Abstract
Purpose: To investigate the characteristics of optical coherence tomography (OCT) and aqueous humor cytokine differences between acute and chronic central serous chorioretinopathy (CSC) and to evaluate the relevance of these findings.
Methods: This was a cross-sectional, observational study. Patients with CSC were divided into acute and chronic groups based on the symptom duration and were compared with normal controls. Best-corrected visual acuity (BCVA), central macular thickness (CMT), subfoveal choroidal thickness (CT), hyperreflective foci (HF), and cytokines including vascular endothelial growth factor (VEGF), interleukin (IL)-6, IL-8, IL-10, interferon-inducible protein-10 (IP-10), and monocyte chemoattractant protein-1 (MCP-1) were used as comparison metrics.
Results: A total of 62 patients (62 eyes) with CSC (22 with acute CSC and 40 with chronic CSC) and 35 patients as controls were included in this study. The chronic CSC group had significantly older average ages and worse BCVA than the acute CSC group (both p < 0.05). Both CSC groups showed significant increases in CMT and CT (both p < 0.05). In chronic CSC, the CMT was thinner, with more HF in the neuroretina (p = 0.034). VEGF levels were significantly higher in patients with chronic CSC than in those with acute CSC and controls (p < 0.05). The levels of inflammatory cytokines showed no significant difference between the CSC and control groups. Spearman’s correlation analysis showed that the number of HF was positively correlated with disease duration (r = 0.311, p = 0.014), logMAR BCVA (r = 0.487, P < 0.001) and MCP-1 levels (r = 0.256, p = 0.045).
Conclusions: Chronicity of CSC could lead to upregulation of VEGF. HF was associated with a more severe visual impairment in CSC patients and had relations with the levels of MCP-1.
Introduction
Central serous chorioretinopathy (CSC) is characterized by localized retinal detachment at the posterior pole, resulting from the hyperpermeability of the choroidal vessels and dysfunction of the retinal pigment epithelium (RPE).Citation1 Currently, CSC is proposed to have two distinct subcategories, namely acute and chronic forms, based on the duration of the retinal detachment and characteristic changes on multimodal images.Citation2 There is no consensus on the duration threshold for distinguishing acute and chronic forms of CSC in most published papers, generally set within six months.Citation3
Spectral-domain optical coherence tomography (SD-OCT) was necessary to identify and assess CSC owing to the ability to visualize the retinal substructure.Citation4 Variable microstructural features, including subretinal fluid (SRF), pigment epithelium detachment (PED), and RPE changes have been well studied in the acute and chronic forms of CSC.Citation5 Hyperreflective foci (HF) have also been detected in the detached neuroretina and subretinal space in eyes with CSC. The number of these foci tends to increase with disease progression and is related to worse visual acuity.Citation6,Citation7 However, the origin and element of HF remain controversial.
In addition to morphological changes, some studies have targeted the discrepancy in cytokines expression between acute and chronic CSC.Citation8–11 The vascular endothelial growth factor (VEGF) family and inflammatory cytokines, such as interleukin-6 (IL-6), IL-8, interferon-inducible protein-10 (IP-10), and monocyte chemoattractant protein-1 (MCP-1) are discussed in the molecular pathogenesis of CSC. However, it remains controversial whether the inflammatory or angiogenic cytokines are upregulated from the acute to the chronic form of CSC. Additionally, the comparison of cytokines between the different types of CSC and normal controls has not been fully elucidated.
A correlation between HF and aqueous humor inflammatory cytokines has been observed in macular edema.Citation12,Citation13 However, little is known about the correlation in patients with acute and chronic CSC. In this study, we evaluated the levels of VEGF and inflammatory cytokines in the aqueous humor of patients with acute CSC, chronic CSC, and normal controls. In addition, we analyzed the association between cytokines and morphological changes in OCT to better understand the progression of CSC.
Materials and methods
Patients diagnosed with CSC between May 2019 and May 2021 in the Zhejiang Provincial People’s Hospital and affiliated eye hospital of Wenzhou Medical University were included in this cross-sectional observational study, in accordance with the principles of the Declaration of Helsinki. Patients undergoing cataract surgeries were recruited as controls for comparison. This study was approved by the institutional review board of the Eye Hospital of Wenzhou Medical University. Informed signed consent was obtained from participants.
Patients
All subjects underwent comprehensive ophthalmic evaluation with a slit lamp, intraocular pressure examination, best-corrected visual acuity (BCVA) testing, SD-OCT, optical coherence tomography angiography (OCTA), fundus fluorescein angiography (FFA) and indocyanine green angiography (ICGA). The diagnosis of CSC was based on clinical features and multimodal images.Citation1–3 Acute CSC () was characterized by dome-shaped serous retinal detachment (SRD) with small focal PED and leakage through the RPE alterations on FFA, with a duration of less than 6 months. Conversely, chronic CSC () was diagnosed based on the diffuse lesions of the RPE with multiple hyperfluorescence leakage points on FFA and symptoms lasting more than 6 months. Patients who were diagnosed with CSC and accepted intravitreal anti-VEGF injection for the first time were included. Patients were excluded if they had macular neovascularization (MNV), age-related macular degeneration, polypoidal choroidal vasculopathy, glaucoma, high myopia, or serious systemic disease. Patients with a history of Diamox, psycho-regulatory and corticosteroid medications use, photodynamic therapy, laser treatment, anti-VEGF therapy, or excessive consumption of caffeine were also excluded from this study.
Figure 1. Multimodal images from the left eye of a 38-year-old male patient with acute CSC. (A) FFA showed focal hyperfluorescence leakage points at macular (white arrow). (B) ICGA showed dilated choroidal vessels at the leakage area (white circle). (C) B-Scan OCT showed serous retinal detachment at macular with slight hyperreflective foci in the outer neuroretina (white arrow).
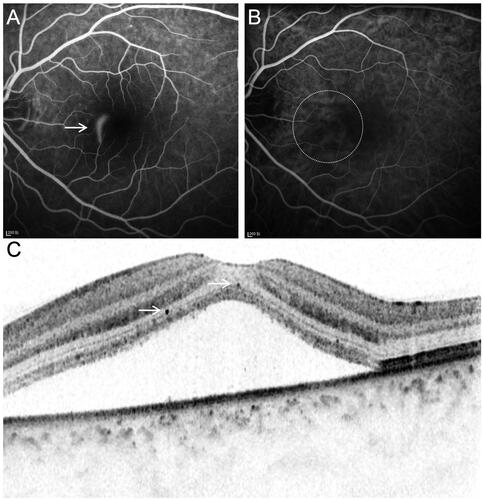
Figure 2. Multimodal images from the right eye of a 53-year-old male patient with chronic CSC. (A) FFA showed diffuse hyperfluorescence leakage points. (B) ICGA showed dilated choroidal vessels at the leakage area. (C) B-Scan OCT showed serous retinal detachment at macular with moderate hyperreflective foci.
The control group included patients who had undergone cataract surgery. The exclusion criterion for controls was a history of severe oculopathy and systemic disease, such as glaucoma, high myopia, unstable cardiovascular diseases, diabetes, and autoimmune disease.
Optical coherence tomography measurements
The measurement parameters of SD-OCT included central macular thickness (CMT), subfoveal choroidal thickness (CT), and HF. All measurements were manually conducted at the macular central fovea using horizontal SD-OCT B-scan images. CMT was measured from the internal limiting membrane to Bruch’s membrane. CT was measured from Bruch’s membrane to the inner edge of the sclera. The average values of the three times measurements were available for the analysis.
HF was defined as isolated hyperreflective dots with clear demarcation in the neuroretina on OCT, which was 20–50 µm in diameter ( and Citation2).Citation14 HF were manually counted at a length of 3000 µm centered on the fovea by two experienced masked ophthalmologists. In addition, the patients were classified into two grades based on the number of HF. A slight grade represented a small number (0–5 HF). A moderate grade represented approximately six or more HF.
Collection and analysis of aqueous humor cytokines
Aqueous humor samples were collected before intravitreal injection of anti-VEGF drugs or before cataract surgery. After topical anesthesia, approximately 0.05 ml of aqueous humor was withdrawn using a 30-gauge insulin syringe from the anterior chamber.Citation15 The sample was immediately kept in a sterile Eppendorf tube and frozen at −84 °C until assayed. Cytokines including VEGF, IL-6, IL- 8, IL-10, IP-10, and MCP-1 were analyzed using Luminex200 (BIO-RAD, Hercules, CA, USA) with the Luminex Performance Assay kit (LXSAHM-06, RnD) and calculated using the standard curve. All the steps were conducted at room temperature under dark illumination to avoid the light-induced degradation of the samples.
Statistical analysis
Statistical analyses were performed using SPSS 20.0 (IBM SPSS Inc., Chicago, IL, USA). A P value less than 0.05 was considered statistically significant. Quantitative data are expressed as means and standard deviations. Categorical variables were compared using the chi-square test. Continuous variables and nonparametric variables between the acute CSC and chronic CSC groups were compared using independent t-tests and Mann–Whitney U-tests. One-way analysis of variance (ANOVA) was used to compare the continuous variables, and the Kruskal–Wallis test was used to compare cytokine levels among the three groups. Correlations between variables were assessed using Spearman’s correlation analysis.
Results
Twenty-two patients (22 eyes) with acute CSC, 40 patients (40 eyes) with chronic CSC, and 35 controls (35 eyes) were included in this study. The demographic data for each group are presented in . There were significant differences in age (p < 0.001), symptom duration (p < 0.001), and BCVA (p = 0.011) between the acute and chronic CSC groups. There was no significant difference in sex between the CSC and control groups (χ2=0.252, p = 0.882).
Table 1. Demographic data and clinical characteristics of patients with CSC and controls.
More hyperreflective foci in patients with chronic CSC
OCT parameters are listed in . CMT and CT in acute and chronic CSC eyes were significantly higher than those in controls (p < 0.001). Further analysis revealed that CMT in the acute CSC group was higher than that in the chronic CSC group (p = 0.033). However, there was no significant difference in subfoveal CT between the acute and chronic CSC groups (p = 0.917).
Table 2. OCT characteristics of patients with CSC and controls.
The total number of HF in chronic CSC eyes was 5.58 ± 3.80 (range, 0–13), which was more than that in the acute CSC eyes, 3.91 ± 2.22 (range, 0–8) (p = 0.034). According to the number of HF, patients were then classified into two grades. In the acute CSC group, 17 (77.3%) eyes were graded as slight (range, 0–5) and five (22.7%) as moderate (range, 6–13). In the chronic CSC group, 17 (42.5%) eyes were graded as slight and 23 (57.5%) as moderate. There was a statistically significant difference in the grade of HF number between the acute and chronic CSC groups (χ2=6.93, p = 0.008).
Upregulated levels of VEGF in patients with chronic CSC
As shown in , six aqueous humor cytokines (VEGF, IL-6, IL-8, IL-10, IP-10, and MCP-1) were analyzed in controls and patients with CSC. We noted significant differences in the concentrations of VEGF (p = 0.004) among the three groups. Aqueous humor levels of VEGF were significantly higher in the chronic CSC group than in the control and acute CSC groups (p < 0.05, ). However, VEGF levels did not differ significantly between patients with acute CSC and controls (p = 0.749). The other cytokines showed no significant differences among the three groups.
Figure 3. Box-and-whisker plot of vascular endothelial growth factor levels in the aqueous humor in the acute CSC, chronic CSC, and control groups.
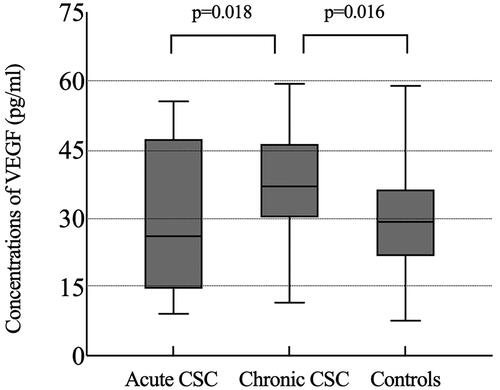
Table 3. Cytokines levels of patients with CSC and controls.
Upregulated levels of IL-8 in CSC patients with moderate HF
Cytokine levels in patients with CSC classified by the number of HF are compared in . IL-8 levels were significantly higher in patients with moderate HF than in those with slight HF (p = 0.047). However, the higher levels of VEGF, IL-6, IP-10, and MCP-1 and lower levels of IL-10 in patients with moderate HF were not statistically different from those in patients with slight HF.
Table 4. Cytokines levels of CSC patients with slight or moderate number of HF.
OCT characteristics associated with disease progression
We evaluated correlations between age, disease duration, BCVA, and OCT characteristics (). Notably, the number of HF was positively correlated with disease duration (r = 0.311, p = 0.014) and logMAR BCVA (r = 0.487, P < 0.001) in patients with acute and chronic CSC. Subfoveal CT was correlated with age (r=–0.281, p = 0.027). Other OCT characteristics were not correlated with age, disease duration, or BCVA.
Table 5. Correlation between the OCT characteristics and age, duration of the disease, or logMAR BCVA in the patients with CSC.
Cytokines associated with disease progression
For the entire CSC cohort, there were strong positive correlations between IL-6 and IL-8 concentrations (r = 0.686, p < 0.001), IL-6 and IP-10 concentrations (r = 0.671, p < 0.001), IL-6, and MCP-1 concentrations (r = 0.693, p < 0.001), IL-8 and IP-10 concentrations (r = 0.719, P < 0.001), IL-8 and MCP-1 concentrations (r = 0.605, p < 0.001), and IP-10 and MCP-1 concentrations (r = 0.540, p < 0.001).
Disease duration, logMAR BCVA, and the number of HF were correlated with multiple cytokines in patients with CSC (). Disease duration was positively correlated with IL-6 (r = 0.260, p = 0.041), IL-8 (r = 0.306, p = 0.018) and IP-10 concentrations (r = 0.337, p = 0.008). The logMAR BCVA was positively correlated with MCP-1 concentrations (r = 0.269, p = 0.034). The number of HF was positively correlated with MCP-1 concentrations (r = 0.256, p = 0.045). Moreover, cytokines levels were not correlated with age and other OCT characteristics.
Table 6. Correlation between duration of the disease, logMAR BCVA, HF and six cytokine concentrations in the patients with CSC.
Discussion
This study aimed to describe the differences in retinal microstructural and cytokines levels between acute and chronic CSC patients. First, we noted lower central retinal thickness and higher HF with worse visual acuity in chronic CSC patients. In addition, VEGF levels were significantly increased in chronic CSC patients compared with acute CSC patients and controls, whereas inflammatory cytokines, including IL-6, IL-8, IP-10, and MCP-1, were elevated, but the difference was not statistically significant. Moreover, an increased number of HF was associated with some upregulated inflammatory cytokines in CSC.
In CSC, adjacent hyperperfusion and focal choriocapillary ischemia are currently considered the primary underlying pathogenic mechanisms.Citation16,Citation17 The pathological processes leading to choroidal abnormalities may include autonomic dysregulation, inflammation, abnormal angiogenesis, and the complement system.Citation2,Citation18 Recently, several studies have reported cytokine levels in patients with CSC. Lim et al.Citation10 found that VEGF was correlated with symptom duration in a small sample size study. However, they did not detect differences in VEGF levels between patients with chronic CSC and controls. Terao et al.Citation8 revealed that inflammatory cytokines were significantly increased along with CSC progression. Angiogenic cytokines such as VEGF almost reached a significant increase in chronic CSC. In contrast, Jung et al.Citation9 found no significant difference in cytokine levels between acute and chronic CSC patients. The conflicting findings of aqueous humor cytokines in previous reports indicated that the role of cytokines in CSC remains to be further studied.
On this basis, we analyzed the discrepancy of angiogenic and inflammatory cytokines among controls, acute and chronic CSC patients. In this study, VEGF levels were significantly higher in the chronic CSC group than in the acute CSC and control groups. VEGF, angiogenic cytokines, can theoretically modify choroidal hyperpermeability.Citation19 Therefore, anti-VEGF injection has been proposed as a treatment for CSC, although it is still off-label.Citation2 Further analysis of our results indicated that the distribution of VEGF in chronic CSC showed great differences from the lowest to the highest. Hence, anti-VEGF treatment for CSC should be selectively conducted and considered with some biomarkers, such as flat irregular PED.Citation20
Classic inflammation is generally not involved in the onset of CSC which would be induced by corticosteroids. There was no significant difference in inflammatory cytokine levels between the CSC and control groups in this study. However, IL-6, IL-8, IP-10, and MCP-1 levels tended to be upregulated in chronic CSC patients compared to those in acute CSC patients. These results indicate that persistent SRF in chronic CSC may have an impact on immune regulation of the retina and choroid, which gradually leads to the alteration of these inflammatory cytokines.
In CSC, HF is mainly distributed in the outer neuroretina and more frequently detected in the chronic form and deemed as a sign of poor visual prognosis.Citation6,Citation21,Citation22 The presence of HF in SD-OCT has been proposed as a biomarker of the inflammatory response in several ocular fundus diseases.Citation23,Citation24 Some have also considered it to be correlated with the migration of RPE cells, lipoprotein exudation from the choriocapillaris, or degeneration of outer segments.Citation14 In this study, we found the level of IL-8 was elevated in the patients with a moderate number of HF compared to those with a slight number of HF. IL-8 is produced by retinal endothelial and glial cells and is involved in ischemic angiogenesis.Citation10 It plays a role in chemotaxis and activation of inflammatory cells such as neutrophils and T lymphocytes.Citation9 In addition, the number of HF showed a positive correlation with MCP-1, which is related to leukostasis due to hypoxic retinas.Citation25 The activation and aggregation of inflammatory cells induced by MCP-1 lead to endothelial damage of capillaries, which may further affect the permeability of choroidal vessels.Citation26 This may suggest that inflammatory cytokines participate in the formation of HF in CSC.
This study has several limitations. First, this was a cross-sectional, observational study. Further research with follow-up data is needed to evaluate the prognostic value of HF and cytokines. Second, the difference in the average age among the three groups was caused by the clinical characteristics of acute and chronic CSC. To avoid the influence of age, we assessed the relationship between cytokine levels and age and found no significant correlations. Moreover, cytokines were obtained from the aqueous humor, not vitreous, to represent the intraocular levels.
In conclusion, we assessed the discrepancy between acute and chronic CSC based on cytokines and OCT characteristics. Our results indicated that the higher expression of VEGF in chronic CSC might be evidence for individualized treatment. Additionally, HF as a biomarker was increased in chronic CSC and related to the upregulation of MCP-1. The pathogenesis and clinical value of cytokines in CSC still need further investigation.
Author contributions
C Zhang, J Mao and L Shen: conceived and designed the experiments; J Mao: performed the treatment; S Zhang, Z Zheng, Z Zhang, and Z Xiang: collected the data; C Zhang: analyzed the data and wrote the paper; Y Chen and L Shen: approved the final version of the manuscript.
Ethical statement
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and the national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by the Institutional Review Board of the Affiliated Eye Hospital of Wenzhou Medical University (No.121-K-107-01).
Disclosure statement
No conflict of interest was reported by the author(s).
Data availability statement
The data used to support the findings of this study were supplied by Lijun Shen under license and so cannot be made freely available. Requests for access to these data should be made to Lijun Shen ([email protected]).
Additional information
Funding
References
- Kaye R, Chandra S, Sheth J, Boon CJF, Sivaprasad S, Lotery A. Central serous chorioretinopathy: an update on risk factors, pathophysiology and imaging modalities. Prog Retin Eye Res. 2020; 79:100865. doi: 10.1016/j.preteyeres.2020.100865.
- van Rijssen TJ, van Dijk EHC, Yzer S, Ohno-Matsui K, Keunen JEE, Schlingemann RO, Sivaprasad S, Querques G, Downes SM, Fauser S, et al. Central serous chorioretinopathy: towards an evidence-based treatment guideline. Prog Retin Eye Res. 2019; 73:100770. doi: 10.1016/j.preteyeres.2019.07.003.
- Daruich A, Matet A, Dirani A, Bousquet E, Zhao M, Farman N, Jaisser F, Behar-Cohen F. Central serous chorioretinopathy: recent findings and new physiopathology hypothesis. Prog Retin Eye Res. 2015; 48:82–118. doi: 10.1016/j.preteyeres.2015.05.003.
- Iglicki M, Busch C, Loewenstein A, Fung AT, Invernizzi A, Mariussi M, Arias R, Gabrielle PH, Cebeci Z, Okada M, et al. Underdiagnosed optic disk pit maculopathy: spectral domain optical coherence tomography features for accurate diagnosis. Retina. 2019;39(11):2161–2166. doi: 10.1097/IAE.0000000000002270.
- Nkrumah G N, Paez-Escamilla M, Singh SR, Rasheed MA, Maltsev D, Guduru A, Chhablani J. Biomarkers for central serous chorioretinopathy. Ther Adv Ophthalmol. 2020; 12:2515841420950846. doi: 10.1177/2515841420950846.
- Yalcinbayir O, Gelisken O, Akova-Budak B, Ozkaya G, Gorkem Cevik S, Yucel AA. Correlation of spectral domain optical coherence tomography findings and visual acuity in central serous chorioretinopathy. Retina. 2014;34(4):705–712. doi: 10.1097/IAE.0000000000000001.
- Lee H, Lee J, Chung H, Kim HC. Baseline spectral domain optical coherence tomographic hyperreflective foci as a predictor of visual outcome and recurrence for central serous chorioretinopathy. Retina. 2016;36(7):1372–1380. doi: 10.1097/IAE.0000000000000929.
- Terao N, Koizumi H, Kojima K, Yamagishi T, Nagata K, Kitazawa K, Yamamoto Y, Yoshii K, Hiraga A, Toda M, et al. Association of upregulated angiogenic cytokines with choroidal abnormalities in chronic central serous chorioretinopathy. Invest Ophthalmol Vis Sci. 2018;59(15):5924–5931. doi: 10.1167/iovs.18-25517.
- Jung SH, Kim KA, Sohn SW, Yang SJ. Cytokine levels of the aqueous humour in central serous chorioretinopathy. Clin Exp Optom. 2014;97(3):264–269. doi: 10.1111/cxo.12125.
- Lim JW, Kim MU, Shin MC. Aqueous humor and plasma levels of vascular endothelial growth factor and interleukin-8 in patients with central serous chorioretinopathy. Retina. 2010;30(9):1465–1471. doi: 10.1097/IAE.0b013e3181d8e7fe.
- Shin MC, Lim JW. Concentration of cytokines in the aqueous humor of patients with central serous chorioretinopathy. Retina. 2011;31(9):1937–1943. doi: 10.1097/IAE.0b013e31820a6a17.
- Lee H, Jang H, Choi YA, Kim HC, Chung H. Association between soluble CD14 in the aqueous humor and hyperreflective foci on optical coherence tomography in patients with diabetic macular edema. Invest Ophthalmol Vis Sci. 2018;59(2):715–721. doi: 10.1167/iovs.17-23042.
- Li M, Li J, Chen K, Wang J, Sheng M, Li B. Association between inflammatory factors in the aqueous humor and hyperreflective foci in patients with intractable macular edema treated with antivascular endothelial growth factor. Dis Markers. 2021; 2021:5552824. doi: 10.1155/2021/5552824.
- Fragiotta S, Abdolrahimzadeh S, Dolz-Marco R, Sakurada Y, Gal-Or O, Scuderi G. Significance of hyperreflective foci as an optical coherence tomography biomarker in retinal diseases: characterization and clinical implications. J Ophthalmol. 2021; 2021:6096017. doi: 10.1155/2021/6096017.
- Kitazawa K, Sotozono C, Koizumi N, Nagata K, Inatomi T, Sasaki H, Kinoshita S. Safety of anterior chamber paracentesis using a 30-gauge needle integrated with a specially designed disposable pipette. Br J Ophthalmol. 2017;101(5):548–550. doi: 10.1136/bjophthalmol-2016-309650.
- Shinojima A, Kawamura A, Mori R, Fujita K, Yuzawa M. Findings of optical coherence tomographic angiography at the choriocapillaris level in central serous chorioretinopathy. Ophthalmologica. 2016;236(2):108–113. doi: 10.1159/000448436.
- Jeong S, Kang W, Noh D, van Hemert J, Sagong M. Choroidal vascular alterations evaluated by ultra-widefield indocyanine green angiography in central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2022;260(6):1887–1898. doi: 10.1007/s00417-021-05461-0.
- Schellevis RL, van Dijk EHC, Breukink MB, Altay L, Bakker B, Koeleman BPC, Kiemeney LA, Swinkels DW, Keunen JEE, Fauser S, et al. Role of the complement system in chronic central serous chorioretinopathy: a genome-wide association study. JAMA Ophthalmol. 2018;136(10):1128–1136. doi: 10.1001/jamaophthalmol.2018.3190.
- Nourinia R, Ahmadieh H, Nekoei E, Malekifar P, Tofighi Z. Changes in central choroidal thickness after treatment of diabetic macular edema with intravitreal bevacizumab correlation with central macular thickness and best-corrected visual acuity. Retina. 2018;38(5):970–975. doi: 10.1097/IAE.0000000000001645.
- Mao J, Zhang C, Zhang S, Liu C, Chen N, Tao J, She X, Zheng Z, Lv Z, Shen L. Predictors of anti-VEGF efficacy in chronic central serous chorioretinopathy based on intraocular cytokine levels and pigment epithelium detachment subtypes. Acta Ophthalmol. 2022;100(7):e1385–e1394.
- Song IS, Shin YU, Lee BR. Time-periodic characteristics in the morphology of idiopathic central serous chorioretinopathy evaluated by volume scan using spectral-domain optical coherence tomography. Am J Ophthalmol. 2012;154(2):366–375.e4. doi: 10.1016/j.ajo.2012.02.031.
- Iglicki M, Busch C, Zur D, Okada M, Mariussi M, Chhablani JK, Cebeci Z, Fraser-Bell S, Chaikitmongkol V, Couturier A, et al. Dexamethasone implant for diabetic macular edema in naive compared with refractory eyes: the international retina group real-life 24-month multicenter study. The IRGREL-DEX study. Retina. 2019;39(1):44–51. doi: 10.1097/IAE.0000000000002196.
- Ding X, Hu Y, Yu H, Li Q. Changes of optical coherence tomography biomarkers in macular edema secondary to retinal vein occlusion after anti-VEGF and anti-inflammatory therapies. Drug Des Devel Ther. 2022; 16:717–725. doi: 10.2147/DDDT.S351683.
- Iglicki M, González DP, Loewenstein A, Zur D. Next-generation anti-VEGF agents for diabetic macular oedema. Eye. 2022;36(2):273–277. doi: 10.1038/s41433-021-01722-8.
- Joo JH, Kim H, Shin JH, Moon SW. Aqueous humor cytokine levels through microarray analysis and a sub-analysis based on optical coherence tomography in wet age-related macular degeneration patients. BMC Ophthalmol. 2021;21(1):399. doi: 10.1186/s12886-021-02152-6.
- Hodge DL, Reynolds D, Cerbán FM, Correa SG, Baez NS, Young HA, Rodriguez-Galan MC. MCP-1/CCR2 interactions direct migration of peripheral B and T lymphocytes to the thymus during acute infectious/inflammatory processes. Eur J Immunol. 2012;42(10):2644–2654. doi: 10.1002/eji.201242408.
