z, Nelson JD, Nichols JJ, Tsubota K, et al. TFOS dews ii definition and classification report. Ocul Surf. 2017;15(3):276–83. doi:10.1016/j.jtos.2017.05.008.
- Pavesio CE, Decory HH. Treatment of ocular inflammatory conditions with loteprednol etabonate. Br J Ophthalmol. 2008;92(4):455–59. doi:10.1136/bjo.2007.132621.
- Sy A, O’Brien KS, Liu MP, Cuddapah PA, Acharya NR, Lietman TM, Rose-Nussbaumer J. Expert opinion in the management of aqueous deficient dry eye disease (DED). BMC Ophthalmol. 2015;15:133. doi:10.1186/s12886-015-0122-z.
- Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CSA phase 3 study group. Ophthalmology. 2000;107:631–39.
- Leonardi A, Van Setten G, Amrane M, Ismail D, Garrigue J-S, Figueiredo FC, Baudouin C. Efficacy and safety of 0.1% cyclosporine a cationic emulsion in the treatment of severe dry eye disease: a multicenter randomized trial. Eur J Ophthalmol. 2016;26(4):287–96. doi:10.5301/ejo.5000779.
- Baudouin C, Figueiredo FC, Messmer EM, Ismail D, Amrane M, Garrigue JS, Bonini S, Leonardi A. A randomized study of the efficacy and safety of 0.1% cyclosporine a cationic emulsion in treatment of moderate to severe dry eye. Eur J Ophthalmol. 2017;27(5):520–30. doi:10.5301/EJO.5000952.
- Perez VL, Pflugfelder SC, Zhang S, Shojaei A, Haque R. Lifitegrast, a novel integrin antagonist for treatment of dry eye disease. Ocul Surf. 2016;14(2):207–15. doi:10.1016/j.jtos.2016.01.001.
- Holland EJ, Luchs J, Karpecki PM, Nichols KK, Jackson MA, Sall K, Tauber J, Roy M, Raychaudhuri A, Shojaei A. Lifitegrast for the treatment of dry eye disease: results of a phase iii, randomized, double-masked, placebo-controlled trial (OPUS-3). Ophthalmology. 2017;124(1):53–60. doi:10.1016/j.ophtha.2016.09.025.
- Godin M, Gupta P. Lifitegrast ophthalmic solution in the treatment of signs and symptoms of dry eye disease: design, development, and place in therapy. Clin Ophthalmol. 2017;11:951–57. doi:10.2147/OPTH.S117188.
- Hubner S, Dejager L, Libert C, Tuckermann JP. The glucocorticoid receptor in inflammatory processes: transrepression is not enough. Biol Chem. 2015;396(11):1223–31. doi:10.1515/hsz-2015-0106.
- Cain DW, Cidlowski JA. Immune regulation by glucocorticoids. Nat Rev Immunol. 2017;17(4):233–47. doi:10.1038/nri.2017.1.
- Kersey JP, Broadway DC. Corticosteroid-induced glaucoma: a review of the literature. Eye. 2006;20(4):407–16. doi:10.1038/sj.eye.6701895.
- Pleyer U, Ursell PG, Rama P. Intraocular pressure effects of common topical steroids for post-cataract inflammation: are they all the same? Ophthalmol Ther. 2013;2(2):55–72. doi:10.1007/s40123-013-0020-5.
- Jung HH, Ji YS, Sung MS, Kim KK, Yoon KC. Long-term outcome of treatment with topical corticosteroids for severe dry eye associated with Sjogren’s syndrome. Chonnam Med J. 2015;51(1):26–32. doi:10.4068/cmj.2015.51.1.26.
- Daull P, Feraille L, Barabino S, Cimbolini N, Antonelli S, Mauro V, Garrigue JS. Efficacy of a new topical cationic emulsion of cyclosporine A on dry eye clinical signs in an experimental mouse model of dry eye. Exp Eye Res. 2016;153:159–64. doi:10.1016/j.exer.2016.10.016.
- Brignole-Baudouin F, Riancho L, Ismail D, Deniaud M, Amrane M, Baudouin C. Correlation between the inflammatory marker HLA-DR and signs and symptoms in moderate to severe dry eye disease HLA-DR quantification and DED characteristics. Invest Ophthalmol Vis Sci. 2017;58(4):2438–48. doi:10.1167/iovs.15-16555.
- Strong B, Farley W, Stern ME, Pflugfelder SC. Topical cyclosporine inhibits conjunctival epithelial apoptosis in experimental murine keratoconjunctivitis sicca. Cornea. 2005;24:80–85.
- Chast F, Lemare F, Legeais JM, Batista R, Bardin C, Renard G. [cyclosporine 2% eye drops preparation]. J Fr Ophtalmol. 2004;27:567–76.
- Donnenfeld E, Pflugfelder SC. Topical ophthalmic cyclosporine: pharmacology and clinical uses. Surv Ophthalmol. 2009;54(3):321–38. doi:10.1016/j.survophthal.2009.02.002.
- Klee CB, Ren H, Wang X. Regulation of the calmodulin-stimulated protein phosphatase, calcineurin. J Biol Chem. 1998;273:13367–70.
- Crabtree GR, Olson EN. NFAT signaling: choreographing the social lives of cells. Cell. 2002;109:S67–79.
- Barabino S, Chen Y, Chauhan S, Dana R. Ocular surface immunity: homeostatic mechanisms and their disruption in dry eye disease. Prog Retin Eye Res. 2012;31(3):271–85. doi:10.1016/j.preteyeres.2012.02.003.
- Zhou L, Beuerman RW, Chan CM, Zhao SZ, Li XR, Yang H, Tong L, Liu S, Stern ME, Tan D. Identification of tear fluid biomarkers in dry eye syndrome using iTRAQ quantitative proteomics. J Proteome Res. 2009;8(11):4889–905. doi:10.1021/pr900686s.
- Zhou L, Beuerman RW. Tear analysis in ocular surface diseases. Prog Retin Eye Res. 2012;31(6):527–50. doi:10.1016/j.preteyeres.2012.06.002.
- Soria J, Acera A, Duran JA, Boto-de-Los-Bueis A, Del-Hierro-Zarzuelo A, Gonzalez N, Reigada R, Suarez T. The analysis of human conjunctival epithelium proteome in ocular surface diseases using impression cytology and 2D-DIGE. Exp Eye Res. 2018;167:31–43. doi:10.1016/j.exer.2017.03.006.
- Perumal N, Funke S, Pfeiffer N, Grus FH. Proteomics analysis of human tears from aqueous-deficient and evaporative dry eye patients. Sci Rep. 2016;6:29629. doi:10.1038/srep29629.
- Chen Y, Chauhan SK, Shao C, Omoto M, Inomata T, Dana R. IFN-gamma-expressing th17 cells are required for development of severe ocular surface autoimmunity. J Immunol. 2017;199(3):1163–69. doi:10.4049/jimmunol.1602144.
- Okanobo A, Chauhan SK, Dastjerdi MH, Kodati S, Dana R. Efficacy of topical blockade of interleukin-1 in experimental dry eye disease. Am J Ophthalmol. 2012;154(1):63–71. doi:10.1016/j.ajo.2012.01.034.
- Dursun D, Wang M, Monroy D, Li DQ, Lokeshwar BL, Stern ME, Pflugfelder SC. A mouse model of keratoconjunctivitis sicca. Invest Ophthalmol Vis Sci. 2002;43:632–38.
- Barabino S, Shen L, Chen L, Rashid S, Rolando M, Dana MR. The controlled-environment chamber: a new mouse model of dry eye. Invest Ophthalmol Vis Sci. 2005;46(8):2766–71. doi:10.1167/iovs.04-1326.
- French decree n° 2013-118, dated February 01, 2013 publishing the European directive 2010/63/ue. J. Offic. Rep. Fr. 2013; text 24 out of 130. 2013.
- Barabino S, Antonelli S, Cimbolini N, Mauro V, Bouzin M. The effect of preservatives and antiglaucoma treatments on the ocular surface of mice with dry eye. Invest Ophthalmol Vis Sci. 2014;55(10):6499–504. doi:10.1167/iovs.14-14548.
- Lemp MA. Report of the national eye institute/industry workshop on clinical trials in dry eyes. Clao J. 1995;21:221–32.
- Zheng X, de Paiva CS, Li DQ, Farley WJ, Pflugfelder SC. Desiccating stress promotion of th17 differentiation by ocular surface tissues through a dendritic cell-mediated pathway. Invest Ophthalmol Vis Sci. 2010;51(6):3083–91. doi:10.1167/iovs.09-3838.
- Kodati S, Chauhan SK, Chen Y, Dohlman TH, Karimian P, Saban D, Dana R. Ccr7 is critical for the induction and maintenance of th17 immunity in dry eye disease. Invest Ophthalmol Vis Sci. 2014;55(9):5871–77. doi:10.1167/iovs.14-14481.
- Chen Y, Chauhan SK, Lee HS, Saban DR, Dana R. Chronic dry eye disease is principally mediated by effector memory th17 cells. Mucosal Immunol. 2014;7(1):38–45. doi:10.1038/mi.2013.20.
- De Paiva CS, Chotikavanich S, Pangelinan SB, Pitcher JD 3rd, Fang B, Zheng X, Ma P, Farley WJ, Siemasko KF, Niederkorn JY, et al. Il-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol. 2009;2(3):243–53. doi:10.1038/mi.2009.5.
- Liu R, Gao C, Chen H, Li Y, Jin Y, Qi H. Analysis of th17-associated cytokines and clinical correlations in patients with dry eye disease. PLoS One. 2017;12(4):e0173301. doi:10.1371/journal.pone.0173301.
- Stern ME, Schaumburg CS, Dana R, Calonge M, Niederkorn JY, Pflugfelder SC. Autoimmunity at the ocular surface: pathogenesis and regulation. Mucosal Immunol. 2010;3(5):425–42. doi:10.1038/mi.2010.26.
- De Paiva CS, Pangelinan SB, Chang E, Yoon KC, Farley WJ, Li DQ, Pflugfelder SC. Essential role for c-jun n-terminal kinase 2 in corneal epithelial response to desiccating stress. Arch Ophthalmol. 2009;127(12):1625–31. doi:10.1001/archophthalmol.2009.316.
- Chotikavanich S, de Paiva CS, Li de Q, Jj C, Bian F, Wj F, Pflugfelder SC. Production and activity of matrix metalloproteinase-9 on the ocular surface increase in dysfunctional tear syndrome. Invest Ophthalmol Vis Sci. 2009;50(7):3203–09. doi:10.1167/iovs.08-2476.
- Hyeon J, Cho SY, Hong ME, Kang SY, Do I, Im YH, Cho EY. Nanostring ncounter(r) approach in breast cancer: a comparative analysis with quantitative real-time polymerase chain reaction, in situ hybridization, and immunohistochemistry. J Breast Cancer. 2017;20(3):286–96. doi:10.4048/jbc.2017.20.3.286.
- Thomassen MJ, Antal JM, Divis LT, Wiedemann HP. Regulation of human alveolar macrophage inflammatory cytokines by tyloxapol: a component of the synthetic surfactant exosurf. Clin Immunol Immunopathol. 1995;77:201–05.
- Ghio AJ, Marshall BC, Diaz JL, Hasegawa T, Samuelson W, Povia D, Kennedy TP, Piantodosi CA. Tyloxapol inhibits NF-kappa b and cytokine release, scavenges HOCI, and reduces viscosity of cystic fibrosis sputum. Am J Respir Crit Care Med. 1996;154(3 Pt 1):783–88. doi:10.1164/ajrccm.154.3.8810619.
- Daull P, Guenin S, Hamon de Almeida V, Garrigue JS. Anti-inflammatory activity of CKC-containing cationic emulsion eye drop vehicles. Mol Vis. 2018;24:459–70.
- Lee HS, Hattori T, Park EY, Stevenson W, Chauhan SK, Dana R. Expression of toll-like receptor 4 contributes to corneal inflammation in experimental dry eye disease. Invest Ophthalmol Vis Sci. 2012;53(9):5632–40. doi:10.1167/iovs.12-9547.
- Redfern RL, Barabino S, Baxter J, Lema C, McDermott AM. Dry eye modulates the expression of toll-like receptors on the ocular surface. Exp Eye Res. 2015;134:80–89. doi:10.1016/j.exer.2015.03.018.
- Eslani M, Movahedan A, Afsharkhamseh N, Sroussi H, Djalilian AR. The role of toll-like receptor 4 in corneal epithelial wound healing. Invest Ophthalmol Vis Sci. 2014;55(9):6108–15. doi:10.1167/iovs.14-14736.
- Shen L, Barabino S, Taylor AW, Dana MR. Effect of the ocular microenvironment in regulating corneal dendritic cell maturation. Arch Ophthalmol. 2007;125(7):908–15. doi:10.1001/archopht.125.7.908.
- Veldhoen M, Stockinger B. Tgfbeta1, a “jack of all trades”: the link with pro-inflammatory il-17-producing t cells. Trends Immunol. 2006;27(8):358–61. doi:10.1016/j.it.2006.06.001.
- Okamura T, Morita K, Iwasaki Y, Inoue M, Komai T, Fujio K, Yamamoto K. Role of tgf-beta3 in the regulation of immune responses. Clin Exp Rheumatol. 2015;33:S63–69.
- Sugaya S, Sakimoto T, Shoji J, Sawa M. Regulation of soluble interleukin-6 (il-6) receptor release from corneal epithelial cells and its role in the ocular surface. Jpn J Ophthalmol. 2011;55(3):277–82. doi:10.1007/s10384-011-0002-x.
- Kucherenko AM, Pampukha VM, Drozhzhyna GI, Livshits LA. Il1beta, il6 and il8 gene polymorphisms involvement in recurrent corneal erosion in patients with hereditary stromal corneal dystrophies. Tsitol Genet. 2013;47:42–45.
- Choi W, Noh H, Yeo A, Jang H, Ahn HK, Song YJ, Lee HK. The effect of TNF-α blocker hl036337 and its best concentration to inhibit dry eye inflammation. Korean J Ophthalmol. 2016;30(4):302–08. doi:10.3341/kjo.2016.30.4.302.