ABSTRACT
Purpose: To evaluate rotational stability, tilt and decentration of a new monofocal IOL with a 7.0 mm optic and frame haptics.
Methods: Prospective post-market clinical follow-up study at the Kepler University Hospital Linz, Austria. An Aspira-aXA (HumanOptics, Germany) was implanted in 74 eyes of 42 cataract patients. The lens was manufactured with toric markings. IOL rotational stability was evaluated by comparing its position at the end of surgery (EoS) versus 1 day, 1 week, 1 month and 4 months postoperatively. IOL tilt and decentration were measured using a Scheimpflug camera at 1 week, 1 month and 4 months.
Results: Median absolute IOL rotation was 1.42 degrees (n = 52; mean = 2.18 ± 2.23°) within 1 day after surgery and was significantly higher compared to all later intervals (median <1.0 degree; P = .001). At the 4 months follow-up, IOL rotation was within 5.0 degrees in 85% of the eyes (n = 40) and within 10.0 degrees in 98% (n = 46) of the eyes. The only eye with an IOL rotation of ≥ 10.ty0 degrees (EoS vs. 1 day) had an AL of 26.45 mm. At the last follow-up, the IOL vertical and horizontal tilt referenced to the pupillary axis was in average less than 1.5 degrees in both eyes (n = 54; maximum 5.85°). Decentration in both meridians was on average less than 0.10 mm in both eyes (maximum 0.30 mm).
Conclusion: The one-piece Aspira-aXA IOL showed good and stable positioning within the capsular bag over a 4 months period.
Introduction
Despite the existence of a plethora of intraocular lens (IOL) designs, most foldable IOLs available for cataract surgery have a fixed optic diameter of 6.0 mm. This might limit IOL options for patients with large pupils and/or vitreoretinal diseases. For instance, the occurrence of pseudophakic photic phenomena might occur more frequently in large pupils when the IOL optic diameter is only slightly smaller than the pupil size causing optical distortions.Citation1 But even smaller pupils can suffer from optical distortion from oblique incident light rays. Hence, implantation of an IOL with a large optic might contribute to reduce these undesired optical images. From a vitreoretinal perspective, it has been shown that the creation of a larger capsulorhexis with implantation of a 3-piece IOL with a 7.0 mm optic (versus a 6.0 mm optic) contributed to a larger anterior capsule opening after cataract surgery in patients with diabetes mellitus facilitating the postoperative view of the vitreous and retina.Citation2
None of these stated advantages, however, can predict the performance of an IOL in terms of postoperative stability which is crucial for achieving optimal visual outcomes. Rotational stability is particularly relevant for toric IOLs since every degree of misalignment reduces the IOL effectiveness and can lead to unpredictable outcomes. The effect of IOL decentration larger than 0.4 mm and tilt more than 5.0 degrees can also severely deteriorate visual performance of premium IOLs such as aberration-correcting and multifocal IOLs. Nonetheless, all IOLs are not equal and their post-operative behavior in the capsular bag is highly dependent on their unique design. Several factors have been reported to contribute to IOL stability including IOL material properties,Citation3 IOL overall diameter,Citation4 haptics design combined with axial length (AL),Citation5,Citation6 IOL surface treatmentCitation3 and haptic texture.Citation7
Therefore, measurement of the postoperative stability of a new lens design is essential to ensure that the IOL platform is adequate for the development of premium IOLs. The purpose of the current study was to evaluate the rotational stability, tilt and decentration of a new monofocal aberration-free IOL with a 7.0 mm optic zone and frame haptics (Aspira-aXA, HumanOptics AG, Erlangen, Germany) after implantation in cataract patients. The Aspira-aXA has been granted a CE-mark approval for use in 2016 but was launched to the market in 2018.
Methods
This prospective observational study was conducted at the Kepler University Hospital Linz, Austria and all patients provided written informed consent before enrolment in the study. The study adhered to the tenets of the declaration of Helsinki and was approved by the local ethics committee (Nr: 1024/2017).
Patients scheduled to have monocular or binocular cataract surgery were consecutively included when their dilated pupil size was larger than 5.5 mm. Only patients with availability, willingness, and sufficient cognitive awareness to comply with examination procedures were included in the study. The obtainable diopter range for the study lenses was between +10.0 and +25.0 D, therefore required IOL power had to be within this range. Eyes with previous corneal or intraocular surgery, uncontrolled glaucoma with IOP greater than 24.0 mmHg, dry macular degeneration, amblyopia and any other ocular pathology other than cataract were excluded.
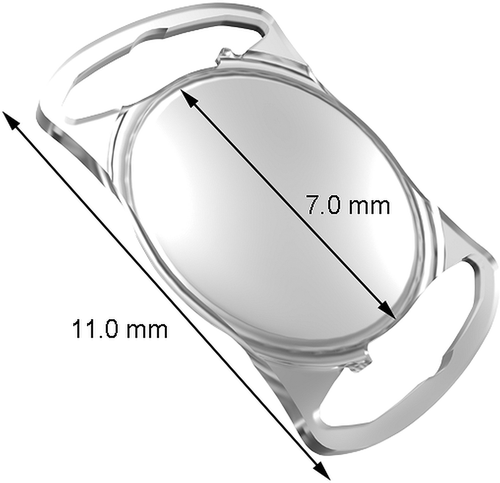
The Aspira-aXA IOL (), a one-piece monofocal hydrophilic acrylic posterior chamber IOL with cut-out haptics was implanted in 74 eyes of 42 patients. The lens has an aspheric aberration-free clear optic diameter of 7.0 mm and an overall diameter of 11.0 mm. Its posterior surface is designed with a 360-degree lens epithelial cell barrier. All IOLs were non-toric but manufactured with a toric marking in order to assess the rotational stability.
Surgical technique
All surgeries were performed between February 2018 and August 2019 by three surgeons (MB, PL, RS) under topical anesthesia. After a clear corneal incision (2.0 mm to 2.5 mm), the anterior chamber was filled with a dispersive OVD [Polyvisc 2.0%, Polytech Domilens GmbH, Rossdorf, Germany]. A creation of a continuous curvilinear capsulorhexis slightly smaller than the IOL optic diameter (approximately 6.5 mm) was performed in all cases. After a standard phacoemulsification procedure, the capsular bag was filled with a cohesive OVD [Amvisc (Bausch & Lomb, Rochester, NY, USA) or Healon 5, (Johnson & Johnson, New Brunswick, NJ, USA)]. All IOLs were implanted vertically (80° to 120°) using the ViscoJet 2.2 injector (Medicel AG, Wolfhalden, Switzerland) in combination with the Autoloading Safeloader system (HumanOptics AG, Erlangen, Germany). The procedure of all surgeries was recorded. We paid attention to remove carefully the OVD from behind the IOL at the end of surgery.
Preoperative and postoperative examinations
Preoperatively, all patients had an ophthalmologic examination including medical history, slit lamp examination, funduscopy, tonometry, Scheimpflug tomography (Pentacam HR, Oculus Optikgeräte GmbH, Wetzlar, Germany), partial coherence interferometry and/or swept source OCT biometry (IOLMaster 500 and 700, Carl Zeiss AG, Oberkochen, Germany), monocular manifest refraction, and corrected distance visual acuity (CDVA).
Postoperatively, patients were examined at 1 day, 1 week, 1 month and 4 months after surgery. Examinations included funduscopy, tonometry, Scheimpflug tomography and keratometry. Corrected distance visual acuity (CDVA) was measured at the 1 month and 4 months visits using the standard ETDRS chart (Precision Vision, Woodstock, IL, USA).
Assessment of IOL rotation
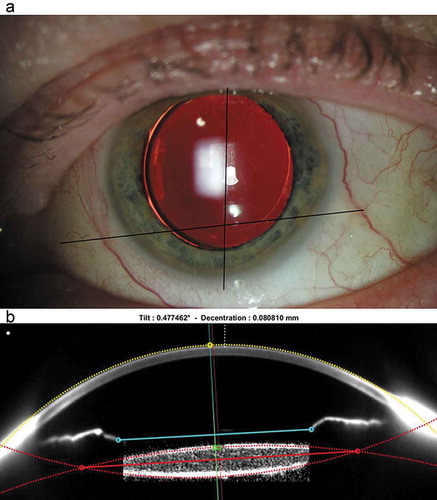
IOL position was assessed at the end of surgery (EoS), 1 day, 1 week, 1 month and 4 months after surgery. An intraoperative image at the EoS (screenshot of the surgical video) was taken (baseline) and retro-illumination slit lamp images were taken at each follow-up. To asses rotational stability of the IOL, we used the method according to Wolffsohn and BuckhurstCitation8 using Adobe Photoshop version CS5 (San Jose, CA, USA). In brief, for all images a reference axis was drawn by joining two critical details (e.g. Axenfeld-loops, limbal vessels) that were clearly identifiable and sufficiently far apart. The IOL orientation axis was drawn by connecting the two toric markers placed at the periphery of the optics or haptic insertion (see ). The angle between the reference and the comparison axis was then calculated and tracked throughout the study course. The change of IOL orientation at follow-up stages 1 day, 1 week, 1 month, and 4 months referenced to the respective value at EoS was defined as the angle of rotation of the IOL. The entire analysis was performed by the same investigator.
Figure 2. (a) Example of rotational stability evaluation performed with Photoshop. The reference axis is drawn by joining two critical details (e.g. Axenfeld-loops, limbal vessels) and the comparison axis is drawn by joining the IOL toric markings. (b) Example of tilt and decentration evaluation with customized Matlab code demonstrated with a raw image of Pentacam. For study data, a geometric distortion correction of the image was applied before data analysis
Assessment of IOL tilt and decentration
Tilt and decentration of the IOL were evaluated from images taken with the Pentacam Scheimpflug imaging system at the horizontal and vertical meridians. The raw images were directly processed via a customized code written in Matlab 9.8 R2020a (The MathWorks, Inc., Natick, MA; USA) according to the methods of Rosales and Marcos and de Castro et al.Citation9,Citation10 For all pictures a geometric distortion correction was applied before image processing, in order to address a Pentacam related image-compression along the z-axis. The customized code was able to detect the edges of the cornea, visible iris segments and the IOL and to fit the curves of these areas ().
The IOL axis (thin red line) was defined as the perpendicular line crossing the IOL center. The radius (yellow dotted line) of the central cornea and its intersection with the pupil center (thin blue line) determined the reference axis (yellow line). The distance between the reference axis and the IOL center was defined as decentration (in mm). The angle between the IOL axis and the reference axis was defined as horizontal and vertical tilt (in degree).
Statistical analysis
Data analysis was performed using Analyse-it® software, version 4.65.2 (Analyse-it Software, Ltd., Leeds, UK). Data were checked first for normal distribution using the Shapiro Wilk test. Variables such as axial length of the eye, age, IOL power, refraction, visual acuity, IOP, degrees of IOL rotation, tilt and decentration were summarized with explorative statistics (n, mean, standard deviation, range and median). Refraction was expressed in diopters (D) and visual acuity in logMAR units for analysis.
Tilt and decentration are displayed as plots. Absolute outcome values of tilt and decentration along the x and y axis are reported. Student’s t-test and analysis of variance (ANOVA) were used for comparison of normally distributed values, and the non-parametric Wilcoxon-test and Friedman-test (repeated measures) were used for comparisons between arbitrarily distributed data. Potential correlations between IOL rotation/tilt/decentration and AL were calculated using Spearman’s correlation coefficient. For all statistical tests, a P-value less than 0.05 was considered statistically significant.
Results
The study cohort comprised 74 eyes of 42 patients (19 females and 23 males). The mean age at the time of surgery was 70.17 ± 7.47 years (median 71.5, range 55.0 to 87.0) and mean IOL power was 21.66 ± 2.28 D (median 22.0, range 16.50 to 25.00). Preoperative clinical data are shown in . All patients completed the 1-month follow-up examinations (n = 74 eyes) and 66 eyes were available at the 4-month follow-up. One patient (two eyes) was lost to follow up, one patient (two eyes) dropped out due to health issues and two patients (four eyes) because of difficulties to comply with examination procedures. All parameters were arbitrarily distributed with the exception of age (p = .019) and ACD (p = .40).
Table 1. Preoperative patient demographics; values are expressed as mean ± SD and median (range)
Rotational stability
Due to blurry images, insufficient mydriatic conditions (toric markings not visible) and non-visible vessels not all intra- and postoperative images could be evaluated for rotational stability. The available respective numbers which could be evaluated at the follow-up examinations are listed in .
Table 2. IOL rotation at different follow-up stages to the entire study population
All IOLs were implanted in vertical direction (80° to 120°). Median absolute IOL rotation was 1.42 within 1 day after surgery and was significantly higher compared to all other time-points (median < 1.0 degree; P = .001). and show the rotation values from the end of surgery (EoS) versus 1 day and 4 months postoperatively and in between follow-ups. Larger amounts of rotation seemed to mostly take place between EoS and day one, whereas rotation between all later points in time happened to a significantly lower degree. At the 4 months follow-up, median IOL rotation was 1.54 degrees (mean = 2.34 ± 2.39°). IOL rotation was within 5.0 degrees in 85% of the eyes (n = 40) and within 10.0 degrees in 98% of the eyes (n = 46).
Figure 3. IOL rotational stability boxplot for all eyes from end of surgery (EoS) to each follow-up and in between follow-ups
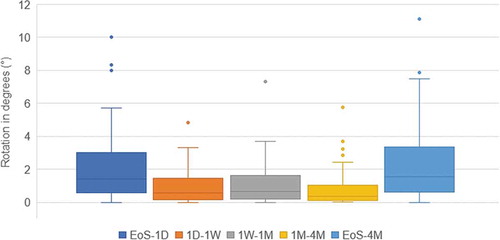
Only in one eye IOL rotation exceeded 10.0 degrees (11.13°) from EoS to 4 months. The majority of rotation occurred within the first day postoperatively (10.0°) and decreased to less than 1.0 degree thereafter. This eye had an AL of 26.45 mm and experienced a slight IOP spike following the surgery (23.0 mmHg). After medication, the IOP returned to a normal value one week postoperatively (18 mmHg).
There was no correlation between AL and absolute rotation from EoS to 4 months (Spearmans’s r = 0.132; p = .38).
Tilt and decentration
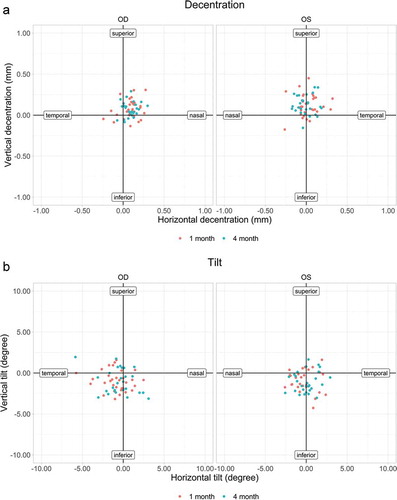
Due to insufficient image quality (measurement not possible or visible iris segments not sufficient) not all postoperative Scheimpflug images could be evaluated. Tilt and decentration were evaluated for right (OD) and left (OS) eyes separately as well as for the horizontal (0°) and vertical (90°) meridian. shows mean absolute tilt and decentration values for OD and OS in both meridians (only values available for the 1 week, 1 month and 4 months follow-ups are shown). There were no significant differences between all measurements. ) show tilt and decentration at 1 month (n = 64) and 4 months (n = 54) postoperatively. All IOLs had < 6.0° of tilt (maximum 5.84°) and less than 0.4 mm of decentration (maximum 0.34 mm). At the last follow-up, most IOLs were decentred superior (77% OD and 89% OS) and nasally (88% OD and 52% OS). The majority of the IOLs were tilted inferior (73% OD and 79% OS). In the right eyes (58%), the IOL tended to tilt temporal while in the left eyes (52%) the IOL tended to tilt nasally. There was no significant correlation between tilt and AL (horizontal meridian: Spearman’s r = −0.09, p = .51; vertical meridian: Spearman’s r = 0.13, p = .36) or decentration and AL (horizontal meridian: Spearman’s r = −0.12, p = .38; vertical meridian: Spearman’s r = −0.02, p = .91) after 4 months.
Table 3. Mean (± SD) absolute tilt and decentration values for the right eyes (n = 24) and left eyes (n = 21)
Visual outcome
Mean CDVA was −0.06 ± 0.10 after one month and −0.08 ± 0.13 after 4 months. Median CDVA was −0.10 (range: −0.30–0.20) after one month and −0.10 (range: −0.30–0.60) after 4 months. CDVA was significantly better at the 4-months visit (p = .02).
Complications
Intraoperative complications included one case where the capsulorhexis ran out and a Brian Little maneuver had to be performed, and another case with mild iris bleeding at the end of surgery. However, these adverse events were not IOL related and did not have any impact on postoperative outcomes. Postoperative complications were not observed.
Discussion
Postoperative IOL stability inside the capsular bag is critical for achieving optimal visual outcomes. Therefore, the measurement of rotation, tilt and decentration of an IOL with a new design is an important clinical endpoint. In this prospective study we evaluated the performance of a new monofocal IOL with a 7.0 mm optic diameter and cut-out haptics (Aspira-aXA) after implantation in cataract patients.
There are numerous factors influencing the biomechanical stability inside the capsular bag including surgical techniques, IOL material properties, haptic design, IOL size as well as the inherent patient’s variability of the capsular bag and wound healing. AL could be taken as marker for capsular bag size, as there is evidence that significant IOL rotation is more likely to occur in myopic or high myopic eyes as the capsular bag increases with elongation of the AL.Citation5 Zhu et al. found a high degree of IOL rotation in myopic eyes which was however significantly greater in eyes implanted with C-loop haptics (AcrySof Toric, Alcon, Freiburg, Germany) than plate haptics (AT Torbi 709 M, Carl Zeiss Meditec AG, Jena, Germany) IOLs, especially when they were placed vertically.Citation5 The mean IOL rotation 3-month postoperatively (vs. end of surgery) was 8.00 ± 3.60° for the AcrySof vs. 4.42 ± 3.24° for the AT Torbi, with IOL rotations above 10.0° observed in 25.81% and 9.68% of the cases, respectively. The authors suggested that the interaction between the capsular bag and the haptics was a factor that could influence postoperative IOL rotational stability. Unlike C-loop haptic design, plate-haptic design without gaps between haptics and optic provide sufficient friction between the surfaces of the capsular bag and the haptics in eyes with elongated AL. Thus, the IOL is supported directly and firmly through its four corners from the surrounding capsular bag, resulting in better rotational stability than C-loop haptics IOLs. Therefore, plate-haptic toric IOLs might be a better choice for the correction of astigmatism in myopic eyes. Other findings suggest that the capsular bag might be of ellipsoid shape rather than circular, which could have contributed to high degrees of rotation (15° to 45°) found within 24 hours after surgery with a plate haptic IOL (Lentis Mplus Toric, Oculentis GmbH, Berlin, Germany) positioned along the vertical meridian in myopic eyes.Citation11
Besides studies which have specifically addressed the challenge of IOL stability in myopic eyes, most studies on IOL rotational stability reported a postoperative mean or median lens rotation of less than five degrees.Citation12,Citation13 However, the different methods and different time-points used to measure baseline and follow-up rotation make direct data comparison across studies flawed. Time-points could vary between intended axis,Citation14 immediately at the end of surgery (EoS),Citation7 after 1 hour,Citation12 within 24 hours,Citation15 after 1 or 2 weeks,Citation16 to even several months postoperatively.Citation17 In the current study, we measured the IOL position at the EoS and used an objective method to measure IOL rotation over a period of 4 months. Our results show a median absolute IOL rotation of 1.42° within 1 day after surgery and decrease to less than 1.0° thereafter. This outcome is in line with the comparable literature showing that the early postoperative period, and in particular the first hour, is critical for IOL rotation.Citation4,Citation7 Underlying reasons for this early rotation might be remaining OVD behind the IOL, myopia and concomitant greater capsular bag sizeCitation18 but also insufficient IOL-capsulorhexis overlap (<0.5 mm).Citation5,Citation6,Citation19 In our study, the use of different OVDs for filling the capsular bag did not have an impact on the rotation stability. Overall, only in one IOL, rotation exceeded 10.0 degrees within one day of surgery (EoS vs. 1 day). This eye experienced a slight IOP spike after surgery, had an axial length of 26.45 mm, an anterior chamber depth of 3.60 mm and required an IOL power of 18.0 D with a target refraction of −2.63 D. Since the contralateral eye rotated only by 2.57° for the same time-point, myopia could be excluded as a favorizing factor for the rotation. Furthermore, we did not find any correlation between AL and absolute IOL rotation. Overall, our results show a good rotational stability of the Aspira-aXA with 85% of lenses having less than 5.0° rotation and 98% less than 10.0° rotation.
Several features of the Aspira-aXA may have contributed to these good outcomes. The four-point non-angulated haptic design combined with the large optic of the Aspira-aXA provide a large surface of contact between the IOL and the capsular bag. Furthermore, as reported by Vandekerckhove,Citation3 IOL surface treatment is also thought to be an important factor contributing to rotational stability. The author compared the rotational stability of the PhysIOL Ankoris monofocal toric IOL versus the PhysIOL FineVision Pod FT trifocal toric IOL (PhysIOL SA, Liège, Belgium). Both IOLs share the same hydrophilic acrylic material and identical design, except for the lens surface which is polished for the Ankoris and unpolished for the Pod FT. Results showed significantly better rotational stability with the unpolished trifocal IOL because rough surfaces produce greater friction with the capsular bag, preventing IOL rotation. The Aspira-aXA lens has also an unpolished surface which may have also contributed to its good rotational stability. Furthermore, the center thickness of a 7.0 mm optic is increased in volume compared to a 6.0 mm optic, giving the lens a higher resistance to optic deformation (personal communication with the manufacturer).
In the same context, Schartmuller et al. suggested that the good rotational stability of the Hoya Vivinex XY1 IOL (Hoya Corporation, Tokyo, Japan) which never exceeded 5° in the early postoperative periodCitation7 was likely due to its unique frosted anterior and posterior haptic surfaces, favorizing friction with the capsular bag. In a comparative study, the same authors also found that the total diameter of C-loop haptics IOLs was another influencing factor impacting rotational stability, with the 13.0 mm IOLs providing better outcomes than the 12.5 mm IOLs.Citation4
Tilt and decentration of the IOL might induce higher-order aberrations and reduce the overall visual performance of the lens.Citation20,Citation21 Clinical studies that measure the IOL tilt and decentration in a pseudophakic eye have used different measurement methods.Citation22 Kimura et al. for instance used the second generation AS-OCT (Casia 2, Tomey, Nagoya, Japan)Citation23 while others used Purkinje imagesCitation24 or Scheimpflug imaging systems.Citation25 These measurements lead to different calculations of tilt and decentration values. It has to be noted that for any image-processing of tilt and decentration from raw images, a geometric distortion correction has to be considered, as different devices may result in different distortions. The Pentacam used in this study usually shows an image-compression along the z-axis, while other devices such as the Casia 2 may show an image-extension along the z-axis.
Not only did some of the studies not consider direction but only absolute values, but they also used different reference axes (i.e. pupillary axis, corneal axis). Despite these difficulties in data comparison, it has been shown that decentration up to 0.40 mm and tilt up to 7.0 degrees do not influence visual performance.Citation26,Citation27
According to modern schematic model eyes (e.g. the Liou-Brennan eye) the crystalline lens is tilted and decentered.Citation23,Citation24,Citation28 When taking the visual axis as reference point, the Liou-Brennan eye describes a characteristic tilt and decentration direction, which does vary when other reference markers are used.Citation28 While Kimura et alCitation23 found both parameters to be inferotemporal for the IOL and the natural lens, Mester and colleaguesCitation24 found the natural lens and the IOL to tilt superotemporal whereas the natural lens decentered inferotemporal and the IOL superonasal. Since some of the reported values as well as our results are small numbers close to zero, we find it rather difficult to precisely determine the direction, which is certainly affected by the visual axis and subsequent eye position during fixation that leads to a certain amount of measurement artifacts. Nonetheless, the minimal tilt and decentration values reported in this study with the pupillary axis as reference point are in line with the values described in the literature.Citation26 Furthermore, similar to Baumeister et al, we found that IOL tilt and decentration were stable over timeCitation29 suggesting a good capsular bag stability of the Aspira-aXA likely due to its unique design. We also did not notice differences in tilt and decentration values between eyes with different axial lengths. This is in agreement with the recent findings of Meng et al. who compared C-loop haptics and plate-haptics within myopes and non-myopes in terms of tilt and decentration.Citation6 While plate-haptics were more stable in myopic eyes, there was no increase of tilt and decentration with greater axial length within this group.
The results show a good visual performance in terms of distance visual acuity with only two eyes out of 66 achieving a CDVA worse than 0.10 logMAR at the 4-months visit. One of the eyes was diagnosed with Sicca Syndrome, a condition which is likely to explain the unsatisfying subjective outcome. Overall, no postoperative complications were observed during the course of the study.
The main limitation of this study is that the orientation of the IOLs at the end of surgery was left vertical (80° to 120°) rather than randomly assigned to vertical, horizontal, or oblique orientation. Therefore, we could not evaluate whether different IOL orientations would have resulted in different outcomes.
In conclusion, the Aspira-aXA shows good clinical outcomes and additionally offers the ophthalmologist a better view on the fundus for further examinations or surgeries. It is versatile in application, not only from the vitreoretinal perspective. Its good performance in terms of rotational stability, tilt and decentration suggest that the Aspira-aXA could be a good platform for the development of toric and multifocal IOLs.
Meeting presentation
Preliminary versions of this study were presented at the Congress of the ESCRS (Bolz et al), Paris, France, September 2019 and at the virtual ESCRS (K.Waser et al), Amsterdam, October 2020.
Acknowledgments
We would like to thank Mr. Johannes Heberle for his support regarding the customized Matlab code.
Disclosure statement
This work was supported by HumanOptics in form of financial aid for administration and monitoring services of the house intern ars ophthalmica study center. None of the authors has a financial or proprietary interest in any material or method mentioned.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
- Bournas P, Drazinos S, Kanellas D, Arvanitis M, Vaikoussis E. Dysphotopsia after cataract surgery: comparison of four different intraocular lenses. Ophthalmologica. 2007;221(6):378–83. doi:https://doi.org/10.1159/000107496.
- Takamura Y, Tomomatsu T, Yokota S, Matsumura T, Takihara Y, Inatani M. Large capsulorhexis with implantation of a 7.0 mm optic intraocular lens during cataract surgery in patients with diabetes mellitus. J Cataract Refract Surg. 2014;40(11):1850–56. doi:https://doi.org/10.1016/j.jcrs.2014.02.039.
- Vandekerckhove K. Rotational stability of monofocal and trifocal intraocular toric lenses with identical design and material but different surface treatment. J Refract Surg. 2018;34(2):84–91. doi:https://doi.org/10.3928/1081597X-20171211-01.
- Schartmüller D, Schwarzenbacher L, Meyer EL, Schriefl S, Leydolt C, Menapace R. Comparison of long-term rotational stability of three commonly implanted intraocular lenses. Am J Ophthalmol. 2020;220:72–81. doi:https://doi.org/10.1016/j.ajo.2020.07.019.
- Zhu X, Meng J, He W, Rong X, Lu Y. Comparison of the rotational stability between plate-haptic toric and C-loop haptic toric IOLs in myopic eyes. J Cataract Refract Surg. 2020;46(10):1353–59. doi:https://doi.org/10.1097/j.jcrs.0000000000000259.
- Meng J, He W, Rong X, Miao A, Lu Y, Zhu X. Decentration and tilt of plate-haptic multifocal intraocular lenses in myopic eyes. Eye Vis (Lond). 2020;7(1):17. doi:https://doi.org/10.1186/s40662-020-00186-3.
- Schartmuller D, Schriefl S, Schwarzenbacher L, Leydolt C, Menapace R. True rotational stability of a single-piece hydrophobic intraocular lens. Br J Ophthalmol. 2019;103(2):186–90. doi:https://doi.org/10.1136/bjophthalmol-2017-311797.
- Wolffsohn JS, Buckhurst PJ. Objective analysis of toric intraocular lens rotation and centration. J Cataract Refract Surg. 2010;36(5):778–82. doi:https://doi.org/10.1016/j.jcrs.2009.12.027.
- Rosales P, Marcos S. Pentacam Scheimpflug quantitative imaging of the crystalline lens and intraocular lens. J Refract Surg. 2009;25:421–28.
- De Castro A, Rosales P, Marcos S. Tilt and decentration of intraocular lenses in vivo from Purkinje and Scheimpflug imaging validation study. J Cataract Refract Surg. 2007;33(3):418–29. doi:https://doi.org/10.1016/j.jcrs.2006.10.054.
- Amigo A, Bonaque-Gonzalez S. Is the capsular bag perimeter round or elliptical? J Ophthalmic Vis Res. 2016;11(2):159–61. doi:https://doi.org/10.4103/2008-322X.183925.
- Hirnschall N, Maedel S, Weber M, Findl O. Rotational stability of a single-piece toric acrylic intraocular lens: a pilot study. Am J Ophthalmol. 2014;157(2):405–411 e401. doi:https://doi.org/10.1016/j.ajo.2013.09.032.
- Patel CK, Ormonde S, Rosen PH, Bron AJ. Postoperative intraocular lens rotation: a randomized comparison of plate and loop haptic implants. Ophthalmology. 1999;106(11):2190–95. discussion 2196. doi:https://doi.org/10.1016/S0161-6420(99)90504-3.
- De Silva DJ, Ramkissoon YD, Bloom PA. Evaluation of a toric intraocular lens with a Z-haptic. J Cataract Refract Surg. 2006;32(9):1492–98. doi:https://doi.org/10.1016/j.jcrs.2006.04.022.
- Gyongyossy B, Jirak P, Schonherr U. Long-term rotational stability and visual outcomes of a single-piece hydrophilic acrylic toric IOL: a 1.5-year follow-up. Int J Ophthalmol. 2017;10(4):573–78. doi:https://doi.org/10.18240/ijo.2017.04.12.
- Zuberbuhler B, Signer T, Gale R, Haefliger E. Rotational stability of the AcrySof SA60TT toric intraocular lenses: a cohort study. BMC Ophthalmol. 2008;8(1):8. doi:https://doi.org/10.1186/1471-2415-8-8.
- Kwartz J, Edwards K. Evaluation of the long-term rotational stability of single-piece, acrylic intraocular lenses. Br J Ophthalmol. 2010;94(8):1003–06. doi:https://doi.org/10.1136/bjo.2009.163485.
- Lee BS, Chang DF. Comparison of the rotational stability of two toric intraocular lenses in 1273 consecutive eyes. Ophthalmology. 2018;125(9):1325–31. doi:https://doi.org/10.1016/j.ophtha.2018.02.012.
- Torquetti L. Toric intraocular lens rotation related to the capsulorhexis. J Cataract Refract Surg. 2015;41(2):483. doi:https://doi.org/10.1016/j.jcrs.2014.12.046.
- Barbero S, Marcos S, Jiménez-Alfaro I. Optical aberrations of intraocular lenses measured in vivo and in vitro. JOSA A. 2003;20(10):1841–51. doi:https://doi.org/10.1364/JOSAA.20.001841.
- Langenbucher A, Omidi P, Eppig T, Szentmáry N, Menapace R, Hoffmann P. Combination of lens decentration and tilt in phakic and pseudophakic eyes-optical simulation of defocus, astigmatism and coma. Ophthalmologe. 2020. doi:https://doi.org/10.1007/s00347-020-01235-x
- Baumeister M, Kohnen T. Assessment of centration and position stability of modern intraocular lenses after cataract surgery. Klin Monbl Augenheilkd. 2010;227(8):611–16. doi:https://doi.org/10.1055/s-0029-1245563.
- Kimura S, Morizane Y, Shiode Y, Hirano M, Doi S, Toshima S, Fujiwara A, Shiraga F. Assessment of tilt and decentration of crystalline lens and intraocular lens relative to the corneal topographic axis using anterior segment optical coherence tomography. PLoS One. 2017;12(9):e0184066. doi:https://doi.org/10.1371/journal.pone.0184066.
- Mester U, Sauer T, Kaymak H. Decentration and tilt of a single-piece aspheric intraocular lens compared with the lens position in young phakic eyes. J Cataract Refract Surg. 2009;35(3):485–90. doi:https://doi.org/10.1016/j.jcrs.2008.09.028.
- Sasaki K, Sakamoto Y, Shibata T, Nakaizumi H, Emori Y. Measurement of postoperative intraocular lens tilting and decentration using Scheimpflug images. J Cataract Refract Surg. 1989;15(4):454–57. doi:https://doi.org/10.1016/S0886-3350(89)80071-9.
- Chang PY, Lian CY, Wang JK, Su PY, Wang JY, Chang SW. Surgical approach affects intraocular lens decentration. J Formosan Med Assoc = Taiwan Yi Zhi. 2017;116(3):177–84. doi:https://doi.org/10.1016/j.jfma.2016.04.003.
- Piers PA, Weeber HA, Artal P, Norrby S. Theoretical comparison of aberration-correcting customized and aspheric intraocular lenses. J Refract Surg. 2007;23(4):374–84. doi:https://doi.org/10.3928/1081-597X-20070401-10.
- Liou HL, Brennan NA. Anatomically accurate, finite model eye for optical modeling. J Opt Soc Am A Opt Image Sci Vis. 1997;14(8):1684–95. doi:https://doi.org/10.1364/JOSAA.14.001684.
- Baumeister M, Neidhardt B, Strobel J, Kohnen T. Tilt and decentration of three-piece foldable high-refractive silicone and hydrophobic acrylic intraocular lenses with 6-mm optics in an intraindividual comparison. Am J Ophthalmol. 2005;140(6):1051–58. doi:https://doi.org/10.1016/j.ajo.2005.07.026.