ABSTRACT
Purpose: Many intraocular pressure (IOP)-lowering medications contain benzalkonium chloride (BAK), a preservative associated with unfavorable outcomes.
A formulation of latanoprost 0.005% ophthalmic without BAK is approved by the FDA and indicated for reduction of IOP in patients with open-angle glaucoma or ocular hypertension. We present two preclinical studies of latanoprost 0.005% BAK-free vs latanoprost with BAK; one examining plasma and ocular tissue pharmacokinetics (PK) in New Zealand white rabbits, and one comparing in vivo IOP-lowering efficacy in healthy beagles.
Methods: In the PK study, one drop of treatment (latanoprost BAK-free or latanoprost with BAK) was instilled into both eyes of rabbits in each treatment group (n = 18). At 0.25, 0.5, 1, 4, 6, and 24 hours postdose, three rabbits per study group underwent terminal blood and tissue collection.
In the IOP study, in the first dosing period, both eyes of each beagle received either 1 drop latanoprost BAK-free or latanoprost with BAK, once daily for 10 days. After a 10-day washout period, a second 10-day dosing period was conducted and latanoprost BAK-free or latanoprost with BAK were dosed in the opposite eyes, respectively. IOP measurements were taken at 1, 6, and 12 hours postdose.
Results: The maximum plasma concentration for latanoprost BAK-free and latanoprost with BAK occurred 0.25 hours after administration (174.1 vs 217.2 pg/mL, respectively). Area under the concentration time curve from zero to infinity was highest in aqueous humor for latanoprost BAK-free and latanoprost with BAK (133.1 vs 119.6 hr·ng/mL, respectively) and was not estimable in vitreous humor. In beagles, once-daily administration of latanoprost BAK-free or latanoprost with BAK led to a significant reduction in IOP vs baseline (P < .001); there was no difference between groups (P > .05).
Conclusions: Latanoprost BAK-free showed comparable activity in reducing IOP, and comparable plasma and ocular PK parameters to latanoprost with BAK.
Introduction
Prostaglandin selective FP receptor agonists are ophthalmic medications used to reduce intraocular pressure (IOP).Citation1 Latanoprost 0.005% (50 µg/mL) ophthalmic solution (Xalatan®, Pharmacia & Upjohn Co, New York, NY) is a colorless, isotonic, sterile, buffered aqueous solution that contains 0.02% benzalkonium chloride (BAK) as a preservative.Citation2 The concentration of BAK in IOP-lowering medications ranges from 0.004% to 0.02%.Citation3–5
The use of BAK as a preservative is associated with a number of unfavorable outcomes, including decreased epithelial cell integrity, increased epithelial cell apoptosis, eye irritation, and increased risk for ocular allergies and delayed hypersensitivity reactions.Citation6 Patients with glaucoma often require the use of IOP-lowering medications for years, leading to an increased risk of developing ocular surface disease. This results in an unmet need for a medication that can lower IOP in patients with open-angle glaucoma (OAG) or ocular hypertension (OHT) without the use of BAK as a preservative.
Latanoprost 0.005% ophthalmic emulsion without BAK (Xelpros™, Sun Pharmaceutical Industries, Inc., Cranbury, NJ) is a prostaglandin F2α analog that is approved by the Food and Drug Administration and indicated for the reduction of elevated IOP in patients with OAG or OHT.Citation2 This formulation of latanoprost 0.005% BAK-free uses potassium sorbate as a preservative,Citation2 which helps eliminate many of the issues that are seen in medications that use BAK.Citation7
Here we present the results of two preclinical studies; one that examines the plasma and ocular tissue pharmacokinetics (PK) of latanoprost 0.005% BAK-free compared to latanoprost 0.005% ophthalmic solution in New Zealand white rabbits (herein referred to as PK study), and one that compares the in-vivo efficacy of latanoprost 0.005% BAK-free vs latanoprost 0.005% in healthy beagles (herein referred to as IOP study).
Materials and methods
Experimental design
In the PK study, 36 male New Zealand white rabbits, bred at RAJ Biotech (Pune, India), were received from Laboratory Animal Resources (Tandalja, Vadodara, India). The rabbits were 5–6 months old and weighing 1.8–2.5 kg at the time of randomization to be included in the study. A single drop (30 µL) of either latanoprost 0.005% BAK-free or latanoprost 0.005% with BAK (herein referred to as reference) was instilled into both eyes of the rabbits (n = 18 rabbits for each group). At 0.25, 0.5, 1, 4, 6, and 24 hours postdose, three rabbits each per study group underwent terminal blood and tissue collection.
In the IPO study, six healthy male beagle dogs were received from Laboratory Animal Resources (Tandalja, Vadodara, India). For inclusion in this two-period, crossover study, the dogs were aged 2–4 years old and weighed between 10–20 kg at study initiation. In the first dosing period, the left and right eyes of each animal were administered a single drop (30 µL) of latanoprost 0.005% BAK-free or reference, respectively, once daily for 10 days. After a washout period of 10 days, the second dosing period of 10 days was carried out; latanoprost 0.005% BAK-free or reference was dosed in the opposite eyes, respectively.
All animals were housed under standard conditions and allowed an acclimation period of approximately one week between receipt and starting the treatment phases of both studies. The general health and ocular condition of each animal in both studies was evaluated by a veterinarian prior to initiation of treatment. The study plans were reviewed and approved by the Institutional Animal Ethics Committee and Committee for the Purpose of Control and Supervision of Experiment on Animals and their recommendations for animal care and handling were followed. The beagles were not euthanized in the course of, or following the completion of, this study.
Sample collection
In the PK study, at each postdose time point, three rabbits per study group were sacrificed by carbon dioxide asphyxiation, and blood samples as well as aqueous and vitreous humors were immediately collected.
For blood and plasma analysis, approximately 1500 µL of blood was collected from the ear vein into a microcentrifuge tube containing 20 µL of 10% potassium EDTA. Plasma was separated from the collected blood samples by centrifugation at approximately 3000 rpm for 10 minutes at 10°C and stored into a labeled collection vial. The samples were stored at −65°C (± 10°C) in a deep freezer and transferred to test site for analysis.
Ocular tissues (ie, conjunctiva, cornea, lens, iris and ciliary body, retina, sclera [with choroid], optic nerve, and eye lid) from both eyes were dissected and adherent tissues trimmed off. Samples from the right and left eyes of the animal were prepared and analyzed separately.
The free acid of latanoprost was selected as the analyte, as latanoprost is rapidly hydrolyzed to the active acidic metabolite following ocular administration (data on file). Tissues were rinsed with phosphate buffer saline (pH 7.4 ± 0.2) and blotted on Whatman filter paper to facilitate blood removal. The tissues were individually weighed and prepared under a constant cooling ice bath to a 5% w/v with phosphate buffer saline. The tissues were chopped and homogenized at 26000 rpm for at least 1 minute before being placed in 5 mL cryovials or 15 mL centrifuge tubes and stored in a deep freezer at −65°C (± 10°C) until transferred for analysis.
Aqueous and vitreous humors and ocular tissues were analyzed for analyte concentrations using validated liquid chromatography with tandem mass spectrometry methods. The lower limit of quantitation (LLOQ) was established as 0.101 ng/mL for conjunctiva, optic nerve, eye lid, sclera, and aqueous humor, and 50.3 pg/mL for iris and ciliary body, lens, plasma, retina, cornea, and vitreous humor. The chromatographic conditions were hypersil columns BDSC-18 (250 x 4.6 mm x 5 cm) with a flow rate of 1.5 mL/min, injection volume of 100 mcl, run time of 15 minutes, and UV detection at 297 nm, with mobile phase as buffer:methanol:acetonitrile 75:20:5 (Acetate buffer pH 3.4). For aqueous humor samples, mobile phase was modified to methanol:water 1:1 containing octane sulfonic acid sodium and dibutylamine with pH 3.0 with orthophosphoric acid.
Statistical analyses
Pharmacokinetic parameters, including elimination half-life (t1/2), time to maximum concentration, maximum observed concentration (Cmax), area under the concentration time curve from zero to time of last non-zero concentration, and area under the concentration time curve from zero to infinity (AUC0-inf) were determined from the concentration data based on a noncompartmental analysis using WinNonlin® Software version 5.3 (Pharsight Corporation, St. Louis, MO, USA).
Intraocular pressure efficacy measurements
In the IOP study, pretreatment IOP was measured using a Pneumatonometer Model 30 Classic™ (Reichert, New York, USA) twice daily prior to the study at 8:00 am and 8:00 pm and the values averaged to obtain the baseline IOP measurement. For each IOP measurement, the beagle was restrained manually without sedation in the head-up position and received one drop of 0.5% proparacaine as a topical anesthetic prior to the pneumotonometer being placed on the cornea. The probe was then placed on the cornea for 10–15 seconds and, for each time point, seven consecutive measurements were taken and averaged to obtain a single value.
During the drug administration phases, which started 48 hours after the baseline readings began, the 8:00 am IOP measurements were obtained and then the drug was administered; additional IOP measurements were taken at 1, 6, and 12 hours postdose. After the study drug administration phase, IOP measurements were obtained once daily at 9:00 am. Pupil size, blepharospasm, and conjunctival hyperemia were also measured before, during, and after the medication administration periods.
Statistical analyses
After subtracting the initial baseline IOP readings, the percent reduction of IOP during the treatment period was calculated and the difference between latanoprost 0.005% BAK-free and reference was tested statistically. Changes in IOP were analyzed using the repeated-measures analysis of variance followed by Bonferroni posttest as compared with their initial respective values and were determined to be significant if P < .05. For the plasma-concentration time data, the data was analyzed by unpaired t-test to each respective time point, with P < .05 considered as statistically significant.
Results
Pharmacokinetic study
Plasma pharmacokinetics
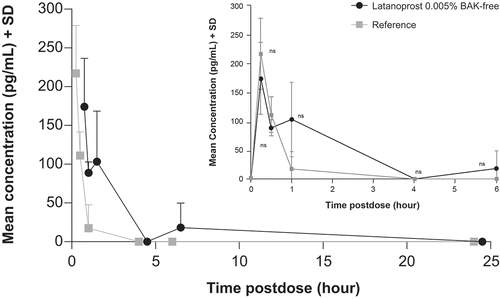
Following administration of a single dose of latanoprost 0.005% BAK-free and reference, the plasma Cmax was reached 0.25 hours postdose, at 174.1 and 217.2 pg/mL, respectively. For both treatments, there was a slow decline of latanoprost acid, as shown in and . Results of the plasma PK parameters are shown in .
Table 1. Mean ± standard deviation plasma concentration-time data of latanoprost acid after ocular administration of 1 drop of latanoprost 0.005% BAK-free or reference in New Zealand white rabbits
Table 2. Mean plasma pharmacokinetic profile of latanoprost acid after ocular administration of 1 drop of latanoprost 0.005% BAK-free or reference in New Zealand white rabbits
Ocular pharmacokinetics
Overall, the percent of samples with quantifiable plasma and tissue concentrations ranged from 36% to 100%. One hour after administration of one drop of latanoprost 0.005% BAK-free, higher levels of latanoprost acid were seen in the aqueous humor vs vitreous humor, and the cornea was the tissue with the highest level of latanoprost acid. After administration of the reference drug at 1-hour postdose, the aqueous humor and cornea also had the highest levels of latanoprost acid ().
Table 3. Ocular fluid and tissue concentrations ± standard deviation of latanoprost acid after ocular administration of 1 drop of latanoprost 0.005% BAK-free or reference in New Zealand white rabbits
Following administration of latanoprost 0.005% BAK-free, the t1/2 for latanoprost acid in the aqueous humor, vitreous humor, and ocular tissues was 1.4 to 41.4 hours. Following administration of reference drug, the t1/2 in the same tissues was 1.5 to 27.4 hours. The AUC(0-inf) after latanoprost 0.005% BAK-free administration was highest in the aqueous humor (133.1 hr·ng/mL); after reference drug administration, the highest AUC(0-inf) was also seen in the aqueous humor (119.6 hr·ng/mL) ().
Table 4. Mean pharmacokinetic parameters of latanoprost acid in ocular fluid and tissue after ocular administration of 1 drop of latanoprost 0.005% BAK-free or reference in New Zealand white rabbits
Intraocular pressure study
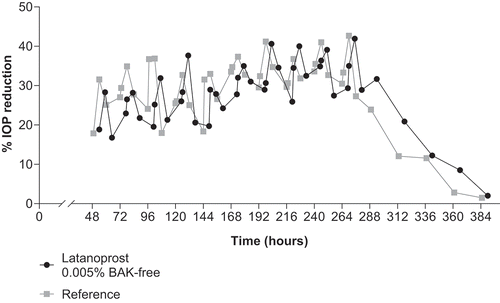
Once daily administration of latanoprost 0.005% BAK-free and reference led to a significant reduction in IOP vs baseline (P < .001); however, there was no difference between the two treatment groups (P > .05; ). The maximum reduction in IOP was seen at 6 and 12 hours after administration, and IOP remained decreased for up to 48 hours after treatment was discontinued ( and ).
Table 5. Mean ± standard error of the mean intraocular pressure in beagles after ocular administration of latanoprost 0.005% BAK-free or reference
Figure 2. Mean IOP ± standard error of the mean of IOP during and after once daily administration of latanoprost 0.005% BAK-free and reference in beagles
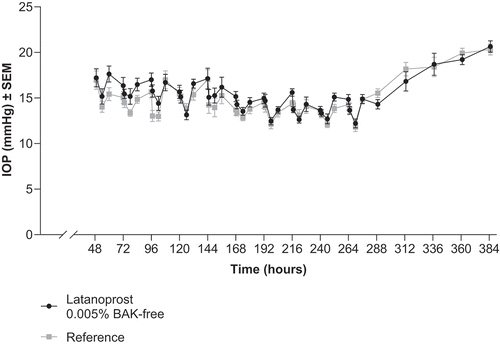
Figure 3. Mean + standard error of the mean percent of IOP decrease during and after once-daily administration of latanoprost 0.005% BAK-free and reference in beagles
Administration of both latanoprost 0.005% BAK-free and reference resulted in significant decreases in pupillary diameter vs baseline (P < .001). All animals in both test groups developed extreme miosis within 1 hour of drug administration. Mild conjunctival hyperemia was observed in several eyes treated with latanoprost 0.005% BAK-free and reference. There were no observed cases of blepharospasm or aqueous flare in either treatment group.
Discussion
Patients with OAG or OHT often use IOP-lowering medication for years, if not decades, leading to an increased risk of cellular death. Prostaglandin analogues (PGAs) are the first step of treatment in glaucoma management as their efficacy and tolerability is superior to other drug classes.Citation8 Treatment PGAs, such as latanoprost 0.005%, achieves higher reduction of IOP (25%–33%) vs beta-adrenergic antagonists, alpha-adrenergic antagonists, and parasympathomimetic agents (all 20%–25%), and topical carbonic anhydrase inhibitors (15%–20%).Citation8
BAK is toxic to the conjunctival and corneal epithelium; the extent of the cellular damage is both time- and dose-dependent.Citation6,Citation9 A decrease in goblet cell density, resulting in decreased mucin production and tear film stability, has also been associated with BAK.Citation9 Limiting the ocular exposure to BAK may help prevent loss of goblet cells as well as damage to the corneal and conjunctival epithelial cells.Citation6,Citation9 Preventing destruction of goblet cells, as well as maintaining ocular surface integrity help maintain tear film integrity, which in turn helps prevent symptoms of dry eye disease.Citation10 Reducing the amount of BAK in IOP-lowering medication improves tear-break up time, corneal staining scores, and conjunctival hyperemia in patients with glaucoma.Citation11 Patients with glaucoma may cycle through multiple treatment options to find the best one to lower their IOP. Studies show switching a patient from a BAK-containing PGA to a BAK-free PGA significantly improves corneal staining (P < .001).Citation12 Reducing, or even delaying, exposure to BAK in patients with glaucoma can help protect their ocular surface, therefore lengthening the time before patients begin to experience adverse effects from BAK.
For the reduction of IOP, latanoprost 0.005% BAK-free was found to be comparable to reference in healthy male beagle dogs. The group mean of maximal intraocular decrease in each animal during the treatment period was 42% and 43% for latanoprost 0.005% BAK-free and reference, respectively. This supports the use of latanoprost 0.005% BAK-free as an alternative to formulations with BAK with no loss in efficacy.
In the current study, latanoprost was absorbed into the eye with the highest concentrations found in the anterior tissues and the lowest concentrations in the posterior tissues. The analyte levels in the tissues vs the aqueous humor were very low but consistently above the LLOQ for both latanoprost 0.005% BAK-free and reference. These results are comparable to aqueous humor concentration of latanoprost in humans. After administration of one drop latanoprost (1 of 6 different formulations), the concentration ± SE of latanoprost free acid ranged from 1.9 ± 0.5 to 21.3 ± 11.7 over 6 hours.Citation13 For the anterior and periocular tissues, the calculable tissue to aqueous humor ratios obtained were most likely observable because these tissues were directly exposed to the study drugs. For the other tissues, the samples were below the quantification limit. The comparability of tissue concentrations is limited by the fact that the exposures of the ocular tissues to latanoprost 0.005% BAK-free and reference, except in the aqueous humor, was intrinsically very low.
Although there is a large variation in the tissue concentration values, this is not uncommon in preclinical PK studies where data are derived from different animals by terminal sacrifice method. In fact, similarly high variation are seen in a comparative study with bimatoprost in rabbits. This study demonstrated standard deviations (SDs) >50% of the mean for the Cmax in aqueous humor.Citation14 Additionally, there is no significant difference in ocular pharmacokinetics between latanoprost 0.005% BAK-free and reference; as also confirmed from a comparable pharmacodynamics effect of the two products.
The use of BAK in PGAs helps increase the penetration of the active molecule into the tear film by disrupting the lipid membrane of the cells. The micelle formulation of latanoprost BAK-free helps encapsulate the medication and allows it to solubilize and penetrate the tear film. In addition to our study, a PK study of preservative-free latanoprost 0.005% vs BAK-preserved latanoprost 0.005% in 120 rabbits who received either a single dose or multidose of the study drug showed no significant difference in AUC0-12h values (P > .05 for both).Citation15 The results of these PK studies support the BAK-free formulation achieving comparable tissue penetration and concentration as the formulation with BAK. Our study expands upon the results in the study by Zhou et al., by including the PK profile for latanoprost 0.005% BAK-free and reference in all ocular tissues.
A limitation of these studies is the methodology used; results from preclinical studies are often not predictive of ocular tissue penetration in a clinical setting. However, the corresponding clinical PK studies are not able to be ethically carried out as they may damage healthy subject’s eyes. Additionally, while we were able to accurately measure the mean PK profile and, by extension, additional PK profiles, because the rabbits were euthanized in order to obtain the tissue for measurements, we were not able to calculate the corresponding SD for the additional PK values.
Conclusion
Latanoprost 0.005% BAK-free demonstrates comparable efficacy to latanoprost 0.005% with BAK, while ocular tissue concentrations and pharmacokinetics remains similar. These studies support the use of latanoprost 0.005% BAK-free for the treatment of OAG or OHT.
Declaration of interests
AH and AJ are both employees of Sun Pharma Advanced Research Company, Ltd.
Statement of ethics
The study plans were reviewed and approved by the Institutional Animal Ethics Committees in India, and their recommendations for animal care and handling were followed.
Acknowledgments
The authors would like to thank Dr. Vinod Burade of Sun Pharmaceutical Industries, Ltd., for his assistance with the manuscript. Writing and editorial assistance was provided by Jennifer Meyering, RN, MS, CMPP, of AlphaBioCom (King of Prussia, PA), with funding provided by Sun Pharmaceutical Industries, Inc (Princeton, NJ).
Additional information
Funding
References
- Lim K, Nau C, O’Byrne M, Hodge D, Toris C, McLaren J, Johnson D. Mechanism of action of bimatoprost, latanoprost, and travoprost in healthy subjects. A Crossover Study. Ophthalmology. 2008;115:790–795 e794. doi:10.1016/j.ophtha.2007.07.002.
- Xalatan® (latanoprost ophthalmic solution) 0.005%. Full prescribing information. New York (NY): Pharmacia & Upjohn Co; 2012.
- Timoptic® 0.25% and 0.5% (timolol maleate ophthalmic solution). Full prescribing information. Whitehouse Station (NJ): Merck & Co., Inc; 2005.
- Iopidine (apraclonidine hydrochloride ophthalmic solution). Full prescribing information. Fort Worth (TX): Alcon Laboratories; 2004.
- Betagan(r) (levobunolol hydrochloride ophthalmic solution, usp). Full prescribing information. Irvine (Ca): Allergan; 2017.
- Pisella P, Pouliquen P, Baudouin C. Prevalence of ocular symptoms and signs with preserved and preservative free glaucoma medication. Br J Ophthalmol. 2002;86(4):418–23. doi:10.1136/bjo.86.4.418.
- Ammar D, Noecker R, Kahook M. Effects of benzalkonium chloride-preserved, polyquad-preserved, and sofzia-preserved topical glaucoma medications on human ocular epithelial cells. Adv Ther. 2010;27(11):837–45. doi:10.1007/s12325-010-0070-1.
- Prum B Jr., Rosenberg L, Gedde S, Mansberger SL, Stein JD, Moroi SE, Herndon LW, Lim MC, Williams RD. Primary open-angle glaucoma preferred practice pattern® guidelines. Ophthalmology. 2016;123:P41–P111. doi:10.1016/j.ophtha.2015.10.053.
- Wilson W, Duncan A, Jay J. Effect of benzalkonium chloride on the stability of the precorneal tear film in rabbit and man. Br J Ophthalmol. 1975;59(11):667–69. doi:10.1136/bjo.59.11.667.
- Craig J, Nelson J, Azar D, Belmonte C, Bron A, Chauhan S, de Paiva C, Gomes J, Hammitt K, Jones L, et al. TFOS DEWS II report executive summary. Ocul Surf. 2017;15(4):802–12. doi:10.1016/j.jtos.2017.08.003.
- Walimbe T, Chelerkar V, Bhagat P, Joshi A, Raut A. Effect of benzalkonium chloride-free latanoprost ophthalmic solution on ocular surface in patients with glaucoma. Clin Ophthalmol. 2016;10:821–27. doi:10.2147/OPTH.S102976.
- Lopes N, Gracitelli C, Chalita M, de Faria N. Ocular surface evaluation after the substitution of benzalkonium chloride preserved prostaglandin eye drops by a preservative-free prostaglandin analogue. Med Hypothesis Discov Innov Ophthalmol. 2019;8:52–56.
- Sekine Y, Shimada M, Satake S, Okubo M, Hisaka A, Hara T, Honjo M, Aihara M. Pharmacokinetic analysis of intraocular penetration of latanoprost solutions with different preservatives in human eyes. J Ocul Pharmacol Ther. 2018;34(3):280–86. doi:10.1089/jop.2017.0091.
- Shafiee A, Bowman LM, Hou E, Hosseini K. Ocular pharmacokinetics of bimatoprost formulated in durasite compared to bimatoprost 0.03% ophthalmic solution in pigmented rabbit eyes. Clin Ophthalmol. 2013;7:1549–56. doi:10.2147/OPTH.S48766.
- Zhou T, Miao Y, Li Z, Lu P, Liang Z, Yang J, He J, Xia H, Zhang Z, Zhang J. A comparative ocular pharmacokinetics study of preservative-free latanoprost unit-dose eye drops and a benzalkonium chloride-preserved branded product following topical application to rabbits. J Ocul Pharmacol Ther. 2020;36:522–28. doi:10.1089/jop.2019.0102.