Abstract
Purpose: The purpose of this paper is to investigate how the imbalance of neurogenic factor (NGF) and its precursor (pro-NGF) mediates structural and functional impairment of retinal neurovascular unit (RNVU) that plays a role in retinal degenerative diseases.
Methods: A literature search of electronic databases was performed.
Results: The pro-apoptotic effect of pro-NGF and the pro-growth effect of NGF are essential for the pathological and physiological activities of RNVU. Studies show that NGF-based treatment of retinal degenerative diseases, including glaucoma, age-related macular degeneration, retinitis pigmentosa, and diabetic retinopathy, has achieved remarkable efficacy.
Conclusions: RNVU plays a complex and multifaceted role in retinal degenerative diseases. The exploration of the differential signaling expression of proNGF-NGF homeostasis under physiological and pathological conditions, and the corresponding pathological processes induced by its regulation, has prompted us to focus on earlier retinal neuroprotective therapeutic strategies to prevent retinal degenerative diseases.
Introduction
Retinal degenerative diseases, including age-related macular degeneration (AMD), retinitis pigmentosa (RP), diabetic retinopathy (DR) and glaucoma, are the leading causes of impaired vision and blindness.Citation1 Treatments of retinal degenerative diseases have mostly focused on advanced complications or anatomical correlates, such as lowering IOP, improving fundus microcirculation, and nutritional support at late stages when vision is already profoundly compromised.Citation2–4 Therefore, a better understanding of the pathogenesis of retinal degeneration in its early stages is warranted to provide timely and effective prevention and intervention strategies.
In addition to neurons in the neuronal nuclear layer which consists of multiple layers from retinal pigment epithelium (RPE) to ganglion cell layer (GCL), the retinal neurovascular unit (RNVU) also includes glial cells and retinal microvessels.Citation5 As a multicellular structure, RNVU plays an important role in the physiological function and homeostasis of the retina through interactions between the cells of the RNVU.Citation6 Ischemia, hyperglycemia, inflammation, oxidative stress, aging, genetics, and other factors have been implicated in the development of retinal degenerative diseases by disrupting the structural and/or functional coupling of the RNVU.
Neurotrophin nerve growth factor (NGF) was firstly described in 1950s as a key mediator in neuronal survival and differentiation.Citation7 More recently, it was reported that the balanced expression of NGF, its own precursors and its receptors are highly involved in the physiology and pathology of the retinal microvasculature through the regulation of axonal growth, synapse formation, and the synthesis and release of neurotransmitters and neuropeptides.Citation8 The impact of NGF on the structural and functional changes in the RNVU suggests a potential role in the pathogenesis of retinal degeneration and sheds light on the development of novel NGF-based therapies for treating retinal degenerative diseases.
This review summarizes the current understanding of the involvement of NGF in the pathophysiology of the RNVU, as well as perspectives on implementing NGF-based therapeutics for treating retinal degenerative diseases at their onset.
Structure of RNVU and its necessity in the physiological retinal function
Structural and functional damage to the neuro-glial-vascular unit underlies many neurodegenerative and neuroinflammatory diseases. This concept was first applied to studies of several diseases in which the blood-brain barrier is disrupted, such as stroke, cerebral infarction, Parkinson’s disease, and Alzheimer’s disease.Citation9,Citation10 NVU refers to the functional coupling and interdependence between neurons, glial cells and vascular system.Citation11 From an anatomical and physiological perspective, the eye is considered an extension of the brain. In analogy to the NVU of the brain, the structure of the RNVU consists of retinal neurons, glial cells, and retinal microvasculature, which are distributed in the corresponding 10 layers of the retina.Citation12,Citation13 The structure of the retina and RNVU is shown in .
Figure 1. Structure of retina with magnified view of retinal neurovascular unit (NVU). RPE, retinal pigment epithelium; R and C: rod and cone; OLM: outer limiting membrane; ONL: outer nuclear layer; OPL: outer plexus layer; INL: inner nucleus; IPL: inner plexus layer; GCL: ganglion cell layer; NFL: nerve fiber layer; ILM: internal limiting membrane.
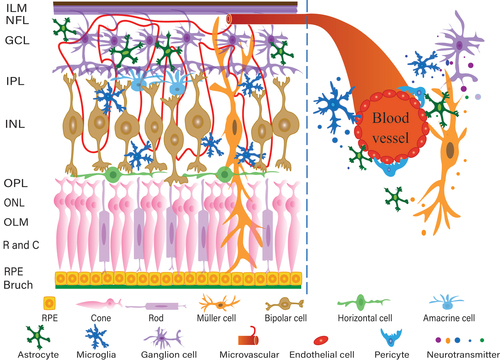
There are five types of retinal neurons: photoreceptors, horizontal cells, bipolar cells, amacrine cells, and ganglion cells. Photoreceptors predominantly comprise cones and rods and are located in the outer nuclear layer (ONL). The cell bodies of horizontal, bipolar, and anaplastic cells are primarily located in the internal nuclear layer (INL), whereas retinal ganglion cells (RGCs) are distributed in the ganglion cell layer (GCL).Citation14 The outer plexiform layer (OPL) and the inner plexiform layer (IPL) are the sites where these three levels of neurogenic synapses are connected.Citation15 The internal limited membrane (ILM) brings together the synapses of ganglion cells, which are sent by the optic nerve to the visual cortex to complete the transmission of visual signals.
The glial component of the RNVU consists of macroglia (astrocytes and Müller cells), microglia, and oligodendrocytes, which surround the neurons, form retinal defense structures, and help maintain retinal homeostasis. Glial cells are the interface between neurons and the vascular system and are therefore key regulators of functional communication between them.Citation16 Müller cells, the only cells that span the entire width of the retina, reside between the inner boundary membrane (ILM) and outer membrane (OLM) and are in contact with almost every cell type in the retina. The unique position of Müller cells allows them to perform various functions necessary for retinal homeostasis, including converting glycogen to lactate, prompting the synthesis of retinoid from retinol, modulating the concentration of extracellular ions to regulate plasma membrane polarization, maintaining extracellular pH, controlling neurotransmission, recycling transmitters, and transporting metabolites.Citation17,Citation18 Astrocytes are present only in the nerve fiber layer (NFL) and GCL, and provide metabolic and mechanical support for neurons.Citation19,Citation20 Most nutrients, waste products, ions, water, and other molecules are transported between vessels and neurons via macroglia, and disruption of their functional imbalance can result in inflammation and apoptosis. Microglia are highly specialized phagocytes that constantly monitor local synaptic activity and remove dead cells and metabolic debris.Citation20 In chronic retinal disease, they secrete large amounts of proinflammatory cytokines that affect the normal function of the neurovascular units.Citation21
It is now well recognized that retinal microvasculature is distributed from the NFL to the INL but absent in the ONL.Citation22,Citation23 Telopods of endothelial cells, pericytes, and a small number of macroglia aggregate to form the retinal microvasculature. The physiological demands of retinal neurons determine the filling of vessels and changes in their luminal diameter, vasodilation, and vascular narrowing.Citation24,Citation25 The retinal microvasculature reciprocally provides nutrients and oxygen to the RNVU and excretes waste products from the tissue to meet the high demand for oxygen consumption by the retina, a metabolically active neurovascular tissue.Citation26,Citation27
The normal function of RNVU relies on the delicate coordination of various cell types inside RNVU, which supports the nutrition and metabolism of the retina and provide a suitable environment for neural signaling.Citation28 Neurons are the most critical cells for neurological function, acting as “pacemakers”. When subtle changes occur in the retinal environment, neurons receive signals and establish synaptic connections with neighboring glial cells to complete communication with the vasculature and regulate the stability of the intraretinal environment. Therefore, the neuroglial-vascular coupling of the RNVU is essential for retinal homeostasis.
RNVU changes in retinal degenerative diseases
Structural disruption of the RNVU and abnormal signal transmission are the main pathological mechanisms of retinal degenerative diseases.Citation29 Oxidative stress, apoptosis, inflammation, and endoplasmic reticulum stress activate the NF-κB proinflammatory pathway and caspase apoptotic pathway,Citation30,Citation31 leading to pathological changes in the retinal microvasculature, neurodegeneration and disruption of blood retinal barrier function.Citation32,Citation33 Changes in the retinal microvasculature are mainly manifested by thickening of the basement membrane, loss of pericytes and endothelial cells, formation of microaneurysms, and retinal leakage.Citation33 Recent studies have demonstrated that neurodegeneration occurs prior to the appearance of visible microvascular lesions.Citation34 Neuronal apoptosis is an early pathological feature of retinal degenerative disease. Activation of the NF-κB proinflammatory pathway is a major cause of RGC death.Citation35 Damage to any component of the RNVU can lead to impairment of the entire coupling mechanism, resulting in local inflammation or apoptosis, paralysis of the entire neurovascular unit, progressive retrograde axonal degeneration, and impaired messaging.
Expression of nerve growth factors and their signaling pathways
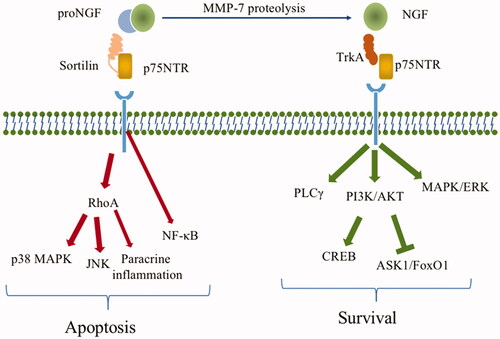
Nerve Growth Factor-β (NGF) was first isolated by Nobel Prize winners Srita Levi Montalcini and Stanley Cohen in 1956 and is the earliest discovered member of the neurotrophic factor family.Citation36 In the early 1980s, three NGF homologous growth factors were identified, namely brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and neurotrophin-4/5 (NT-4/5).Citation37 NGF is considered a powerful and highly selective growth factor for sympathetic and sensory neurons and cells from the neural crest. It is involved in the survival, differentiation, and function of peripheral and central nervous cells through interaction with specific cell receptors, regulating the gene expression of neurons. The biological effects of NGF also extend to the immune and endocrine systems.Citation38–42 NGF is produced from its precursor pro-NGF by the hydrolysis of matrix metalloproteinase-7 (MMP-7) and fibrinolytic protein.Citation43 Studies have shown that pro-NGF is not only hydrolyzed into mature NGF but is also secreted out of the cell to exert its unique biological activities.Citation44 P75 neurotrophin receptor (p75NTR) is a member of the tumor necrosis factor receptor superfamily (TNFRSF), also known as TNFRSF16, which plays a pro-apoptotic role by binding to its high-affinity ligand pro-NGF. This process requires the participation of its co-receptor sortilin, which specifically binds pro-NGF and increases the affinity of p75NTR to pro-NGF.Citation45–47 The formed Pro-NGF/sortilin/p75NTR apoptotic signal complex is involved in the regulation of synaptic inhibition, cell death, and neurodegeneration.Citation48
As an upstream signal initiator, the Pro-NGF/sortilin/p75NTR signal axis induces the activation of a series of downstream signaling pathways. For example, the complex-mediated activation of RhoA kinase and paracrine inflammatory response further activate the p38 MAPK and JNK pathways and participate in cell apoptosis.Citation49 Activated MAPK contributes to NF-κB activation and promotes the release of proinflammatory cytokines, including IL-1β, IL-6, IL-8, and TNF-α.Citation50–54 This complex can also interact with apoptotic pathways, resulting in changes in Bax, Bcl-2 and Caspase-3 levels.Citation55
Mature NGF mediates its growth-promoting biological function via the tropomyosin kinase receptor A (TrkA).Citation56 NGF binds to high-affinity TrkA receptors and low-affinity p75NTR to activate the phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT), phospholipase C-γ (PLCγ), and mitogen-activated protein kinase/extracellular signal-associated kinase (MAPK/ERK) pathways.Citation57–60 Activation of PI3K/AKT further activates the cAMP response element binding protein (CREB) pathway and inhibits the apoptosis signal-regulating kinase 1/ Forkhead box O1 (ASK1/FoxO1) pathway.Citation61,Citation62 All these factors contribute to neuronal survival, synaptic growth, ganglion cell survival, and other neurotrophic effects. The expression of nerve growth factors and their signaling pathways are shown in .
Figure 2. Expression map of nerve growth factor and its signaling pathway. ProNGF is hydrolysed by MMP-7 and fibronectin to produce mature NGF. p75NTR binds to its high-affinity ligand proNGF to exert a pro-apoptotic effect, a process that requires the involvement of its co-receptor sortilin and formation of the proNGF/sortilin/p75NTR apoptotic signaling complex. The sortilin/p75NTR signaling axis acts as an upstream signal initiator RhoA kinase and NF-κB, which are involved in apoptosis and inflammatory responses. proNGF binds to the high-affinity TrkA receptor and the low-affinity receptor p75NTR to activate the PI3K/AKT, PLCγ and MAPK/ERK pathways, which are involved in the proliferation, growth, and survival of retinal neurons and microvascular cells.
Nerve growth factor is involved in the pathology and physiology of RNVU
Neurotrophic factors have been shown to be locally produced by retinal neurons and glial cells, or paracrine and recycled from the superior colliculus back to the retina.Citation63,Citation64 However, a similar superior colliculus transport mechanism has not been found for NGF, which raises the possibility that NGF is most likely synthesized and secreted locally in retinal neurons and glial cells.Citation65,Citation66 NGF plays a crucial role in the embryonic development of the vertebrate visual system.Citation67 As early as 1976, American medical scientist J.E. Turner found that a single 200 BU intraocular injection of NGF at optic nerve dissection significantly accelerated the response of goldfish RGCs to axon dissection.Citation68 Jian Q. et al. found that the levels of NGF and TrkA were augmented in parallel with the NGF/TrkA signaling downstream components, such as PI3K and Akt, in rat retinal Müller cells transplanted from the subretinal cavity of rat bone marrow mesenchymal stem cells (rBMSCs), suggesting that activation of this pathway plays an important role in the protection of photoreceptors and RGCs.Citation69
Immunohistochemical staining of the rat retina have shown universal expression of NGF across normal retinal neural tissues from the RPE to the GCL, with the highest abundance in RPEs, RGCs, and Müller cells.Citation70,Citation71 NGF receptors, including high-affinity TrkA and low-affinity receptor p75NTR, are present in NGF-responsive neurons and glial cells, respectively.Citation72–74 In addition to its neuroprotective effects, NGF may prevent pericyte loss and the formation of acellular occluded capillaries in rats developing diabetic retinopathy.Citation75,Citation76 The downstream PI3K/Akt pathway activated by NGF/TrkA is an important intracellular signaling pathway involved in a range of cellular responses of retinal microvascular endothelial cells and pericytes, such as cell proliferation, survival, apoptosis, cytoskeleton construction, vesicle transport, glucose transport, and platelet function.Citation77,Citation78 NGF is not only an important regulator of neuronal and glial survival and death in the eye, but also mediates the development, survival, and regeneration of microvascular cells. NGF-mediated effects are achieved through neuro-glial-vascular coupling.Citation79
In animal models, pro-NGF labelling was found to be widely distributed across retinal tissues, including RPE, photoreceptor outer segments, ONL, INL, IPL, GCL, microglia, and Müller cells.Citation71,Citation80,Citation81 In addition, an increase in the release of pro-NGF and p75NTR and a reduction in the production of NGF were observed in retinal tissues of animal models of retinal degenerative diseases.Citation82 For example, in a diabetic retinopathy (DR) mouse model, upregulation of the proNGF-p75NTR axis induces apoptosis of pericytes, mediates ligand-dependent production of inflammatory cytokines, and destroys vascular units, resulting in damage to the blood-retinal barrier.Citation83 In contrast, elevated NGF levels attenuated pericyte damage and reduced neointimal formation by decreasing VEGF expression.Citation84 NGF and inflammatory mediators are released into the vitreous, leading to local activation of retinal RGCs and Müller cells in a Reelin-deficient mouse model.Citation85 The degeneration of retinal photoreceptor cells also results from reduced NGF.Citation86 The formation of peroxynitrite induced by ischemia, hyperglycemia, inflammation, oxidative stress, and aging has been shown to impairs the production and activity of MMP-7. This further leads to the accumulation of pro-NGF in RNVU cells and a decrease in NGF levels. Pro-NGF activation and reduced bioavailability of NGF can activate downstream signaling pathways and induce inflammation, Müller cell and astrocyte gliosis, microglial phagocytosis impairment, microvascular degeneration, in RNVU tissues.
Potential NGF-based therapies in the treatment of retinal degenerative diseases
Retinitis pigmentosa
Retinitis pigmentosa (RP) is caused by hereditary retinal photoreceptor dystrophy, with photoreceptor apoptosis as the final event in the disease process.Citation87 In recent years, RP has been implicated in neuronal cell death, neuronal morphological changes and migration, retinal nerve fiber atrophy, altered glial metabolism and morphology,Citation17,Citation88 and reduction in the caliber of both small retinal arteries and veins.Citation89 RP is a disease accompanied by RNVU damage.
NGF is produced by retinal nerve cells and stored to maintain photoreceptor survival and prevent photoreceptor degeneration.Citation90 Studies have demonstrated that short-term topical application of NGF improves best-corrected visual acuity (BCVA), macular focal electroretinogram recordings (fERG), and Goldmann visual field tests in a subset of RP patients.Citation91 Further studies revealed that intraocular injection of NGF prevented photoreceptor degeneration and regulated retinoid expression in rats with RP.Citation92 Platon-Corchado et al.Citation93 investigated the interaction between pro-NGF/p75NTR signaling in RP-related photoreceptor degeneration processes and demonstrated that p75NTR antagonists have potential therapeutic value in RP treatment. Furthermore, NGF and anti-vascular endothelial growth factor (αVEGF) in combination significantly increased retinal cell survival, retinoid expression, and neuropil growth in the photoreceptors. In the same study, NGF/α-VEGF also upregulated Bcl-2 mRNA, downregulated Bax mRNA, and upregulated Trk-A mRNA.Citation94
Glaucoma
Glaucoma is a neurodegenerative disease associated with abnormal sensitivity to intraocular pressure (IOP). Elevated IOP suppresses retinal output neurons, leading to progressive degeneration of RGCs and axons, as well as damage to the optic nerve.Citation95,Citation96 Damaged RGCs or astrocytes can release damage-associated molecular patterns (DAMPs) that trigger retinal inflammatory responses.Citation97 Narrowing of the fundus and disturbances in blood flow have been recognized as the major causes of progressive visual field loss in patients with glaucoma.Citation98–100 A variety of mechanisms have been proposed to explain the pathogenesis of glaucoma, such as neuroinflammation, loss of neurotrophic factors, destruction of the axonal cytoskeleton, and disruption of the neurovascular unit, including vascular disorders and coupling imbalances.Citation101,Citation102
In pathological conditions such as glaucoma, elevated IOP disrupts the balanced expression of pro-NGF, NGF, and its receptors and induces apoptosis in RGCs.Citation62 Serum NGF levels were found to be significantly lower in patients with glaucoma than in healthy controls.Citation103,Citation104 In a mouse model of glaucoma, NGF eye drops plus ultrasound contrast agent (UCA) intervention significantly reduced IOP, elevated transient visually evoked potentials, enhanced activity of RGCs, and diminished apoptosis and autophagy.Citation105 In clinics, administration of NGF drops for glaucoma significantly reduced RGC apoptosis and showed long-term improvements in visual field, optic nerve function, contrast sensitivity, and visual acuity, suggesting neuroprotective effects of NGF in clinics.Citation106 In another clinical trial of patients with open-angle glaucoma, application of 80 μg/mL recombinant human nerve growth factor (rhNGF) eye drops significantly improved visual function and retinal structural measurements compared to the placebo group.Citation107 Lin et al. demonstrated that the protective effect of NGF on glaucomatous retinal RGCs was achieved through inhibition of endoplasmic retinal stress.Citation108
Diabetic retinopathy
Diabetic retinopathy (DR) destroys all the components of the neurovascular unit.Citation109 Traditionally, DR is considered to be primarily a microvascular disease characterized by morphological changes in the microvasculature, thickening of the basement membrane, absence of tight junctions between the endothelium, early and selective loss of pericytes, increased vascular permeability, capillary occlusion, and microaneurysms.Citation110 Recent studies have pointed out that disruption of the neuroglial-vascular unit and imbalance in its coupling mechanism (coupling) play a key role in the early onset of DR.Citation33 A high-glucose environment leads to dopaminergic neuronal dysfunctionCitation111 and progressive structural and functional alterations in both macro- and microglia.Citation112 In contrast, inflammatory factors that are currently thought to play a key role in the development of DR are more closely related to microglial activation.Citation113
A growing body of evidence supports the relationship between pro-NGF/NFG imbalance, inflammation, and neurological and microvascular degeneration in early diabetic retinopathy.Citation114 Diabetes-induced formation of peroxynitrite was observed in parallel with impaired MMP-7 production and activity, pro-NGF accumulation, and reduced NGF levels in the analysis of samples from DR patients and experimental models.Citation80 Inhibition of p75 (NTR) blunted pro-NGF-induced retinal inflammation, abrogated diabetes-induced glial fibrillary acidic protein expression, reduced ganglion cell loss, and increased vascular permeability.Citation115 Elsherbiny et al. found that carbamazepine, a neuroprotective agent, attenuates diabetes-induced neuronal loss and reduces diabetes-induced inflammation by activating NGF/PI3K/Akt.Citation116 Troullinaki et al. revealed the anti-apoptotic effect of NGF in the hypoxic retinal endothelium, suggesting that NGF could be a potential target for the treatment of proliferative retinal diseases.Citation117 Postball administration of NGF attenuates visual damage in DR rats by reducing the loss of RGCs but does not affect the retinal microvasculature.Citation118 Recently, studies on the repair of neurovascular units by restoring the balance between pro-NGF and NGF have been recognized as a promising approach for the treatment of DR.
Age-related macular degeneration
Age-related macular degeneration (AMD) is characterized by neuroinflammation of retinal neurons, gliosis of Müller cells and astrocytes, and impairment of microglial phagocytosis, which reduces the bioavailability of nerve growth factors and ultimately leads to pathological changes in the retinal pigment epithelium and choroid.Citation119,Citation120 AMD is primarily a result of structural and functional alterations in the RPE.Citation121 The macula is divided into four regions: the central concave avascular zone, central concave zone, paracentral concave zone, and pericentral concave zone. The macula accounts for only 3% of the retinal surface area but has the highest oxygen consumption, with extensive capillary distribution in all regions except the central concave avascular zone.Citation122 There are primarily two types of AMD, wet and dry, which are categorized based on the presence and absence of neovascularization, respectively.Citation123 Therefore, it is unlikely that nerve damage does not involve the microvasculature, particularly in wet AMD. In addition, a growing body of optical coherence tomography angiography (OCTA) evidence suggests that altered choroidal capillary (choriocapillaris) blood flow plays a dominant role in the pathogenesis of dry AMD.Citation120
Only a few studies have investigated the direct correlation between NGF and AMD. Among these limited studies, Telegina et al. reported increased NGF staining in Müller cells of OXYS rats, which developed advanced AMD-like retinopathy at 18 months of age.Citation124 Activation and dysregulation of proinflammatory signaling pathways, such as JNK, MAPK, and NF-κB, are more frequently observed in AMD. As discussed in the previous sections, these signaling pathways are highly associated with the structural and functional degeneration of retinal components, including neuroinflammation in the retina, axonal cytoskeleton reconstruction, impaired axonal signaling, abnormal expression of electrical signals, and neurovascular unit lesions, including vascular disorders.Citation125–128 Are these pathways mediated by an upstream pro-NGF-NGF imbalance? There is ample evidence for this, and more studies are needed to enrich the theoretical body of the close relationship between pro-NGF, NGF and AMD.
Conclusion
RNVU plays a complex and multifaceted role in retinal degenerative diseases, and there is evolving recognition of the protective role of NGF in this pathological process. In addition to traditional pharmacological and surgical interventions that primarily target retinopathy at late stages, novel retinal neuroprotective therapeutics that focus on neurovascular coupling are a promising strategy to prevent retinal degeneration at early stages. The expression of pro-NGF/NGF has been correlated with the differential regulation of multiple signaling pathways involved in retinal degeneration. Interestingly, researchers have used a number of NGF-based therapies that have neuroprotective effects and prevent degeneration of the RNVU: (1) the use of eye drops containing NGF or the injection of NGF into or behind the eye to increase mature retinal NGF.Citation129 For example, intravitreal injection (ivt) or eye drop (ed) administration of rhNGF has been shown to restore retinal and optic nerve damage, reduce RGC loss, stimulate axonal regeneration in a rat model,Citation130,Citation131 significantly increase retinal thickness, and reduce apoptosis of retinal photoreceptors.Citation132,Citation133 Similarly, one study demonstrated that topical application of rhNGF twice daily for three weeks significantly improved the survival of retinal RGCs in an animal model of optic neuropathy, increased the number of subretinal RGCs, promoted axon survival, and inhibited astrocyte activity in the optic nerve, thus providing optic nerve protection.Citation134 (2) TrkA agonists that activate the NGF/TrkA signaling pathway and promote cell survival in RNVU.Citation135 (3) Silencing of the pro-NGF signaling pathway using p75NTR antagonists such as LM11A-31 to block the binding of p75NTR to pro-NGF with no effect on the binding of NGF to TrkA.Citation136 (4) Fibrin activators that convert pro-NGF into mature NGF.Citation137
The role of the balance between NGF and its precursors in the physiology of RNVU and the pathological processes involved in retinal degenerative disease are not fully understood.Citation114,Citation138 More studies are needed to understand the differences in the distribution of genes involved in this signaling axis in different retinal tissues, as well as the different activation signals due to different pathologies. The main challenges in NGF neuroprotection are to reduce off-target effects and provide safe therapeutic levels of neuroprotective drugs. NGF therapy is mostly in its early stages, and further clinical studies are needed to prove its clinical benefits. In addition, NGF has been administered in saline or other aqueous delivery vehicles in experimental and clinical trials to date despite its low aqueous solubility. Studies are encouraged to explore other ophthalmic drug delivery tools that are more compatible with the physiochemical properties and pharmacokinetic profile of NGF and the accessibility of current ophthalmic equipment instruments. The source of NGF biologics should also focus on more natural ingredients, such as cord blood serum,Citation139,Citation140 ginsenosides,Citation141 Astragalus membranaceus,Citation142 and saffron.Citation143 Furthermore, it should be pondered: is NVU dysfunction the end result of a degenerative retinal disease process or a causative factor? How are glial cells involved in vasoactive factor release, hemodynamics, metabolic adaptation, and microvascular constriction and dilation? Are NGFs really the most upstream signals in disease development, and what other signaling pathways are potentially involved in the event? Therefore, there is a need to fully elucidate the role of NVU dysfunction in neurodegenerative diseases and the specific mechanisms by which imbalances in NGF and pro-NGF are involved in their pathology. New knowledge in this area may open new avenues for the prevention, early diagnosis, and treatment of degenerative diseases in humans.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Bourne RR, Stevens GA, White RA, Smith JL, Flaxman SR, Price H, Jonas JB, Keeffe J, Leasher J, Naidoo K, et al. Causes of vision loss worldwide, 1990–2010: a systematic analysis. Lancet Glob Health. 2013;1(6):e339–e349. doi:https://doi.org/10.1016/S2214-109X(13)70113-X
- Schuster AK, Erb C, Hoffmann EM, Dietlein T, Pfeiffer N. The diagnosis and treatment of glaucoma. Dtsch Arztebl Int. 2020;117(13):225–234. doi:https://doi.org/10.3238/arztebl.2020.0225.
- Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, Wong TY. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106–e116. doi:https://doi.org/10.1016/S2214-109X(13)70145-1.
- Virgili G, Michelessi M, Parodi MB, Bacherini D, Evans JR. Laser treatment of Drusen to prevent progression to advanced age-related macular degeneration. Cochrane Database Syst Rev. 2015;10(10):CD006537. doi:https://doi.org/10.1002/14651858.CD006537.
- Yu C, Roubeix C, Sennlaub F, Saban DR. Microglia versus monocytes: distinct roles in degenerative diseases of the retina. Trends Neurosci. 2020;43(6):433–449. doi:https://doi.org/10.1016/j.tins.2020.03.012.
- Usui Y. Elucidation of pathophysiology and Novel treatment for diabetic macular edema derived from the concept of neurovascular unit. 2020;JMA J. 3(3):201–207. doi:https://doi.org/10.31662/jmaj.2020-0022.
- Levi-Montalcini R, Hamburger V. Selective growth stimulating effects of mouse sarcoma on the sensory and sympathetic nervous system of the chick embryo. J Exp Zool. 1951;116(2):321–361. doi:https://doi.org/10.1002/jez.1401160206.
- Minnone G, De Benedetti F, Bracci-Laudiero L. NGF and its receptors in the regulation of inflammatory response. Int J Mol Sci. 2017;18(5):1028. doi:https://doi.org/10.3390/ijms18051028.
- Zhou Y, Chen Q, Wang Y, Wu H, Xu W, Pan Y, Gao S, Dong X, Zhang JH, Shao A. Persistent neurovascular unit dysfunction: pathophysiological substrate and trigger for late-onset neurodegeneration after traumatic brain injury. 2020;Front Neurosci. 14(6):581. doi:https://doi.org/10.3389/fnins.2020.00581
- Reed MJ, Damodarasamy M, Banks WA. The extracellular matrix of the blood-brain barrier: structural and functional roles in health, aging, and Alzheimer’s disease. Tissue Barriers. 2019;7(4):1651157.doi:https://doi.org/10.1080/21688370.2019.1651157
- Noonan JE, Lamoureux EL, Sarossy M. Neuronal activity-dependent regulation of retinal blood flow. Clin Exp Ophthalmol. 2015;43(7):673–682. doi:https://doi.org/10.1111/ceo.12530.
- Hammes HP. Diabetic retinopathy: hyperglycaemia, oxidative stress and beyond. Diabetologia. 2018;61(1):29–38. doi:https://doi.org/10.1007/s00125-017-4435-8.
- Vecino E, Rodriguez FD, Ruzafa N, Pereiro X, Sharma SC. Glia-neuron interactions in the mammalian retina. Prog Retin Eye Res. 2016;51(3):1–40.
- Meng C, Gu C, He S, Su T, Lhamo T, Draga D, Qiu Q. Pyroptosis in the retinal neurovascular unit: new insights into diabetic retinopathy. Front Immunol. 2021;12(10):763092.doi:https://doi.org/10.3389/fimmu.2021.763092
- Xiang M, Zhou H, Nathans J. Molecular biology of retinal ganglion cells. Proc Natl Acad Sci USA. 1996;93(2):596–601. doi:https://doi.org/10.1073/pnas.93.2.596.
- Bilimoria PM, Stevens B. Microglia function during brain development: new insights from animal models. 2015;Brain Res. 1617(8):7–17.
- Jones BW, Pfeiffer RL, Ferrell WD, Watt CB, Marmor M, Marc RE. Retinal remodeling in human retinitis pigmentosa. Exp Eye Res. 2016;150(9):149–165. doi:https://doi.org/10.1016/j.exer.2016.03.018.
- Bringmann A, Grosche A, Pannicke T, Reichenbach A. GABA and glutamate uptake and metabolism in retinal glial (Müller) cells. Front Endocrinol. 2013;4:48. doi:https://doi.org/10.3389/fendo.2013.00048.
- Volgyi B. Molecular biology of retinal ganglion cells. Cells. 2020;9(11):2483.
- Masland RH. The neuronal organization of the retina. Neuron. 2012;76(2):266–280. doi:https://doi.org/10.1016/j.neuron.2012.10.002.
- Osman AM, Rodhe J, Shen X, Dominguez CA, Joseph B, Blomgren K. The Secretome of microglia regulate neural stem cell function. Neuroscience. 2019;405(5):92–102.
- Chan G, Balaratnasingam C, Yu PK, Morgan WH, McAllister IL, Cringle SJ, Yu DY. Quantitative morphometry of perifoveal capillary networks in the human retina. Invest Ophthalmol Vis Sci. 2012;53(9):5502–5514. doi:https://doi.org/10.1167/iovs.12-10265.
- Tawfik A, Samra YA, Elsherbiny NM, Al-Shabrawey M. Implication of hyperhomocysteinemia in Blood Retinal Barrier (BRB) dysfunction. Biomolecules. 2020;10(8):1119. doi:https://doi.org/10.3390/biom10081119.
- Muoio V, Persson PB, Sendeski MM. The neurovascular unit – concept review. Acta Physiol. 2014;210(4):790–798. doi:https://doi.org/10.1111/apha.12250.
- Kowiański P, Lietzau G, Steliga A, Waśkow M, Moryś J. The astrocytic contribution to neurovascular coupling–still more questions than answers? Neurosci Res. 2013;75(3):171–183. doi:https://doi.org/10.1016/j.neures.2013.01.014.
- Hill J, Rom S, Ramirez SH, Persidsky Y. Emerging roles of pericytes in the regulation of the neurovascular unit in health and disease. J Neuroimmune Pharmacol. 2014;9(5):591–605.
- Abcouwer SF, Lin CM, Shanmugam S, Muthusamy A, Barber AJ, Antonetti DA. Minocycline prevents retinal inflammation and vascular permeability following ischemia-reperfusion injury. J Neuroinflammation. 2013;10(12):149.
- Yang S, Zhang J, Chen L. The cells involved in the pathological process of diabetic retinopathy. Biomed Pharmacother. 2020;132(12):110818.
- Sinclair SH, Schwartz SS. Diabetic retinopathy-an underdiagnosed and undertreated inflammatory, neuro-vascular complication of diabetes. Front Endocrinol. 2019;10(12):843.
- Cecilia OM, Jose Alberto CG, Jose NP, Ernesto German CM, Ana Karen LC, Luis Miguel RP, Ricardo Raul RR, Adolfo Daniel RC. Oxidative stress as the main target in diabetic retinopathy pathophysiology. J Diabetes Res. 2019;2019(8):8562408. doi:https://doi.org/10.1155/2019/8562408
- Kowluru RA, Abbas SN. Diabetes-induced mitochondrial dysfunction in the retina. Invest Ophthalmol Vis Sci. 2003;44(12):5327–5334. doi:https://doi.org/10.1167/iovs.03-0353.
- Diaz-Coranguez M, Ramos C, Antonetti DA. The inner blood-retinal barrier: cellular basis and development. Vision Res. 2017;139(10):123–137.
- Nian S, Lo ACY, Mi Y, Ren K, Yang D. Neurovascular unit in diabetic retinopathy: pathophysiological roles and potential therapeutical targets. 2021;Eye Vis. 8(1):15. doi:https://doi.org/10.1186/s40662-021-00239-1.
- Simo R, Stitt AW, Gardner TW. Neurodegeneration in diabetic retinopathy: does it really matter? Diabetologia. 2018;61(9):1902–1912. doi:https://doi.org/10.1007/s00125-018-4692-1.
- Kim SJ, Yoo WS, Choi M, Chung I, Yoo JM, Choi WS. Increased O-GlcNAcylation of NF-κB enhances retinal ganglion cell death in streptozotocin-induced diabetic retinopathy. Curr Eye Res. 2016;41(2):249–257. doi:https://doi.org/10.3109/02713683.2015.1006372.
- Tessarollo L. Pleiotropic functions of neurotrophins in development. Cytokine Growth Factor Rev. 1998;9(2):125–137. doi:https://doi.org/10.1016/s1359-6101(98)00003-3.
- Lewin GR, Barde YA. Physiology of the neurotrophins. Annu Rev Neurosci. 1996;19(1):289–317. doi:https://doi.org/10.1146/annurev.ne.19.030196.001445.
- Sacchetti M, Bruscolini A, Lambiase A. Neurotrophic factors and nerve growth factor in ocular allergy. Curr Opin Allergy Clin Immunol. 2019;19(5):510–516. doi:https://doi.org/10.1097/ACI.0000000000000555.
- Bradshaw RA, Mobley W, Rush RA. Nerve growth factor and related substances: a brief history and an introduction to the international NGF meeting series. Int J Mol Sci. 2017;18(6):1143. doi:https://doi.org/10.3390/ijms18061143.
- Cowan WM. Viktor Hamburger and Rita Levi-Montalcini: the path to the discovery of nerve growth factor. Annu Rev Neurosci. 2001;24(1):551–600. doi:https://doi.org/10.1146/annurev.neuro.24.1.551.
- Rush RA, Chie E, Liu D, Tafreshi A, Zettler C, Zhou XF. Neurotrophic factors are required by mature sympathetic neurons for survival, transmission and connectivity. Clin Exp Pharmacol Physiol. 1997;24(8):549–555. doi:https://doi.org/10.1111/j.1440-1681.1997.tb02089.x.
- Aloe L. Nerve growth factor and neuroimmune responses: basic and clinical observations. Arch Physiol Biochem. 2001;109(4):354–356. doi:https://doi.org/10.1076/apab.109.4.354.4235.
- Nguyen NM, Song KM, Choi MJ, Ghatak K, Kwon MH, Ock J, Yin GN, Ryu JK, Suh JK. Inhibition of proNGF and p75NTR pathway restores erectile function through dual angiogenic and neurotrophic effects in the diabetic mouse. 2019;J Sex Med. 16(3):351–364. doi:https://doi.org/10.1016/j.jsxm.2019.01.013.
- Fahnestock M, Yu G, Coughlin MD. ProNGF: a neurotrophic or an apoptotic molecule? Prog Brain Res. 2004;146:101–110.
- Pallottini V, Colardo M, Tonini C, Martella N, Strimpakos G, Colella B, Tirassa P, Bartolomeo SD, Segatto M. ProNGF/p75NTR axis drives fiber type specification by inducing the fast-glycolytic phenotype in mouse skeletal muscle cells. Cells. 2020;9(10):2232. doi:https://doi.org/10.3390/cells9102232.
- Fahnestock M, Shekari A. ProNGF and neurodegeneration in Alzheimer’s Disease. Front Neurosci. 2019;13(2):129.
- Zhu MC, Xiong P, Li GL, Zhu M. Could lung cancer exosomes induce apoptosis of natural killer cells through the p75NTR-proNGF-sortilin axis? Med Hypotheses. 2017;108(10):151–153. doi:https://doi.org/10.1016/j.mehy.2017.09.003.
- Shen L-L, Mañucat-Tan NB, Gao S-H, Li W-W, Zeng F, Zhu C, Wang J, Bu X-L, Liu Y-H, Gao C-Y, et al. The ProNGF/p75NTR pathway induces tau pathology and is a therapeutic target for FTLD-tau. Mol Psychiatry. 2018;23(8):1813–1824. doi:https://doi.org/10.1038/s41380-018-0071-z.
- Al-Gayyar MM, Mysona BA, Matragoon S, Abdelsaid MA, El-Azab MF, Shanab AY, Ha Y, Smith SB, Bollinger KE, El-Remessy AB. Diabetes and overexpression of proNGF cause retinal neurodegeneration via activation of RhoA pathway. PLoS One. 2013;8(1):e54692. doi:https://doi.org/10.1371/journal.pone.0054692.
- Correction: ProNGF-p75NTR axis plays a proinflammatory role in inflamed joints: a novel pathogenic mechanism in chronic arthritis. RMD Open. 2017;4(1):e000441. doi: https://doi.org/10.1136/rmdopen-2017-000441
- Ki YW, Park JH, Lee JE, Shin IC, Koh HC. JNK and p38 MAPK regulate oxidative stress and the inflammatory response in chlorpyrifos-induced apoptosis. Toxicol Lett. 2013;218(3):235–245. doi:https://doi.org/10.1016/j.toxlet.2013.02.003.
- Ahmad I, Yue WY, Fernando A, Clark JJ, Woodson EA, Hansen MR. p75NTR is highly expressed in vestibular schwannomas and promotes cell survival by activating nuclear transcription factor κB. Glia. 62(10):1699–1712. doi:https://doi.org/10.1002/glia.22709.
- Dai WL, Yan B, Bao YN, Fan JF, Liu JH. 2020. Suppression of peripheral NGF attenuates neuropathic pain induced by chronic constriction injury through the TAK1-MAPK/NF-κB signaling pathways. Cell Commun Signal. 18(1):66. doi:https://doi.org/10.1186/s12964-020-00556-3.
- Manti S, Brown P, Perez MK, Piedimonte G. 2017. The role of neurotrophins in inflammation and allergy. Vitam Horm. 104:313–341. doi:https://doi.org/10.1016/bs.vh.2016.10.010.
- Luo M, Shi X, Guo Q, Li S, Zhang Q, Sun X, Piao F. 2021. 2,5-Hexanedione induced apoptosis in rat spinal cord neurons and VSC4.1 cells via the proNGF/p75NTR and JNK pathways. Biosci Rep. 41(4):BSR20204264.
- Wong HLX, Qin HY, Tsang SW, Zuo X, Che S, Chow CFW, Li X, Xiao HT, Zhao L, Huang T, et al. Early life stress disrupts intestinal homeostasis via NGF-TrkA signaling. Nat Commun. 2019;10(1):1745. doi:https://doi.org/10.1038/s41467-019-09744-3.
- Moosavi F, Hosseini R, Saso L, Firuzi O. Modulation of neurotrophic signaling pathways by polyphenols. Drug Des Devel Ther. 2016;10(12):23–42. doi:https://doi.org/10.2147/DDDT.S96936
- Gudasheva TA, Logvinov IO, Nikolaev SV, Antipova TA, Povarnina PY, Seredenin SB. Dipeptide mimetics of different NGF and BDNF loops activate PLC-γ1. Dokl Biochem Biophys. 2020;494(1):244–247. doi:https://doi.org/10.1134/S1607672920050075.
- Hou Y, Jia L, Zhang Y, Ji W, Li H. Activation of the NGF/TrkA signaling pathway attenuates diabetic erectile dysfunction. Oncotarget. 2017;8(62):105692–105702. doi:https://doi.org/10.18632/oncotarget.22389.
- Fudalej E, Justyniarska M, Kasarełło K, Dziedziak J, Szaflik JP, Cudnoch-Jędrzejewska A. Neuroprotective factors of the retina and their role in promoting survival of retinal ganglion cells: a review. Ophthalmic Res. 2021;64(3):345–355. doi:https://doi.org/10.1159/000514441.
- Luo D, Zhao J, Cheng Y, Lee SM, Rong J. N-Propargyl Caffeamide (PACA) ameliorates dopaminergic neuronal loss and motor dysfunctions in MPTP Mouse Model of Parkinson’s Disease and in MPP(+)-induced neurons via promoting the conversion of proNGF to NGF. Mol Neurobiol. 2018;55(3):2258–2267. doi:https://doi.org/10.1007/s12035-017-0486-6.
- Liu H, Wang W, Li X, Huang C, Zhang Z, Yuan M, Li X. High hydrostatic pressure induces apoptosis of retinal ganglion cells via regulation of the NGF signalling pathway. Mol Med Rep. 2019;19(6):5321–5334. doi:https://doi.org/10.3892/mmr.2019.10206
- von Bartheld CS. Neurotrophins in the developing and regenerating visual system. Histol Histopathol. 1998;13(2):437–459. doi:https://doi.org/10.14670/HH-13.437.
- Quigley HA, McKinnon SJ, Zack DJ, Pease ME, Kerrigan-Baumrind LA, Kerrigan DF, Mitchell RS. Retrograde axonal transport of BDNF in retinal ganglion cells is blocked by acute IOP elevation in rats. Invest Ophthalmol Vis Sci. 2000;41(11):3460–3466.
- Carmignoto G, Maffei L, Candeo P, Canella R, Comelli C. Effect of NGF on the survival of rat retinal ganglion cells following optic nerve section. J Neurosci. 1989;9(4):1263–1272. doi:https://doi.org/10.1523/JNEUROSCI.09-04-01263.1989.
- Yip HK, Grafstein B. Effect of nerve growth factor on regeneration of goldfish optic axons. Brain Res. 1982;238(2):329–339. doi:https://doi.org/10.1016/0006-8993(82)90108-1.
- Tirassa P, Rosso P, Iannitelli A. Ocular Nerve Growth Factor (NGF) and NGF eye drop application as paradigms to investigate NGF neuroprotective and reparative actions. Methods Mol Biol. 2018;1727:19–38.
- Turner JE, Delaney RK. Retinal ganglion cell response to axotomy and nerve growth factor in the regenerating visual system of the newt (Notophthalmus viridescens): an ultrastructural morphometric analysis. Brain Res. 1979;171(2):197–212. doi:https://doi.org/10.1016/0006-8993(79)90327-5.
- Jian Q, Li Y, Yin ZQ. Rat BMSCs initiate retinal endogenous repair through NGF/TrkA signaling. Exp Eye Res. 2015;132(3):34–47. doi:https://doi.org/10.1016/j.exer.2015.01.008.
- Garcia TB, Hollborn M, Bringmann A. Expression and signaling of NGF in the healthy and injured retina. Cytokine Growth Factor Rev. 2017;34(4):43–57. doi:https://doi.org/10.1016/j.cytogfr.2016.11.005.
- Chakrabarti S, Sima AA, Lee J, Brachet P, Dicou E. Nerve growth factor (NGF), proNGF and NGF receptor-like immunoreactivity in BB rat retina. Brain Res. 1990;523(1):11–15. doi:https://doi.org/10.1016/0006-8993(90)91630-y.
- Bothwell M. Tissue localization of nerve growth factor and nerve growth factor receptors. Curr Top Microbiol Immunol. 1991;165:55–70. doi:https://doi.org/10.1007/978-3-642-75747-1_4.
- Wang H, Wang R, Thrimawithana T, Little PJ, Xu J, Feng ZP, Zheng W. The nerve growth factor signaling and its potential as therapeutic target for glaucoma. Biomed Res Int. 2014;2014:759473. doi:https://doi.org/10.1155/2014/759473.
- Ng DS, Chiang PP, Tan G, Cheung CG, Cheng CY, Cheung CY, Wong TY, Lamoureux EL, Ikram MK. Retinal ganglion cell neuronal damage in diabetes and diabetic retinopathy. Clin Exp Ophthalmol. 2016;44(4):243–250. doi:https://doi.org/10.1111/ceo.12724.
- Hammes HP, Federoff HJ, Brownlee M. Nerve growth factor prevents both neuroretinal programmed cell death and capillary pathology in experimental diabetes. Mol Med. 1995;1(5):527–534.
- Retamales-Ortega R, Orostica L, Vera C, Cuevas P, Hernandez A, Hurtado I, Vega M, Romero C. Role of Nerve Growth Factor (NGF) and miRNAs in Epithelial Ovarian Cancer. Int J Mol Sci. 2017;18(3):507. doi:https://doi.org/10.3390/ijms18030507.
- Ji Z, Luo J, Su T, Chen C, Su Y. miR-7a targets insulin receptor substrate-2 gene and suppresses viability and invasion of cells in diabetic retinopathy mice via PI3K-Akt-VEGF pathway. Diabetes Metab Syndr Obes. 2021;14(2):719–728. doi:https://doi.org/10.2147/DMSO.S288482.
- Xie Y, Shi X, Sheng K, Han G, Li W, Zhao Q, Jiang B, Feng J, Li J, Gu Y. PI3K/Akt signaling transduction pathway, erythropoiesis and glycolysis in hypoxia. Mol Med Rep. 2019;19(2):783–791.
- Ishitsuka K, Ago T, Arimura K, Nakamura K, Tokami H, Makihara N, Kuroda J, Kamouchi M, Kitazono T. Neurotrophin production in brain pericytes during hypoxia: a role of pericytes for neuroprotection. Microvasc Res. 2012;83(3):352–359. doi:https://doi.org/10.1016/j.mvr.2012.02.009.
- Ali TK, Al-Gayyar MM, Matragoon S, Pillai BA, Abdelsaid MA, Nussbaum JJ, El-Remessy AB. Diabetes-induced peroxynitrite impairs the balance of pro-nerve growth factor and nerve growth factor, and causes neurovascular injury. Diabetologia. 2011;54(3):657–668. doi:https://doi.org/10.1007/s00125-010-1935-1.
- Wei Y, Wang HZ, Zhang FK, Zao JP, Jiang XH, Lu QJ, Gao EJ, Wang NL. Enhanced expression of proneurotrophins in elevated introcular pressure-induced rat retinal ischemia. Chin Med J. 2012;125(21):3875–3879.
- Al-Gayyar MM, Matragoon S, Pillai BA, Ali TK, Abdelsaid MA, El-Remessy AB. Epicatechin blocks pro-nerve growth factor (proNGF)-mediated retinal neurodegeneration via inhibition of p75 neurotrophin receptor expression in a rat model of diabetes. Diabetologia. 54(3):669–680. doi:https://doi.org/10.1007/s00125-010-1994-3.
- Mohamed R, Shanab AY, El Remessy AB. 2017. Deletion of the Neurotrophin Receptor p75(NTR) prevents diabetes-induced retinal acellular capillaries in streptozotocin-induced mouse diabetic model. J Diabetes Metab Disord Control. 4(6):129.
- Saleh I, Maritska Z, Parisa N, Hidayat R. Inhibition of receptor for advanced glycation end products as new promising strategy treatment in diabetic retinopathy. Open Access Maced J Med Sci. 2019;7(23):3921–3924. doi:https://doi.org/10.3889/oamjms.2019.759.
- Balzamino BO, Esposito G, Marino R, Keller F, Micera A. Changes in vitreal protein profile and retina mRNAs in Reeler mice: NGF, IL33 and Muller cell activation. PLoS One. 2019;14(2):e0212732. doi:https://doi.org/10.1371/journal.pone.0212732.
- Sahin K, Gencoglu H, Akdemir F, Orhan C, Tuzcu M, Sahin N, Yilmaz I, Juturu V. Lutein and zeaxanthin isomers may attenuate photo-oxidative retinal damage via modulation of G protein-coupled receptors and growth factors in rats. Biochem Biophys Res Commun. 2019;516(1):163–170. doi:https://doi.org/10.1016/j.bbrc.2019.06.032.
- Punzo C, Xiong W, Cepko CL. Loss of daylight vision in retinal degeneration: are oxidative stress and metabolic dysregulation to blame? J Biol Chem. 2012;287(3):1642–1648. doi:https://doi.org/10.1074/jbc.R111.304428.
- Asakawa K, Ishikawa H, Uga S, Mashimo K, Kondo M, Terasaki H. Histopathological changes of inner retina, optic disc, and optic nerve in rabbit with advanced retinitis Pigmentosa. Neuroophthalmology. 2016;40(6):286–291. doi:https://doi.org/10.1080/01658107.2016.1229339.
- Lu B, Morgans CW, Girman S, Lund R, Wang S. Retinal morphological and functional changes in an animal model of retinitis pigmentosa. Vis Neurosci. 2013;30(3):77–89. doi:https://doi.org/10.1017/S0952523813000011.
- Rocco ML, Balzamino BO, Petrocchi Passeri P, Micera A, Aloe L. Effect of purified murine NGF on isolated photoreceptors of a rodent developing retinitis pigmentosa. PLoS One. 2015;10(4):e0124810. doi:https://doi.org/10.1371/journal.pone.0124810.
- Falsini B, Iarossi G, Chiaretti A, Ruggiero A, Manni L, Luigi M, Galli-Resta L, Corbo G, Abed E. Abed E. NGF eye-drops topical administration in patients with retinitis pigmentosa, a pilot study. J Transl Med. 2016;14(9):8. doi:https://doi.org/10.1186/s12967-015-0750-3.
- Rocco ML, Calza L, Aloe L. NGF and Retinitis Pigmentosa: structural and molecular studies. Adv Exp Med Biol. 2021;1331:255–263. doi:https://doi.org/10.1007/978-3-030-74046-7_17.
- Platon-Corchado M, Barcelona PF, Jmaeff S, Marchena M, Hernandez-Pinto AM, Hernandez-Sanchez C, Saragovi HU, de la Rosa EJ. p75NTR antagonists attenuate photoreceptor cell loss in murine models of retinitis pigmentosa. Cell Death Dis. 2017;8(7):e2922. doi:https://doi.org/10.1038/cddis.2017.306.
- Rocco ML, Balzamino BO, Esposito G, Petrella C, Aloe L, Micera A. NGF/anti-VEGF combined exposure protects RCS retinal cells and photoreceptors that underwent a local worsening of inflammation. Graefes Arch Clin Exp Ophthalmol. 2017;255(3):567–574. doi:https://doi.org/10.1007/s00417-016-3567-8.
- Calkins DJ. Critical pathogenic events underlying progression of neurodegeneration in glaucoma. Prog Retin Eye Res. 2012;31(6):702–719. doi:https://doi.org/10.1016/j.preteyeres.2012.07.001.
- Burgoyne CF. A biomechanical paradigm for axonal insult within the optic nerve head in aging and glaucoma. Exp Eye Res. 2011;93(2):120–132. doi:https://doi.org/10.1016/j.exer.2010.09.005.
- Wei X, Cho KS, Thee EF, Jager MJ, Chen DF. Neuroinflammation and microglia in glaucoma: time for a paradigm shift. J Neurosci Res. 2019;97(1):70–76. doi:https://doi.org/10.1002/jnr.24256.
- Galassi F, Sodi A, Ucci F, Renieri G, Pieri B, Baccini M. Ocular hemodynamics and glaucoma prognosis: a color Doppler imaging study. Arch Ophthalmol. 2003;121(12):1711–1715. doi:https://doi.org/10.1001/archopht.121.12.1711.
- Newman A, Andrew N, Casson R. Review of the association between retinal microvascular characteristics and eye disease. Clin Exp Ophthalmol. 2018;46(5):531–552. doi:https://doi.org/10.1111/ceo.13119.
- Kawasaki R, Wang JJ, Rochtchina E, Lee AJ, Wong TY, Mitchell P. Retinal vessel caliber is associated with the 10-year incidence of glaucoma: the Blue Mountains Eye Study. Ophthalmology. 2013;120(1):84–90. doi:https://doi.org/10.1016/j.ophtha.2012.07.007.
- Wareham LK, Calkins DJ. The neurovascular unit in glaucomatous neurodegeneration. Front Cell Dev Biol. 2020;8(7):452.
- Syc-Mazurek SB, Libby RT. Axon injury signaling and compartmentalized injury response in glaucoma. Prog Retin Eye Res. 2020;73(12):100769. doi:https://doi.org/10.1016/j.preteyeres.2019.07.002.
- Lambiase A, Centofanti M, Micera A, Manni GL, Mattei E, De Gregorio A, de Feo G, Bucci MG, Aloe L. Nerve growth factor (NGF) reduces and NGF antibody exacerbates retinal damage induced in rabbit by experimental ocular hypertension. Graefes Arch Clin Exp Ophthalmol. 1997;235(12):780–785. doi:https://doi.org/10.1007/BF02332863.
- Oddone F, Roberti G, Micera A, Busanello A, Bonini S, Quaranta L, Agnifili L, Manni G. Exploring serum levels of brain derived neurotrophic factor and nerve growth factor across glaucoma stages. PLoS One. 2017;12(1):e0168565. doi:https://doi.org/10.1371/journal.pone.0168565.
- Ke Y, Huang L, Chen B, Sima J, Cao J, Li Q. Effects of ultrasound contrast agent-mediated nerve growth factor on apoptosis of retinal ganglion cells in mice with glaucoma. Comput Math Methods Med. 2021;2021(11):6084496.
- Lambiase A, Aloe L, Centofanti M, Parisi V, Báo SN, Mantelli F, Colafrancesco V, Manni GL, Bucci MG, Bonini S, et al. Experimental and clinical evidence of neuroprotection by nerve growth factor eye drops: implications for glaucoma. Proc Natl Acad Sci USA. 2009;106(32):13469–13474. doi:https://doi.org/10.1073/pnas.0906678106.
- Beykin G, Stell L, Halim MS, Nuñez M, Popova L, Nguyen BT, Groth SL, Dennis A, Li Z, Atkins M, et al. Phase 1b randomized controlled study of short course topical recombinant human Nerve Growth Factor (rhNGF) for neuroenhancement in glaucoma: safety, tolerability, and efficacy measure outcomes. Am J Ophthalmol. 2022;234:223–234. doi:https://doi.org/10.1016/j.ajo.2021.11.002.
- Lin B, Zhang X, Xu X. Nerve growth factor protects retinal ganglion cells related to inhibiting endoplasmic reticulum stress by inhibiting IRE1-JNK-CHOP signaling pathway. Ocul Immunol Inflamm. 2021;4(1):1–6.
- Gardner TW, Davila JR. The neurovascular unit and the pathophysiologic basis of diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2017;255(1):1–6. doi:https://doi.org/10.1007/s00417-016-3548-y.
- Beltramo E, Porta M. Pericyte loss in diabetic retinopathy: mechanisms and consequences. Curr Med Chem. 2013;20(26):3218–3225. doi:https://doi.org/10.2174/09298673113209990022.
- Aung MH, Park HN, Han MK, Obertone TS, Abey J, Aseem F, Thule PM, Iuvone PM, Pardue MT. Dopamine deficiency contributes to early visual dysfunction in a rodent model of type 1 diabetes. J Neurosci. 2014;34(3):726–736. doi:https://doi.org/10.1523/JNEUROSCI.3483-13.2014.
- Sorrentino FS, Allkabes M, Salsini G, Bonifazzi C, Perri P. The importance of glial cells in the homeostasis of the retinal microenvironment and their pivotal role in the course of diabetic retinopathy. Life Sci. 2016;162(10):54–59.
- Kinuthia UM, Wolf A, Langmann T. Microglia and inflammatory responses in diabetic retinopathy. Front Immunol. 2020;11(11):564077. doi:https://doi.org/10.3389/fimmu.2020.564077.
- Mohamed R, El-Remessy AB. Imbalance of the nerve growth factor and its precursor: implication in diabetic retinopathy. J Clin Exp Ophthalmol. 2015;06(05):483. doi:https://doi.org/10.4172/2155-9570.1000483.
- Mysona BA, Al-Gayyar MM, Matragoon S, Abdelsaid MA, El-Azab MF, Saragovi HU, El-Remessy AB. Modulation of p75(NTR) prevents diabetes- and proNGF-induced retinal inflammation and blood-retina barrier breakdown in mice and rats. Diabetologia. 2013;56(10):2329–2339. doi:https://doi.org/10.1007/s00125-013-2998-6.
- Elsherbiny NM, Abdel-Mottaleb Y, Elkazaz AY, Atef H, Lashine RM, Youssef AM, Ezzat W, El-Ghaiesh SH, Elshaer RE, El-Shafey M, et al. Carbamazepine alleviates retinal and optic nerve neural degeneration in diabetic mice via nerve growth factor-induced PI3K/Akt/mTOR activation. Front Neurosci. 2019;13(13(11):1089.
- Troullinaki M, Alexaki VI, Mitroulis I, Witt A, Klotzsche-von Ameln A, Chung KJ, Chavakis T, Economopoulou M. Nerve growth factor regulates endothelial cell survival and pathological retinal angiogenesis. J Cell Mol Med. 2019;23(4):2362–2371. doi:https://doi.org/10.1111/jcmm.14002.
- Wang QC, Sheng W, Yi CJ, Lv H, Cheng B. Retrobulbarly injecting nerve growth factor attenuates visual impairment in streptozotocin-induced diabetes rats. Int Ophthalmol. 2020;40(12):3501–3511. doi:https://doi.org/10.1007/s10792-020-01537-8.
- Telegina DV, Kozhevnikova OS, Kolosova NG. Changes in retinal glial cells with age and during development of age-related macular degeneration. Biochemistry. 2018;83(9):1009–1017. doi:https://doi.org/10.1134/S000629791809002X.
- Edwards MM, McLeod DS, Bhutto IA, Villalonga MB, Seddon JM, Lutty GA. Idiopathic preretinal glia in aging and age-related macular degeneration. Exp Eye Res. 2016;150:44–61.
- Felszeghy S, Viiri J, Paterno JJ, Hyttinen JMT, Koskela A, Chen M, Leinonen H, Tanila H, Kivinen N, Koistinen A, et al. Loss of NRF-2 and PGC-1α genes leads to retinal pigment epithelium damage resembling dry age-related macular degeneration. Redox Biol. 2019;20(1):1–12. doi:https://doi.org/10.1016/j.redox.2018.09.011.
- Hudson N, Cahill M, Campbell M. Inner blood-retina barrier involvement in dry age-related macular degeneration (AMD) pathology. Neural Regen Res. 2020;15(9):1656–1657. doi:https://doi.org/10.4103/1673-5374.276332.
- Ambati J, Fowler BJ. Mechanisms of age-related macular degeneration. Neuron. 2012;75(1):26–39. doi:https://doi.org/10.1016/j.neuron.2012.06.018.
- Telegina DV, Kolosova NG, Kozhevnikova OS. Immunohistochemical localization of NGF, BDNF, and their receptors in a normal and AMD-like rat retina. BMC Med Genomics. 2019;12(S2):48. doi:https://doi.org/10.1186/s12920-019-0493-8.
- Wang S, Liu Y, Liu Y, Li C, Wan Q, Yang L, Su Y, Cheng Y, Liu C, Wang X, et al. Reversed senescence of retinal pigment epithelial cell by coculture with embryonic stem cell via the TGFbeta and PI3K pathways. Front Cell Dev Biol. 2020;8(11):588050.
- AnandBabu K, Sen P, Angayarkanni N. Oxidized LDL, homocysteine, homocysteine thiolactone and advanced glycation end products act as pro-oxidant metabolites inducing cytokine release, macrophage infiltration and pro-angiogenic effect in ARPE-19 cells. PLoS One. 2019;14(5):e0216899. doi:https://doi.org/10.1371/journal.pone.0216899.
- Kyosseva SV. Targeting MAPK signaling in age-related macular degeneration. Ophthalmol Eye Dis. 2016;8(6):23–30.
- Du H, Sun X, Guma M, Luo J, Ouyang H, Zhang X, Zeng J, Quach J, Nguyen DH, Shaw PX, et al. JNK inhibition reduces apoptosis and neovascularization in a murine model of age-related macular degeneration. Proc Natl Acad Sci USA. 2013;110(6):2377–2382. doi:https://doi.org/10.1073/pnas.1221729110.
- Lambiase A, Aloe L. Nerve growth factor delays retinal degeneration in C3H mice. Graefe’s Arch Clin Exp Ophthalmol. 1996;234(S1):S96–S100. doi:https://doi.org/10.1007/BF02343055.
- Rosso P, Fico E, Mesentier-Louro LA, Triaca V, Lambiase A, Rama P, Tirassa P. NGF eye administration recovers the TrkB and glutamate/GABA marker deficit in the adult visual cortex following optic nerve crush. Int J Mol Sci. 2021;22(18):10014. doi:https://doi.org/10.3390/ijms221810014.
- Mesentier-Louro LA, Rosso P, Carito V, Mendez-Otero R, Santiago MF, Rama P, Lambiase A, Tirassa P. Nerve growth factor role on retinal ganglion cell survival and axon regrowth: effects of ocular administration in experimental model of optic nerve injury. Mol Neurobiol. 2019;56(2):1056–1069. doi:https://doi.org/10.1007/s12035-018-1154-1.
- Sacchetti M, Mantelli F, Rocco ML, Micera A, Brandolini L, Focareta L, Pisano C, Aloe L, Lambiase A. Recombinant human nerve growth factor treatment promotes photoreceptor survival in the retinas of rats with Retinitis Pigmentosa. Curr Eye Res. 2017;42(7):1064–1068. doi:https://doi.org/10.1080/02713683.2017.1279634.
- Aloe L, Rocco ML, Balzamino BO, Esposito G, Micera A. Retrobulbar administration of purified anti-nerve growth factor in developing rats induces structural and biochemical changes in the retina and cornea. Int J Ophthalmol. 2021;14(2):209–216. doi:https://doi.org/10.18240/ijo.2021.02.05.
- Guo L, Davis BM, Ravindran N, Galvao J, Kapoor N, Haamedi N, Shamsher E, Luong V, Fico E, Cordeiro MF. Topical recombinant human Nerve growth factor (rh-NGF) is neuroprotective to retinal ganglion cells by targeting secondary degeneration. Sci Rep. 2020;10(1):3375. doi:https://doi.org/10.1038/s41598-020-60427-2.
- Cattaneo A, Capsoni S. Painless Nerve Growth Factor: a TrkA biased agonist mediating a broad neuroprotection via its actions on microglia cells. Pharmacol Res. 2019;139(1):17–25.
- Elshaer SL, Alwhaibi A, Mohamed R, Lemtalsi T, Coucha M, Longo FM, El-Remessy AB. Modulation of the p75 neurotrophin receptor using LM11A-31 prevents diabetes-induced retinal vascular permeability in mice via inhibition of inflammation and the RhoA kinase pathway. Diabetologia. 2019;62(8):1488–1500. doi:https://doi.org/10.1007/s00125-019-4885-2.
- Wyganowska-Świątkowska M, Matthews-Kozanecka M, Matthews-Brzozowska T, Skrzypczak-Jankun E, Jankun J. Can EGCG alleviate symptoms of down syndrome by altering proteolytic activity? Int J Mol Sci. 2018;19(1):248. doi:https://doi.org/10.3390/ijms19010248.
- Barcelona PF, Sitaras N, Galan A, Esquiva G, Jmaeff S, Jian Y, Sarunic MV, Cuenca N, Sapieha P, Saragovi HU. p75NTR and its ligand ProNGF activate paracrine mechanisms etiological to the vascular, inflammatory, and neurodegenerative pathologies of diabetic retinopathy. J Neurosci. 2016;36(34):8826–8841. doi:https://doi.org/10.1523/JNEUROSCI.4278-15.2016.
- Ciavarella C, Buzzi M, Bergantin E, Di Marco S, Giannaccare G, Campos E, Bisti S, Versura P. Effects of Cord Blood Serum (CBS) on viability of retinal Müller glial cells under in vitro injury. PLoS One. 2020.15(6):e0234145. doi:https://doi.org/10.1371/journal.pone.0234145.
- Di Marco S, Riccitelli S, Di Paolo M, Campos E, Buzzi M, Bisti S, Versura P. Cord blood serum (CBS)-based eye drops modulate light-induced neurodegeneration in albino rat retinas. Biomolecules. 2020;10(5):678. doi:https://doi.org/10.3390/biom10050678.
- Wang L, Cao T, Chen H. Treatment of glaucomatous optic nerve damage using ginsenoside Rg1 mediated by ultrasound targeted microbubble destruction. Exp Ther Med. 2018;15(1):300–304.
- Wu Q, Gu X, Liu X, Yan X, Liao L, Zhou J. Astragalus membranaceus injection protects retinal ganglion cells by regulating the nerve growth factor signaling pathway in experimental rat traumatic optic neuropathy. Evid Based Complement Alternat Med. 2020;2020:1–12. doi:https://doi.org/10.1155/2020/2429843.
- Hill D, Compagnoni C, Cordeiro MF. Investigational neuroprotective compounds in clinical trials for retinal disease. Expert Opin Investig Drugs. 2021;30(5):571–577. doi:https://doi.org/10.1080/13543784.2021.1896701.
