ABSTRACT
Corneal pathologies are a major cause of blindness and visual impairment, especially in the developing world. However, not only is there a global shortage of donor corneal tissue, but a significant proportion of these blinding pathologies also carry an unfavourable long-term prognosis for conventional corneal transplantation. In the last few decades, there has been a spurt of research on developing alternate approaches to address corneal blindness, including stem cell therapy. After the discovery of epithelial stem cells at the limbus, successful cell-based approaches to treat severe ocular surface disease were developed and have subsequently become widely practised across the world. More recently, mesenchymal stem cells were identified near the epithelial stem cells at the limbus, providing a unique opportunity to develop regenerative therapies for both corneal epithelial and stromal pathologies. This review firstly emphasises on qualifying limbal stem cells as either epithelial or mesenchymal and then summarises all the existing knowledge on both cell types and their individual roles in corneal regeneration. The review describes the history, indications, techniques, and outcomes of the different methods of limbal epithelial stem cell transplantation and elaborates on the potential applications of limbal mesenchymal stem cell therapy.
Introduction
The cornea is a very delicate part of the anterior eye that focusses incident light onto the light-sensitive retina by acting as a transparent refractive lens (). Since it is exposed to the external environment, the cornea is constantly at risk of getting damaged. Although there are redundant layers of physical and biological defences to prevent such damage, even small changes in the clarity, smoothness or shape of the cornea can adversely impact its optical functions and result in visual symptoms. Corneal diseases are an important cause of visual impairment and blindness that affect millions worldwide, particularly in the developing world.Citation1
Figure 1. Normal Appearance of the Cornea and Limbus. The healthy cornea is optically transparent (a) and surrounded by the annular limbus which separates it from the opaque sclera. The limbus contains the palisades of Vogt (b) which are finger-like projections of the stroma. The optical coherence tomography (OCT) image of the normal cornea shows a smooth uniform stratified epithelium (c) with underlying compact stroma. The OCT angiography image of the normal limbus shows linear anastomosing hair-pin loops of the limbal capillaries (d) with adjacent avascular corneal stroma.
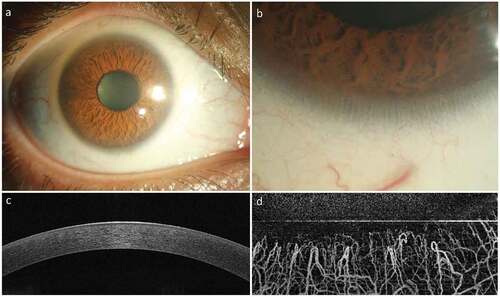
The corneal surface is especially delicate and consists of tightly packed stratified squamous epithelium arranged in a regular fashion ()). Underneath the epithelium is the corneal stroma made of regularly arranged collagen lamellae, interspersed by proteoglycans and scantily populated by mesenchymal cells called keratocytes. The epithelial surface has an extremely high turnover (4–7 days) as mature superficial cells exposed to the environment are continuously shed and replaced instantly by younger cells. The corneal stroma, on the other hand, being relatively acellular has a very slow turnover rate.Citation2 Although their symptoms may overlap, corneal epithelial and stromal diseases have traditionally been considered to have distinct pathophysiology. The burden of visual impairment and blindness is also shared unequally by these two entities, stromal opacification being by far the more common cause. In the last three decades much attention has been paid to the limbus (), the annular transition zone between the clear cornea and the opaque sclera, and its role in maintaining corneal homeostasis. Two distinct populations of adult stem cells, epithelial and mesenchymal, have been discovered at the limbus and their role in corneal regeneration has been extensively studied. This review discusses the known role and future potential of limbal epithelial and mesenchymal stem cells in corneal regeneration and in addressing the problem of blindness and visual impairment due to corneal diseases.
Limbal epithelial stem cells
The mechanisms of corneal epithelial renewal or the reasons for its dysfunction, termed ocular surface disease, were not well understood until about three decades ago. The concept that stem cells could be present peripherally in the limbal palisades of Vogt ()) was first suggested by Davanger and Evensen in 1971.Citation3 It was becoming clear at that time that corneal transplantation alone did not work in eyes with severe ocular surface disease and Thoft proposed peri-limbal conjunctival transplantation as an alternative.Citation4,Citation5 Conjunctival transplantation actually showed better results than conventional penetrating keratoplasty (PK) in a series of eyes with chemical burns.Citation4 At that point in time, it was proposed and widely believed that the corneal surface epithelialized from conjunctival cells.Citation6
However, subsequently, Thoft and colleagues realized that the limbal epithelium was much more similar to the corneal epithelium than to the conjunctival epithelium.Citation7 This prompted them to propagate the x-y-z hypothesis of corneal epithelial homeostasis. This hypothesis proposed that epithelial cells from the limbus/peripheral cornea migrated centripetally and differentiated to replace the more mature cells in the central cornea.Citation8 Finally in 1986, Schermer et al. in a seminal paper demonstrated the presence of putative stem cells in the basal layers of the limbal epithelium which were slow-cycling, label-retaining and were capable of proliferation in vitro. They proposed that these basal epithelial cells of the limbus transform into transient amplifying cells which in turn give rise to terminally differentiated cells of the corneal epithelium.Citation9 Cotsarelis et al. further elaborated that injury to the central cornea leads to rapid proliferation of otherwise slow-cycling limbal basal epithelial cells.Citation10 Thus, the limbus was established as the central dogma of corneal epithelial regeneration, a paradigm that holds true till this day.10 These important discoveries in the late 1980s led to the coinage of the term ‘limbal stem cells’ to denote the corneal epithelial stem cells and the clinical term ‘limbal stem cell deficiency’ to denote severe ocular surface disease due to limbal stem cell failure.
Limbal epithelial stem cell deficiency
Limbal epithelial stem cell deficiency or LSCD occurs because of direct damage to the limbal epithelial stem cells or to their microenvironment, the niche. As a consequence, the corneal epithelium is unable to regenerate or heal and the corneal surface gets gradually replaced with conjunctival epithelium. This process of conjunctivalization is usually accompanied by neovascularization and scarring with eventual loss of corneal clarity and deterioration of vision ()). The compromised cornea in eyes with LSCD is also at risk of developing infections, persistent epithelial defects, and even stromal melting and perforation. The most common method for clinical diagnosis of LSCD is slit-lamp biomicroscopy.Citation11 The clinical features suggestive of LSCD are superficial corneal vascularization, diffuse fluorescein staining of the corneal surface with or without persistent epithelial defects, conjunctivalization of the corneal surface and absence of limbal palisades of Vogt ()).
Figure 2. Clinical Appearance of Limbal Epithelial Stem Cell Deficiency (LSCD). LSCD is characterized clinically by loss of corneal clarity because of superficial neovascularization (a), positive fluorescein staining (b), presence of conjunctival cytokeratin (CK) markers on impression cytology (CK19) and immunohistochemistry (c), altered epithelial cell morphology with sub-basal fibrosis on in vivo confocal microscopy (d), replacement of the dark hyporeflective corneal epithelial phenotype with bright and thick conjunctival phenotype on optical coherence tomography (OCT, E) and loss of normal limbal vascular architecture on OCT angiography (F).
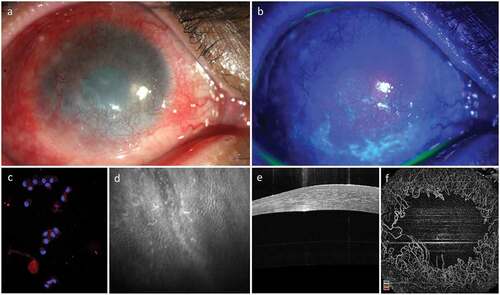
More objective methods that have been used to confirm the clinical diagnosis are impression cytology and immunohistochemistry, in vivo confocal microscopy and anterior segment optical coherence tomography (AS-OCT, ).Citation12 LSCD can be clinically classified as partial or total depending on the amount of surface conjunctivalization of the cornea. Partial LSCD is characterized by incomplete limbal damage with focal conjunctivalization and presence of healthy-looking limbus in other quadrants. Total LSCD is characterized by conjunctivalization of the entire corneal surface and complete loss of the limbal palisades in all quadrants. A staging system for LSCD based on clinical presentation has also been devised recently taking the total surface area of corneal conjunctivalization and the limbal involvement into account.Citation13 Depending on laterality, LSCD can be further classified into unilateral or bilateral. Here, it is important to consider that an affected individual may have a unilateral total/partial LSCD or bilateral LSCD with either total/partial LSCD in both the eyes or total LSCD in one eye and partial LSCD in the other eye.
Causes of LSCD
The aetiologies for LSCD are varied and can be summarized under the broad categories of traumatic, inflammatory, immunological, hereditary, and idiopathic (). Of all the listed aetiologies, ocular burns are the commonest cause of both unilateral and bilateral LSCD.Citation14 Whereas immunological and hereditary diseases usually cause bilateral LSCD, although the presentation may be asymmetrical. There have been few reports on medical management of limited or partial LSCD,Citation15,Citation16 but total LSCD is typically a condition that requires surgical management when visually significant.
Table 1. Aetiological classification of limbal stem cell deficiency.
Limbal epithelial stem cell transplantation (LSCT)
Following the discovery of the limbal epithelial stem cells (LESCs), Tseng and colleagues conducted the first pre-clinical trial in rabbits to show that limbal transplantation was far more effective than conjunctival transplantation in improving corneal clarity in experimentally induced LSCD.Citation17 This paved the way for the “proof-of-concept” human clinical studies and set the ball rolling for an interesting two decades of technical evolution in the surgical approaches to LSCT. Depending on the source of the donor limbal tissue, LSCT can either be autologous (source: healthy fellow eye in unilateral LSCD) or allogeneic (source: healthy eye of one or more living related/unrelated donors or cadaveric donors). The clinical outcomes of LSCT can be classified as anatomical and functional. The anatomical outcome is usually subjectively defined as the regeneration of a stable, epithelialized and avascular corneal surface. But the anatomical outcome can also be objectively defined in terms of restoration of the normal corneal phenotype seen on impression cytology, in vivo confocal microscopy or AS-OCT. On the other side, the functional outcome is more objectively assessed with respect to the improvement in visual acuity.Citation12 Different techniques of limbal epithelial transplantation (e.g. conjunctival-limbal autografting (CLAu), cultivated limbal epithelial transplantation (CLET), simple limbal epithelial transplantation (SLET)), their comparison, limitations, and future potential are described in the following sections.
Conjunctival-limbal autografting (CLAu)
In 1989, Kenyon and Tseng described the original technique of autologous LSCT in a series of cases with acute and chronic unilateral chemical burns.Citation18 Initially called limbal autograft transplantation (LAT), the current universally accepted terminology for this technique is conjunctival-limbal autografting (CLAu). This involves harvesting up to six clock-hours of donor limbal tissue from the healthy contralateral donor eye and transplanting the two limbal lenticules of three clock-hours each to the affected eye.Citation18 The outcomes of this technique are summarized in .Citation18–Citation28 Although CLAu is largely effective in reversing LSCD and establishing a stable corneal surface, there are concerns about the impact of such extensive dissection on the healthy eye as there have been reports of iatrogenic LSCD in the donor areas.Citation29,Citation30 However, Cheung et al. in their recent study dismissed these concerns by demonstrating no evidence of LSCD in 51 donor eyes from which 4 clock-hours (2 segments of 2 clock-hours each) of limbal tissue had earlier been harvested for transplantation.Citation31 Busin et al. also demonstrated that limbal epithelial stem cells repopulated the donor site as early as 1-year post-operatively.Citation32 Interestingly, Kheirkhah et al. showed the restoration of the entire corneal surface in a case of total LSCD with a modified technique of minimal CLAu using only 60 degrees (2 clock-hours) of limbal tissue instead of the usual 4–6 clock-hours.Citation33 This interesting report not only questioned the need for large-sized grafts but also supported the notion that there is some redundancy in the limbal stem cell reserve of a normal eye and that a minimal number of limbal stem cells can sustain the entire corneal surface in the long term.Citation33
Table 2. Clinical outcomes of conjunctival-limbal autografting (CLAu) for unilateral limbal epithelial stem cell deficiency (LSCD).
Cultivated limbal epithelial transplantation (CLET)
In 1997, Pellegrini and associates described autologous CLET, a technique in which a transplantable epithelial sheet is cultivated ex vivo on a fibrin scaffold in the laboratory by using less than one clock-hour of limbal tissue.Citation34 Following this, several groups have published their outcomes of this technique, however the cultivation protocols are quite variable across groups as summarized in .Citation35–Citation48 Broadly there are two techniques of limbal cultivation: a) the suspension culture, where the biopsied limbal tissue is first digested enzymatically to separate the cells from the extracellular matrix and then the cell suspension is placed in culture over a suitable substrate; and b) explant culture where the limbal tissue fragment is sectioned into smaller pieces and placed directly on the substrate without enzymatic digestion. Additionally, the constituents of the culture medium may or may not contain animal-derived products or xenobiotic materials. Xenogeneic constituents of a limbal culture system can be in the form of murine feeder cells, bovine serum, or animal-derived growth factors.Citation49 This is one of the major limitations of CLET because use of xenogeneic material carries some risk of disease transmission due to both known and unknown pathogens.Citation49 The use of xenogeneic materials makes regulatory approvals difficult and when considered along with the costs of establishing and maintaining a clinical grade laboratory, together these factors make CLET quite an expensive procedure.Citation50
Table 3. Techniques and clinical outcomes of autologous-cultivated limbal epithelial transplantation (CLET) for unilateral Limbal Stem Cell Deficiency (LSCD).
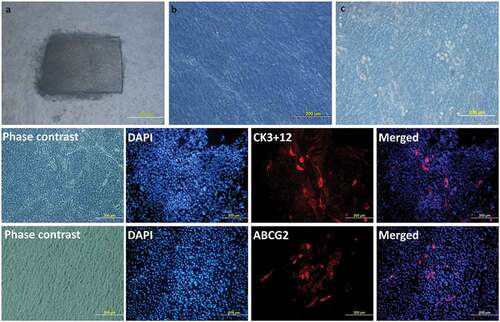
However, Mariappan et al. have developed completely xeno-free protocol for limbal epithelial cell cultivation.Citation51 In this protocol, the donor limbal tissue was transported to the laboratory in human corneal epithelium (HCE) medium and shredded into small pieces under strict aseptic conditions. A human amniotic membrane (hAM) prepared and preserved by the eye bank was used as a carrier. A 3 cm x 4 cm hAM sheet was de-epithelialized using TrypLE® for 30 minutes. The shredded bits of limbal tissue were explanted over the centre of de-epithelized hAM with the basement membrane side-up ()). A submerged explant culture system without a feeder-cell layer was used under standard culture conditions using autologous serum. A confluent monolayer of cells, ready for clinical transplantation, usually formed on the hAM in 10 to 14 days ()).
Figure 3. Limbal Explant Culture on the amniotic membrane: After attaching the explants to the hAM membrane, slow growth can be seen around the explant till the whole membrane is fully covered. Day 2 (a), Day 9 (b), Day 15 (c). Phenotypic marker expression by LESCs. LESCs showing positive expression of the CK3 + 12, their native phenotypic marker and ABCG2, stem cell marker, on day 9 of culture.
Simple limbal epithelial transplantation (SLET)
Sangwan et al., in 2012 described an innovative approach called SLET.Citation52 In this procedure, a one-clock-hour limbal tissue is harvested from the donor eye, cut into multiple small pieces and secured on the recipient eye over an hAM graft.Citation52 This is akin to in vivo cultivation, as the cornea re-epithelializes using the natural environment of the ocular surface instead of laboratory reagents in a petri-dish. Several large studies have subsequently established the long-term safety and efficacy of SLET in the treatment of severe ocular surface disease and LSCD, particularly in chemical burns.Citation53–Citation56 The clinical outcomes of SLET for unilateral LSCD are summarized in .Citation53–Citation56 Since SLET works very differently from CLAu or CLET it may be worthwhile to discuss its mechanism of action. Serial photographic imaging of eyes using fluorescein staining has clearly shown the multi-directional growth of epithelial cells from each transplant within the first few days which gradually coalesced to form a confluent stratified sheet of epithelium on the corneal surface by 10–14 days.Citation57 Amescua et al. used high-resolution AS-OCT to show that this epithelial proliferation occurs on the hAM scaffold and the hAM itself becomes the new basement membrane for the regenerated epithelium ().Citation58 Histopathological and immunohistochemical analysis of excised corneal buttons from eyes undergoing PK after SLET also confirmed that the corneal surface epithelium was of corneal phenotype (CK3+, CK12+, CK19−, MUC5AC−) and also that there was focal retention of stem cells (ABCG2+ and Δp63α+) in the basal epithelial layer of the newly regenerated epithelium.Citation53 A recent study used a combination of impression cytology and in vivo confocal microscopy to show that clinically successful post-SLET cases did indeed demonstrate features suggestive of a normal corneal epithelial phenotype.Citation59
Table 4. Clinical outcomes of autologous simple limbal epithelial transplantation (SLET) for Unilateral Limbal Stem Cell Deficiency (LSCD).
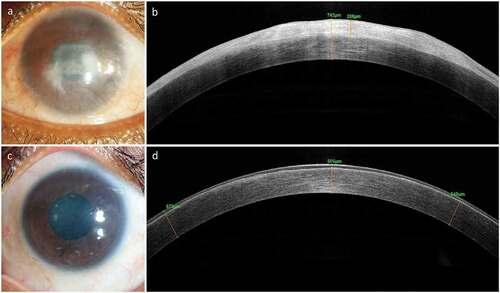
Figure 4. Clinical Outcome of Simple Limbal Epithelial Transplantation (SLET). The top row shows the pre-operative appearance of affected eye with opacification and vascularization of the corneal surface (a) and a thick bright hyperreflective conjunctival epithelial phenotype seen on optical coherence tomography (OCT, b). The same eye one-year post-operative shows remarkably improved corneal clarity, absence of vascularization and opacification (c) and smooth dark hyporeflective corneal epithelial phenotype on OCT imaging (d).
Comparison of CLAu, CLET and SLET in unilateral LSCD
There are no randomised controlled clinical trials that have compared the outcomes of CLAu, CLET and SLET. However, a recent systematic review compared the different techniques of autologous LSCT in patients with unilateral LSCD due to mostly ocular surface burns.Citation60 The review included 22 non-comparative case series comprising 1023 eyes. At 1.75 years post-operatively, autologous LSCT for unilateral LSCD showed overall anatomical and functional success rates of 69% and 60%, respectively. However, the review reported that the anatomical and functional success rates of SLET (78%; 68.6%) and CLAu (81%; 74.4%) were significantly better than those of CLET (61.4%; 53%). The review concluded that although all techniques of autologous LSCT are safe and effective for the treatment of unilateral LSCD, existing evidence clearly indicates that clinical outcomes are better with SLET and CLAu as compared to CLET.Citation60
Allogeneic LSCT for bilateral LSCD
The regenerative techniques that are performed for bilateral LSCD include CLAL (conjunctival-limbal allograft), KLAL (kerato-limbal allograft), allogeneic CLET, and allogeneic SLET. The clinical outcomes of CLAL/KLAL for patients with bilateral LSCD are mentioned in .Citation61–Citation66 The clinical outcomes of allogeneic CLET for bilateral LSCD are mentioned in .Citation37,Citation38,Citation67,Citation68 Unlike autologous LSCT, it is difficult to compare the different techniques of allogeneic LSCT because the indications are diverse and systemic immunosuppression regimens used are not uniform and vary widely across most studies. Like autologous CLET, different groups have used diverse cell cultivation protocols, which is an additional confounder that makes this comparison difficult. A recent review summarized the outcomes of allogeneic SLET for bilateral LSCD in Stevens-Johnson syndrome, mucous membrane pemphigoid, ocular burns and severe chronic allergy in 30 eyes of 29 patients.Citation57 The patients underwent either living-related or cadaveric allogeneic SLET with an identical postoperative immunosuppression regimen. The median follow-up was 28 months (range: 13–66 months) with no significant difference at baseline between the living-related and cadaveric groups. One year post-operatively, the median best-corrected visual acuity had improved from hand-motions to 20/80 and more than 60% of operated eyes had a visual recovery of 20/60 or better, irrespective of the source of donor tissue. Overall allogeneic SLET was successful in 83.3% eyes at final follow-up. Kaplan Meier survival analysis showed statistically similar 5-year cumulative survival probability of 90% in the living related and 82% in the cadaveric donor group.Citation57
Table 5. Clinical outcomes of conjunctival limbal allografts (CLAL)/Keratolimbal allograft (KLAL) for Bilateral Limbal Stem Cell Deficiency (LSCD).
Table 6. Clinical Outcomes of Allogeneic Cultivated Limbal Epithelial Transplantation (CLET) for Bilateral Limbal Stem Cell Deficiency (LSCD).
Summary, limitations, and future of LSCT for LSCD
The main limitation of LSCT for unilateral and bilateral LSCD is that irrespective of the surgical technique, source of donor tissue, or post-operative care, about 20–30% of all cases develop a recurrence of LSCD over the long term. The risk factors of failure have been studied in detail and are usually related to the severity of the pathology.Citation35,Citation42,Citation53 Those with extensive conjunctival fibrosis and with co-morbidities like corneal stromal damage tend to do poorly. It is also well recognized that dryness, exposure and eyelid pathologies (entropion, ectropion and trichiasis) are relative contraindications for LSCT. Corneal epithelial regeneration also does not always translate into visual gain, as evident from the data presented in the tables, because the primary pathology may have rendered the underlying stromal matrix irregular or severely scarred. Those with bilateral LSCD particularly need concomitant or sequential keratoplasty for optimal visual restoration. In such situations, it is recommended that the surface reconstruction is performed first followed by the corneal transplantation in a sequentially staged manner.Citation67,Citation69 Overall, significant advances have been made in the last 2–3 decades in the management of severe ocular surface disease due to LSCD. What was once considered an incurable cause of corneal blindness due to the poor outcomes with keratoplasty, is now treatable with a variety of different techniques of LSCT with 70–80% success rates.Citation60 This is a phenomenal example of bench-to-bedside translation that hardly has any parallels in other branches of medicine.
With a better understanding of the limbal stem cell niche using advanced imaging techniques and development of markers specific for the epithelial stem cells, it is likely that clinical outcomes will only improve with time. Since limbal epithelial stem cells (LESCs) are morphologically indistinct from transiently amplifying cells or corneal epithelial cells, much attention has been devoted to developing markers that will help in identifying them. The LESCs were initially identified in suspension cultures as holoclones (clones with properties of long-term proliferation, self-renewal, and clonogenicity).Citation70 Later, p63α (more specifically termed as deltaNp63α) was reported to be essential for maintaining migration and proliferation of LESCs.Citation71 Further studies reported the expression of ATP-binding cassette transporter super-family G member 2 (ABCG2) in side population of limbal epithelium,Citation72 whereas CCAAT enhancer binding protein delta (C/EBPδ) was co-expressed along with deltaNp63α in mitotically inactive limbal stem cells and reported to regulate cell cycle and auto-renewal of human LESCs.Citation73 Most recently in 2014, ATP-binding cassette, sub-family B, member 5 (ABCB5) was discovered as a limbal stem cell gene which is essential for the development and repair of cornea and specifically identifies label-retaining stem cell population in limbus and not in cornea.Citation74 While the jury is still out on whether a single marker or a combination of the above-mentioned markers should be used to selectively identify the actual LESCs, the ability to quantify the amount of stem cells being transplanted will help standardize LSCT procedures, particularly CLET. It is also likely that predictable gene-editing combined with CLET may help treat the rare hereditary forms of LSCD using the patient’s own cells.Citation75,Citation76
Limbal mesenchymal/stromal stem cells
Mesenchymal stem/stromal cells (MSCs) belong to a diverse population of non-hematopoietic fibroblast-like cells which are plastic adherent and phenotypically defined as CD90+ CD105+ CD73+ CD29+ CD34− CD45−.Citation77 These cells are mostly derived from the adult tissues and therefore have exceptional genetic stability and fewer ethical concerns compared to induced pluripotent stem cells (iPSCs) and embryonic stem cells, respectively.Citation78 They can repair injured tissue either by direct differentiation or by secretion of trophic factors.Citation79 MSCs can be easily isolated from different sources including bone marrow (BM-MSCs), dental pulp (DPSCs), adipose tissue (ADSCs), and umbilical cord (UC-MSCs) and cultured in vitro due to their plastic adherence property. The MSCs induce the modulation of immune response and tissue repair through inflammatory cytokines and growth factors.Citation79
Recently MSCs have been identified and successfully isolated from the corneal stroma and limbus ().Citation80–Citation83 A distinct population of cells with mesenchymal characteristics (e.g., multipotent differentiation, clonal growth and expression of MSC-specific markers) sub-adjacent to the limbal basement membrane has been reported to support the viability and potency of the limbal epithelial stem cells. They were initially identified as ‘side population’ in the flow cytometry and termed as corneal stromal stem cells (CSSCs) in 2005.Citation80 These limbal MSCs were also thought to be present at the anterior peripheral (limbal) stroma in the cornea and therefore were termed as peripheral and limbal corneal stromal cells (PLCSCs).Citation81 These were reported to conform to all the criteria (plastic adherent, CD105+, CD73+, CD90+, CD45−, CD34−, CD14−, CD11b−, CD79α−, and HLA-DR−, differentiate into osteoblasts, adipocytes and chondroblasts in vitro) specified by the International Society for Cell Therapy (ISCT) for MSCs.Citation77,Citation78 Limbal niche cells isolated from the limbal stroma were reported to generate progenitors with MSC-like characteristics, indicating their potential role in healing of the wounded cornea.Citation82–Citation84 Later, in 2014, these cells were isolated from the limbal stroma and termed as limbus-derived stromal/mesenchymal stem cells (LMSCs).Citation85 These LMSCs have the potential to: a) differentiate into various cell types including keratocytes, b) secrete normal corneal extra-cellular matrix (ECM) components like collagen I, lumican and keratocan in a regular lamellar pattern, c) remodel the diseased corneal stroma, d) suppress inflammation and angiogenesis and e) restore normal corneal transparency.Citation82,Citation85 Owing to the fact that these LMSCs can be obtained by biopsy and expanded in vitro to give large number of cells, these possess potential therapeutic application for cell-based therapy for corneal stromal pathologies.Citation83
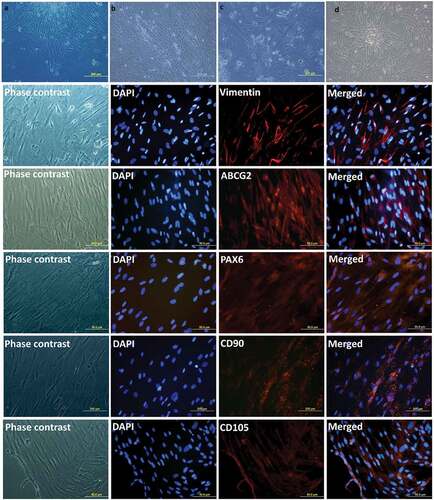
Figure 5. Expansion of Limbal Mesenchymal Stem Cells (LMSCs). Phase-contrast images of the cultured LMSCs on Day 3 (a) and Day 15 (b) of primary culture; end of passages 1 (c) and 2 (d). Phenotypic expression of LMSCs. The LMSCs show positive expression of the markers for mesenchymal-lineage: Vimentin, CD90 and CD105; and are also positive for stem cell markers ABCG2 and PAX-6.
CSSC/LMSCs and corneal stromal wound healing
Corneal stromal opacification can either be due to genetic abnormalities or acquired factors like traumatic corneal injuries and ulceration. The role of CSSC/LMSCs have been evaluated in experimental animal models of both congenital opacities (Lumican null mice)Citation84 and traumatic injury (ulceration with a mechanical rotating burr).Citation86 Du et al. in 2009 isolated the CSSCs and injected them directly into the corneal stroma of the Lumican-null mice.Citation84 Lumican, being a critical proteoglycan component of the corneal stromal ECM, helps in stromal organization and in maintaining corneal transparency. Lumican null mice develop corneal opacity (due to disorganization of the stromal collagen) resembling corneal scars.Citation84 The injected cells survived for months in wild-type mice without inducing a T-cell-mediated response. Remarkably, in Lumican null mice, thickness of the corneal stroma and defects of collagen fibril were restored to that of the wild-type mice, post injection of human CSSCs.Citation84
Acquired corneal stromal opacities are more common and develop due to a fibrotic response to injury or inflammation. Boote et al. in 2012 reported that penetration of epithelial basement membrane during wounding is a decisive factor for inducing suitable ultrastructural alterations in the stroma leading to opacity and scars.Citation86 They used a rotating mechanical burr to mechanically induce the injury. OCT and x-ray scattering were used to measure corneal opacity, diameter of collagen and order of matrix; electron microscopy to image proteoglycans; and q-PCR to evaluate transcript expression of quiescent and fibrotic genes. They observed that debridement of corneal epithelium while leaving the basement membrane intact resulted in a significant increase in matrix disorder but minimal corneal opacity at 8 weeks. Whereas epithelial debridement along with basement membrane resulted in presence of atypically large proteoglycans in the subepithelial stroma, enhanced disorder in matrix, scattering of light, collagen diameter and upregulation of fibrotic markers 2 to 4 weeks post-injury. Using the similar mouse model of corneal scarring, Basu et al. in 2014 transplanted the LMSCs (expanded from clinically repeatable, small and superficial limbal biopsies obtained from human cadaveric corneo-scleral rims) into wounded mouse corneas which stopped the formation of scars containing abnormal ECM components.Citation85 Moreover, these LMSCs prompted replacement of the depleted stroma with structured and organized lamellar layers resembling the native stroma.Citation85
Further studies in this direction revealed the significance of secreted factors like hepatocyte growth factor (HGF). HGF is critical for potentiating the tissue repair function of MSCs and is itself able to restore corneal transparency post corneal injury.Citation87 This suggests direct implication of HGF in development of HGF-based therapy for corneal injuries.88 HGF is a pleiotropic growth factor secreted by MSCs and reported to induce proliferation of epithelial cells, morphogenesis, motility, and angiogenesis in different organs through phosphorylation of its receptor c-Met.Citation88 This results in mitogenic, motogenic, and morphogenic activities in different types of cells.Citation88,Citation89 HGF treatment has been reported to improve and accelerate corneal epithelial repair by suppressing corneal inflammation and enhancing the proliferation of corneal epithelial cells (CECs) in mouse model of corneal injury.Citation90 MSC-conditioned medium containing HGF along with other growth factors and interleukins has been shown to significantly increase the wound closure rate of corneal stromal cells, in vitro. This further results in accelerated corneal wound healing due to enhanced viability and migration of stromal keratocytes and formation of desirable ECM.Citation91 Most recently, Mittal et al. have reported that MSCs modulate corneal alloimmunity and promote graft survival via secretion of HGF.Citation92 Previous studies have also shown that other growth factors like transforming growth factor-β (TGFβ) and platelet-derived growth factor (PDGF) also play important roles in corneal stromal wound healing. Bone marrow cells can also move to wounded site for repair along with limbal stromal cells and may help to prevent fibrosis.Citation93–Citation95
Clinical applications of LMSCs
A pilot clinical study by Basu et al. has been examining the safety and efficacy of both autologous and allogeneic LMSCs in corneal stromal regeneration in superficial corneal pathologies like scars, ulcers and burns (https://clinicaltrials.gov/ct2/show/NCT02948023).Citation96–Citation98 The LMSCs were derived from the limbal biopsies obtained from human cadaveric corneo-scleral rims using a well-established xeno-free ex vivo cultivation technique.Citation85 Briefly, the procedure involved collection of the limbal ring from serologically tested and clinically accepted cadaveric corneas from the eye bank, followed by dissection of the limbal ring into small pieces and overnight enzymatic digestion. The cells thus obtained are cultured in low serum (2%) medium and passaged thrice for elimination of epithelial cells from the culture. These LMSCs were delivered using an onlay technique using fibrin glue. During transplantation, the corneal epithelium was removed with a surgical sponge and 100 µl of stromal cells (5 x 103 cells/µl, diluted with the thrombin component of fibrin glue (TISSEL, Baxter)) were applied to the debrided corneal stroma, and a bandage contact lens was placed over the cornea. Post-operatively, the patient received topical antibiotics and comprehensive ophthalmic evaluation including scheimpflug imaging, anterior segment OCT scanning and slit-lamp biomicroscopy was performed.Citation96,Citation97 The patients did not receive any topical corticosteroids but when examined at 6-weeks, 3-months, 6-months, and 1-year, post-operatively; the eyes receiving LSSCs demonstrated significantly better uncorrected and best-corrected visual acuity with improved corneal clarity and reduced opacification and vascularization as compared to the controls.Citation96,Citation97 The LMSCs have certain inherent advantages over the MSCs derived from other sources like bone marrow or dental pulp, including but not limited to less invasive and painful extraction procedures as compared to bone marrow and dental pulp extraction.Citation98
Controversies and future challenges
It has been around three decades since the role of LESCs in corneal epithelial renewal was established, but some questions continue to remain unanswered. Clinicians have often wondered how some eyes continue to maintain a clear central cornea despite having obvious clinical signs of peripheral LSCD. These observations have called into question the role of LESCs in corneal epithelial homeostasis.Citation99 There have been controversial reports suggesting that oligopotent epithelial stem cells may be distributed throughout the mammalian ocular surface,Citation100 giving rise to the concept of corneal stem cells (CSCs) as opposed to LESCs. Nasser et al., recently reported that committed corneal cells have the potential to dedifferentiate, repopulate the stem cell pool, and correctly re-construct the corneo-scleral junction in the presence of intact stroma.Citation101
The complexity and costs of CLET as a technique of LSCT have also been called into question, particularly with recent reports suggesting that it may not offer any clinical benefit above and beyond that of other techniques like CLAu and SLET.Citation60 The proposed advantage that CLET results in amplification of not only corneal epithelial cells but also LESCs in culture may not be the case. Perhaps the discovery of a specific marker for LESCs will help in this cause. While ABCB5 has been reported as a limbal stem cell geneCitation74 and ABCG2+/ABCB5+double-positive LESCs have been reported to be highly proliferative,Citation102 the search for a suitable marker specifically identifying LMSCs is also ongoing. Since a single marker is not able to discern between LESCs and LMSCs, using a possible combination of established markers (e.g., ABCG2, ΔNp63α, CEBPδ, and ABCB5) for LESCs and an additional subset of mesenchymal markers for LMSCs (e.g., CD90, CD73, and CD166) is recommended. Finally, the major advantage of limbal stem cells (both epithelial and stromal) lies in their potentially autologous clinical applications which substantially reduce ethical concerns and risk of graft rejection. However, the ethical issues associated with the use of allogeneic tissue, particularly concerning living-related donors need to be addressed appropriately with specific guidelines.Citation103
Conclusions
The limbus harbours both epithelial and mesenchymal stem cells. The LESCs reside in the basal layer of the limbal epithelium in the palisades of Vogt whereas LMSCs are present in the limbal stroma sub-adjacent to the limbal basement membrane. The LESCs, and perhaps also the LMSCs, play a vital role in maintaining normal corneal homeostasis and their dysfunction leads to a clinical state of stem cell deficiency. This is perhaps the only known clinical stem cell-deficient condition other than cancer/chemotherapy-induced bone marrow stem cell deficiency. Great progress has been made in this area, with limbal epithelial stem cell therapy being successfully practised worldwide using different surgical approaches. Unlike LESCs, the role of LMSCs in normal homeostasis is not yet completely understood. There is some speculation that the mesenchymal cells are vital to the stromal niche that maintains or modulates the function of the epithelial stem cells. There is also no clinically known state of limbal or corneal mesenchymal stem cell deficiency, and therefore the clinical application of LMSCs differs principally from that of LESCs. However, the therapeutic potential of the LMSCs along with other sources of MSCs in the treatment of blinding corneal stromal pathologies is being enthusiastically explored through pre-clinical and early clinical studies. Since both these cell types are concurrently the subject of intense research and clinical applications, the authors feel that the term ‘Limbal Stem Cells’, should, therefore, be qualified by adding the cell type, epithelial or mesenchymal.
Sources of support
Department of Science and Technology (No. IFA14-LSBM-104), and Science and Engineering Research Board (No. SERB/EMR/2017/005086), Govt. of India.
Acknowledgments
Sachin Shukla acknowledges support from Department of Science and Technology, Govt. of India (No. IFA14-LSBM-104). Sayan Basu acknowledges support from Science and Engineering Research Board (No. SERB/EMR/2017/005086), Govt. of India.
Disclosure statement
Authors declare that there is no conflict of interest.
Additional information
Funding
References
- Flaxman SR, Bourne RRA, Resnikoff S, Ackland P, Braithwaite T, Cicinelli MV, Das A, Jonas JB, Keeffe J, Kempenet JH, et al. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(12): e1221-e1234. doi: 10.1016/S2214-109X(17)30393-5.
- Hanna C, Bicknell DS, O’brien JE. Cell turnover in the adult human eye. Arch Ophthalmol. 1961;65:695–98. doi:10.1001/archopht.1961.01840020697016.
- Davanger M, Evensen A. Role of the pericorneal papillary structure in renewal of corneal epithelium. Nature. 1971;229(5286):560–61. doi:10.1038/229560a0.
- Brown SI, Bloomfield SE, Pearce DB. A follow-up report on transplantation of the alkali-burned cornea. Am J Ophthalmol. 1974;77(4):538–42. doi:10.1016/0002-9394(74)90468-1.
- Thoft RA. Conjunctival transplantation as an alternative to keratoplasty. Ophthalmology. 1979;86:1084–92.
- Shapiro MS, Friend J, Thoft RA. Corneal re-epithelialization from the conjunctiva. Invest Ophthalmol Vis Sci. 1981;21:135–42.
- Kinoshita S, Kiorpes TC, Friend J, Thoft RA. Limbal epithelium in ocular surface wound healing. Invest Ophthalmol Vis Sci. 1982;23:73–80.
- Thoft RA, Friend JT. The X, Y, Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci. 1983;24:1442–43.
- Schermer A, Galvin S, Sun TT. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986;103(1):49–62. doi:10.1083/jcb.103.1.49.
- Cotsarelis G, Cheng SZ, Dong G, Sun TT, Lavker RM. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57:201–09.
- Jawaheer L, Anijeet D, Ramaesh K. Diagnostic criteria for limbal stem cell deficiency-a systematic literature review. Surv Ophthalmol. 2017;62(4):522–32. doi:10.1016/j.survophthal.2016.11.003.
- Le Q, Xu J, Deng SX. The diagnosis of limbal stem cell deficiency. Ocul Surf. 2018;16(1):58–69. doi:10.1016/j.jtos.2017.11.002.
- Deng SX, Borderie V, Chan CC, Dana R, Figueiredo FC, Gomes JAP, Pellegrini G, Shimmura S, Kruse FE. The International limbal stem cell deficiency working group. global consensus on definition, classification, diagnosis, and staging of limbal stem cell deficiency. Cornea. 2019;38(3):364–75. doi:10.1097/ICO.0000000000001820.
- Vazirani J, Nair D, Shanbhag S, Wurity S, Ranjan A, Sangwan V. Limbal stem cell deficiency - demography and underlying causes. Am J Ophthalmol. 2018;188:99–103. doi:10.1016/j.ajo.2018.01.020.
- BY K, KM R, Bakhtiari P, Chan CC, Welder JD, Holland EJ, Basti S, Djalilian AR. Medically reversible limbal stem cell disease: clinical features and management strategies. Ophthalmology. 2014;121(10):2053–58. doi:10.1016/j.ophtha.2014.04.025.
- Tan JC, Tat LT, Coroneo MT. Treatment of partial limbal stem cell deficiency with topical interferon alpha-2b and retinoic acid. Br J Ophthalmol. 2016;100(7):944–48. doi:10.1136/bjophthalmol-2015-307411.
- Tsai RJ, Sun TT, Tseng SC. Comparison of limbal and conjunctival autograft transplantation in corneal surface reconstruction in rabbits. Ophthalmology. 1990;97:446–55.
- Kenyon KR, Tseng SC. Limbal autograft transplantation for ocular surface disorders. Ophthalmology. 1989;96:709–22.
- Rao SK, Rajagopal R, Sitalakshmi G, Padmanabhan P. Limbal autografting: comparison of results in the acute and chronic phases of ocular surface burns. Cornea. 1999;18:164–71.
- Shimazaki J, Shimmura S, Tsubota K. Donor source affects the outcome of ocular surface reconstruction in chemical or thermal burns of the cornea. Ophthalmology. 2004;111(1):38–44. doi:10.1016/j.ophtha.2003.02.003.
- Ozdemir O, Tekeli O, Ornek K, Arslanpence A, Yalcindag NF. Limbal autograft and allograft transplantations in patients with corneal burns. Eye. 2004;18(3):241–48. doi:10.1038/sj.eye.6700640.
- Santos MS, Gomes JA, Hofling-Lima AL, Rizzo LV, Romano AC, Belfort R Jr. Survival analysis of conjunctival limbal grafts and amniotic membrane transplantation in eyes with total limbal stem cell deficiency. Am J Ophthalmol. 2005;140(2):223–30. doi:10.1016/j.ajo.2005.03.022.
- Wylegala E, Dobrowolski D, Tarnawska D, Janiszewska D, Gabryel B, Malecki A, Siekiera U. Limbal stem cells transplantation in the reconstruction of the ocular surface: 6 years experience. Eur J Ophthalmol. 2008;18:886–90.
- Miri A, Al-Deiri B, Dua HS. Long-term outcomes of autolimbal and allolimbal transplants. Ophthalmology. 2010;117(6):1207–13. doi:10.1016/j.ophtha.2009.10.028.
- Baradaran-Rafii A, Eslani M, Jamali H, Karimian F, Tailor UA, Djalilian AR. Postoperative complications of conjunctival limbal autograft surgery. Cornea. 2012;31(8):893–99. doi:10.1097/ICO.0b013e31823f095d.
- Burcu A, Yalniz-Akkaya Z, Ozdemir MF, Erdem E, Onat MM, Ornek F. Surgical rehabilitation following ocular chemical injury. Cutan Ocul Toxicol. 2014;33(1):42–48. doi:10.3109/15569527.2013.796477.
- Barreiro TP, Santos MS, Vieira AC, de Nadai Barros J, Hazarbassanov RM, Gomes JA. Comparative study of conjunctival limbal transplantation not associated with the use of amniotic membrane transplantation for treatment of total limbal deficiency secondary to chemical injury. Cornea. 2014;33(7):716–20. doi:10.1097/ICO.0000000000000139.
- Moreira PB, Magalhaes RS, Pereira NC, Oliveira LA, Sousa LB. Limbal transplantation at a tertiary hospital in Brazil: a retrospective study. Arq Bras Oftalmol. 2015;78(4):207–11. doi:10.5935/0004-2749.20150054.
- Basti S, Mathur U. Unusual intermediate-term outcome in three cases of limbal autograft transplantation. Ophthalmology. 1999;106(5):958–63. doi:10.1016/S0161-6420(99)00516-3.
- Miri A, Said DG, Dua HS. Donor site complications in autolimbal and living-related allolimbal transplantation. Ophthalmology. 2011;118(7):1265–71. doi:10.1016/j.ophtha.2010.11.030.
- Cheung AY, Sarnicola E, Holland EJ. Long-Term Ocular Surface Stability in Conjunctival Limbal Autograft Donor Eyes. Cornea. 2017;36(9):1031–35. doi:10.1097/ICO.0000000000001260.
- Busin M, Breda C, Bertolin M, Bovone C, Ponzin D, Ferrari S, Barbaro V, Elbadawy HM. Corneal epithelial stem cells repopulate the donor area within 1 year from limbus removal for limbal autograft. Ophthalmology. 2016;123(12):2481–88. doi:10.1016/j.ophtha.2016.08.018.
- Kheirkhah A, Raju VK, Tseng SC. Minimal conjunctival limbal autograft for total limbal stem cell deficiency. Cornea. 2008;27(6):730–33. doi:10.1097/QAI.0b013e31815cea8b.
- Pellegrini G, Traverso CE, Franzi AT, Zingirian M, Cancedda R, De Luca M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349(9057):990–93. doi:10.1016/S0140-6736(96)11188-0.
- Sangwan VS, Basu S, Vemuganti GK, Sejpal K, Subramaniam SV, Bandyopadhyay S, Krishnaiah S, Gaddipati S, Tiwari S, Balasubramanian D, et al. Clinical outcomes of xeno-free autologous cultivated limbal epithelial transplantation: a 10-year study. Br J Ophthalmol. 2011;95(11):1525–29. doi:10.1136/bjophthalmol-2011-300352.
- Sangwan VS, Matalia HP, Vemuganti GK, Fatima A, Ifthekar G, Singh S, Nutheti R, Rao GN. Clinical outcome of autologous cultivated limbal epithelium transplantation. Indian J Ophthalmol. 2006;54(1):29–34. doi:10.4103/0301-4738.21611.
- Shimazaki J, Higa K, Morito F, Dogru M, Kawakita T, Satake Y, Shimmura S, Tsubota K. Factors influencing outcomes in cultivated limbal epithelial transplantation for chronic cicatricial ocular surface disorders. Am J Ophthalmol. 2007;143(6):945–53. doi:10.1016/j.ajo.2007.03.005.
- Pauklin M, Fuchsluger TA, Westekemper H, Steuhl KP, Meller D. Midterm results of cultivated autologous and allogeneic limbal epithelial transplantation in limbal stem cell deficiency. Dev Ophthalmol. 2010;45:57–70. doi:10.1159/000315020.
- Schwab IR. Cultured corneal epithelia for ocular surface disease. Trans Am Ophthalmol Soc. 1999;97:891–986.
- Schwab IR, Reyes M, Isseroff RR. Successful transplantation of bioengineered tissue replacements in patients with ocular surface disease. Cornea. 2000;19:421–26.
- Rama P, Bonini S, Lambiase A, Golisano O, Paterna P, De Luca M, Pellegrini G. Autologous fibrin-cultured limbal stem cells permanently restore the corneal surface of patients with total limbal stem cell deficiency. Transplantation. 2001;72:1478–85.
- Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, Pellegrini G. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363(2):147–55. doi:10.1056/NEJMoa0905955.
- Di Iorio E, Ferrari S, Fasolo A, Bohm E, Ponzin D, Barbaro V. Techniques for culture and assessment of limbal stem cell grafts. Ocul Surf. 2010;8:146–53.
- Prabhasawat P, Ekpo P, Uiprasertkul M, Chotikavanich S, Tesavibul N. Efficacy of cultivated corneal epithelial stem cells for ocular surface reconstruction. Clin Ophthalmol. 2012;6:1483–92. doi:10.2147/OPTH.S33951.
- Sejpal K, Ali MH, Maddileti S, Basu S, Ramappa M, Kekunnaya R, Vemuganti GK, Sangwan VS. Cultivated limbal epithelial transplantation in children with ocular surface burns. JAMA Ophthalmol. 2013;131(6):731–36. doi:10.1001/jamaophthalmol.2013.2308.
- Zakaria N, Possemiers T, Dhubhghaill SN, Leysen I, Rozema J, Koppen C, Timmermans JP, Berneman Z, Tassignon MJ. Results of a phase I/II clinical trial: standardized, non-xenogenic, cultivated limbal stem cell transplantation. J Transl Med. 2014;12:58. doi:10.1186/1479-5876-12-58.
- Ganger A, Vanathi M, Mohanty S, Tandon R. Long-term outcomes of cultivated limbal epithelial transplantation: evaluation and comparison of results in children and adults. Biomed Res Int. 2015;2015:480983. doi:10.1155/2015/480983.
- Fasolo A, Pedrotti E, Passilongo M, Marchini G, Monterosso C, Zampini R, Bohm E, Birattari F, Franch A, Barbaro V. Safety outcomes and long-term effectiveness of ex vivo autologous cultured limbal epithelial transplantation for limbal stem cell deficiency. Br J Ophthalmol. 2017;101(5):640–49. doi:10.1136/bjophthalmol-2015-308272.
- Schwab IR, Johnson NT, Harkin DG. Inherent risks associated with manufacture of bioengineered ocular surface tissue. Arch Ophthalmol. 2006;124(12):1734–40. doi:10.1001/archopht.124.12.1734.
- Tseng SC. Regulation and clinical implications of corneal epithelial stem cells. Mol Biol Rep. 1996;23:47–58.
- Mariappan I, Maddileti S, Savy S, Tiwari S, Gaddipati S, Fatima A, Sangwan VS, Balasubramanian D, Vemuganti GK. In vitro culture and expansion of human limbal epithelial cells. Nat Protoc. 2010;5(8):1470–79. doi:10.1038/nprot.2010.115.
- Sangwan VS, Basu S, MacNeil S, Balasubramanian D. Simple limbal epithelial transplantation (SLET): a novel surgical technique for the treatment of unilateral limbal stem cell deficiency. Br J Ophthalmol. 2012;96(7):931–34. doi:10.1136/bjophthalmol-2011-301164.
- Basu S, Sureka SP, Shanbhag SS, Kethiri AR, Singh V, Sangwan VS. Simple limbal epithelial transplantation: long-term clinical outcomes in 125 cases of unilateral chronic ocular surface burns. Ophthalmology. 2016;123(5):1000–10. doi:10.1016/j.ophtha.2015.12.042.
- Vazirani J, Ali MH, Sharma N, Gupta N, Mittal V, Atallah M, Amescua G, Chowdhury T, Abdala-Figuerola A, Ramirez-Miranda A, et al. Autologous simple limbal epithelial transplantation for unilateral limbal stem cell deficiency: multicentre results. Br J Ophthalmol. 2016;100(10):1416–20. doi:10.1136/bjophthalmol-2015-307348.
- Gupta N, Joshi J, Farooqui JH, Mathur U. Results of simple limbal epithelial transplantation in unilateral ocular surface burn. Indian J Ophthalmol. 2018;66(1):45–52. doi:10.4103/ijo.IJO_602_17.
- Basu S, Mohan S, Bhalekar S, Singh V, Sangwan V. Simple limbal epithelial transplantation (SLET) in failed cultivated limbal epithelial transplantation (CLET) for unilateral chronic ocular burns. Br J Ophthalmol. 2018;102(12):1640–45. doi:10.1136/bjophthalmol-2017-311506.
- Shanbhag SS, Patel CN, Goyal R, Rao Donthineni P, Singh V, Basu S. Simple limbal epithelial transplantation (SLET): review of indications, surgical technique, mechanism, outcomes, limitations and impact. Indian J Ophthalmol. In Press;2019. doi: 10.4103/ijo.IJO_117_19.
- Amescua G, Atallah M, Nikpoor N, Galor A, Perez VL. Modified simple limbal epithelial transplantation using cryopreserved amniotic membrane for unilateral limbal stem cell deficiency. Am J Ophthalmol. 2014;158(469–75.e2). doi:10.1016/j.ajo.2014.06.002.
- Prabhasawat P, Luangaram A, Ekpo P, Lekhanont K, Tangpagasit W, Boonwong C, Inthasin N, Chirapapaisan C. Epithelial analysis of simple limbal epithelialtransplantation in limbal stem cell deficiency by in vivo confocal microscopy and impression cytology. Cell Tissue Bank. 2019;20(1):95–108. doi:10.1007/s10561-018-09746-3.
- Shanbhag SS, Nikpoor N, Rao Donthineni P, Singh V, Basu S. Autologous limbal stem cell transplantation: a systematic review of clinical outcomes with different surgical techniques. Br J Ophthalmol. 2019. doi:10.1136/bjophthalmol-2019-314081.
- Holland EJ. Epithelial transplantation for the management of severe ocular surface disease. Trans Am Ophthalmol Soc. 1996;94:677–743.
- Tsubota K, Satake Y, Kaido M, Shinozaki N, Shimmura S, Bissen-Miyajima H, Shimazaki J. Treatment of severe ocular-surface disorders with corneal epithelial stem-cell transplantation. N Engl J Med. 1999;340(22):1697–703. doi:10.1056/NEJM199906033402201.
- Ilari L, Daya SM. Long-term outcomes of keratolimbal allograft for the treatment of severe ocular surface disorders. Ophthalmology. 2002;109:1278–84.
- Solomon A, Ellies P, Anderson DF, Touhami A, Grueterich M, Espana EM, Ti SE, Goto E, Feuer WJ, Tseng SC. Long-term outcome of keratolimbal allograft with or without penetrating keratoplasty for total limbal stem cell deficiency. Ophthalmology. 2002;109:1159–66.
- Maruyama-Hosoi F, Shimazaki J, Shimmura S, Tsubota K. Changes observed in keratolimbal allograft. Cornea. 2006;25(4):377–82. doi:10.1097/01.ico.0000176608.65708.27.
- Shi W, Gao H, Wang T, Xie L. Combined penetrating keratoplasty and keratolimbal allograft transplantation in comparison with corneoscleral transplantation in the treatment of severe eye burns. Clin Exp Ophthalmol. 2008;36(6):501–07. doi:10.1111/j.1442-9071.2008.01802.x.
- Basu S, Fernandez MM, Das S, Gaddipati S, Vemuganti GK, Sangwan VS. Clinical outcomes of xeno-free allogeneic cultivated limbal epithelial transplantation for bilateral limbal stem cell deficiency. Br J Ophthalmol. 2012;96(12):1504–09. doi:10.1136/bjophthalmol-2012-301869.
- Koizumi N, Inatomi T, Suzuki T, Sotozono C, Kinoshita S. Cultivated corneal epithelial stem cell transplantation in ocular surface disorders. Ophthalmology. 2001;108:1569–74.
- Basu S, Mohamed A, Chaurasia S, Sejpal K, Vemuganti GK, Sangwan VS. Clinical outcomes of penetrating keratoplasty after autologous cultivated limbal epithelial transplantation for ocular surface burns. Am J Ophthalmol. 2011;152:917–924.e911.
- Pellegrini G, Golisano O, Paterna P, Lambiase A, Bonini S, Rama P, De Luca M. Location and clonal analysis of stem cells and their differentiated progeny in the human ocular surface. J Cell Biol. 1999;145:769–82. doi:10.1083/jcb.145.4.769.
- Di IE, Barbaro V, Ruzza A, Ponzin D, Pellegrini G, De LM. Isoforms of DeltaNp63 and the migration of ocular limbal cells in human corneal regeneration. Proc Natl Acad Sci USA. 2005;102:9523–28. doi:10.1073/pnas.0503437102.
- Watanabe K, Nishida K, Yamato M, Umemoto T, Sumide T, Yamamoto K, Maeda N, Watanabe H, Okano T, Tano Y. Human limbal epithelium contains side population cells expressing the ATP-binding cassette transporter ABCG2. FEBS Lett. 2004;565:6–10. doi:10.1016/j.febslet.2004.03.064.
- Barbaro V, Testa A, Di IE, Mavilio F, Pellegrini G, De LM. C/EBPdelta regulates cell cycle and self-renewal of human limbal stem cells. J Cell Biol. 2007;177:1037–49. doi:10.1083/jcb.200703003.
- Ksander BR, Kolovou PE, Wilson BJ, Saab KR, Guo Q, Ma J, McGuire SP, Gregory MS, Vincent WJ, Perez VL, et al. ABCB5 is a limbal stem cell gene required for corneal development and repair. Nature. 2014;511:353–57. doi:10.1038/nature13426.
- Hirsch T, Rothoeft T, Teig N, Bauer JW, Pellegrini G, De Rosa L, Scaglione D, Reichelt J, Klausegger A, Kneisz D, et al. Regeneration of the entire human epidermis using transgenic stem cells. Nature. 2017;551:327–32. doi:10.1038/nature24487.
- Murauer EM, Koller U, Pellegrini G, De Luca M, Bauer JW. Advances in Gene/Cell Therapy in Epidermolysis Bullosa. Keio J Med. 2015;64:21–25. doi:10.2302/kjm.2014-0013-RE.
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8(4):315–17. doi:10.1080/14653240600855905.
- Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, Deans RJ, Krause DS, Keating A. Clarification of the nomenclature for MSC: the International Society for Cellular Therapy position statement. Cytotherapy. 2005;7(5):393–95. doi:10.1080/14653240500319234.
- Sahu A, Foulsham W, Amouzegar A, Mittal SK, Chauhan SK. The therapeutic application of mesenchymal stem cells at the ocular surface. Ocul Surf. 2019;17(2):198–207. doi:10.1016/j.jtos.2019.01.006.
- Funderburgh JL, Funderburgh ML, Du Y. Stem Cells in the Limbal Stroma. Ocul Surf. 2016;14:113–20. doi:10.1016/j.jtos.2015.12.006.
- Branch MJ, Hashmani K, Dhillon P, Jones DR, Dua HS, Hopkinson A. Mesenchymal stem cells in the human corneal limbal stroma. Invest Ophthalmol Vis Sci. 2012;53(9):5109–16. doi:10.1167/iovs.11-8673.
- Li GG, Zhu YT, Xie HT, Chen SY, Tseng SC. Mesenchymal stem cells derived from human limbal niche cells. Invest Ophthalmol Vis Sci. 2012;53(9):5686–97. doi:10.1167/iovs.12-10300.
- Du Y, Funderburgh ML, Mann MM, SundarRaj N, Funderburgh JL. Multipotent stem cells in human corneal stroma. Stem Cells. 2005;23:1266–75. doi:10.1634/stemcells.2004-0256.
- Du Y, Carlson EC, Funderburgh ML, Birk DE, Pearlman E, Guo N, Kao WW, Funderburgh JL. Stem cell therapy restores transparency to defective murine corneas. Stem Cells. 2009;27(7):1635–42. doi:10.1002/stem.91.
- Basu S, Hertsenberg AJ, Funderburgh ML, Burrow MK, Mann MM, Du Y, Lathrop KL, Syed-Picard FN, Adams SM, Birk DE et al Human limbal biopsy-derived stromal stem cells prevent corneal scarring. Sci Transl Med. 2014;6:266ra172. doi:10.1126/scitranslmed.3009644.
- Boote C, Du Y, Morgan S, Harris J, Kamma-Lorger CS, Hayes S, Lathrop KL, Roh DS, Burrow MK, Hiller J, et al. Quantitative assessment of ultrastructure and light scatter in mouse corneal debridement wounds. Invest Ophthalmol Vis Sci. 2012;53(6):2786–95. doi:10.1167/iovs.11-9305.
- Mittal SK, Omoto M, Amouzegar A, Sahu A, Rezazadeh A, Katikireddy KR, Shah DI, Sahu SK, Chauhan SK. Restoration of Corneal Transparency by Mesenchymal Stem Cells. Stem Cell Reports. 2016;7:583–90. doi:10.1016/j.stemcr.2016.09.001.
- Nakamura T. Structure and function of hepatocyte growth factor. Prog Growth Factor Res. 1991;3:67–85.
- Matsumoto K, Nakamura T. Hepatocyte growth factor: molecular structure, roles in liver regeneration, and other biological functions. Crit Rev Oncog. 1992;3:27–54.
- Omoto M, Suri K, Amouzegar A, Li M, Katikireddy KR, Mittal SK, Chauhan SK. Hepatocyte growth factor suppresses inflammation and promotes epithelium repair in corneal injury. Mol Ther. 2017;25:1881–88. doi:10.1016/j.ymthe.2017.04.020.
- Jiang Z, Liu G, Meng F, Wang W, Hao P, Xiang Y, Wang Y, Han R, Li F, Wang L, et al. Paracrine effects of mesenchymal stem cells on the activation of keratocytes. Br J Ophthalmol. 2017;101:1583–90. doi:10.1136/bjophthalmol-2016-310012.
- Mittal SK, Foulsham W, Shukla S, Elbasiony E, Omoto M, Chauhan SK. Mesenchymal stromal cells modulate corneal alloimmunity via secretion of hepatocyte growth factor. Stem Cells Transl Med. 2019. doi:10.1002/sctm.19-0004.
- Singh V, Jaini R, Torricelli AA, Santhiago MR, Singh N, Ambati BK, Wilson SE. TGFβ and PDGF-B signaling blockade inhibits myofibroblast development from both bone marrow-derived and keratocyte-derived precursor cells in vivo. Exp Eye Res. 2014;121:35–40. doi:10.1016/j.exer.2014.02.013.
- Singh V, Agrawal V, Santhiago MR, Wilson SE. Stromal fibroblast-bone marrow-derived cell interactions: implications for myofibroblast development in the cornea. Exp Eye Res. 2012;98:1–8. doi:10.1016/j.exer.2012.03.006.
- Singh V, Barbosa FL, Torricelli AA, Santhiago MR, Wilson SE. Transforming growth factor β and platelet-derived growth factor modulation of myofibroblast development from corneal fibroblasts in vitro. Exp Eye Res. 2014;120:152–60. doi:10.1016/j.exer.2014.01.003.
- Basu S, Damala M, Singh V. Limbal stromal stem cell therapy for acute and chronic superficial corneal pathologies: early clinical outcomes of the funderburgh technique. Invest Ophthalmol Vis Sci. 2017;58(8):3371. doi:10.1167/iovs.16-20610.
- Funderburgh JL, Basu S, Damala M, Tavakkoli F, Sangwan VS, Singh V. Limbal stromal stem cell therapy for acute and chronic superficial corneal pathologies: one-year outcomes. Invest Ophthalmol Vis Sci. 2018;59(9):3455. doi:10.1167/iovs.17-23678.
- Mitragotri N, Damala M, Singh V, Basu S. Limbal Stromal Stem Cells in Corneal Wound Healing: current Perspectives and Future Applications. In Alió J, Alió del Barrio J, Arnalich-Montiel F, editors. Corneal Regeneration. Essentials in Ophthalmology. Cham: Springer; 2019.
- Dua HS, Miri A, Alomar T, Yeung AM, Said DG. The role of limbal stem cells in corneal epithelial maintenance: testing the dogma. Ophthalmology. 2009;116(5):856–63. doi:10.1016/j.ophtha.2008.12.017.
- Majo F, Rochat A, Nicolas M, Jaoudé GA, Barrandon Y. Oligopotent stem cells are distributed throughout the mammalian ocular surface. Nature. 2008;456:250–54. doi:10.1038/nature07406.
- Nasser W, Amitai-Lange A, Soteriou D, Hanna R, Tiosano B, Fuchs Y, Shalom-Feuerstein R. Corneal-committed cells restore the stem cell pool and tissue boundary following injury. Cell Rep. 2018 Jan 9;22(2):323–31. doi:10.1016/j.celrep.2017.12.040.
- Kim EK, Lee GH, Lee B, Maeng YS. Establishment of novel limbus-derived, highly proliferative abcg2+/abcb5+ limbal epithelial stem cell cultures. Stem Cells Int. 2017:7678637. doi:10.1155/2017/7678637.
- Behaegel J, Ní Dhubhghaill S, Draper H. Ethical issues in living-related corneal tissue transplantation. J Med Ethics. 2019. medethics-2018-105146 doi:10.1136/medethics-2018-105146
