ABSTRACT
Purpose
The objective of this study is to present Color Doppler imaging (CDI) features of the lacrimal sac in normal and diseased states.
Methods
Prospective study was performed on 20 lacrimal sacs of 20 eyes of 10 patients who underwent Color Doppler imaging at a tertiary care Dacryology service over a period of 6 months. All the patients were subjected to Duplex doppler scanning of the lacrimal sacs. Of the 20 lacrimal drainage systems studied, 8 were normal, 8 had primary acquired nasolacrimal duct obstruction (PANDO) and 4 were that of acute dacryocystitis (AcDac). Patient demographics, clinical presentation, duration of the disease and Color Doppler vascular characteristics like peri-sac vascular flow, peak systolic velocity (PSV), end-diastolic velocity (EDV), resistivity index (RI), arterial spectral waveforms and sac dimensions and wall thickness were analyzed.
Results
The vascular flow around the lacrimal sac was increased with higher flow velocities in PANDO as compared to normal and grossly enhanced in AcDac. Flow disturbances were also quite discernible in AcDac. The mean PSV and EDV were 9 & 3.87 cm/sec, 13.07 & 4.63 cm/sec and 18 & 8.5 cm/sec in normal, PANDO and AcDac, respectively. The mean vascular resistivity index increased in patients with PANDO (0.67) and decreased in AcDac (0.53) as compared to the normal (0.57). The arterial spectral waveforms in PANDO and AcDac showed low pulsatility, but the systolic peaks were sharper with more continuous forward flow through diastole in AcDac. This reflects vascular dilatation and reduced resistance to flow in AcDac.
Conclusion
Characteristic Color Doppler flow parameters can be demonstrated in patients with PANDO and acute dacryocystitis. Color Doppler techniques have the potential to enhance the understanding of lacrimal drainage pathophysiology.
Introduction
Color Doppler imaging (CDI) studies the changes in sound frequency caused by moving blood and has been commonly employed to determine the vascular flow characteristics of cerebrovascular, hepatic and peripheral vasculature.Citation1,Citation2 Blood vessels larger than 1 mm diameter are usually accessible to CDI. The flow characteristics of defined areas are obtained by pulse wave Doppler and are then superimposed on pulse-echo image to obtain a color-coded map of the anatomical regions of interest. Hence, this technique is also called as Duplex scanning.Citation1 The frequency and velocity spectral information is assessed automatically by the computer software using the Doppler equations.
CDI has been used in ophthalmology to assess the vascular characteristics in orbital tumors, Graves’ disease, retinal vascular occlusions, ischemic optic neuropathy, ocular ischemic syndrome and giant cell arteritis.Citation3–5 The utility of ultrasound B-scan has been explored for lacrimal drainage disorders but the same is not true of Color Doppler imaging.Citation6 There are no focused studies on the utility of CDI for lacrimal drainage, although a single study has evaluated its role in lacrimal sac tumors.Citation7,Citation8 The current study has attempted to assess the Color Doppler imaging characteristics of the lacrimal sac vasculature in states of health and disease.
Methods
Institutional review board and Ethics committee approval of L.V. Prasad Eye Institute was obtained. The study was conducted in accordance with the Declaration of Helsinki. Prospective study was performed on 20 lacrimal sacs of 20 eyes of 10 patients who underwent color doppler imaging at a tertiary care Dacryology service over a period of 6 months. All the patients were subjected to Duplex doppler scanning of the lacrimal sacs. Of the 20 lacrimal drainage systems studied, 8 were normal, 8 had primary acquired nasolacrimal duct obstruction (PANDO) and 4 were that of acute dacryocystitis.
Scanning was performed using LOGIQ S7 system (GE Healthcare, Chicago, USA) using a 5–15 MHz frequency, broad-spectrum linear matrix-array transducer (ML6-15, GE Healthcare, Chicago USA), which has applications for assessing small and focused anatomical regions. All patients were examined in the supine position. Following the application of a conductive gel, the transducer was directly placed over the skin corresponding to the lacrimal fossa region. The probe was then moved in transverse and cranio-caudal directions. Baseline evaluation of the lacrimal sacs was first performed in B-mode ultrasound (frequency – 15 MHz) to assess the sac dimensions, wall thickness, qualitative assessment of sac contents and surrounding structures. Color doppler ultrasound (frequency – 10 MHz, pulse repetition frequency – 3.2 kHz) was used to assess the peri-sac vasculature and the flow characteristics. Pulse wave doppler (frequency – 5 MHz, pulse repetition frequency – 3.7 kHz) was then performed to obtain peri-sac arterial spectral waveforms to assess the direction of flow, pulsatility, systolic peaks and forward flow through diastole. The color gain assessment took into account the potential blooming artifacts. Patient demographics, clinical presentation, duration of the disease and color doppler features like peri-sac vascular flow, peak systolic velocity (PSV), end-diastolic velocity (EDV), resistivity index (RI), arterial spectral waveforms and sac dimensions and wall thickness were analyzed. Resistivity index was calculated as RI = PSV- EDV/PSV.
Results
Twenty lacrimal sacs of ten patients with a mean age of 42 years (range: 6–67 years) were studied. The mean age of the PANDO group was 47.5 years (range: 25–67 years) and that of the acute dacryocystitis (AcDac) group was 34 (range: 6–51). Female: male ratio was 7:3. All the diseased eyes (8 PANDO and 4 AcDac) presented with epiphora and in addition, the AcDac patients had swelling and discharge. The mean duration of disease was 18 months in the PANDO group and 4.75 days in the AcDac group. The radiological features can be divided groupwise as follows:
Normal lacrimal sacs
The asymptomatic eyes with patent lacrimal drainage system showed well-defined hypoechoic lacrimal sacs with variable internal reflectivity. The mean sac dimensions measured by ultrasound were 5.3 × 3.8 mm (range: 5–7 × 3–4 mm) with a wall thickness of 0.4–0.7 mm at randomly selected points. Peri-sac vasculature was discernible with a mean peak systolic velocity of 9 cm/sec (range: 7–11.1 cm/sec) and mean end-diastolic velocity of 3.87 cm/sec (range: 2.8–5.4 cm/sec). The mean resistivity index was 0.57 (range: 0.49–0.65).
Lacrimal sacs in PANDO
The lacrimal sacs in PANDO were larger as compared to normal but remained as well-defined hypoechoic structures with multiple internal echoes of fluid and debris. The mean lacrimal sac dimensions were 9 × 6.6 mm (range: 6–17 × 5–10 mm). The mean wall thickness was 1.4 mm (range: 1.1–1.8 mm), nearly double that of the normal. Color doppler revealed increased vascular flow with moderate to high velocities without any internal flow disturbances. The mean peak systolic velocity was 13.07 cm/sec (range: 8.8–19.6 cm/sec) and the mean end-diastolic velocity was 4.63 cm/sec (range: 2.8–9.3 cm/sec). The mean resistivity index (0.67 [range: 0.53–0.72]) was increased as compared to the normal. The arterial spectral waveforms demonstrated low pulsatility, broad systolic peak and forward flow through diastole.
Lacrimal sacs in acute dacryocystitis
The lacrimal sacs in acute dacryocystitis were grossly larger as compared to those with PANDO and normal but largely remained as well-defined hypoechoic structures with large variations in internal echoes of fluid and debris. There was evidence of edema and sludge-like collection in the peri-sac areas. mean lacrimal sac dimensions were 11.5 × 9.75 mm (range: 10–12 × 5–13 mm). The mean wall thickness was 1.2 mm (range: 1.1–1.3 mm), nearly double that of the normal, but less than those with PANDO. Color doppler revealed grossly enhanced vascular flow with high vascular flow as compared to PANDO and normal. There was evidence of internal flow disturbances at multiple points within the vasculature. The mean peak systolic velocity was 18.8 cm/sec (range: 9.3–28 cm/sec) and the mean end-diastolic velocity was 8.5 cm/sec (range: 4.4–14.3 cm/sec). The mean resistivity index (0.53 [range: 0.49–0.58]) was much less as compared to the PANDO and normal. The arterial spectral waveforms demonstrated low pulsatility, sharper systolic peaks and persistent forward flow through diastole as compared to the PANDO group.
Discussion
The current study performed focused analysis of color doppler features of the lacrimal sac and peri-sac vasculature. The lacrimal sacs with PANDO were demonstrated to have larger dimensions and higher wall thickness as compared to the normal. Although the vascular flow was enhanced in both the PANDO and AcDac groups as compared to the normal; the PSV, EDV were far higher in cases of AcDac. In addition, the mean vascular resistivity index was the least in sacs with AcDac.
Color Doppler Imaging (CDI) is an advanced and specialized form of ultrasound that studies the vascular flow characteristics. As compared to the routine ultrasound, the CDI analyses the blood rather than solid tissues and the target has to be interrogated multiple times with multiple pulses to obtain meaningful data.Citation2 The flow images are colored, the color scale is calibrated in cm/sec and usually demonstrates the flow towards the probe as red-orange-yellow and the flow away from the probe in shades of blue (). Different colors at a focal point within the vessel lumen represent flow disturbances. Fast Fourier transformation techniques are utilized to obtain the Doppler shift information, which is then quantitatively represented as Doppler spectral waveforms.Citation1
Figure 1. Color Doppler imaging of lacrimal sac vasculature in a patient with left-sided PANDO. Note the vascular flow characteristics in the normal right lacrimal sac and compare it with that of the left-dilated lacrimal sac (arrow) with enhanced flow and thicker walls (hyper-reflective area denoted by tip of the arrow)
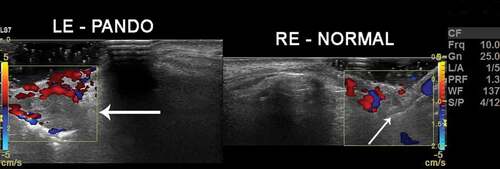
Figure 2. Color Doppler imaging in a patient with PANDO. Note the color scale calibrations and enhanced flow volumes. Vs – peak systolic velocity, Vd – end-diastolic velocity and RI – resistivity index. Note a comparatively high RI values in PANDO. The arrow demonstrates the lacrimal sac and its tip corresponds to the sac wall. Note the thickened wall. The graph below the image represents arterial spectral waveform which shows high systolic peaks (yellow +1 mark) and follow through diastole (yellow +2 mark)
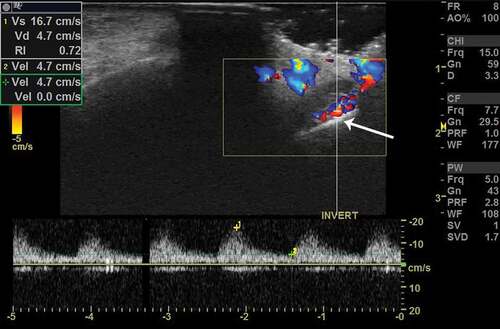
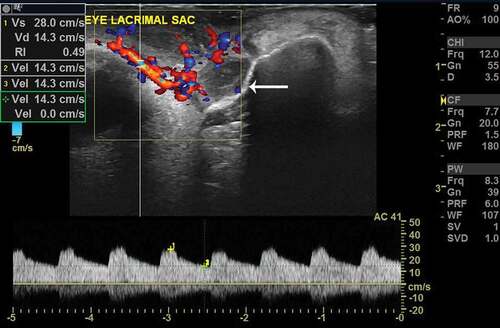
Figure 3. Color Doppler imaging in a patient with acute dacryocystitis. Note the color scale calibrations and grossly enhanced flow volumes. Also note the very high Vs (peak systolic velocity) and Vd (end-diastolic velocity) and quite reduced RI in comparison to that of PANDO in . The arrow demonstrates the lacrimal sac and its tip corresponds to the sac wall. Note the much dilated sac and much thickened wall as compared to PANDO and normal in figure 1. The arterial spectral waveform has higher and sharper systolic peaks (yellow +1 mark) with continuous and persistent follow through of the diastole (yellow +2 mark) as compared to that of PANDO in fig 2
The understanding of the lacrimal vascular flow characteristics can be enhanced with careful analysis of CDI features of ocular and orbital vasculature. The normal flow velocity waveform in ophthalmic artery resembles that of the internal carotid with high systolic and low diastolic flow.Citation3 The peak systolic velocity (PSV) in superior ophthalmic vein was recorded as −7.6 ± 1.8 cm/sec.Citation3 The PSV and EDV of central retinal artery and posterior ciliary arteries were 9.6 ± 1.4 & 2.4 ± 0.8 cm/sec and 11.3 ± 2.2 & 3.6 ± 1.2 cm/sec, respectively.Citation3 In comparison, the current study data of peri-sac blood flow from healthy subjects clearly demonstrate the similarities with the posterior ciliary circulation. The diastolic flow in lacrimal sac vasculature was enhanced in PANDO but grossly enhanced in acute dacryocystitis, which indicates vascular dilatation and lowering of the resistance in the infective stage. The enhanced blood flow in AcDac can be explained secondary to infection and inflammation of the sac and peri-sac tissues.
There are numerous interesting observations in the current study that merit further discussion. First, there was not only a demonstrable increase in the dimensions of the lacrimal sacs in acute dacryocystitis but also well-defined areas of fluid and sludge-like collection in the peri-sac areas. The radiological findings from this study validate the inclusion of peri-sac features in the clinical definition of acute dacryocystitis.Citation9 Secondly, it was interesting that in cases of PANDO, there was not only an increase in the wall thickness and resistivity index but also an increased systolic and diastolic flow as compared to normal. This could reflect the dilatation of the lacrimal sac vasculature. However, unlike acute dacryocystitis, there is no acute infection or acute inflammation that can possibly explain this phenomenon. The lacrimal sac vasculature specifically cavernous bodies has been linked to the etiopathogenesis of PANDO and this has been proposed based on histopathology, ultrastructural studies and tear proteomic assessment.Citation10,Citation11 The findings of CDI in PANDO lend support to the vascular theory of PANDO. Further detailed angiography studies would be needed to prove this link convincingly.
Limitations of the current study include smaller sample size, measurements at random points of peri-sac vasculature, depth ambiguity, Doppler artifacts and individual variations in velocities and measurements leading to a wider range. However, the strengths of this study are focused duplex scanning of lacrimal vasculature in both healthy and diseased states.
In conclusion, the current study is one of its kind to assess the CDI features of healthy lacrimal sacs and those with PANDO and acute dacryocystitis and demonstrated specific flow characteristics in each group. The diastolic flow is enhanced in acute dacryocystitis reflecting vascular dilatation and reduced resistance. There is a need to further explore this modality to better understand the pathophysiology of lacrimal drainage disorders, specially etiopathogenesis of primary acquired nasolacrimal duct obstruction.
Disclosure
Mohammad Javed Ali receives royalties from Springer for the textbook “Principles and Practice of Lacrimal Surgery” and Atlas “Atlas of Lacrimal Drainage Disorders”.
Additional information
Funding
References
- Bhargava S, Bhargava SK, Srivastava AK. Doppler principle and instrumentation. In: Bhargava S editor. Textbook of color Doppler imaging. 3rd. New Delhi: Jaypee Brothers Medical Publishers; 2019. p. 13–21.
- Evans DH, Jensen JA, Nielsen MB. Ultrasonic color Doppler imaging. Interface Focus. 2011;1:490–502.
- Lieb WE. Color Doppler imaging of the eye and orbit. Radiol Clin North Am. 1998;36:1059–71.
- Lecler A, Boucenna M, Lafitte F. Usefulness of color Doppler flow imaging in the management of lacrimal gland lesions. Eur Radiol. 2017;27:779–89.
- Williamson TH, Harris A. Color Doppler ultrasound imaging of the eye and orbit. Surv Ophthalmol. 1996;40:255–67.
- Mahesh L, Ali MJ. Imaging modalities for lacrimal disorders. In: MJ A editor. Principles and practice of lacrimal surgery. 2nd. Singapore: Springer; 2018. p. 111–22.
- Singh S, Ali MJ. Congenital dacryocystocele: A major review. Ophthalmic Plast Reconstructive Surg. 2019;35:309–17.
- Liu XY, Liu Y, Xu JM, Chen Q, Xiong W. Color Doppler ultrasound and contrast enhanced ultrasound in the diagnosis of lacrimal apparatus tumors. Oncol Lett. 2018;16:2215–20.
- Ali MJ, Joshi SD, Naik MN, Honavar SG. Clinical profile and management outcome of acute dacryocystitis: two decades of experience in a tertiary eye care center. Semin Ophthalmol. 2015;30:118–23.
- Ali MJ, Paulsen F. Etiopathogenesis of primary acquired nasolacrimal duct obstruction: what we know and what we need to know. Ophthalmic Plast Reconstr Surg. 2019;35:426–33.
- Ali MJ, Patnaik S, Kelkar N, Ali MJ, Kaur I. Alteration of tear cytokine expressions in primary acquired nasolacrimal duct obstruction – potential insights into the etiopathogenesis. Curr Eye Res. 2020;45:435–39.
