Abstract
Purpose
This study examines the incidence of infection and resistance associated with Intracorneal Ring Segment (ICRS) implantation, a common outpatient surgical treatment for correcting refractive errors and corneal ectatic diseases. Although ICRS procedures are typically safe and reversible, there is a low but notable risk of microbial infections, which require prompt and sometimes invasive treatment.
Methods
Three electronic databases, PubMed, Web of Science (WoS), and Scopus, were utilised to search for literature according to PRISMA guidelines to identify infections related to the implantation of ICRS in the cornea between January 2000 and December 2022.
Results
Gram-positive organisms were involved in 86% of cases: 35.7% S. aureus, 25% coagulase-negative staphylococci species, 17.8% streptococci and 7.1% Nocardia species. Less commonly recorded were Gram-negative bacteria (14%), with Pseudomonas aeruginosa (circa 10%) and Klebsiella pneumonia (4%) being the most common Gram-negative bacteria. In rare cases, fungi have also been reported. ICRS-related bacterial infections can be categorised into early or late onset. Early onset infection typically manifests within the first few weeks after implantation and is often associated with contamination during surgery, unhygienic practices, or inadequate sterilisation techniques. On the other hand, late-onset infection may develop months or even years after the initial procedures and may be associated with persistent bacterial colonisation, secondary infections, or prolonged use of prophylactic antibiotics. S aureus is encountered in both early and late-onset infections, while Nocardia species and K. pneumoniae have generally been reported to occur in late-onset infections. In addition, vision recovery from S. aureus infections tends to be poor compared to other bacterial infections.
Conclusion
S. aureus is a predominant pathogen that often requires surgical intervention with poor outcomes. Early infections result from incision gaps and ring segment rubbing, while late infections are linked to prolonged antibiotic use. Further research is needed on novel antimicrobial ICRS to procure the vision.
Introduction
Intrastromal corneal ring segments received FDA approval in 1999 as a pioneering refractive error correction technique.Citation1 These segments, made of polymethylmethacrylate and acrylic materials, serve as spacers within the corneal lamellae. By placing them in the periphery of the cornea, arch length is shortened, leading to flattening of the pericentral cornea without affecting the centre optic zone.Citation2 The implantation of ICRS is facilitated by mechanical or femtosecond laser incisions made at the cornea’s periphery.Citation3,Citation4 Over the years, ICRS has been successfully employed to correct myopia, astigmatism, and particularly corneal ectasia.Citation5
Ectatic corneal disease encompasses conditions characterised by corneal thinning and bulging, resulting in visual disturbances. These conditions include keratoconus, keratoglobus, pellucid marginal disease and ectasia post-laser in-situ keratomileusis.Citation6,Citation7 The prevalence of keratoconus varies among different populations and ethnicities, with estimates of the prevalence of keratoconus ranging from approximately 0.25% in the Netherlands, to 2% in India and over 4% in Iran.Citation6,Citation8,Citation9
While glasses or contact lenses can partially correct the corneal surface irregularities caused by keratoconus, they are limited by patient tolerability and overall quality of correction, particularly as the condition becomes more advanced.Citation10 In such cases, intrastromal ring segments offer a solution by reducing corneal surface irregularities and improving vision.Citation11 Combining ICRS with collagen cross-linking can further enhance outcomes by increasing the mechanical strength of the cornea.Citation12 Early application of ICRS in keratoconus can improve visual acuity and reduce the risk of the need for corneal transplantation.Citation13 Use of ICRS has been reported to reduce the need for corneal transplantation in 74.5% of eyes.Citation5 Additionally, when ICRS used for the correction myopia patient satisfaction rates have been reported as high as 94% one year after ICRS implantation, highlighting the utility of this means of correction for corneal ectatic diseases.Citation14
However, some studies have reported potential complications and limitations associated with ICRS. These include progressive reduction in visual acuity, refractive failure, extrusion and migration.Citation3,Citation15–18 While rare, infections after ICRS implantation are a serious sight-threatening condition.Citation19 In the event of infection following ICRS implantation, various treatment options are available. Antibiotic management is the mainstay, and may include the use of amniotic patch graft, lamellar keratoplasty or penetrating keratoplasty.Citation5,Citation20 The choice of treatment depends on the severity and extent of the infection, as well as individual patient factors. Prompt and appropriate management is crucial to minimize the potential risks to vision and overall ocular health.
ICRS-associated infections can present with various manifestations such as redness and lacrimation, foreign body sensation, infiltrates, ulcers, neovascularization, exudate, perforation, opacities, corneal melting, migration, and extrusion.Citation4,Citation11,Citation18,Citation21 The infectious agents involved in ICRS-related infections include bacteria, fungi, viruses, and parasites. Among these, bacteria are the main etiologic agent, accounting for 70–98% of these infections.Citation19,Citation22,Citation23
Limited access to health care systems and delay in seeking treatment after ICRS implantation can lead to reduced quality of life and increased economic burden due to direct and indirect costs. Delayed presentation to a physician can result in the development of adverse events including descemetocele, neovascularization, inflammation, corneal melting, or thinning.Citation4,Citation18 The aim of this review was to gather information on the overall infection rate of ICRS and the types of pathogens commonly associated with ICRS infection. By providing this information, researchers and clinicians can make more informed decisions to improve clinical outcomes in cases involving ICRS-associated infections.
Methods
The PRISMA guidelines were followed for this systematic review.Citation24
Search databases
Three electronic databases (PubMed, Web of Science, and Scopus) were infections associated after implantation of ICRS in the cornea. A Boolean search of “Intrastromal corneal ring segment” OR “intra corneal ring” AND “keratitis” OR “eye infection” OR “bacterial infection” OR “infect*” were selected as the search terms with results limited to those from January 2000 to December 2022 and published in peer-reviewed journals. Additionally, a snow-ball sampling approach was applied using the reference lists of the selected articles to expand the search. The search was limited to studies that were published in English language.
Inclusion criteria
Articles were included if they reported patients who had been implanted with intracorneal ring segment devices and were diagnosed as infected based on patient symptoms and microbiological confirmation following either conventional or molecular diagnosis. Retrospective or prospective cohort studies, as well as case-control studies, were included if they were published in peer-reviewed journals.
Exclusion criteria
Studies of intracorneal ring segments with complications that did not include cases where infections were presumed but not clinically or microbiologically confirmed. Case reports, systematic reviews, meta-analyses, and articles in languages other than English were also excluded.
Study selection
Articles found from three databases based on the title and abstract was screened and scrutinized. Full text relevant articles were inspected using the inclusion and exclusion criteria based on reported infection and associated pathogens.
Data extraction
Components recorded from the screened articles included author, year of publication, country, nature of study, gender and mean age of the patient, number of devices, depth of incision, use of prophylactic antibiotics, infection rate, associated pathogens, complications, vision recovery, and risk factors ().
Table 1. Characteristics of the studies included placement of ICRS.
Quality assessment in individual studies
The Newcastle-Ottawa Scale (NOS) tool was used to assess the quality of non-randomised studies in three main areas: selection study groups, comparability of groups and assessment of outcome/exposure. Each area was divided into several criteria, and the study was assigned points based on how well it met each criterion. The total number of points were then used to assess the overall quality of the study. A higher number of points indicated a higher quality study 8–10, modern 6–7, while a lower number suggested a lower below 5 quality study.
For observational studies, NOS assessment was performed to analyse potential risk of bias. Bias in research can arise from various sources, including study design, participate selection, and measurement methods. By using the NOS, a systematic evaluation of the risk of bias and methodological quality of the included observational studies was able to be assessed.
The NOS assessment specifically focused on evaluation of external validity (generalizability) and systematic error (bias) in the observational studies. This indicated how well the findings from these studies could be applied to the broader population (external validity) and whether any systematic errors might have affected the results.
Data analysis
Statistical analyses were performed using GraphPad Prism version 9.5.1 (733) software (GraphPad Software, La Jolla, CA, USA). Comparisons were made between retrospective and prospective studies regarding implant type and infection rates using two-way ANOVA with Tukey’s test for multiple comparisons. The study also analyzed correlations between the variables or risk factor variables, surgical incision depth, infection-related risk factors, and prophylactic antibiotic use, correlations were assessed using two-way ANOVA with Tukey’s test. The statistical significance threshold was set at p < 0.05.
Results
Study selection
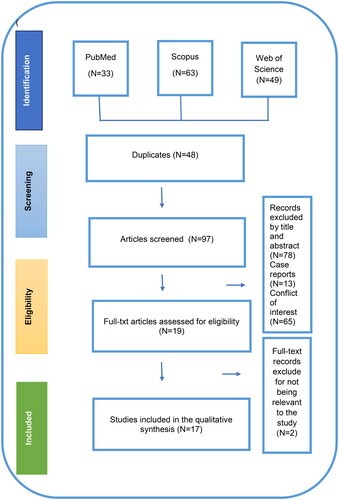
A total of 145 articles that were potentially relevant were identified through the PRISMA search strategy. After removing 48 duplicates, the remaining 97 articles were further investigated by title and abstract. At this stage, 78 articles were excluded due to being not relevant to the study objective. As shown in PRISMA flow diagram (), the final number of articles included for full text review was nineteen. Ultimately, 17 included in the systematic review, as two were excluded due to the outbreak-related to contact lens solution fungal infection or 157due to an associated complication (suture-induced infection). illustrates the detailed screening process.
Study characteristics
The 17 studies included were from the USA (n = 5), Spain (n = 4), Brazil (n = 3), Iran (n = 2), Europe (n = 1), Germany (n = 1) and Egypt (n = 1). Twelve studies were retrospective and five were prospective observational studies. One prospective observational study was reported each from Spain, Brazil, Iran, and two from the USA. Key information of each study included in the analysis are found in .
A total of 7296 devices were implanted across the 17 studies, with 50 reported infections, resulting in an overall infection rate of 0.7%. Among the retrospective studies, out of 6742 implants, there were 45 infections giving an apparent infection rate of 0.7%. In prospective studies involving 554 implants, there were 12 infections, yielding a higher calculated rate of infection of 2.16%. However, 2-way ANOVA statistical analysis indicated no significant difference between the infection rates from retrospective and prospective studies (p = 0.78).
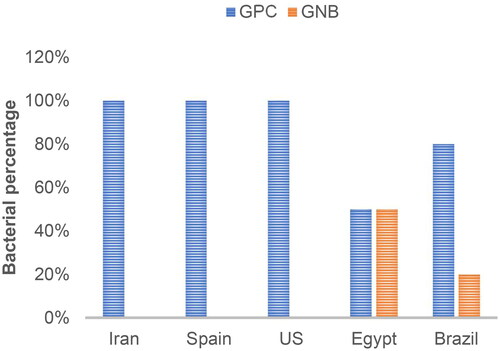
Gram-positive organisms were the primary pathogens, accounting for 85.7% of cases, with Staphylococcus aureus being the main causative agent at 35.7%, followed by coagulase-negative Staphylococcus spp. At 25%, Streptococcus spp. at 17.8%, and Nocardia spp. at 7.1%. Gram-negative organisms accounted for 14.3% of cases, with Pseudomonas spp. at 10.3% and Klebsiella spp. at 3.5%. Gram-positive organism isolation rates were 100% in Iran, Spain, and the US compared to other countries, as shown in .
Figure 2. Breakdown between Gram-positive cocci (GPC) and Gram-negative bacilli (GNB) pathogens identified in ICRS infections across the various countries.
The depth of incision used by surgeons for implant insertion varied. Assessing the infection rates based on the incision depth, an infection rate of 0.6% was observed for implants inserted at a corneal depth of 80%,Citation17,Citation18,Citation25,Citation26 compared to a 0.5% infection rate for ICRS associated with an incision depth of 70%,Citation2,Citation5,Citation21 this was not statically significant (p = 0.78).
Wound gap was the main risk factor for development of infection in ICRS, with an infection rate of approximately 3.9% for those with this feature.Citation2,Citation16,Citation17,Citation20,Citation27 The infection rate associated with other risk factors, such as trauma, diabetes and contact lens wear were lower at 1.1% combined.Citation15,Citation19 However, these infection rates between those associated with wound gaps and other risk factors were not depth of incision, significantly different (p = 0.79).
The choice of prophylactic antibiotic prior to surgery also appeared to influence the infection rate. Patients prescribed aminoglycosides had as infection rate of 1.7%,Citation5,Citation17,Citation19,Citation25 while those prescribed macrolides had a higher infection rate of 13.4%.Citation20 Infection rates for patients prescribed fluroquinolones and cephalosporins were 0.8%, and 0.2% respectively.Citation2,Citation15 However, probably due to the low number of articles, statistical analysis indicated no statistically significant variation between antibacterial agents (p = 0.76).
Progression and management strategies for corneal ectatic disorders
Keratoconus and corneal ectatic disorders are characterized by corneal thinning and visual impairment, with distinct clinical features.Citation7,Citation28 Keratoconus presents as a conical protrusion of the central cornea, while corneal ectatic disorders primarily affect the corneal periphery.Citation29 The onset of keratoconus is progressive in adolescence or early adulthood, whereas ectatic disorders may occur later and progress more rapidly.Citation30,Citation31 Irregularities in the corneal surface associated with ectatic diseases, such as pellucid marginal degeneration and post-LASIK ectasia, can create a favourable environment for microbial adhesion and colonization, potentially increasing the risk of infection.Citation32 A recent 61-year systematic review of LASIK complications reported maximum rates of epithelial defects and microbial keratitis at 14% and 0.034%, respectively.Citation33
The choice of treatment, particularly the use of intracorneal ring segments (ICRS), is influenced by the severity of the condition, with thinner segments preferred for mild cases and thicker segments for moderate to severe cases.Citation34 Complications of ICRS implantation, such as extrusion and refractive failure, vary based on the grade of keratoconus.Citation34 Studies have reported different rates of complications up to 38% but significant improvements in visual acuity UCVA, BSCVA following ICRS implantation, underscoring the need for individualized treatment approaches.Citation2,Citation17,Citation21 The integrity of the cornea plays a crucial role in susceptibility to infections, with The cross-linking therapy of keratoconus corneas maintaining a relatively intact epithelial barrier compared to other ectatic diseases, potentially reducing the risk of microbial infiltration and infection.Citation35
Risk of bias in selected studies
The NOS scores of the selected studies are presented in . Overall, the quality of the studies was moderate, with a retrospective nature and a lack of control groups in all studies contributing to this assessment. There was also inadequate comparability between intervention and control groups. Many studies did not specify the day of infection occurrence or the role of prophylactics and steroids, stating only the minimum and maximum periods after surgery. While most studies discussed gaps and extrusions, (14) only three studies highlighted the insertion packet size as a key factor. However, manual method of surgery and Femto second laser did not show a significant difference in infections. The impact of missing follow-up on the fate of the implant was not addressed in some studies (12). Improper information on implant maintenance instructions or inadequate patient knowledge was noted, with only three studies documenting rubbing as a key responsible factor for infection development. Shallow placement of biomaterial also caused infections in two studies, negatively affecting the quality score. The highest score was found in three studies, with a quality score of 8.Citation14,Citation17,Citation20 The lowest scores of 5 were attributed to four studies.Citation2,Citation3,Citation5,Citation18,Citation19 We chose to grade all studies equally regarding selection item 4, demonstrating that the outcome of interest was not present at the start of the study, due to the unlikely scenario of patients having infections before implantation or being reassessed if they presented with obvious clinical signs of infections such as blepharitis, iritis, limbal infection, and conjunctivitis. No information on identification or culture was taken for isolation of pathogens (6 studies). Four studies did not discuss vision status after keratitis. However, the limited number of articles and the lack of statistical significance in comparing risk factors and infections analyzed diminishes the certainty of the findings.
Table 2. Newcastle-Ottawa Scale for quality assessment of included observational studies.
Discussion
Infections that develop after an ocular implantation of ICRS can have devastating complications, with the potential to permanently threaten vision. This review found that ICRS infections were most frequently caused by Gram-positive bacteria (86%) compared to Gram negative bacteria (14%), with S. aureus being the most commonly identified causative agent. The study also found that a gap as left in the cornea after surgery results in a higher infection rate, as did the use of macrolides as prophylactic antibiotics.
S. aureus can be isolated from the conjunctiva and lids from ≥20% of eyes.Citation36 S. aureus can also commonly be isolated from the fingers and hands as well as other areas of skin.Citation37 In addition, coagulase negative staphylococci can also be isolated from eyes and hands.Citation36,Citation37 It is therefore possible that many of the causative bacteria identified in this study were introduced to the ICRS during surgery, either via the eye or hands. Staphylococci can adhere to surfaces such as contact lenses, which are made out of similar materials to ICRS, in relatively high numbers,Citation38 and so there is a possibility that inadvertent contamination can result in some cells being attached to the ICRS. The relatively low rate of infections caused by Gram-negative bacteria may be associated with their relatively low numbers on the ocular surface and hands,Citation39 making their potential for contamination less likely and so subsequent infection uncommon.
The study highlighted multiple risk factors associated with ICRS infection, including presence of a wound gap, extrusion and shallow placement of the implant, and corneal melting post-implantation. Larger wound gaps can extend the corneal healing time, with breakdowns in the epithelial cell layer and epithelial hyperplasia disrupting fluid dynamics, leading to inflammation and cell death.Citation32,Citation40 This environment favours the increased activity of collagenases and gelatinases, including matrix metalloproteinases, which degrade the extracellular matrix and facilitate bacterial colonization.Citation41,Citation42 Particularly, Staphylococcus aureus can adhere to and invade these damaged tissues more efficiently, aided by matrix metalloproteinases, enhancing its virulence and biofilm formation capabilities.Citation3
High-resolution imaging of ICRS has revealed biofilms on extruded implants, regardless of whether cultures were positive, indicating that extrusion can occur with thinner segments or when the stroma is thinned.Citation3,Citation16 Extrusion may be exacerbated by mechanical stress from rubbing or trauma..Citation43 The shallow placement may not achieve the intended flattening effect on the cornea, reducing ICRS efficacy for treating conditions such as keratoconus or corneal ectasia and lead infection.Citation44 Shallow placement may thin the cornea around the ICRS, weakening it and increasing the risk of infection.Citation45 Corneal melting is a rare but serious ICRS complication, facilitating extrusion or creating a nutritional environment for bacterial growth and subsequent infections requiring prompt intervention.Citation2,Citation16,Citation27
Moreover, the importance of providing wound closure after corneal implantation of ICRSs. The presence of a wound and extrusion of the ICRS has been shown to be major risk factors linked with Gram-positive bacterial ICRS infections.Citation5,Citation17,Citation20,Citation27 This might be similar to the association of trauma with keratitis that has been reported in several epidemiology studies,Citation46 and demonstrates the susceptibility of damaged and exposed corneal stroma to infections.
The study also underscored the importance of using the correct antibiotic for prophylaxis, with the use of macrolides being associated with increased rate of infection compared to other antibiotics. Whilst it is not usual to examine the susceptibilities of normal ocular or skin microbiota to antibiotics, knowing the usual types of microbes associated with ICRS infections and their usual susceptibility to antibiotics is helpful for planning antibiotic prophylaxis as part of these procedures. For example, the macrolide azithromycin used for four or eight days had minimal effect on the numbers of coagulase negative staphylococci isolated from conjunctival swabs than the use of the fluoroquinolone moxifloxacin on the same schedule.Citation47 Also, the ARMOR study from the USA has shown that a large proportion of staphylococci isolated from ocular infections are resistant to oxacillin and azithromycin. As these infections can also be caused by endogenous staphylococci, this correlates to the findings, at least for the macrolide azithromycin, in the current study.Citation48,Citation49 Repeated ocular surface antibiotic use, including prophylaxis, disrupts the eye’s microbiome balance, potentially causing imbalances, antibiotic resistance and ultimately promotes infections.Citation2,Citation17,Citation25,Citation50,Citation51. No statement on susceptibility data and what was initiation risk factors led to PK.
It is important to consider these results in the context of the specific studies mentioned and the populations they investigated. The differences in infection rates emphasize the potential impact of geographic factors such as local microbial ecology and resistance patterns, as well as the effectiveness of different antibiotics in combating infections.Citation20 Clinicians should carefully evaluate the local conditions, including the prevalence of different organisms and their antibiotic susceptibility profiles, when choosing appropriate antibiotic for treatment.Citation19 The findings emphasize the importance of informed decision-making by clinicians, considering both geographic variations and the potential efficacy of different antibiotics in addressing infections.Citation25
Several included studies considered cases of sterile inflammation separately from cases of infectious endophthalmitis, heterogeneously defined as sterile vitritis, non-infectious endophthalmitis, severe acute intraocular inflammation, sterile acute intraocular or ocular inflammation, sterile inflammation of the anterior chamber or uveitis. Due to the non-harmonized definitions used, a meaningful analysis could be performed regarding sterile inflammation.
There are also some limitations to this work. Many studies identified in the literature search primarily focussed on clinical observations, often neglecting critical microbiological aspects for confirmation, identification, and susceptibility. The analyses frequently overlooked organism-related antibiotic resistance and its clinical implications. Moreover, the influence of underlying systemic disease remains unclear. Additionally, there is a lack of consistent and detailed data across all studies, including information on use or choice of prophylactic antibiotics and post infection management. Furthermore, as most included studies were retrospective, various sources of bias could have influenced their results, potentially accounting for the observed heterogeneity in Ottawa scores.
In conclusion, the current study analysis found some potential associations between corneal wounds or prophylactic and ICRS-related infections. This may indicate that contact of the ring with the ocular surface is a significant risk factor. The inability to demonstrate statistical significance for all the findings is likely due to the limited number of papers that describe in detail microbiological analyses of these infections. Therefore, we would encourage future studies to describe these details to provide a more complete picture of the risk factors associated with ICRS-related infections. Despite the relatively low occurrence of ICRS-associated microbial infections and the rarity of bacterial resistance, ensuring optimal visual recovery remains crucial.
In the future, perhaps the development of antimicrobial ICRS could help reduce the incidence of infections, enhance visual outcomes, and mitigate microbial burden. Even then, regular hygiene practices and management are essential, as for all medical devices, including those with antimicrobial coatings, to prevent adverse events, given their foreign nature to the ocular system.
Acknowledgments
This work was partly supported by the NHMRC Ideas Grant (APP1183597) and UNSW Sydney in the form of a Scientia Scholarship provided to MS.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets analysed using core scientific databases. Derived data supporting the findings of this study are available from the corresponding author, Sara M., upon request.
References
- Schanzlin DJ, Asbell PA, Burris TE, Durrie DS. The intrastromal corneal ring segments: phase II results far the correction of myopia. Ophthalmology. 1997;104(7):1067–1078. doi:10.1016/s0161-6420(97)30183-3.
- Zare MA, Hashemi H, Salari MR. Intracorneal ring segment implantation for the management of keratoconus: safety and efficacy. J Cataract Refract Surg. 2007;33(11):1886–1891. doi:10.1016/j.jcrs.2007.06.055.
- Ferrer C, Alió JL, Montañés AU, Pérez-Santonja JJ, del Rio MA, de Toledo JA, Teus MA, Javaloy J. Causes of intrastromal corneal ring segment explantation: clinicopathologic correlation analysis. J Cataract Refract Surg. 2010;36(6):970–977. doi:10.1016/j.jcrs.2009.12.042.
- Carrasquillo KG, Rand J, Talamo JH. Intacs for keratoconus and post-LASIK ectasia: mechanical versus femtosecond laser-assisted channel creation. Cornea. 2007;26(8):956–962. doi:10.1097/ICO.0b013e31811dfa66.
- Kwitko S, Severo NS. Ferrara intracorneal ring segments for keratoconus. J Cataract Refract Surg. 2004;30(4):812–820. doi:10.1016/j.jcrs.2003.12.005.
- Claessens JLJ, Godefrooij DA, Vink G, Frank LE, Wisse RPL. Nationwide epidemiological approach to identify associations between keratoconus and immune-mediated diseases. Br J Ophthalmol. 2021;106(10):1350–1354. doi:10.1136/bjophthalmol-2021-318804.
- Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42(4):297–319. doi:10.1016/s0039-6257(97)00119-7.
- Shilpy N, Shah Z, Singh S, Purohit D. Prevalence of keratoconus in refractive surgery cases in western India. Middle East Afr J Ophthalmol. 2020;27(3):156–159. doi:10.4103/meajo.MEAJO_182_19.
- Hashemi H, Heydarian S, Yekta A, Ostadimoghaddam H, Aghamirsalim M, Derakhshan A, Khabazkhoob M. High prevalence and familial aggregation of keratoconus in an Iranian rural population: a population-based study. Ophthalmic Physiol Opt. 2018;38(4):447–455. doi:10.1111/opo.12448.
- Rabinowitz YS, Li X, Ignacio TS, Maguen E. INTACS inserts using the femtosecond laser compared to the mechanical spreader in the treatment of keratoconus. J Refract Surg. 2006;22(8):764–771. doi:10.3928/1081-597X-20061001-06.
- Alió JL, Artola A, Ruiz-Moreno JM, Hassanein A, Galal A, Awadalla MA. Changes in keratoconic corneas after intracorneal ring segment explantation and reimplantation. Ophthalmology. 2004;111(4):747–751. doi:10.1016/j.ophtha.2003.08.024.
- Sachdev GS, Sachdev R, Sachdev MS. Intra corneal ring segment implantation with lenticule assisted stromal augmentation for crosslinking in thin corneas. Am J Ophthalmol Case Rep. 2020;19:100726–100726. doi:10.1016/j.ajoc.2020.100726.
- Larco P, Larco P, Jr., Torres D, Piñero DP. Intracorneal ring segment implantation for the management of keratoconus in children. Vision (Basel). 2020;5(1):1. doi:10.3390/vision5010001.
- Ruckhofer J, Stoiber J, Alzner E, Grabner G. One year results of European Multicenter Study of intrastromal corneal ring segments. Part 2: complications, visual symptoms, and patient satisfaction. J Cataract Refract Surg. 2001;27(2):287–296. doi:10.1016/S0886-3350(00)00740-9.
- Mounir A, Radwan G, Farouk MM, Mostafa EM. Femtosecond-assisted intracorneal ring segment complications in keratoconus: from novelty to expertise. Clin Ophthalmol. 2018;12:957–964. doi:10.2147/OPTH.S166538.
- Kanellopoulos AJ, Pe LH, Perry HD, Donnenfeld ED. Modified intracorneal ring segment implantations (INTACS) for the management of moderate to advanced keratoconus: efficacy and complications. Cornea. 2006;25(1):29–33. doi:10.1097/01.ico.0000167883.63266.60.
- Shabayek MH, Alió JL. Intrastromal corneal ring segment implantation by femtosecond laser for keratoconus correction. Ophthalmology. 2007;114(9):1643–1652. doi:10.1016/j.ophtha.2006.11.033.
- Nguyen N, Gelles JD, Greenstein SA, Hersh PS. Incidence and associations of intracorneal ring segment explantation. J Cataract Refract Surg. 2019;45(2):153–158. doi:10.1016/j.jcrs.2018.09.021.
- Hofling-Lima AL, Branco BC, Romano AC, Campos MQS, Moreira H, Miranda D, Kwitko S, de Freitas D, Casanova FH, Sartori M, et al. and others. Corneal infections after implantation of intracorneal ring segments. Cornea. 2004;23(6):547–549. doi:10.1097/01.ico.0000126434.95325.24.
- Tabatabaei SA, Soleimani M, Mirghorbani M, Tafti ZF, Rahimi F. Microbial keratitis following intracorneal ring implantation. Clin Exp Optom. 2019;102(1):35–42. doi:10.1111/cxo.12810.
- D’Oria F, Abdelghany AA, Ledo N, Barraquer RI, Alio JL. Incidence and reasons for intrastromal corneal ring segment explantation. Am J Ophthalmol. 2021;222:351–358. doi:10.1016/j.ajo.2020.09.041.
- Spernovasilis N, Maraki S, Kokorakis E, Kofteridis D, Tsilimbaris M, Siganos C, Samonis G. Antimicrobial susceptibility of isolated pathogens from patients with contact lens-related bacterial keratitis in Crete, Greece: a ten-year analysis. Cont Lens Anterior Eye. 2021;44(4):101355. doi:10.1016/j.clae.2020.07.006.
- Teweldemedhin M, Gebreyesus H, Atsbaha AH, Asgedom SW, Saravanan M. Bacterial profile of ocular infections: a systematic review. BMC Ophthalmol. 2017;17(1):212. doi:10.1186/s12886-017-0612-2.
- Rethlefsen ML, Kirtley S, Waffenschmidt S, Ayala AP, Moher D, Page MJ, Koffel JB, PRISMA-S Group PRISMA-S: an extension to the PRISMA statement for reporting literature searches in systematic reviews. Syst Rev. 2021;10(1):39. doi:10.1186/s13643-020-01542-z.
- Mulet ME, Pérez-Santonja JJ, Ferrer C, Alió JL. Microbial keratitis after intrastromal corneal ring segment implantation. J Refract Surg. 2010;26(5):364–369. doi:10.3928/1081597X-20090617-06.
- Miranda D, Sartori M, Francesconi C, Allemann N, Ferrara P, Campos M. Ferrara intrastromal corneal ring segments for severe keratoconus. J Refract Surg. 2003;19(6):645–653. doi:10.3928/1081-597X-20031101-06.
- Kugler LJ, Hill S, Sztipanovits D, Boerman H, Swartz TS, Wang MX. Corneal melt of incisions overlying corneal ring segments: case series and literature review. Cornea. 2011;30(9):968–971. doi:10.1097/ICO.0b013e3182031ca0.
- Tomidokoro A, Oshika T, Amano S, Higaki S, Maeda N, Miyata K. Changes in anterior and posterior corneal curvatures in keratoconus. Ophthalmology. 2000;107(7):1328–1332. doi:10.1016/s0161-6420(00)00159-7.
- Kennedy RH, Bourne WM, Dyer JA. A 48-year clinical and epidemiologic study of keratoconus. Am J Ophthalmol. 1986;101(3):267–273. doi:10.1016/0002-9394(86)90817-2.
- Vega-Estrada A, Alió JL, Plaza-Puche AB. Keratoconus progression after intrastromal corneal ring segment implantation in young patients: five-year follow-up. J Cataract Refract Surg. 2015;41(6):1145–1152. doi:10.1016/j.jcrs.2014.08.045.
- Alió JL, Salem TF, Artola A, Osman AA. Intracorneal rings to correct corneal ectasia after laser in situ keratomileusis. J Cataract Refract Surg. 2002;28(9):1568–1574. doi:10.1016/s0886-3350(01)01275-5.
- Dawson DG, Edelhauser HF, Grossniklaus HE. Long-term histopathologic findings in human corneal wounds after refractive surgical procedures. Am J Ophthalmol. 2005;139(1):168–178. doi:10.1016/j.ajo.2004.08.078.
- Sahay P, Bafna RK, Reddy JC, Vajpayee RB, Sharma N. Complications of laser-assisted in situ keratomileusis. Indian J Ophthalmol. 2021;69(7):1658–1669.
- Sakellaris D, Balidis M, Gorou O, Szentmary N, Alexoudis A, Grieshaber MC, Sagri D, Scholl H, Gatzioufas Z. Intracorneal ring segment implantation in the management of keratoconus: an evidence-based approach. Ophthalmol Ther. 2019;8(Suppl 1):5–14. doi:10.1007/s40123-019-00211-2.
- Volatier TL, Figueiredo FC, Connon CJ. Keratoconus at a molecular level: a review. Anat Rec (Hoboken)). 2020;303(6):1680–1688. doi:10.1002/ar.24090.
- Willcox MD, Power KN, Stapleton F, Leitch C, Harmis N, Sweeney DF. Potential sources of bacteria that are isolated from contact lenses during wear. Optom Vis Sci. 1997;74(12):1030–1038. doi:10.1097/00006324-199712000-00025.
- Kalaiselvan P, Yasir M, Vijay AK, Willcox MD, Tummanapalli S. Longevity of hand sanitisers on fingers. Clin Exp Optom. 2023;106(4):436–442. doi:10.1080/08164622.2022.2040334.
- Vijay AK, Zhu H, Ozkan J, Wu D, Masoudi S, Bandara R, Borazjani RN, Willcox MD. Bacterial adhesion to unworn and worn silicone hydrogel lenses. Optom Vis Sci. 2012;89(8):1095–1106. doi:10.1097/OPX.0b013e318264f4dc.
- Willcox MD. Characterization of the normal microbiota of the ocular surface. Exp Eye Res. 2013;117:99–105. doi:10.1016/j.exer.2013.06.003.
- Samimi S, Leger F, Touboul D, Colin J. Histopathological findings after intracorneal ring segment implantation in keratoconic human corneas. J Cataract Refract Surg. 2007;33(2):247–253. doi:10.1016/j.jcrs.2006.08.059.
- McCaig LF, McDonald LC, Mandal S, Jernigan DB. Staphylococcus aureus–associated skin and soft tissue infections in ambulatory care. Emerg Infect Dis. 2006;12(11):1715–1723. doi:10.3201/eid1211.060190.
- Fabre E, Bureau J, Pouliquen Y, Lorans G. Binding sites for human interleukin 1 α, gamma interferon and tumor necrosis factor on cultured fibroblasts of normal cornea and keratoconus. Curr Eye Res. 1991;10(7):585–592. doi:10.3109/02713689109013850.
- Falgayrettes N, Patoor E, Cleymand F, Zevering Y, Perone J-M. Biomechanics of keratoconus: two numerical studies. PLoS One. 2023;18(2):e0278455. doi:10.1371/journal.pone.0278455.
- Coskunseven E, Kymionis GD, Tsiklis NS, Atun S, Arslan E, Siganos CS, Jankov M, Pallikaris IG. Complications of intrastromal corneal ring segment implantation using a femtosecond laser for channel creation: a survey of 850 eyes with keratoconus. Acta Ophthalmol. 2011;89(1):54–57. doi:10.1111/j.1755-3768.2009.01605.x.
- Schanzlin DJ, Abbott RL, Asbell PA, Assil KK, Burris TE, Durrie DS, Fouraker BD, Lindstrom RL, McDonald JE, Verity SM, et al. and others. Two-year outcomes of intrastromal corneal ring segments for the correction of myopia. Ophthalmology. 2001;108(9):1688–1694. doi:10.1016/s0161-6420(01)00692-3.
- Stapleton F. The epidemiology of infectious keratitis. Ocular Surf. 2023;28:351–363.
- Teberik K, Eski MT, Çalışkan E, Kılınçel Ö, Kaya M, Ankaralı H. Effects of topical azithromycin, moxifloxacin, and povidone iodine on conjunctival bacterial flora in patients undergoing intravitreal injection. Arquivos Brasileiros de Oftalmolog. 2019;82:25–31.
- Asbell PA, Sanfilippo CM, Pillar CM, DeCory HH, Sahm DF, Morris TW. Antibiotic resistance among ocular pathogens in the United States: five-year results from the antibiotic resistance monitoring in ocular microorganisms (ARMOR) surveillance study. JAMA Ophthalmol. 2015;133(12):1445–1454. doi:10.1001/jamaophthalmol.2015.3888.
- Asbell PA, Sanfilippo CM, Sahm DF, DeCory HH. Trends in antibiotic resistance among ocular microorganisms in the United States from 2009 to 2018. JAMA Ophthalmol. 2020;138(5):439–450. doi:10.1001/jamaophthalmol.2020.0155.
- Willcox MDP. Antibiotics and Microbial Keratitis: do We Need to Test for Resistance? Eye Contact Lens. 2020;46(1):1–2. doi:10.1097/ICL.0000000000000682.
- Robertson SM, Curtis MA, Schlech BA, Rusinko A, Owen GR, Dembinska O, Liao J, Dahlin DC. Ocular pharmacokinetics of moxifloxacin after topical treatment of animals and humans. Surv Ophthalmol. 2005;50(Suppl 1): S32–S45. doi:10.1016/j.survophthal.2005.07.001.