Abstract
Purpose
To demonstrate the suitability of using decellularized SMILE (Small-incision Lenticule Extraction) lenticules for culturing and transplanting the corneal endothelium (CE).
Methods
The SMILE lenticules, obtained during refractive surgery, were decellularized by incubating in CE culture medium and fetal bovine serum. Decellularization was confirmed by hematoxylin and eosin staining, DAPI staining, and gel electrophoresis. The amount of DNA per milligram of dry tissue weight was calculated to quantify the residual nuclear content. The transparency of the decellularized lenticules was determined by calculating the modulation transfer function. Immunostaining for stromal collagens and glycosaminoglycan was performed using specific antibodies. Engineered tissue was constructed by culturing the CE cells on lenticules and staining for ZO-1, Na/K ATPase, and N-cadherin. The functionality of the engineered tissues was assessed by transplanting them onto edematous human donor corneas and perfusing for 10 days ex-vivo.
Results
The residual DNA per milligram of dry tissue weight was found to be significantly reduced (p < 0.0001) in serum (0.255 µg/mg) and Opti-MEM (0.140 µg/mg) when compared to fresh lenticules (3.9 µg/mg). Decellularization did not alter the arrangement of the collagen fibers or the transparency of the lenticules. CE cells attached and matured to express ZO-1, Na/K ATPase, and N-cadherin at two weeks after seeding. The engineered tissue upon transplantation significantly reduced the corneal edema (p < 0.05) and the transplanted cells remained intact on the SMILE lenticule post-transplantation.
Conclusion
This study demonstrates the suitability of using SMILE lenticules decellularized using a simple, chemical-free method for engineering the corneal endothelium for transplantation.
Introduction
The cornea is one of the most transplanted tissues globally, accounting for ∼180,000 surgeries in a single year in over 116 countries. Yet, only 1 healthy tissue is available for every 70 that are required leaving nearly 12.7 million people awaiting transplantation.Citation1 Of the total corneal transplants performed in a given year, nearly 40% of them are done to replace the dysfunctional corneal endothelium (CE).Citation2 Engineering the endothelial layer, therefore, would provide a much-needed alternative to donor tissues. Tissue engineering involves two important steps: the first is the generation of the native cells and the second is the material used to construct the tissue. Many synthetic and biological materials have been used for constructing the CE layer.Citation3–9 Of particular interest is the corneal stroma since it retains all the required features of the native tissue allowing for better integration after transplantation. In recent times, lenticules that are extracted during refractive surgery (Small Incision Lenticule Extraction or SMILE) have garnered interest since they provide a cadaver-donor independent source of corneal stroma and are readily available. These lenticules are increasingly being used as implants to correct hyperopia,Citation10,Citation11 presbyopia,Citation12,Citation13 as grafts to treat tears/perforationsCitation14–16 and for constructing corneal equivalents.Citation17,Citation18
For tissue engineering, the biological source must be decellularized because the presence of residual cellular material can trigger the immune system to reject the transplantation or can affect the remodeling following transplantation.Citation19,Citation20 While ensuring sufficient decellularization, it is also important to retain much of the native extracellular matrix (ECM), which is known to regulate cell mitogenesis, differentiation and can induce host tissue remodeling.Citation21–23 In the case of corneal transplantation, the tissue transparency must also be unaffected following decellularization. Several chemical (e.g. detergents), enzymatic (e.g. collagenase, trypsin), and physical methods (e.g. compression, freeze-thawing) have been used to decellularize human and animal corneal tissues.Citation18,Citation24–35 The efficacy of these techniques at removing cellular components varies with tissue thickness but the most common drawbacks reported are the stripping of ECM proteins, compromised optical clarity, and a loss in the tensile strength of the tissue. Further, the residual presence of any of the chemicals used for decellularization can evoke an adverse immune response after transplantation thus requiring extensive washing over several hours or days.
In this paper, we report a simple, chemical-free, yet effective technique for decellularizing the SMILE lenticules, which were used for engineering the human CE (hCE) layer. The engineered hCE layer, when transplanted to the human cornea using an ex-vivo perfusion system, was able to significantly reduce corneal edema thus providing proof for its potential use as a carrier for transplanting cultured hCE cells.
Materials and methods
Materials
Opti-MEM and keratinocyte media (KM) were purchased from Gibco, Thermo fisher Scientific, USA. Fetal bovine serum (FBS) was from Hyclone; chondroitin sulfate and calcium chloride were from Sigma, USA. Epidermal growth factor, insulin, gentamicin, phalloidin, anti-mouse IgG, anti-rabbit IgG, live-dead assay kit (R37601), and anti-ZO-1 antibody were purchased from Invitrogen, USA. NaCl and Cell Tracker Red CMTPX (C34552) were from Thermo fisher Scientific, USA. DNase was purchased from Promega, USA. Collagens 1, 3, 5, 12, anti-keratan sulfate, anti-Na/K ATPase, and anti-N-cadherin antibodies were from Santa Cruz Biotechnology, USA. Collagen 3 antibody was from Abcam, UK, and collagen 4 antibody was from GENEXT Genomics Pvt. Ltd., India. The antibody details are provided in supplementary table 1.
Preparation of SMILE lenticules
Stromal lenticules were obtained from patients undergoing SMILE refractive surgery after obtaining institutional review board approval (LEC 07-17-067) and informed consent. A total of 160 lenticules were collected for the study with an average thickness of 84.519 µm ± 29.2 µm and a diameter of 6.5 mm for all lenticules. The lenticules were rinsed with 0.05% betadine followed by a wash with 1X phosphate-buffered saline (PBS) and then were placed in the following media for decellularization (1) CE medium (Opti-MEM, 8% FBS, 0.08% chondroitin sulfate, 200 mg/L calcium chloride, 5 ng/ml EGF, 50 µg/ml gentamicin), (2) keratinocyte medium(DMEM + F12, 10% FBS, 5ng/ml EGF, 5 mg/ml insulin, 50 µg/ml gentamicin), (3) fetal bovine serum, and (4) 1X phosphate-buffered saline. Lenticules decellularized using 1.5 M NaCl + 5U DNase as per the previous protocol with some modifications served as the positive control.Citation18 Briefly, for NaCl-DNase treatment, 1.5 M NaCl was added to the lenticules for 24 hrs, removed, and replaced with fresh solution for an additional 24 hrs. The NaCl solution was removed and 5 units of DNase were added to the lenticules and incubated for 24hrs. After removal of the DNase, the lenticules were washed in 1xPBS for 24 hrs followed by 3 washes in 1xPBS for 5 mins each. Fresh lenticules and lenticules placed in KM served as negative controls. Following incubation in the different media for the specified time points, the lenticules were taken for further assessment.
DAPI staining of lenticules
After treatment, the lenticules were stained with 4′, 6-diamidino-2-phenylindole (DAPI) for 5 mins, followed by three PBS washes of 5 mins each. The lenticules were mounted using glycerol and images were taken at 5X magnification using the Ziess Airyspace LSM 880 confocal microscope. The images were stitched together to create montages of the lenticules.
Live-dead assay
The end-point of decellularization was 7 days for Opti-MEM medium (37 °C), 1X PBS (4 °C) and KM (37 °C), and 72 hrs for FBS (37 °C). The assay was conducted at 24 hrs for comparison. NaCl-DNase treated lenticule was used as the control for decellularization and fresh lenticuleserved as the negative control. After incubation in the respective media for the defined time points, the lenticules were washed with 1X PBS and checked for keratocyte viability using LIVE-DEAD cytotoxicity assay as per the manufacturer’s recommendations. Images were taken at 10X magnification using the Zeiss Airyspace LSM 880 confocal microscope. Live and dead cells at the end time point of each treatment were quantified by calculating the corrected total cell fluorescence (CTFT) using Image J software. The intensity values for green and red fluorescence were quantified based on the formula: CTFT = Integrated density-(area of selected cells X background mean gray value). Images from four different fields and depths of lenticules were taken for quantification. The quantification was conducted on two biological replicates (N = 2). One-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test was performed on the data using GraphPad Prism V9.0 and p ≤ 0.05 was considered statistically significant.
DNA extraction and quantification
Lenticules, at the end of the incubation period in the different media, were dried at 60 °C for 2 hrs and the dry weight of the lenticules was measured. Dried lenticules were chopped and suspended in 200 µl of lysis buffer containing 1 M Tris pH 8, 10% SDS, and 0.5M EDTA pH 8. After 10 mins of incubation at room temperature, 20 mg/ml Proteinase K (Genei, India) was added and incubated at 56 °C for 2 hrs. 10 µl of 5M NaCl and equal proportions of isopropanol were added and incubated at 4 °C for 10 mins and centrifuged at 12,000 rpm for 15 mins. The supernatant was removed followed by resuspension of the pellet in 70% ethanol and centrifugation at 12,000 rpm for 5 mins. Ethanol was discarded and the air-dried pellet was resuspended in TE buffer (pH 8). DNA was quantified using NanoDrop (Thermo Scientific, USA) and the values were used to calculate DNA concentration per milligram weight of lenticules (N = 3). One-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test was performed on the data using GraphPad Prism V9.0. Significance was set at p < 0.05. Agarose gel electrophoresis was used to visualize the genomic DNA.
Fluorescence staining
The paraffin-embedded sections of fresh lenticule, lenticules placed in PBS for 7 days, Opti-MEM for 7 days, in serum for 72 hrs, and NaCl-DNase treated lenticules were first deparaffinized by heating at 70 °C for 2–5 mins, followed by three xylene washes of 5 mins each, and serial ethanol wash of 100, 90, and 80% for 5 mins each. Antigen retrieval was performed by heating the samples in sodium citrate buffer for 15 mins, followed by staining for specific stromal collagens 1, 3, 4, 5, 12, and keratan sulfate (glycosaminoglycan marker). Lenticule sections stained only with the secondary antibody of mouse and rabbit were used as the control for quantification (Supplementary Figure 1).The intensity of collagen proteins across all the groups was compared by calculating the CTFT value, as described in the earlier section, using Image J software, NIH. The total intensity obtained for each group was subtracted from the intensity obtained for the negative control. Two sections for each treatment condition were used for the quantification of total fluorescence.
To check for residual cytoskeletal components after decellularization, the lenticules were fixed with 4% paraformaldehyde for 10 mins at room temperature, rinsed thrice with PBS, and incubated in 0.1% Triton-X for 5 mins. Next, the cells were incubated in 1:200 diluted phalloidin, washed in PBS, counterstained with DAPI, and imaged using the Zeiss Airyscan LSM 880 confocal microscope. The total intensity for phalloidin staining was quantified from five images at different depths using Image J software, NIH, and plotted to determine the decrease in residual cytoskeletal components in the treated groups. Fresh lenticule was used as the control for comparison. One-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test was performed, and p ≤ 0.05 was considered statistically significant.
Hematoxylin and eosin staining
Sections at the initial time point of 24 hrs and end time points of PBS (7 days), Opti-MEM (7 days), Serum (72 hrs), KM (7 days), and NaCl-DNase were stained with hematoxylin-eosin (H&E) to determine the presence of cells in the lenticules and periodic acid–Schiff base (PAS) staining to visualize collagen.
The thickness of the lenticules was quantified from the H&E sections using the ZEN 3.5 Blue Edition software, Zeiss. Five measurements of the central thickness were taken per lenticule for each treatment group and from two tissue sections each. The results are reported as mean ± SD. One-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test was performed, and a p-value ≤0.05 was considered statistically significant.
Scanning electron microscopy
The structure of the collagen fibers in the treated lenticules was visualized using scanning electron microscope (SEM). The samples were fixed in 4% glutaraldehyde in cacodylate buffer of 7.4 pH for 24 hrs. They were then treated with 1% osmium tetraoxide, followed by three washes using distilled water. Samples were then dehydrated with alcohol in ascending order of 10, 30, 50, 70, and 90% for 20 mins each, followed by 100% alcohol for 20 mins twice. They were then dried at room temperature overnight, gold-sputtered, and imaged using Zeiss EVO 18 microscope.
Optical performance of lenticules
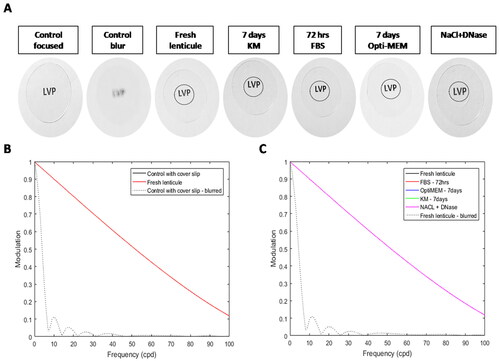
The optical performance of the wet lenticules was determined using two-dimensional modulation transfer function (MTF) using MATLAB software (Nantucket, MA, USA) as reported earlier.Citation8 In-focus and blurred images of the letters "LVP" without any lenticules were used as controls. The normalized power values were plotted as a function of the object frequency. It is expected that in a natural scene, the optical performance will reduce with an increase in object spatial frequency. The analysis determined if the MTF loss with the decellularized lenticules was any greater than or equal to the MTF loss without lenticule.
Human corneal endothelial cell culture on lenticules
Donor corneas, unsuitable for transplantation, were obtained from the Ramayamma International Eye Bank, LV Prasad Eye Institute, Hyderabad, India and handled according to the declaration of Helsinki. The corneas were harvested within 24 hrs of death, preserved in McCarey-Kaufman medium, and used within 4 days of preservation. Cells from 3 donors of ages 1 month, 4.5 months, and 2 years were used for primary cell culture. Details of the donor tissues are included in supplementary table 2. The isolation and culture of hCE cells followed the reported methods.Citation8,Citation36 Briefly, donor tissues were disinfected by washing in PBS containing antibiotics, and Descemet’s membrane along with the CE cells was gently peeled. The peeled tissues were chopped into smaller pieces and digested using collagenase (1 mg/ml) for 2–6 hrs. The cells were dissociated using trypsin-EDTA (0.25%) and then seeded on fibronectin and collagen 1 (FNC)-coated dishes. The culture medium was changed every 2 days until the cells reached confluence and then subcultured by trypsinization. Cells between passages 1 and 3 were seeded at 3000cells/mm2 on fresh SMILE lenticules for 2 weeks and stained for phalloidin, N-cadherin, Na/K ATPase and ZO-1 following which imaging was done using Zeiss Airyscan LSM 880 confocal microscope.
Ex-vivo perfusion
Cells between passages 1 and 3 were seeded on lenticules at a density of 3000cells/mm2 and cultured for 3 days before transplantation. The cells were stained with Cell Tracker Red CMTPX Dye (10 µM) for 45 mins before transplantation. The recipient donor corneas were rinsed thoroughly with PBS and the existing CE cells were removed by incubating intrypsin-EDTA (0.25%) and gently washing the loosened cells. Removal of the cells was confirmed using trypan blue staining after which they were placed in Opti-MEM overnight to induce swelling. The corneal thickness was measured using optical coherence tomography (OCT) before transplantation. The lenticules with or without hCE cells were then carefully transplanted into the donor corneas. Fibrin glue was used to aid with the adhesion of the lenticule to the posterior cornea. The corneas along with the lenticules were mounted onto the artificial anterior chamber connected to a peristaltic pump to maintain a constant flow rate of 2.5 µl/min and pressure of 15 mmHg. This setup was placed in a CO2 incubator and constantly perfused with Opti-MEM medium. The perfusion setup is similar to that used by Patel et al., for injecting CE cells with magnetic beads.Citation37 Silicone oil (PDMS 10 cc 5000 centiStokes (cSt), Micromed, Italy) was added on top of the epithelial surface to reduce the evaporation of media through the anterior cornea. Central corneal thickness was measured 10 days after perfusion using OCT. The experiments were repeated twice (N = 3) and the results are reported as mean ± SD. Student’s t-test was performed using Graphpad prism software and a p-value ≤0.05 was considered to be statistically significant. Details of the donor corneas used for perfusion are given in supplementary table 3.
Results
Decellularization of lenticules
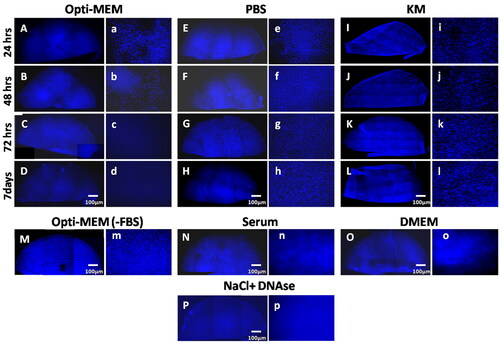
Fresh lenticules placed in Opti-MEM medium, PBS, and KM for different time points (24 hrs, 48 hrs, 72 hrs, and 7 days) were fixed and stained with DAPI to determine the presence of nuclear material (). There was no perceptible difference in DAPI staining at 24 hrs but a reduction in keratocytes was obvious at 48 hrs in lenticules placed in Opti-MEM (). By 7 days, the nuclear material was not visible in lenticules placed in Opti-MEM unlike PBS and KM ( vs. H and L), and was comparable to NaCl-DNase (). Changing the temperature of the medium from 37 to 4 °C or PBS from 4 to 37 °C did not alter the results. Similarly, agitation of lenticules (200 rpm) for 48 hrs did not hasten the degradation or removal of the nuclear material (data not shown).
Next, we wanted to check the component in the Opti-MEM medium that degraded the nuclear components. A few lenticules were placed in Opti-MEM medium without serum. As shown in , there was significant nuclear staining visible even after 7days. Therefore, we narrowed the effect of decellularization to serum in the medium. To confirm, lenticules were incubated in 100% serum which resulted in a significant reduction in DAPI staining () within 72 hrs. Decellularization of the lenticule was also seen in those placed in DMEM containing 2% serum for 7 days ().
Decellularization was further confirmed using H&E staining of the tissue sections after incubation in the respective media until the endpoint as shown in Supplementary Figure 2. Nuclei could not be detected in sections from lenticules placed in Opti-MEM for 7 days, serum for 72 hrs or treated with NaCl-DNase (Supplementary Figures 2(E,G,I)) unlike fresh lenticules, lenticules placed in PBS for 7 days or in KM for 7 days (Supplementary Figures 2(A,C,H)) further confirming the results in .
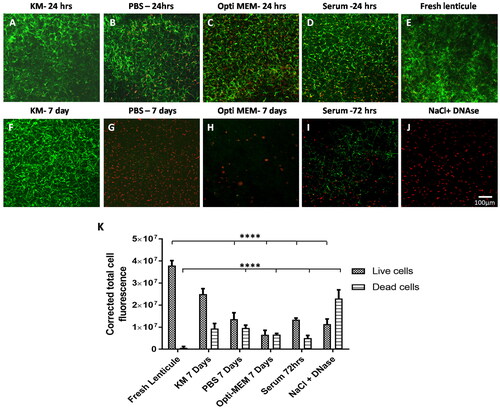
Incubation in Opti-MEM resulted in cell death
As expected, cells in lenticules placed in KM were viable at both 24 hrs and 7 days as can be seen in . More dead cells could be appreciated in lenticules that were placed in Opti-MEM even at 24 hrs when compared to KM ( vs. A)). At 7 days, there were no viable cells left in these lenticules and the staining for dead cells was also scarce indicating that the degraded nuclear material was also removed (). Lenticules placed in serum showed extensive cell death at 72 hrs () and those placed in PBS also had many dead cells at 24 hrs () which significantly increased by 7 days () but the degraded nuclei were still visible. Lenticules treated with NaCl-DNase were almost devoid of live cells () which was the opposite of fresh lenticules () in which the dead cells were very scarce. Quantification of fluorescence intensity of live and dead cells in these lenticules showed a significant reduction in the live cells in lenticules treated with Opti-MEM, serum, or NaCl-DNase when compared to fresh lenticules or lenticules incubated in KM (). Contrarily, the staining for dead cells was significantly more in those treated with NaCl-DNase when compared to fresh lenticules, Opti-MEM, or serum () indicating the presence of residual degraded DNA in these lenticules.
Quantification of residual DNA content
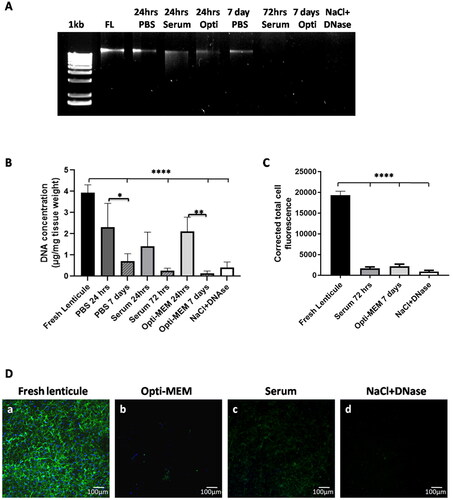
Genomic DNA was seen in fresh lenticule, PBS, serum, and Opti-MEM at 24 hrs. At 7 days, DNA was still present in lenticules placed in PBS (). However, no bands were observed in the case of 7 days Opti-MEM, serum, and NaCl-DNase treated lenticules ().
Calculation of the residual DNA per milligram dry weight of the lenticules indicated a reduction in the following order: 7 days Opti-MEM > 72 hrs serum > NaCl-DNase > 7 days PBS (). The decellularization obtained with serum and Opti-MEM at the respective endpoints was comparable to NaCl-DNase and there was no significant difference between these methods. Though not statistically significant, the DNA in lenticules placed in Opti-MEM (0.140 µg/mg) was much lower when compared to NaCl-DNase (0.419 µg/mg) or PBS (0.718 µg/mg) and comparable to serum (0.255 µg/mg).
Next, we wanted to check the effect of the decellularization technique on the cytoplasmic cell contents. Positive staining for actin cytoskeleton indicated the presence of keratocytes in fresh lenticules. There was a significant reduction (p ≤ 0.0001) in actin staining in lenticules placed in serum (72 hrs) and Opti-MEM (7 days) which was comparable to NaCl-DNase treated lenticules indicating substantial removal of cell cytoplasm along with nuclear components ().
ECM preservation in the treated lenticules
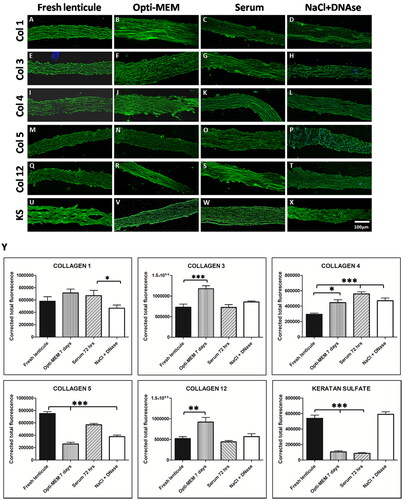
Lenticule sections were immunostained for corneal stroma-specific collagens (collagens 1, 3, 4, 5, and 12) and keratan sulfate at the respective endpoints for serum and Opti-MEM. The lenticules showed positive expression for all the markers indicating that there was no detectable loss of collagens () or glycosaminoglycans () following decellularization. Quantification of the fluorescence intensity showed that there was no significant loss of collagen 1 in Opti-MEM and serum-treated lenticules unlike NaCl-DNase-treated lenticules (p < 0.05) when compared to fresh lenticule. There was a significant reduction in collagen 5 in all the treated lenticules compared to fresh lenticule (p < 0.01) indicating a loss of this collagen protein following decellularization (). On the contrary, there did not seem to be any loss of collagens 3 and 12 in serum and NaCl-DNase-treated lenticules compared to control however, there was increased expression of the same in Opti-MEM treated lenticules (). Interestingly, there was an increase in collagen 4 expression in all the treated lenticules compared to the control (p < 0.05 for control vs. Opti-MEM; p < 0.001 for control vs serum and NaCl-DNase). On the contrary, there was a significant decrease in the expression of the glycoprotein, keratan sulfate, in Opti-MEM and serum lenticules and surprisingly no loss in NaCl-DNase-treated lenticules ().
PAS staining showed intact and homogenous collagen fibers in fresh lenticules, Opti-MEM 7 days, FBS 72 hrs, and KM 7 days (Supplementary Figures 2(a,e,g,h)). Disruption in the collagen fibers could be noted in PBS 7 days and NaCl-DNase treated lenticules (Supplementary Figures 2(c,i)). Scanning electron microscope images showed no difference in the structure of collagen fibers in the treated groups of PBS, KM, serum, and Opti-MEM when compared to the fresh lenticule (Supplementary Figure 3). In NaCl-DNase treated lenticule, large gaps could be noted indicating some loss in the ECM structure (arrows in Supplementary Figure 3).
Thickness of lenticules
The thickness of the lenticules was measured using the H&E stained sections of the lenticules at the respective endpoint of incubation in PBS (7 days), Opti-MEM (7 days), Serum (72 hrs), KM (7 days), and NaCl-DNase. The thickness significantly increased in PBS (112.6 ± 5 µm) and Opti-MEM (117.1 ± 5 µm) when compared to fresh lenticules (96.3 ± 5.7 µm) and was comparable to KM (111.13 ± 13.6 µm; Supplementary Figure 4). No significant difference in thickness was observed between 72 hrs of serum and fresh lenticule. However, a significant reduction in thickness was observed in NaCl-DNase-treated lenticules (77.5 ± 5.5 µm) compared to fresh lenticule, Opti-MEM, and serum (Supplementary Figure 4).
Optical performance of decellularized lenticules
The optical performance of the lenticules was measured as their ability to transmit information from the object to the image with high fidelity. It is expected that swelling would greatly reduce transparency which would, in turn, affect the optical performance of the decellularized tissues. Modulation transfer function or MTF is a good measure of the effective resolution of the image which can be degraded by blur. At higher spatial frequencies the MTF reaches 0 which indicates poor visibility of smaller structures. At lower spatial frequency, the MTF is near 1 indicating the ability to visualize larger structures. A decrease in the object contrast (in this case due to loss of lenticule transparency) can reduce the image contrast resulting in degradation of information reflecting on the optical clarity of the lenticule. The MTF values were calculated for fresh lenticule, 72 hrs serum, 7 days KM, 7 days Opti-MEM, and NaCl-DNase treated lenticules. A focused high-resolution image of the object served as the positive control while an optically blurred image served as the negative control (). As expected, the performance of the lenticules decreased at higher object spatial frequencies. However, this decrease was comparable to the fresh lenticule indicating that there was no degradation of information induced by the decellularization process () confirming that the optical quality of the lenticules is intact. A steeper decline in the negative control indicates that the blur has impaired the optical quality, resulting in poor transmission of information from the object at higher spatial frequencies. The MTF plot for the individual treatment condition compared to the blurred image of fresh lenticule is shown in Supplementary Figure 5.
Growth of corneal endothelium on lenticules
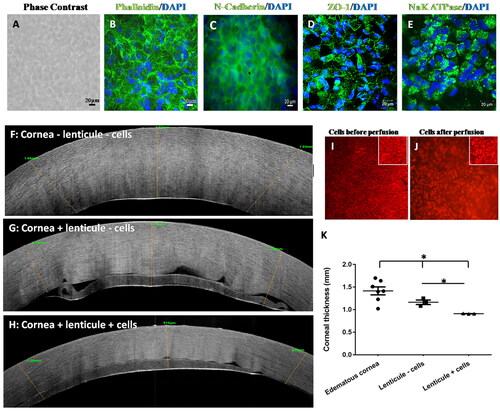
Human CE cells cultured on fresh SMILE lenticules for 14 days were polygonal as can be seen in and expressed N-cadherin (), ZO-1 (), and Na/K ATPase (), which are markers of hCE cells. As expected, there was no noticeable keratocyte nuclear staining at the end of this period. The result indicates that the keratocytes can be removed simultaneously during CE culture since the degradation of keratocyte nuclear contents did not affect the growth of the hCE cells.
SMILE lenticule as a carrier for hCE cell transplantation
Since the hCE cells were able to form a monolayer when seeded on lenticules, we wanted to assess their role as a carrier for the transplantation of the cultured cells. Cells seeded on lenticules were transplanted to edematic corneas, mounted onto artificial anterior chambers, and perfused with CE culture medium for 10 days. The average central corneal thickness was 1.44 ± 0.241 mm before perfusion (), which reduced when perfused with lenticules without cells (p ≤ 0.05) to 1.16 ± 0.085 mm (). A further significant reduction was noted in corneas perfused with lenticules containing cells to 0.80 ± 0.094 mm (p ≤ 0.05; ) suggesting that the hCE cells on lenticules are functional. OCT images of the recipient corneas before perfusion showed the presence of increased spacing between the collagen fibers due to the fluid accumulation in the stroma (). The corneas after perfusion with lenticules without cells were thinner compared to the control, however, fluid spaces between the fibers were still noticeable (). The corneas that were perfused with lenticules containing cells had a compact and thin cornea with an orderly arrangement of collagen fibers (). The presence of the tracker dye showed that the cells remained on the lenticule after 10 days of perfusion as seen in (magnified in inset).
Discussion
Engineering the corneal endothelium for transplantation is an important step toward developing alternatives to cadaver donor tissues and cell injection therapy.Citation38 Several materials have been tried and tested for the same with varying degrees of success. To engineer the whole or parts of the cornea, the following criteria have to be met. The material used to support the cells should be transparent, match the refractive index of the cornea, and integrate with the native tissue in addition to being safe and compatible. The corneal stroma is the material of interest because it meets all of the above requirements. And in this study, SMILE lenticules carved out from the anterior stroma, were used for engineering the CE layer for transplantation. The lenticules were able to support the growth of the hCE cells sufficiently and mature into a functional layer of cells as evidenced by the expression of the markers namely ZO-1 and Na/K ATPase. When transplanted, the cells remained intact on the lenticules for 10 days and were able to reduce the corneal thickness significantly confirming that SMILE lenticules do provide a suitable substrate for engineering the CE layer for transplantation. Interestingly, we also found that the transplanted cells migrated onto the denuded recipient cornea (Supplementary Figure 6) indicating that the cells can repopulate the cornea following transplantation. One major limitation for clinical transplantation would be the diameter of the lenticule, which on average is 6 mm and therefore cannot be used if a larger size of transplant is required. The smaller size would also mean that there will be little tolerance for dislocation or decentration of the lenticule following transplantation. It is also possible that the addition of the lenticules to the posterior cornea could induce a hyperopic shift in the corneal dioptric power similar to what has been reported with DSEK grafts previously.Citation39–41 This is mainly due to the thickness of the lenticules which would alter the posterior radius of curvature of the cornea. The amount of hyperopic shift induced by these lenticules will have to be assessed in detail, in further studies, since the thickness of the lenticules is non-uniform (from center to periphery). However, it can be expected that the current method of decellularization would allow more allogenic lenticules to be available for implantation in the anterior cornea as inlay’s for the correction of presbyopia as demonstrated by Liu et al.,Citation13 using SMILE lenticules.
Any tissue that is used for engineering must have limited residual native DNA content to reduce the chances of rejection following transplantation. Several methods have been employed to decellularize the cornea. Since complete decellularization is difficult to achieve without compromising the tissue integrity, Crapo et al.Citation30 came up with the following recommendation to consider a tissue “sufficiently decellularized” and safe for transplantation: (a) the residual cellular components especially the DNA should be less than 50 ng/mg of the tissue, (b) DNA fragments should be <200 bp, and (c) absence of visible nuclear material within the ECM confirmed using DAPI or H&E staining. Decellularization of SMILE lenticules with serum and Opti-MEM, as shown in this paper, comes very close to meeting the aforementioned criteria. The residual DNA was reduced to ∼4 and 6.3% of the fresh lenticule when treated with Opti-MEM and serum, respectively. This is very similar to the values reported by Yam et al.,Citation18 with the use of 0.1% SDS for decellularization with extensive washing and agitation.
Initial confirmation of decellularization was achieved using DAPI staining which showed that lenticules placed in Opti-MEM were comparable to lenticules decellularized using NaCl-DNase and the data conclusively point to serum as the main factor that facilitates this process. Incubation in 100% FBS decellularized the tissue in a shorter duration and was more effective in removing DNA fragments compared to NaCl-DNase. Similar use of human serum has been reported earlier in porcine corneas where the authors showed that decellularization can be achieved in 24 hrs when used along with electrophoresis.Citation42 This study demonstrated apoptosis (identified using TUNEL staining) to be the mechanism that led to the removal of the keratocytes resulting in the decellularization of the corneal tissue. They further confirmed the results in vitro by demonstrating the death of keratocytes within 12 hrs when cultured in the presence of serum alone. Our results also point to the fact that in addition to degradation, the degraded cell contents were cleared without the need for extensive washing when incubated in Opti-MEM or serum. This leads us to hypothesize that since keratocyte survival is not supported by 100% serum or Opti-MEM, unlike the KM, it results in their death which might trigger the endonucleases present in the serum to degrade the nuclear contents further.
Interestingly, it was noted that much of the cytoskeletal components were also removed when lenticules were placed in Opti-MEM or serum similar to NaCl-DNase treatment indicating that the incubation of lenticules in these media was able to decellularize the lenticules to more than that achieved with extensive treatment with NaCl-DNase which requires agitation and frequent change of reagents. These results taken together point to the use of Opti-MEM or serum for decellularization to be very efficient compared to the use of chemicals or enzymes that require extensive washing to remove residues to ensure their safety for human use. The use of serum will also be more clinically acceptable since many cells used for transplantation are cultured in its presence.
The incubation of lenticules in Opti-MEM or serum did not disturb the arrangement of collagen fibers within the lenticules as was evident from the PAS staining and SEM imaging. The collagen fibers remained compact and homogeneous, unlike the NaCl-DNase-treated lenticules. Quantification of the ECM proteins showed that incubation in serum did not significantly alter the expression of stromal collagens 1, 3, and 12. There was however a reduction in collagen 5 and an increase in collagen 4 in these lenticules. Interestingly, there was increased expression of collagens 3, 4, and 12 in Opti-MEM-treated lenticules. Though we are unsure of the reason for this, it could have to do with the presence of other media components in Opti-MEM in addition to serum. Staining for keratan sulfate indicated that some amount of glycoproteins is retained in lenticules treated with Opti-MEM and serum corroborating with PAS staining. Incubation of lenticules in Opti-MEM led to the swelling of the lenticules by ∼20 µm when compared to fresh lenticule. This was comparable to KM and could be attributed to the swelling induced by glycoproteins within the lenticules or those present in the serum. The lack of change in the thickness of lenticules placed in serum could be due to the shorter incubation period compared to KM or Opti-MEM. On the contrary, NaCl-DNase-treated lenticules were significantly thinner compared to fresh lenticules, Opti-MEM, and serum-treated lenticules. Similar to earlier studies that have reported a significant reduction in collagen staining with the use of nucleases,Citation18 we found a significant reduction in specifically collagens 1 and 5 in these lenticules. Taken together, it can be deduced that while NaCl-DNase is detrimental to the ECM proteins, treatment with Opti-MEM or serum preserves the architecture and ECM proteins of the stroma.
A very interesting result in this study is that the death of keratocytes and the growth of CE cells were occurring simultaneously and it is important to note that the death and degradation of the keratocyte cell components did not affect hCE growth and were not toxic to the cells. This means that prior decellularization of the lenticules is not required for culturing the cells and that both can be achieved simultaneously thus saving time, resources, and effort in the process.
Conclusion
This study provides a proof-of-concept for the use of SMILE lenticules as carriers for engineering and transplanting the corneal endothelial layer. The study also demonstrates a simple, chemical-free yet effective technique for the decellularization of the corneal SMILE lenticules. Importantly, we show that decellularization can be achieved independently or simultaneously during the process of culturing cells thus making it cost-effective, time-saving, and simple compared to other detergent or enzyme-based techniques.
Supplemental Material
Download PDF (1.4 MB)Acknowledgment
Hyderabad Eye Research Foundation, LV Prasad Eye Institute, Hyderabad, Ramayamma International Eye bank, LV Prasad Eye Institute, Hyderabad.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data supporting the findings of this study are available within the article and its supplementary materials.
Additional information
Funding
References
- Gain P, Jullienne R, He Z, Aldossary M, Acquart S, Cognasse F, Thuret G. Global survey of corneal transplantation and eye banking. JAMA Ophthalmol. 2016;134(2):167–173. doi:10.1001/jamaophthalmol.2015.4776.
- Tan DT, Dart JK, Holland EJ, Kinoshita S. Corneal transplantation. Lancet. 2012;379(9827):1749–1761. doi:10.1016/S0140-6736(12)60437-1.
- Ishino Y, Sano Y, Nakamura T, Connon CJ, Rigby H, Fullwood NJ, Kinoshita S. Amniotic membrane as a carrier for cultivated human corneal endothelial cell transplantation. Invest Ophthalmol Vis Sci. 2004;45(3):800–806. doi:10.1167/iovs.03-0016.
- Jumblatt MM, Maurice DM, Schwartz BD. A gelatin membrane substrate for the transplantation of tissue cultured cells. Transplantation. 1980;29(6):498–499. doi:10.1097/00007890-198006000-00013.
- Lange TM, Wood TO, McLaughlin BJ. Corneal endothelial cell transplantation using Descemet’s membrane as a carrier. J Cataract Refract Surg. 1993;19(2):232–235. doi:10.1016/S0886-3350(13)80947-9.
- Mimura T, Yamagami S, Yokoo S, Usui T, Tanaka K, Hattori S, Irie S, Miyata K, Araie M, Amano S. Cultured human corneal endothelial cell transplantation with a collagen sheet in a rabbit model. Invest Ophthalmol Vis Sci. 2004;45(9):2992–2997. doi:10.1167/iovs.03-1174.
- Mohay J, Lange TM, Soltau JB, Wood TO, McLaughlin BJ. Transplantation of corneal endothelial cells using a cell carrier device. Cornea. 1994;13(2):173–182. doi:10.1097/00003226-199403000-00011.
- Ramachandran C, Gupta P, Hazra S, Mandal BB. In vitro culture of human corneal endothelium on non-mulberry silk fibroin films for tissue regeneration. Transl Vis Sci Technol. 2020;9(4):12. doi:10.1167/tvst.9.4.12.
- Koizumi N, Sakamoto Y, Okumura N, Okahara N, Tsuchiya H, Torii R, Cooper LJ, Ban Y, Tanioka H, Kinoshita S. Cultivated corneal endothelial cell sheet transplantation in a primate model. Invest Ophthalmol Vis Sci. 2007;48(10):4519–4526. doi:10.1167/iovs.07-0567.
- Ganesh S, Brar S, Rao PA. Cryopreservation of extracted corneal lenticules after small incision lenticule extraction for potential use in human subjects. Cornea. 2014;33(12):1355–1362. doi:10.1097/ICO.0000000000000276.
- Pradhan KR, Reinstein DZ, Carp GI, Archer TJ, Gobbe M, Gurung R. Femtosecond laser-assisted keyhole endokeratophakia: Correction of hyperopia by implantation of an allogeneic lenticule obtained by SMILE from a myopic donor. J Refract Surg. 2013;29(11):777–782. doi:10.3928/1081597X-20131021-07.
- Lim CH, Riau AK, Lwin NC, Chaurasia SS, Tan DT, Mehta JS. Lasik following small incision lenticule extraction (smile) lenticule re-implantation: A feasibility study of a novel method for treatment of presbyopia. PLOS One. 2013;8(12):e83046. doi:10.1371/journal.pone.0083046.
- Liu YC, Teo EPW, Ang HP, Seah XY, Lwin NC, Yam GHF, Mehta JS. Biological corneal inlay for presbyopia derived from small incision lenticule extraction (smile). Sci Rep. 2018;8(1):1831. doi:10.1038/s41598-018-20267-7.
- Abd Elaziz MS, Zaky AG, El SaebaySarhan AR. Stromal lenticule transplantation for management of corneal perforations; one year results. Graefes Arch Clin Exp Ophthalmol. 2017;255(6):1179–1184. doi:10.1007/s00417-017-3645-6.
- Bhandari V, Ganesh S, Brar S, Pandey R. Application of the smile-derived glued lenticule patch graft in microperforations and partial-thickness corneal defects. Cornea. 2016;35(3):408–412. doi:10.1097/ICO.0000000000000741.
- Wu F, Jin X, Xu Y, Yang Y. Treatment of corneal perforation with lenticules from small incision lenticule extraction surgery: a preliminary study of 6 patients. Cornea. 2015;34(6):658–663. doi:10.1097/ICO.0000000000000397.
- Yin H, Qiu P, Wu F, Zhang W, Teng W, Qin Z, Li C, Zhou J, Fang Z, Tang Q, et al. Construction of a corneal stromal equivalent with smile-derived lenticules and fibrin glue. Sci Rep. 2016;6(:33848. doi:10.1038/srep33848.
- Yam GH, Yusoff NZ, Goh TW, Setiawan M, Lee XW, Liu YC, Mehta JS. Decellularization of human stromal refractive lenticules for corneal tissue engineering. Sci Rep. 2016;6(:26339. doi:10.1038/srep26339.
- Brown BN, Valentin JE, Stewart-Akers AM, McCabe GP, Badylak SF. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials. 2009;30(8):1482–1491. doi:10.1016/j.biomaterials.2008.11.040.
- Xu H, Wan H, Sandor M, Qi S, Ervin F, Harper JR, Silverman RP, McQuillan DJ. Host response to human acellular dermal matrix transplantation in a primate model of abdominal wall repair. Tissue Eng Part A. 2008;14(12):2009–2019. doi:10.1089/ten.tea.2007.0316.
- Vorotnikova E, McIntosh D, Dewilde A, Zhang J, Reing JE, Zhang L, Cordero K, Bedelbaeva K, Gourevitch D, Heber-Katz E, et al. Extracellular matrix-derived products modulate endothelial and progenitor cell migration and proliferation in vitro and stimulate regenerative healing in vivo. Matrix Biol. 2010;29(8):690–700. doi:10.1016/j.matbio.2010.08.007.
- Xu CC, Chan RW, Weinberger DG, Efune G, Pawlowski KS. A bovine acellular scaffold for vocal fold reconstruction in a rat model. J Biomed Mater Res A. 2010;92(1):18–32. doi:10.1002/jbm.a.32279.
- Allen RA, Seltz LM, Jiang H, Kasick RT, Sellaro TL, Badylak SF, Ogilvie JB. Adrenal extracellular matrix scaffolds support adrenocortical cell proliferation and function in vitro. Tissue Eng Part A. 2010;16(11):3363–3374. doi:10.1089/ten.tea.2010.0005.
- Bayyoud T, Thaler S, Hofmann J, Maurus C, Spitzer MS, Bartz-Schmidt KU, Szurman P, Yoeruek E. Decellularized bovine corneal posterior lamellae as carrier matrix for cultivated human corneal endothelial cells. Curr Eye Res. 2012;37(3):179–186. doi:10.3109/02713683.2011.644382.
- Du L, Wu X. Development and characterization of a full-thickness acellular porcine cornea matrix for tissue engineering. Artif Organs. 2011;35(7):691–705. doi:10.1111/j.1525-1594.2010.01174.x.
- Angunawela RI, Riau AK, Chaurasia SS, Tan DT, Mehta JS. Refractive lenticule re-implantation after myopic ReLex: a feasibility study of stromal restoration after refractive surgery in a rabbit model. Invest Ophthalmol Vis Sci. 2012;53(8):4975–4985. doi:10.1167/iovs.12-10170.
- Pang K, Du L, Wu X. A rabbit anterior cornea replacement derived from acellular porcine cornea matrix, epithelial cells and keratocytes. Biomaterials. 2010;31(28):7257–7265. doi:10.1016/j.biomaterials.2010.05.066.
- Ponce Marquez S, Martinez VS, McIntosh Ambrose W, Wang J, Gantxegui NG, Schein O, Elisseeff J. Decellularization of bovine corneas for tissue engineering applications. Acta Biomater. 2009;5(6):1839–1847. doi:10.1016/j.actbio.2009.02.011.
- Choi JS, Williams JK, Greven M, Walter KA, Laber PW, Khang G, Soker S. Bioengineering endothelialized neo-corneas using donor-derived corneal endothelial cells and decellularized corneal stroma. Biomaterials. 2010;31(26):6738–6745. doi:10.1016/j.biomaterials.2010.05.020.
- Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32(12):3233–3243. doi:10.1016/j.biomaterials.2011.01.057.
- Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27(19):3675–3683. doi:10.1016/j.biomaterials.2006.02.014.
- Shafiq MA, Gemeinhart RA, Yue BY, Djalilian AR. Decellularized human cornea for reconstructing the corneal epithelium and anterior stroma. Tissue Eng Part C Methods. 2012;18(5):340–348. doi:10.1089/ten.TEC.2011.0072.
- Gonzalez-Andrades M, de la Cruz Cardona J, Ionescu AM, Campos A, Del Mar Perez M, Alaminos M. Generation of bioengineered corneas with decellularized xenografts and human keratocytes. Invest Ophthalmol Vis Sci. 2011;52(1):215–222. doi:10.1167/iovs.09-4773.
- Wilson SL, Sidney LE, Dunphy SE, Dua HS, Hopkinson A. Corneal decellularization: A method of recycling unsuitable donor tissue for clinical translation? Curr Eye Res. 2016;41(6):769–782. doi:10.3109/02713683.2015.1062114.
- Yoeruek E, Bayyoud T, Maurus C, Hofmann J, Spitzer MS, Bartz-Schmidt KU, Szurman P. Decellularization of porcine corneas and repopulation with human corneal cells for tissue-engineered xenografts. Acta Ophthalmol. 2012;90(2):e125–e131. doi:10.1111/j.1755-3768.2011.02261.x.
- Joyce NC, Zhu CC. Human corneal endothelial cell proliferation: potential for use in regenerative medicine. Cornea. 2004;23(8 Suppl):S8–S19. doi:10.1097/01.ico.0000136666.63870.18.
- Patel SV, Bachman LA, Hann CR, Bahler CK, Fautsch MP. Human corneal endothelial cell transplantation in a human ex vivo model. Invest Ophthalmol Vis Sci. 2009;50(5):2123–2131. doi:10.1167/iovs.08-2653.
- Kinoshita S, Koizumi N, Ueno M, Okumura N, Imai K, Tanaka H, Yamamoto Y, Nakamura T, Inatomi T, Bush J, et al. Injection of cultured cells with a rock inhibitor for bullous keratopathy. N Engl J Med. 2018;378(11):995–1003. doi:10.1056/NEJMoa1712770.
- Jun B, Kuo AN, Afshari NA, Carlson AN, Kim T. Refractive change after Descemet stripping automated endothelial keratoplasty surgery and its correlation with graft thickness and diameter. Cornea. 2009;28(1):19–23. doi:10.1097/ICO.0b013e318182a4c1.
- Holz HA, Meyer JJ, Espandar L, Tabin GC, Mifflin MD, Moshirfar M. Corneal profile analysis after Descemet stripping endothelial keratoplasty and its relationship to postoperative hyperopic shift. J Cataract Refract Surg. 2008;34(2):211–214. doi:10.1016/j.jcrs.2007.09.030.
- Hwang RY, Gauthier DJ, Wallace D, Afshari NA. Refractive changes after Descemet stripping endothelial keratoplasty: a simplified mathematical model. Invest Ophthalmol Vis Sci. 2011;52(2):1043–1054. doi:10.1167/iovs.10-5839.
- Shao Y, Tang J, Zhou Y, Qu Y, He H, Liu Q, Tan G, Li W, Liu Z. A novel method in preparation of acellularporcine corneal stroma tissue for lamellar keratoplasty. Am J Transl Res. 2015;7(12):2612–2629.