Abstract
Purpose
Punctal occlusion using punctal plugs has been successfully used to treat the signs and symptoms of dry eye disease. However, the effects of punctal occlusion on the symptoms of allergic conjunctivitis (AC) have been less well documented. There is some concern among clinicians that punctal occlusion may make signs/symptoms of allergic conjunctivitis worse by trapping allergens on the eye. The objective of this post hoc analysis was to address this question and thus assess the effect of punctal occlusion alone on ocular itching and conjunctival redness associated with AC.
Methods
This was a pooled post hoc analysis of three randomized, double-blind, placebo insert-controlled clinical trials in subjects with AC. Enrolled subjects were generally healthy adults with ocular allergies and a positive skin test reaction to perennial and/or seasonal allergens. The study used a modified version of the traditional conjunctival allergen challenge (CAC) model, which included multiple, repeated allergen challenges following placement of the intracanalicular insert. Subjects were rechallenged on Days 6, 7 and 8; Days 13, 14 and 15; and Days 26, 27 and 28.
Results
The data set included 128 subjects that were administered placebo. Baseline mean (SD) ocular itching and conjunctival redness scores were 3.52 (0.44) and 2.97 (0.39), respectively. On post-insertion Days 7, 14 and 28, mean itching scores were 2.62, 2.26 and 1.91, respectively, representing 26%, 36% and 46% itching reductions, respectively (p < 0.001). On Days 7, 14 and 28, mean conjunctival redness scores were 1.98, 1.90, and 2.08, respectively, representing 33%, 36%, and 30% redness reductions, respectively (p < 0.001).
Conclusions
Based on this post hoc pooled analysis, punctal occlusion with a resorbable hydrogel intracanalicular insert did not worsen ocular itching or conjunctival redness in this patient population.
Introduction
Background
Allergic conjunctivitis (AC) is an inflammatory ocular disorder induced by environmental allergens,Citation1 with seasonal and perennial AC being the most common forms of ocular allergy.Citation2,Citation3 It is estimated that 40% of the world’s population suffers from ocular allergy symptoms, and the global prevalence is increasing.Citation4 Common symptoms of AC include ocular itching and conjunctival hyperemia,Citation2 which may have a significant negative impact on the quality of life of affected individuals.Citation5,Citation6 Considering associated medical expenses and loss of productivity, the economic cost of AC in the US may be as high as $2 billion.Citation5,Citation7,Citation8
Current therapies
Current approaches to treating AC include allergen avoidance, topical antihistamines and mast cell stabilizers (alone or in combination), topical nonsteroidal anti-inflammatory drugs, topical corticosteroids, and topical immunomodulators.Citation9,Citation10 The optimal dosing regimen of corticosteroids, the most effective topical anti-inflammatory drugs, are still a major concern,Citation11 and improper administration has been identified as a significant challenge with eyedrop formulations. Challenges to eye drop therapy may be exacerbated by the need for frequent administration, and proper instillation in eyedrop form can be especially challenging for some patient populations, especially among the elderly.Citation12–15 Administration concerns among older patients include co-morbidities such as tremor, arthritis, dementia or the lack of family support.Citation16
Allergic conjunctivitis and dry eye disease are known to coexist,Citation17–19 and the presence of dry eye disease can exacerbate the signs and symptoms of ocular allergy.Citation20 Ocular allergy may also be a risk factor for dry eye disease,Citation19,Citation21 which adds concerns for patients who wear contact lenses.Citation22 Oral antihistamines have been shown to significantly reduce tear volume, which may lead to inadequate flushing of allergens and exacerbation of dry eye disease.Citation23 Preservatives found in topical drugs can also induce ocular toxicity and may exacerbate ocular surface symptoms.Citation24
The favorable effects of punctal occlusion, using punctal plugs, on the signs and symptoms of other ocular surface disorders, such as dry eye disease, have been well documented;Citation25–33 however, there is limited evidence of its effects on patients with AC despite the co-occurrence of AC and dry eye disease, and its use in AC is not widely accepted. In particular, some clinicians are concerned that punctal occlusion may worsen signs/symptoms by trapping allergens on the eye’s and thus potentially increase the allergic response; however, little evidence exists to suggest this is the case.Citation34 A sustained-release intracanalicular dexamethasone insert (DEXTENZA [dexamethasone ophthalmic insert, 0.4 mg], Ocular Therapeutix, Inc.) has demonstrated safety and efficacy for treating subjects with AC in placebo vehicle-controlled trials,Citation35,Citation36 and is currently indicated for the treatment of ocular itching associated with allergic conjunctivitis.Citation36 The use of an intracanalicular placebo insert (polyethylene glycol-based hydrogel insert that did not contain any drug or active ingredient) working as a punctal plug in these trials has provided the opportunity to assess the changes in ocular itching and conjunctival redness in subjects with AC following punctal occlusion alone in the post hoc pooled analysis presented here. Based on results from the post hoc pooled analysis, punctal occlusion may prove beneficial for a patient population with these overlapping conditions.
Please see the supplementary file for the video abstract of this manuscript.
Materials and methods
Study objectives
The objective of the following pooled, post hoc analysis was to assess the effect of punctal occlusion alone, using a hydrogel insert (placebo insert), on ocular itching and conjunctival redness associated with AC. This study used a modified version of the traditional conjunctival allergen challenge (CAC) model ().Citation37 The standardized CAC design has been used to study allergic conjunctivitis because it ensures a highly reproducible ocular allergic reaction. Using the CAC model, a study drug is administered, and the subject is subsequently challenged with perennial allergens (cat dander, dog dander, dust mites, cockroaches) or seasonal allergens (trees, grasses, and/or ragweed) known to elicit a positive allergic response. After the allergen challenge, assessments of signs and symptoms of AC are performed at various timepoints during the early phase of the allergic response.
Figure 1. Standard vs. modified CAC model. The standard CAC model (Top) consists of a single allergen challenge. The standard CAC model was modified in the present study (Bottom) to include multiple, repeated allergen challenges during subsequent visits. Subjects were repeatedly challenged to induce an acute reaction in the presence of late phase inflammation in addition to the inflammation of the early phase.
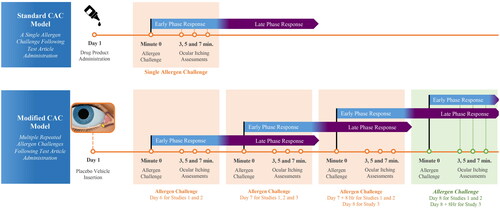
The standard CAC model is characterized by a single allergen challenge; however, for the purpose of studying the dexamethasone ophthalmic insert for AC and to capture the effectiveness of a corticosteroid that acts against both the early and late phases, we modified the standard CAC model in the present study to include multiple, repeated allergen challenges during subsequent visits. Following placement of the intracanalicular insert, subjects were repeatedly challenged to induce an acute reaction in the presence of late phase inflammation in addition to the inflammation of the early phase. These modifications to the standard CAC model allow assessment of ocular itching and redness during the acute allergic response in the presence of late-phase inflammation as it occurs with AC.
Study design
This was a pooled post hoc analysis of three randomized, multicenter, double-blind, placebo vehicle-controlled clinical trials in subjects with AC (). Subjects in each study were screened 7 to 45 days prior to randomization. Insert placement and baseline Ora-CAC® challenge occurred on Day 1. Subjects were then rechallenged on Days 6, 7 and 8; Days 13, 14 and 15; and Days 26, 27 and 28 (Studies 1 and 2 only).
Figure 2. Study design. This schematic shows the design of the three trials included in the present study; however, only data from the placebo insert-treated subjects were included in this post hoc analysis.
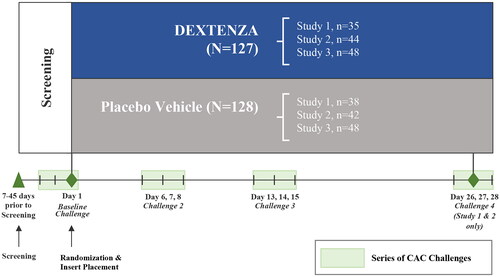
For each study, subjects were randomized 1:1 to receive treatment with active dexamethasone insertCitation36 or placebo insert (both eyes received the same treatment): Study 1 (OTX-14-007, ClinicalTrials.gov Identifier NCT02445326; total N = 73, active treatment, n = 35; PV, n = 38), Study 2 (OTX-15-002, ClinicalTrials.gov Identifier NCT02988882; total N = 86, active treatment, n = 44; PV, n = 42); and Study 3 (CLN-0052, ClinicalTrials.gov Identifier NCT04050865; total N = 96, active treatment, n = 48; PV, n = 48). This post hoc analysis focused solely on pooled data from placebo insert-treated subjects.
Subjects provided signed informed consent prior to participation in any study-related activities. The study protocols and related materials were approved by a commercial Institutional Review Board (Alpha IRB; San Clemente, CA).Citation38 All studies were conducted in accordance with the principles of the Declaration of Helsinki.Citation39
Intracanalicular insert
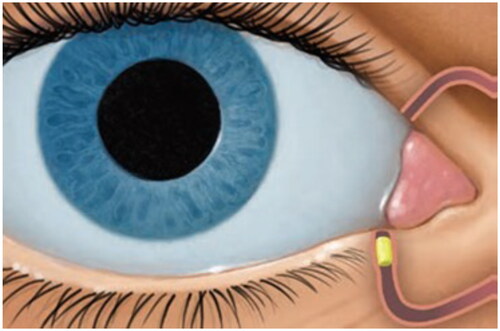
The placebo insert used was a polyethylene glycol-based hydrogel insert and did not contain any drug or active ingredient (Ocular Therapeutix, Inc., Bedford, MA). Once placed into the canaliculus, the insert hydrates, swells to fit the canalicular space and is designed to persist for the duration of the study period before degradation by bulk hydrolysis and resorption through the nasolacrimal tract ().
Figure 3. Mechanism of action. The placebo insert is placed in the punctum, a natural opening in the eye lid, and into the canaliculus. After placement, the insert hydrates, swells to fit the canalicular space and persists for the duration of the study period before degradation by bulk hydrolysis and resorption through the nasolacrimal tract.
Study population
Enrolled subjects were generally healthy adults with a positive history of ocular allergies and a positive skin test reaction to a perennial allergen (cat or dog dander, dust mites, cockroaches) and/or a seasonal allergen (trees, grasses, ragweed) and were required to have a positive bilateral conjunctival allergen challenge with the Ora Conjunctival Allergen Challenge Model (Ora-CAC®; Ora, Inc., Andover, MA). A positive Ora-CAC® was defined as a score >2 for itching and >2 for conjunctival redness for each eye to a qualifying allergen within 10 ± 2 min of the last instillation of allergen. A positive bilateral CAC reaction for at least two out of three time points following the CAC was required with a mean itching score of ≥3 and mean conjunctival redness score ≥2.5 for both eyes at post-CAC assessments during the Phase 3 study.
Efficacy assessments
Changes in ocular itching were determined using the subject self-assessment ocular itching score 3, 5 and 7 min post-allergen challenge using the 5-point Ora Calibra® CAC Itching ScaleCitation40 (0 = none and 4 = incapacitating itch with an irresistible urge to rub with 0.5-unit increments allowed; Ora, Inc., Andover, MA). Assessments of change were made with every challenge. Changes in conjunctival redness were assessed by the investigator, 7, 15 and 20 min post-allergen challenge on Weeks 1, 2 and 4, and using a 0–4 point scale (0 = none and 4 = severe vasodilation with 0.5-unit increments allowed).
Statistical analysis
The statistical analyses for the three phase 3 studies have been described previously.Citation35 These analyses on ocular itching and conjunctival redness scores were performed using descriptive statistics. Statistical significance for changes in these values from baseline were calculated using a pairwise T-test. Since this is an exploratory analysis, the results should be interpreted in a descriptive manner.
Results
Patient demographics and baseline characteristics
The pooled data set consisted of 255 subjects (dexamethasone insert, n = 127; placebo insert, n = 128); however, only data from the placebo insert groups were included in this post hoc analysis. Subject demographics are presented in . At baseline, mean (SD) ocular itching and conjunctival redness scores among placebo insert subjects were 3.52 (0.44) and 2.97 (0.39), respectively.
Table 1. Patient Demographics.
Effect of placebo insert on ocular itching and conjunctival redness scores
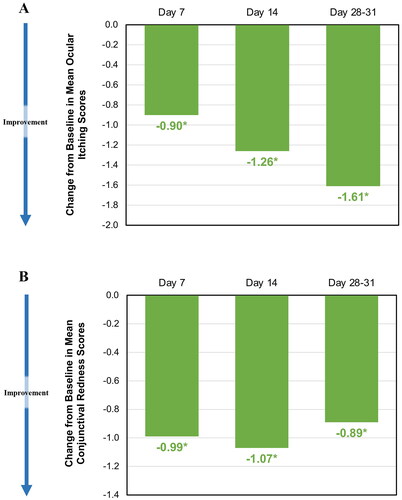
On post-insertion Days 7, 14 and 28, mean ocular itching scores were 2.62, 2.26 and 1.91, respectively, when compared to baseline score (3.52), representing a statistically significant reduction of 26%, 36% and 46%, respectively (p < 0.001). On post-insertion Days 7, 14 and 28 the mean conjunctival redness scores were reduced to 1.98, 1.90, and 2.08, respectively, when compared to baseline score (2.97), representing a statistically significant reduction of 33%, 36%, and 30%, respectively (p < 0.001). show the changes in pooled allergen challenges for ocular itching and redness scores over the course of the pooled studies. show the pooled changes in baseline ocular itching and redness scores over the course of the pooled studies.
Figure 4. (A) Effect of punctal occlusion on pooled ocular itching scores among placebo insert-treated subjects over time. These figures show the change in pooled ocular itching scores among placebo insert-treated subjects for up to 4 weeks. At baseline, mean (SD) ocular itching scores was 3.52 (0.44). On post-insertion Days 7, 14 and 28, mean ocular itching scores were 2.62, 2.26 and 1.91, respectively, representing a significant reduction by 26%, 36% and 46%, respectively (p < 0.001). (B) Effect of punctal occlusion on conjunctival redness scores among placebo insert subjects following repeat allergen challenges. These figures show the pooled changes in ocular redness scores among placebo insert-treated subjects for up to 4 weeks. At baseline, mean (SD) conjunctival redness scores was 2.97 (0.39). On Days 7, 14 and 28 the mean conjunctival redness scores were reduced to 1.98, 1.90, and 2.08, respectively, representing a significant reduction by 33%, 36%, and 30%, respectively (p < 0.001).
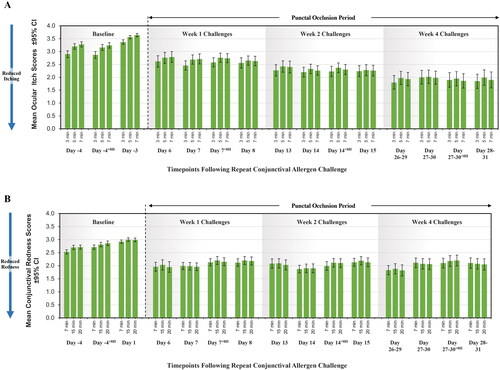
Figure 5. (A) Change from baseline in ocular itching score among placebo insert-treated subjects over time. The mean (SD) baseline ocular itching score was 3.52 (0.44). The change from baseline was calculated as the difference between the mean of 3-, 5-, and 7-min post-CAC ocular itching scores on Days 7, 14 and 28–31 (for each, p < 0.001). Data are for placebo insert-treated subjects only. (B) Change from mean baseline conjunctival redness scores among placebo insert-treated subjects over time. The mean (SD) baseline conjunctival redness score was 2.97 (0.39). Change from baseline was calculated as the difference between the mean of 7, 15 and 20 min post-CAC conjunctival redness scores on Day 1 and Day 7, 14 and 28–31 (for each, p < 0.001). Data are for placebo insert-treated subjects only.
Discussion
While punctal occlusion has been successfully used to treat the signs and symptoms of dry eye disease,Citation25–33 the effects of punctal occlusion on the symptoms of AC are less well-documented. There is some concern among clinicians that punctal occlusion may make signs/symptoms worse by trapping allergens on the surface of the eye for a longer period of time and by potentially increasing the allergic response; however, little evidence exists to suggest this is the case.Citation34,Citation41,Citation42 Likely, allergens on the ocular surface might become diluted due to tear conservation. Further, the hydrogel insert does not completely occlude the punctum during the first few weeks, thus allowing additional drainage compared to a true punctal plug. It is possible that the upper punctum may acclimatize to lower punctal occlusion and, as a result, drains more. The results of this post hoc analysis of pooled data from three randomized, placebo vehicle-controlled studies using a modified repeat CAC model demonstrated that subjects with AC that were administered a placebo insert (n = 128) achieved statistically significant reductions in baseline ocular itching and conjunctival redness after Week 1 which were maintained through Week 4 (for each, p < 0.001). These results demonstrate that punctal occlusion in subjects with AC does not worsen ocular itching or redness.
We used a modified CAC model in the present study. The standard CAC model is characterized by a single allergen challenge. However, to study the dexamethasone ophthalmic insert for AC and capture the effectiveness of a corticosteroid that acts against both the early and late phases, we modified the standard CAC by including multiple, repeated allergen challenges in subsequent visits. After intracanalicular insert placement, the subjects were repeatedly challenged to induce an acute reaction during late phase inflammation, as well as the inflammation of the early phase. These modifications to the standard CAC model allowed us to assess ocular redness and itching during the acute allergic response in the presence of late-phase inflammation, as is the case with AC. Because of these modifications, the modified CAC model sufficiently demonstrates that punctal occlusion does not worsen ocular itching and conjunctival redness in this patient population and may not impact patients clinically. Given that this was a post hoc analysis, any additional testing, such as field testing, is not within the scope of this study, though future studies may provide insights into the mechanism and/or effects on ocular itching and conjunctival redness.
Limitations of this study include: a study design based on a treatment model rather than real world conditions and a pooled post hoc analysis which shows correlation but not designed to show causation. Future prospective studies with a control group (undergoing CAC challenge with no punctal occlusion) can aid in better understanding the role of punctal occlusion in treating AC (if any). Punctal occlusion has not been approved by FDA for the treatment of ocular itching or redness associated with allergic conjunctivitis. Although this was a post hoc analysis, strengths of this analysis include: the number of subjects enrolled in each study was determined a priori by power analyses and the use of the validated Ora-CAC® challenge model which is standardized for allergen exposure and outcome assessment.
Conclusion
There has been uncertainty regarding the effect of punctal occlusion in patients with AC. The results of this pooled post hoc analysis indicate that punctal occlusion with a resorbable hydrogel intracanalicular insert did not worsen ocular itching and conjunctival redness in subjects with AC.
Supplemental Material
Download MP4 Video (86.2 MB)Acknowledgements
The authors acknowledge the editorial assistance of Dr. Carl S. Hornfeldt, Apothekon, Inc., during the preparation of this manuscript.
Disclosure statement
Steven M. Silverstein was a study investigator and reports grants from Ocular Therapeutix, Inc. Michelle A. Sato, and Edward J. Meier were study investigators and have no conflicts of interest to disclose. Stella Dai, Aditi Bauskar, Kennedy Depperschmidt, Nysha Blender, Srilatha Vantipalli, and Rabia Gurses Ozden are employees of Ocular Therapeutix, Inc. Michael H. Goldstein is a consultant for and former employee of Ocular Therapeutix, Inc.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Additional information
Funding
References
- Leonardi A, Castegnaro A, Valerio AL, Lazzarini D. Epidemiology of allergic conjunctivitis: clinical appearance and treatment patterns in a population-based study. Curr Opin Allergy Clin Immunol. 2015;15(5):482–488. doi:10.1097/ACI.0000000000000204.
- Rosario N, Bielory L. Epidemiology of allergic conjunctivitis. Curr Opin Allergy Clin Immunol. 2011;11(5):471–476. doi:10.1097/ACI.0b013e32834a9676.
- Gomes PJ. Trends in prevalence and treatment of ocular allergy. Curr Opin Allergy Clin Immunol. 2014;14(5):451–456. doi:10.1097/ACI.0000000000000100.
- Miyazaki D, Fukagawa K, Okamoto S, Fukushima A, Uchio E, Ebihara N, Shoji J, Namba K, Shimizu Y. Epidemiological aspects of allergic conjunctivitis. Allergol Int. 2020;69(4):487–495. doi:10.1016/j.alit.2020.06.004.
- Dupuis P, Prokopich CL, Hynes A, Kim H. A contemporary look at allergic conjunctivitis. Allergy Asthma Clin Immunol. 2020;16:5. doi:10.1186/s13223-020-0403-9.
- Zhang SY, Li J, Liu R, Lao HY, Fan Z, Jin L, Liang L, Liu Y. Association of allergic conjunctivitis with health-related quality of life in children and their parents. JAMA Ophthalmol. 2021;139(8):830–837. doi:10.1001/jamaophthalmol.2021.1708.
- Bielory L, Delgado L, Katelaris CH, Leonardi A, Rosario N, Vichyanoud P. ICON: diagnosis and management of allergic conjunctivitis. Ann Allergy Asthma Immunol. 2020;124(2):118–134. doi:10.1016/j.anai.2019.11.014.
- Singh K, Axelrod S, Bielory L. The epidemiology of ocular and nasal allergy in the United States, 1988-1994. J Allergy Clin Immunol. 2010;126(4):778–783 e6. doi:10.1016/j.jaci.2010.06.050.
- Labib BA, Chigbu DI. Therapeutic targets in allergic conjunctivitis. Pharmaceuticals (Basel). 2022;15(5):547. doi:10.3390/ph15050547.
- Azari AA, Arabi A. Conjunctivitis: a systematic review. J Ophthalmic Vis Res. 2020;15(3):372–395.
- Leonardi A, Silva D, Perez Formigo D, Bozkurt B, Sharma V, Allegri P, Rondon C, Calder V, Ryan D, Kowalski ML, et al. Management of ocular allergy. Allergy. 2019;74(9):1611–1630. doi:10.1111/all.13786.
- An JA, Kasner O, Samek DA, Levesque V. Evaluation of eyedrop administration by inexperienced patients after cataract surgery. J Cataract Refract Surg. 2014;40(11):1857–1861. doi:10.1016/j.jcrs.2014.02.037.
- Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23(8):1296–1310. doi:10.1016/s0149-2918(01)80109-0.
- Schwartz GF, Hollander DA, Williams JM. Evaluation of eye drop administration technique in patients with glaucoma or ocular hypertension. Curr Med Res Opin. 2013;29(11):1515–1522. doi:10.1185/03007995.2013.833898.
- Burns E, Mulley GP. Practical problems with eye-drops among elderly ophthalmology outpatients. Age Ageing. 1992;21(3):168–170. doi:10.1093/ageing/21.3.168.
- Buchan JC, Cleveland V, Sutton H, Cassels-Brown A. Post-cataract eye drops can be avoided by depot steroid injections. Br J Community Nurs. 2017;22(12):598–601. doi:10.12968/bjcn.2017.22.12.598.
- Hom MM, Nguyen AL, Bielory L. Allergic conjunctivitis and dry eye syndrome. Ann Allergy Asthma Immunol. 2012;108(3):163–166. doi:10.1016/j.anai.2012.01.006.
- Akil H, Celik F, Ulas F, Kara IS. Dry Eye Syndrome and Allergic Conjunctivitis in the Pediatric Population. Middle East Afr J Ophthalmol. 2015;22(4):467–471. doi:10.4103/0974-9233.167814.
- Leonardi A, Modugno RL, Salami E. Allergy and dry eye disease. Ocul Immunol Inflamm. 2021;29(6):1168–1176. doi:10.1080/09273948.2020.1841804.
- Kishimoto T, Ishida W, Nakajima I, Fukuda K, Yamashiro K. Aqueous-deficient dry eye exacerbates signs and symptoms of allergic conjunctivitis in mice. Int J Mol Sci. 2022;23(9):4918.
- Villani E, Rabbiolo G, Nucci P. Ocular allergy as a risk factor for dry eye in adults and children. Curr Opin Allergy Clin Immunol. 2018;18(5):398–403. doi:10.1097/ACI.0000000000000471.
- Markoulli M, Kolanu S. Contact lens wear and dry eyes: challenges and solutions. Clin Optom (Auckl)). 2017;9:41–48. doi:10.2147/OPTO.S111130.
- Fraunfelder FT, Sciubba JJ, Mathers WD. The role of medications in causing dry eye. J Ophthalmol. 2012;2012:285851. doi:10.1155/2012/285851.
- Zhou X, Zhang X, Zhou D, Zhao Y, Duan X. A narrative review of ocular surface disease related to anti-glaucomatous medications. Ophthalmol Ther. 2022;11(5):1681–1704. doi:10.1007/s40123-022-00557-0.
- Brissette AR, Mednick ZD, Schweitzer KD, Bona MD, Baxter SA. Punctal plug retention rates for the treatment of moderate to severe dry eye: a randomized, double-masked, controlled clinical trial. Am J Ophthalmol. 2015;160(2):238–242 e1. doi:10.1016/j.ajo.2015.05.013.
- Burgess PI, Koay P, Clark P. SmartPlug versus silicone punctal plug therapy for dry eye: a prospective randomized trial. Cornea. 2008;27(4):391–394. doi:10.1097/ICO.0b013e318160d030.
- Chen F, Wang J, Chen W, Shen M, Xu S, Lu F. Upper punctal occlusion versus lower punctal occlusion in dry eye. Invest Ophthalmol Vis Sci. 2010;51(11):5571–5577. doi:10.1167/iovs.09-5097.
- Kaido M, Ishida R, Dogru M, Tsubota K. Visual function changes after punctal occlusion with the treatment of short BUT type of dry eye. Cornea. 2012;31(9):1009–1013. doi:10.1097/ICO.0b013e31823f8cfc.
- Mansour K, Leonhardt CJ, Kalk WW, Bootsma H, Bruin KJ, Blanksma LJ, Sjogren W, Sjögren Workgroup Lacrimal punctum occlusion in the treatment of severe keratoconjunctivitis Sicca caused by Sjogren syndrome: a uniocular evaluation. Cornea. 2007;26(2):147–150. doi:10.1097/01.ico.0000244877.30997.6a.
- Nava-Castaneda A, Tovilla-Canales JL, Rodriguez L, Tovilla YPJL, Jones CE. Effects of lacrimal occlusion with collagen and silicone plugs on patients with conjunctivitis associated with dry eye. Cornea. 2003;22(1):10–14. doi:10.1097/00003226-200301000-00003.
- Qiu W, Liu Z, Zhang Z, Ao M, Li X, Wang W. Punctal plugs versus artificial tears for treating dry eye: a comparative observation of their effects on contrast sensitivity. J Ocul Biol Dis Infor. 2012;5(1):19–24. doi:10.1007/s12177-012-9094-x.
- Rabensteiner DF, Boldin I, Klein A, Horwath-Winter J. Collared silicone punctal plugs compared to intracanalicular plugs for the treatment of dry eye. Curr Eye Res. 2013;38(5):521–525. doi:10.3109/02713683.2013.765487.
- Roberts CW, Carniglia PE, Brazzo BG. Comparison of topical cyclosporine, punctal occlusion, and a combination for the treatment of dry eye. Cornea. 2007;26(7):805–809. doi:10.1097/ICO.0b013e318074e460.
- Karpecki PM. Plug into dry eye therapy. 2017. https://www.reviewofoptometry.com/article/plug-into-dry-eye-therapy
- Torkildsen G, Abelson MB, Gomes PJ, McLaurin E, Potts SL, Mah FS. Vehicle-controlled, phase 2 clinical trial of a sustained-release dexamethasone intracanalicular insert in a chronic allergen challenge model. J Ocul Pharmacol Ther. 2017;33(2):79–90. doi:10.1089/jop.2016.0154.
- DEXTENZA® (Dexamethasone Ophthalmic Insert) 0.4 mg for intracanalicular use [prescribing information]. Bedford, MA: Ocular Therapeutix, Inc; 2021.
- Abelson MB, Chambers WA, Smith LM. Conjunctival allergen challenge. A clinical approach to studying allergic conjunctivitis. Arch Ophthalmol. 1990;108(1):84–88. doi:10.1001/archopht.1990.01070030090035.
- McLaurin EB, Evans D, Repke CS, Sato MA, Gomes PJ, Reilly E, Blender N, Silva FQ, Vantipalli S, Metzinger JL, et al. Phase 3 randomized study of efficacy and safety of a dexamethasone intracanalicular insert in patients with allergic conjunctivitis. Am J Ophthalmol. 2021;229:288–300. doi:10.1016/j.ajo.2021.03.017.
- World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194.
- Rodriguez JD, Johnston PR, Ousler GW, Smith LM, Abelson MB. Automated grading system for evaluation of ocular redness associated with dry eye. Clin Ophthalmol. 2013;7:1197–1204. doi:10.2147/OPTH.S39703.
- Jehangir N, Bever G, Mahmood SM, Moshirfar M. Comprehensive review of the literature on existing punctal plugs for the management of dry eye disease. J Ophthalmol. 2016;2016:9312340. doi:10.1155/2016/9312340.
- Bourkiza R, Lee V. A review of the complications of lacrimal occlusion with punctal and canalicular plugs. Orbit. 2012;31(2):86–93. doi:10.3109/01676830.2011.648802.
