Abstract
Purpose
This study assesses the diluted Schirmer method’s effectiveness in collecting tears from dry eye syndrome patients, aiming to identify the most suitable tear collection technique for them.
Methods
A prospective study. Tear samples were collected from patients with dry eye syndrome and healthy individuals using two methods: (1) Direct Schirmer Method: Schirmer strips were directly inserted into the eye to collect tears. (2) Diluted Schirmer Method: After instilling physiological saline into the eye and waiting for 30 s to ensure thorough mixing with tears, Schirmer strips were used for collection. Tear samples from both groups were analyzed and compared for total protein and cytokine levels (IL-1β, IL-6, IL-8, TNF-α).
Results
(1) The study included 32 participants: 16 with dry eye syndrome (4 males, 12 females, average age 34.92 ± 10.13 years) and 16 healthy controls (5 males, 11 females, average age 32.25 ± 9.87 years). (2) The diluted Schirmer method produced a significantly larger tear volume compared to the direct method (p < 0.05), with lower Visual Analogue Scale (VAS) scores indicating less discomfort (p < 0.05). (3) The average total protein content of the two groups was 51.70 ± 3.166 ng measured by Direct Schirmer method, and the average total protein content of the Diluted Schirmer method was 50.05 ± 3.263 ng. There was no statistical difference between the two groups. (t = 1.051, p = 0.3098) (4) The concentrations of total tear protein and various cytokines measured by both methods were higher in the dry eye group compared to the normal group, with statistically significant differences (p < 0.05). Both methods reflected consistent changes in tear protein profiles.
Conclusion
The diluted Schirmer method can comfortably collect an adequate volume of tear samples in a short time and consistently reflect changes in tear proteins, making it an effective method for tear collection in patients with dry eye syndrome.
Introduction
Tears are an exocrine fluid produced by the lacrimal glands, forming a tear film in conjunction with mucins and lipids. This film performs several functions: it acts as a barrier, provides lubrication, and has antibacterial and immunoregulatory activities.Citation1 Proteins in tears are essential for the stability of the ocular surface environment, offering anti-inflammatory properties, immune regulation, and aiding in ocular surface repair.Citation2–4 Alterations in tear proteins can indicate the state of the ocular surface microenvironment and correlate with various pathologies, such as dry eye syndrome, diabetes, and multiple sclerosis.Citation5 Therefore, tear analysis and protein profiling, emerging fields of study, present significant application potential. The method of tear collection is a critical step that determines whether comprehensive and in-depth tear analysis can be conducted.
Currently, the primary methods for collecting tears are the capillary tube method and the Schirmer method. While both methods have pros and cons, their use in studying dry eye disease poses considerable challenges: (1) Dry eye syndrome patients produce a lower volume of tears. The limited tear volume collected via capillary tubes or Schirmer method makes it challenging to conduct multi-parameter testing on a single sample; (2) Placing a capillary tube or Schirmer paper directly on the dry ocular surface increases patient discomfort and stimulates reflex tear secretion, compromising test result accuracy.
In response to the challenge of insufficient tear collection volume from patients with dry eye syndrome, some researchers have explored the diluted Schirmer method,Citation6 pre-diluting with saline solution before collection with Schirmer paper. However, it’s unclear whether this method affects test outcomes or could replace the traditional Schirmer’s method, as studies or reports are lacking.
Consequently, our team aims to validate the effectiveness of the diluted Schirmer method for tear collection in dry eye patients. By collecting tears from the same patient using both the diluted Schirmer method and the direct Schirmer method, followed by sensitive protein quantification and flow cytometry analysis, we will compare changes in total tear proteins and cytokines to evaluate the performance of the diluted Schirmer method in collecting tears from patients with dry eye syndrome.
Materials and methods
Population
This study was conducted in compliance with the principles of the Declaration of Helsinki, and approval was obtained from The Medical Ethics Committee of the Second Affiliated Hospital of Zhejiang Chinese Medical University (Approval No.: 2023-086-01). Informed consent was obtained from all study subjects prior to conducting procedures.
Patients who visited the ophthalmology department of the Second Affiliated Hospital of Zhejiang Chinese Medical University between June and December 2023 were selected. A total of 32 patients were included in this study, comprising 16 dry eye patients (4 males, 12 females) with an average age of 34.92 ± 10.13 years, and 16 normal individuals (5 males, 11 females) with an average age of 32.25 ± 9.87 years.
Inclusion Criteria: (1) Dry Eye Group: Diagnosed based on the “Chinese Consensus on Dry Eye: Definition and Classification (2020)”Citation7 with an Ocular Surface Disease Index (OSDI) ≥13 points, Noninvasive Tear Break-Up Time (NIBUT) ≤10 s, Schirmer I test (SIt) ≤10mm/5 min, or the presence of dry eye-related symptoms, OSDI ≥ 13 points; NIBUT10-12s, SIt > 5mm/5 min and < 10mm/5 min, positive corneal fluorescein staining (FL ≥ 5spots). (2) Control Group: No history of dry eye or ocular diseases, no history of eye surgery, no history of wearing corneal contact lenses, no history of eye allergies. OSDI < 13, NIBUT > 10s, and SIt > 10mm/5 min.
Exclusion Criteri:Citation8 (1) Any ocular or systemic condition that could affect the observations of this trial; (2) Individuals who have undergone ocular surgery within the last 3 months; (3) Recent contact lens wear within the past month; (4) Use of antibiotics, immunosuppressants, or the application of antibiotic, steroid, or artificial tear eye drops within the last 2 weeks; (5) Severe cardiac, liver, or renal dysfunction; (6) Pregnancy or lactation; (7) Patients considered unsuitable for the study by the researchers, including those unable or unwilling to comply with the protocol requirements.
Experimental methods
Eligible participants underwent various examinations, including medical history review, OSDI questionnaire completion, ocular assessments via slit-lamp biomicroscopy and fundoscopy, and dry eye tests such as tear meniscus height measurement, tear film break-up time analysis, corneal fluorescein staining, meibomian gland evaluation through infrared imaging, Schirmer I test, and tear collection. Examinations were scheduled 10 min apart, ensuring a 60-minute gap between tear collection and the Schirmer I test to maintain procedural integrity.Citation8–9 An ophthalmic technician conducted all the eye examinations, while an ophthalmologist was responsible for tear collection. To minimize the effects of circadian rhythms and environmental factors, the examinations were scheduled between 8:00 AM and 10:00 AM in the same examination room with controlled temperature and humidity.
Ocular Surface Disease Index (OSDI): Assesses subjective symptoms, including photophobia, foreign body sensation, eye pain, and fluctuation of vision among 12 indicators over the past week, with each item scored 0–4. The OSDI score = total of all item scores × 25/number of questions, with a total score ranging from 0 to 100.
Tear Meniscus Height (TMH): Utilizes the corneal topographer (OCULUS Keratograph 5M, Oculus, Germany) with an integrated measurement tool to measure the tear meniscus height directly below the center of the pupil three times.
Noninvasive Tear Break-Up Time (NIBUT):Citation10–11 Utilizes the corneal topographer to project 22 concentric circles of infrared light onto the patient’s corneal surface, instructing the patient to blink twice and then keep the eyes open as long as possible. The instrument automatically records the first break-up time NIBUT(f) and the average break-up time NIBUT (av), and displays the location of the tear film break-up.
Fluorescein Staining Score (FL) (using a 12-point system): The cornea is divided into four quadrants, with each quadrant scored from 0 to 3. The scoring criteria are: 0 points: No staining; 1 point: 1-30 punctate stains (mild); 2 points: >30 punctate stains, no merging (moderate); 3 points: Merging punctate stains, filaments, and ulcers (severe).
Meibomian Gland Loss (MGL) Score:Citation12 Evaluated using meibomian gland infrared photography and Meibo-Scan enhancement processing system. Score 0: No loss of meibomian gland. Score 1: Loss of meibomian gland area is less than one-third (<1/3). Score 2: Loss of meibomian gland area is one-third (≥1/3) to two-thirds (≤2/3). Score 3: Loss of meibomian gland area is greater than two-thirds (>2/3).
Schirmer I Test (SIt): A Schirmer tear test strip (Tianjin Jingming New Technological Development Co., Ltd.) is positioned at the outer third junction of the lower conjunctival sac without topical anesthesia. Patients close their eyes for 5 minutes before the wetted length of the strip is measured. |
Tear Collection: Tears were collected from both groups of patients 60 minutes after completing the aforementioned examinations.
Day 1 (direct Schirmer Method): participants were asked to look upward, and a Schirmer strip (Tianjin Jingming New Technology Development Co., Ltd.) was carefully placed on the outer 1/3 of the lower eyelid of the right eye, avoiding contact with the eyeball. The strip was removed after 5 minutes with the eyes closed, placed carefully into an EP tube, labeled, and stored at −80 °C.
Day 2 (diluted Schirmer Method): the diluted Schirmer method was performed at approximately the same time as the previous day for each participant: 20µL of preservative-free saline was instilled into the lower fornix of the right eye using a micropipette (Eppendorf, Germany). The participants were instructed to close their eyes for 30 seconds immediately after. Then, a Schirmer strip was placed on the outer 1/3 of the lower eyelid of the right eye, avoiding contact with the eyeball. The strip was removed after 5 minutes with the eyes closed, labeled, and stored at −80 °C.
Visual Analogue Scale (VAS) for Pain Assessment:Citation13 Post-tear collection, patients evaluated their pain using a 10 cm VAS, ranging from "No pain (0)" to "Worst possible pain (10)." Marks on the scale reflected pain intensity and discomfort, with the total score indicating the level of pain experienced.
Protein quantification (BCA assay)
The BCA assay involves the reaction of the BCA reagent with proteins, catalyzed by copper ions, to produce a purple complex. The absorbance at 562 nm reflects the protein concentration. From each group, 8 patients (16 samples total) were chosen for analysis. The experimental procedure involves placing the entire tear filter paper strip into a protein lysis buffer. After centrifugation and protein elution, the supernatant is collected. Protein lysis buffer is then added to dilute the sample to 22 µL, followed by vortexing to resuspend the protein. This results in a diluted protein sample. The total protein concentration in the sample is then measured using the Micro BCA™ Protein Assay Kit. Finally, the protein concentration is multiplied by the sample volume to obtain the total tear protein amount.
Cytokine profiling (CBA analysis)
This is a technique for the quantitative detection of various soluble intracellular proteins. It uses a flow cytometer to measure microspheres encoded with different fluorophores, enabling the simultaneous detection of multiple cytokines. It offers the advantages of multi-parameter detection from a single sample, requiring a small sample volume, high sensitivity, and good stability. These features make the CBA technique particularly suitable for biomarker detection in small-volume samples such as tears. The remaining 8 patients (16 tear samples) were selected for the detection of IL-1β, IL-6, IL-8, and TNF-α. To ensure the same tear volume, the tear filter papers were cut to the same length, and then the procedures were performed according to the reagent instructions. A 25 µL aliquot of the centrifuged sample supernatant was added to the prepared Beads working solution (22 µL beads diluent + 0.5 µL of each type of beads), mixed thoroughly, and incubated in the dark for 1 h. Then, 25 µL of the prepared PE working solution (22 µL reagent diluent + 0.5 µL of each type of PE) was added to the sample tube, mixed thoroughly, and incubated in the dark for 2 h to form the "beads antibody-antigen in sample-PE antibody" complex. After washing with Wash Buffer, the samples were analyzed using a flow cytometer (FACS CantoTM II, BD Bioscience, San Jose, CA, USA). The accompanying software, FCAP Array v3 (BD Bioscience, San Jose, CA, USA), was used to generate standard curves and calculate the concentrations of each parameter.
Statistical analysis
Statistical analysis was performed using SPSS 25.0 software. The Shapiro-Wilk test was first used to assess normality. When the measurement data followed a normal distribution, the mean ± standard deviation (Mean ± SD) was used. For data with homogeneous variances, the independent sample T-test and paired sample T-test were used; for data with heterogeneous variances, Welch’s t-test was used. When the data did not follow a normal distribution, the median (P25, P75) was used, and non-parametric tests (Wilcoxon rank-sum test) were applied. A P value of less than 0.05 was considered statistically significant.
Results
Tear volume
There is a quantitative relationship between the wetted length of the filter paper strip and the tear volume.Citation14 Therefore, for simplicity, this study uses the length of the tear filter paper to represent the tear sample volume. In the tear samples included in the study, the diluted Schirmer method showed a maximum length of 30 mm, a minimum length of 16 mm, and an average length of 21.5 ± 3.87 mm. The direct Schirmer method showed a maximum length of 22 mm, a minimum length of 5 mm, and an average length of 11.75 ± 5.91 mm. Comparing the two methods, the diluted Schirmer method collected significantly more tears (paired T-test t = 4.821, p < 0.001). Two cases of dry eye patients using the direct Schirmer method resulted in strip lengths of less than 5 mm, making detection impossible, so they were excluded.
Total tear protein content
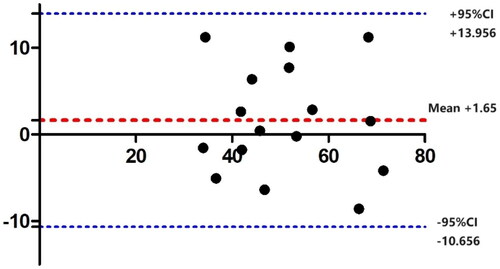
The average total protein content using the direct Schirmer method was 51.70 ± 3.166 ng, and using the diluted Schirmer method was 50.05 ± 3.263 ng. There was no statistically significant difference between the two groups (paired T-test t = 1.051, p = 0.3098). The Bland-Altman plot showed that all data were within the 95% confidence interval, indicating that the two methods had good consistency. In the direct Schirmer method, the dry eye group had an average total protein content of 62.34 ± 2.701 ng, while the normal group had 41.06 ± 1.816 ng. The dry eye group was significantly higher than the normal group (Welch’s t-test t = 6.540, p < 0.0001). In the diluted Schirmer method, the dry eye group had an average total protein content of 59.79 ± 3.656 ng, while the normal group had 40.32 ± 2.281 ng. Again, the dry eye group was significantly higher than the normal group (Welch’s t-test t = 4.519, p = 0.0009) ().
Cytokine levels in tears
The standard curve of each cytokine can be seen in .
Figure 2. Bland–Altman plot for protein content of the two collection methods: the x-axis represents the mean protein content of the two methods, and the y-axis represents the density difference between the two methods. The mean difference between the two methods is 1.65 ng, with an upper limit of 13.956 ng and a lower limit of -10.656 ng. All data fall within the 95% confidence interval.
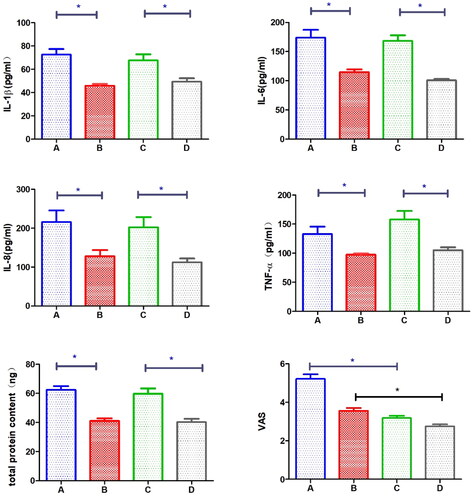
IL-1β: Using the direct Schirmer method, the IL-1β concentration in the dry eye group was 72.67 ± 13.43μg/ml, while in the normal group it was 45.82 ± 4.74μg/ml (independent samples T-test t = 5.228, p = 0.0012). Using the diluted Schirmer method, the IL-1β concentration in the dry eye group was 67.66 ± 14.78μg/ml, while in the normal group it was 49.29 ± 8.57μg/ml (independent samples T-test t = 2.838, p = 0.0251). Both methods showed that the IL-1β concentration was significantly higher in the dry eye group than in the normal group, with a statistically significant difference.
IL-6: Using the direct Schirmer method, the IL-6 concentration in the dry eye group was 173.7 ± 38.69μg/ml, while in the normal group it was 114.8 ± 13.58μg/ml (independent samples T-test t = 4.237, p = 0.0039). Using the diluted Schirmer method, the IL-6 concentration in the dry eye group was 168.2 ± 27.69μg/ml, while in the normal group it was 100.7 ± 6.558μg/ml (independent samples T-test t = 7.677, p = 0.0001). Both methods showed that the IL-6 concentration was significantly higher in the dry eye group than in the normal group, with a statistically significant difference.
IL-8: Using the direct Schirmer method, the IL-8 concentration in the dry eye group was 215.5 ± 84.59μg/ml, while in the normal group it was 127.9 ± 44.35μg/ml (independent samples T-test t = 2.987, p = 0.0203). Using the diluted Schirmer method, the IL-8 concentration in the dry eye group was 203.3 ± 73.65μg/ml, while in the normal group it was 112.3 ± 27.61μg/ml (independent samples T-test t = 4.354, p = 0.0033). Both methods showed that the IL-8 concentration was significantly higher in the dry eye group than in the normal group, with a statistically significant difference.
TNF-α: Using the direct Schirmer method, the TNF-α concentration in the dry eye group ranged from 104.8 to 141.5μg/ml, while in the normal group it ranged from 91.41 to 102.0μg/ml (Wilcoxon rank-sum test Z = 2.987, p = 0.0156). Using the diluted Schirmer method, the TNF-α concentration in the dry eye group ranged from 125.3 to 198.7μg/ml, while in the normal group it ranged from 93.96 to 116.7μg/ml (Wilcoxon rank-sum test Z = 2.987, p = 0.0156). Both methods showed that the TNF-α concentration was significantly higher in the dry eye group than in the normal group, with a statistically significant difference ().
Comfort of tear collection
The Visual Analog Scale (VAS) for pain showed an average score of 4.34 ± 0.96 for the direct Schirmer method and 3.03 ± 1.12 for the diluted Schirmer method. Paired T-test indicated a statistically significant difference (paired T-test t = 6.672, p < 0.001). In the dry eye group, the VAS score was 5.22 ± 0.24 for the direct Schirmer method and 3.19 ± 0.12 for the diluted Schirmer method (paired T-test t = 6.911, p < 0.0001). In the normal group, the VAS score was 3.56 ± 0.14 for the direct Schirmer method and 2.75 ± 0.12 for the diluted Schirmer method (paired T-test t = 3.569, p = 0.0028). Both comparisons showed that the VAS scores for the diluted Schirmer method were lower than those for the direct Schirmer method, with the difference being more significant in the dry eye group ().
Discussion
Tears are an essential component of the tear film and are crucial for the structural integrity of the ocular surface. Normal tear fluid comprises water, proteins, sugars, inorganic salts, and lipids, along with a complex array of substances including lysozyme, various immunoglobulins, complement, and lactoferrin, among others. Notably, tear proteins play a key role in maintaining the stability of the ocular surface environment. To date, 1543 proteins have been identified in tear fluid, which are involved in anti-inflammatory, antiviral, and immune responses in the eye, aiding in the repair of ocular surface tissue damage and the establishment of tear film surface tension.Citation15–16 Studies by Solomon A et al. have shown that the activity of specific proteins such as IL-1β, TNF-α, and MMP-9 is significantly elevated in patients with dry eye, making them biomarkers for the condition.Citation17–18 Additionally, biomarkers for various systemic diseases, such as cancer, multiple sclerosis, diabetes, Parkinson’s disease, and Alzheimer’s disease, can also be detected through tear analysis.Citation19–20 Therefore, tear analysis holds significant value in the diagnosis, prognosis, and treatment guidance of diseases.
Despite the significant potential of tear fluid analysis, it is not yet a standard clinical diagnostic test. The collection of tear fluid remains one of the biggest challenges for researchers.Citation19–20 First, due to the limited volume of human tears, especially in patients with dry eye syndrome who suffer from reduced tear production, obtaining an adequate sample via capillary tubes or the direct Schirmer method proves difficult. In extreme cases, such as with patients suffering from Sjögren’s syndrome dry eye, traditional methods may not collect any sample at all. Additionally, while tear collection should be non-invasive and straightforward, the direct use of Schirmer strips or capillary tubes may distress patients, triggering reflex tears and altering tear composition, thus compromising test accuracy. Therefore, efficient tear collection is crucial for accurate tear analysis and research.
This study introduces and evaluates an enhanced tear collection method (diluted Schirmer method), assessing its efficiency in sample volume, patient comfort, and protein detection. To our knowledge, this topic has not been previously reported in the literature.
In this study, the length of the tear filter paper was used to represent the collected tear volume, as the two can be interconverted. According to literature, the average specific wetted volume can be obtained by measuring the weight of the tear absorbed by the filter paper, converting it to microliters, and then dividing by the wetted length. For Schirmer test values (5 min), the average specific wetted volume is 0.578 µL/mm. Therefore, comparing the length of the filter paper to represent the tear volume is a simple and feasible method. Results from this experiment show that, within the same time frame, the diluted Schirmer method collected significantly more tears than the direct Schirmer method. Additionally, in the direct Schirmer method, two samples were excluded due to insufficient volume for detection. This indicates that the diluted Schirmer method can collect a larger tear volume in a shorter time, making it especially suitable for patients with conditions like dry eye, where tear volume is low, and reducing the risk of detection failure due to insufficient sample volume.
This study also objectively scored the comfort level of the tear collection methods. The results showed that the VAS score for the diluted Schirmer method was significantly lower than that of the direct Schirmer method, particularly in the dry eye group, with a statistically significant difference (p < 0.001). Our team believes that placing Schirmer filter paper directly on a dry ocular surface causes significant foreign body sensation or even pain, negatively affecting the patient’s experience and more likely triggering reflex tear secretion. In contrast, the diluted Schirmer method significantly reduces this discomfort, achieving better patient cooperation and effectively avoiding the impact of reflex tear secretion.
This study used high-sensitivity protein quantification techniques to measure the total protein content in tears collected by two methods. The results showed that both methods effectively detected the total protein content in tears, with a paired T-test showing no significant difference between the two (p > 0.05). This indicates that the diluted Schirmer method has no significant impact on the detection of total tear protein content. We believe the possible reason is that although the protein concentration in the eye is reduced after dilution, a larger volume of tear fluid is removed, similar to flushing out the accumulated tear fluid in the conjunctival sac with physiological saline, resulting in no significant reduction in the total protein content on the filter paper. However, a more accurate explanation requires further experimental validation, which is also the future research direction of our team.
Additionally, comparing the test results between the dry eye group and the normal group, the total protein content measured by both methods was significantly higher in the dry eye group (p < 0.05), indicating that the dilution Schirmer method can effectively reflect the increase in tear protein content in patients with dry eye. This is consistent with previous reports. Jung et al.Citation21 suggested that dry eye patients have over 1700 types of tear proteins, more than 100 types higher than healthy individuals, and considered that the most important pathogenesis of dry eye is the immune-inflammatory response caused by abnormal proteins in tears. Alur et al.Citation22 found that in tear-deficient dry eye, the number of proteins related to inflammatory responses, cell death, and injury responses significantly increased.
IL-1β, IL-6, IL-8, and TNF-α, cytokines closely linked to dry eye syndrome, were analyzed using flow cytometric bead array technology in this study. Concentrations of these cytokines were significantly elevated in the dry eye group compared to controls (p < 0.05). Previous research has established cytokines as key players in the development and progression of dry eye syndrome. IL-1β has various functions, including the induction of cyclooxygenase-2(COX-2),phospholipaseA2, prostaglandins, and platelet-activating factors,Citation23 and can increase the levels of IL-6 and cell adhesion molecules on mesenchymal and endothelial cells; IL-6, as an inducer, along with IL-1 and TNF-α, promotes inflammatory responses, and the level of tear IL-6 in patients with dry eye syndrome negatively correlates with ocular surface parameters such as symptom scores, TBUT, and Schirmer I test;Citation24 IL-8 regulates endogenous immunity and promotes angiogenesis, thereby inducing the migration of neutrophils and T lymphocytes. High levels of IL-8 may be a strong signal causing the typical symptoms of dry eye syndrome;Citation25 TNF-α, mainly secreted by macrophages, plays a key role in inflammatory responses and cell-mediated immunity, and its high expression can induce a series of ocular surface inflammatory responses, thus promoting the development of dry eye syndrome.Citation26 Unlike prior studies using mixed samples, this study’s diluted Schirmer method enabled simultaneous testing of multiple cytokines in a single tear sample by increasing sample volume.
This study validated a tear collection method suitable for dry eye patients. It not only addresses the difficulty of sampling due to low tear volume and reduces patient discomfort, but also shows consistent tear protein changes in dry eye populations, providing an effective diagnostic basis for the disease’s occurrence and progression. Therefore, the diluted filter paper method is particularly suitable for dry eye patients requiring tear collection and analysis.
This study also has potential sources of error. For instance, during the direct filter paper method, dry eye patients may experience significant discomfort, leading to reflex tear secretion. Additionally, in the diluted filter paper method, instilling saline into the patient’s eye may also stimulate additional tear production. However, these additional tears can vary due to many factors, including the intensity of the stimulus, the condition of the eye, and individual physiological responses. As there is no standard measurement method available in the current literature, our team has implemented several measures to reduce reflex tear production, such as diluting with saline (with a pH similar to tears and free of microorganisms), instilling into the lower conjunctival sac to avoid contact with the cornea, and having patients close their eyes and quickly complete the collection after dilution. Therefore, considering that reflex tears may occur in all subjects, are minimal in volume, and difficult to measure accurately, we did not include this error in our data analysis. In future research, we will more thoroughly consider the impact of reflex tear secretion and aim to minimize errors in our experimental design.
Other limitations should also be noted, such as the small sample size and limited detection indicators, as well as the lack of different dilution concentration groups. In the future, the sample size can be expanded, more tear detection indicators can be added, and concentration levels can be subdivided to further explore the experimental methods comprehensively and in depth.
In summary, the diluted Schirmer method can comfortably collect more tear samples from patients with dry eye syndrome, providing effective detection results that reflect disease trends. As the focus shifts towards personalized and preventive medicine, this study’s findings are expected to bolster tear collection methods and advance related research.
Acknowledgements
Our sincere thanks go to Dr. Hu and Dr. Hou for their dedication to data collection. We also extend our appreciation to Professor Xinchang Wang and Professor Xiuming Jin for their valuable guidance.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Data availability statement
The data that support the findings of this study are available from the corresponding author, [Xinchang Wang], upon reasonable request.
Additional information
Funding
References
- Pflugfelder SC, Stern ME. Biological functions of tear film. Exp Eye Res. 2020;197:108115. doi:10.1016/j.exer.2020.108115.
- Herber S, Grus FH, Sabuncuo P, Augustin AJ. Two-dimensional analysis of tear protein patterns of diabetic patients. Electrophoresis. 2001;22(9):1838–1844. doi:10.1002/1522-2683(200105)22:9<1838::AID-ELPS1838>3.0.CO;2-7.
- Evans V, Vockler C, Friedlander M, Walsh B, Willcox MD. Lacryglobin in human tears, a potential marker for cancer. Clin Exp Ophthalmol. 2001;29(3):161–163. doi:10.1046/j.1442-9071.2001.00408.x.
- Devos D, Forzy G, de Seze J, Caillez S, Louchart P, Gallois P, Hautecoeur P. Silver stained isoelectrophoresis of tears and cerebrospinal fluid in multiple sclerosis. J Neurol. 2001;248 (8):672–675. doi:10.1007/pl00007833.
- Koo BS, Lee DY, Ha HS, Kim JC, Kim CW. Comparative analysis of the tear protein expression in blepharitis patients using two-dimensional electrophoresis. J Proteome Res. 2005;4(3):719–724. doi:10.1021/pr0498133.
- Zhang S, Wang H, Mou N, Li MX. Study on the correlation between clinical indicators and IL-1β, IL-6, MMP-9 in tears of patients with dry eye related to primary Sjögren’s syndrome. J Med Res. 2021;50(10):88–92.
- Wang C, Xu Q. Clinical significance and detection methods of biomarkers in tears. Chin J Ophthalmol Otorhinolaryngology. 2023;23(4):333–337.
- Asian Dry Eye Society (China Branch). Cross-strait medicine and health exchange association ophthalmology committee ocular surface and tear disease group, Chinese medical doctor association ophthalmology branch ocular surface and dry eye group. Consensus of Chinese experts on the definition and classification of dry eye. Chin J Ophthalmol. 2020;56(6):418–422.
- Wan XC, Zhang H, Shen Y, Zhou SY, Yang P, Zhou XJ, Gu H, Le QY, Xu JJ, Zhou XT, et al. Preliminary comparative study on the rapid detection of domestic tear MMP9 test kits. Chin J Ophthalmol. 2023;59(4):272–278.
- Yokoi N, Georgiev GA. Tear-film-oriented diagnosis for dry eye. Jpn J Ophthalmol. 2019;63(2):127–136. doi:10.1007/s10384-018-00645-4.
- Yuan J, Han X, Ding JJ, et al. Clinical study on the tear film break-up patterns in patients with dry eye. J Nanjing Med Univ (NatSci). 2022;42(08):1183–1187.
- Corneal Disease Group, Ophthalmology branch of the Chinese Medical Association. Expert consensus on the clinical diagnosis and treatment of dry eye (2013). Chin J Ophthalmol. 2013;49(1):73–75.
- Huskisson EC. Multidimensional pain scales: Visual Analogue Scale (VAS) for pain, Numeric Rating Scales (NRS), and other pain scales. A Compendium of Tests, Scales and Questionnaires. 2020;358–361. doi:10.4324/9781003076391-100.
- Holly FJ, Laukaitis SJ, Esquivel ED. Kinetics of lacrimal secretion in normal human subjects. Curr Eye Res. 1984;3(7):897–910. doi:10.3109/02713688409167207.
- De Souza GA, De Godoy Lyris MF, Mann M. Identification of 491 proteins in the tear fluid proteome reveals a large number of proteases and protease inhibitors. Genome Biol. 2006;7(8):R72. doi:10.1186/gb-2006-7-8-r72.
- Wang Q, Zhang H, Wei RH, Dong LJ. Research progress of tear proteomics in dry eye. J Armed Police Logist Acad (Medical Edition). 2020;29(11):88–92. doi:10.16548/j.2095-3720.2020.11.024.
- Solomon A, Dursun D, Liu Z, Xie Y, Macri A, Pflugfelder SC. Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci. 2001;42(10):2283–2292.
- Svitova TF, Lin MC. Dynamic interfacial properties of human tear-lipid films and their interactions with model-tear proteins in vitro. Adv Colloid Interface Sci. 2016;233:4–24. doi:10.1016/j.cis.2015.12.009.
- Yang M, Chung Y, Lang S, Yawata N, Seah LL, Looi A. The tear cytokine profile in patients with active Graves’ orbitopathy. Endocrine. 2018;59(2):402–409. doi:10.1007/s12020-017-1467-2.
- Gijs M, Arumugam S, van de Sande N, Webers CAB, Sethu S, Ghosh A, Shetty R, Vehof J, Nuijts RMMA. Pre-analytical sample handling effects on tear fluid protein levels. Sci Rep. 2023;13(1):1317. doi:10.1038/s41598-023-28363-z.
- Jung JH, Ji YW, Hwang HS, Oh JW, Kim HC, Lee HK, Kim KP. Proteomic analysis of human lacrimal and tear fluid in dry eye disease. Sci Rep. 2017;7(1):13363. doi:10.1038/s41598-017-13817-y.
- Aluru SV, Shweta A, Bhaskar S, Geetha K, Sivakumar RM, Utpal T, Padmanabhan P, Angayarkanni N. Tear fluid protein changes in dry eye syndrome associated with rheumatoid arthritis: a proteomic approach. Ocul Surf. 2017;15(1):112–129. doi:10.1016/j.jtos.2016.09.005.
- Huang ZF, Massey JB, Via DP. Differential regulation of cyclooxygenase-2 (COX-2) mRNA stability by interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) in human in vitro differentiated macrophages. Biochem Pharmacol. 2000;59(2):187–194. doi:10.1016/S0006-2952(99)00312-3.
- Yoon KC, Jeong IY, Park YG, Yang SY. Interleukin-6 and tumor necrosis factor-alpha levels in tears of patients with dry eye syndrome. Cornea. 2007;26(4):431–437. doi:10.1097/ICO.0b013e31803dcda2.
- Xia L, Zhang S, Zhou J, et al. A crucial role for B and T lymphocyte attenuator in preventing the development of CD4+ T cell-mediated herpetic stromal keratitis. Mol Vis. 2010;16:2071–2083.
- Lee SY, Han SJ, Nam SM, Yoon SC, Ahn JM, Kim TI, Kim EK, Seo KY. Analysis of tear cytokines and clinical correlations in Sjögren syndrome dry eye patients and non-Sjögren syndrome dry eye patients. Am J Ophthalmol. 2013;156(2):247–253.e1. doi:10.1016/j.ajo.2013.04.003.