ABSTRACT
A Late Cretaceous (Maastrichtian) assemblage of snakes from the Maevarano Formation of the Mahajanga Basin, northwestern Madagascar, constitutes the only fossil record of snakes from the island. The assemblage, which lived in a highly seasonal, semi-arid climate, includes only archaic forms belonging to the Madtsoiidae and Nigerophiidae, and therefore no representatives of extant Malagasy clades. A large sample of exquisitely preserved vertebrae and several ribs are assigned to Madtsoia madagascariensis, a long (almost 8 m), heavy-bodied ambush predator inferred to have subdued its prey via constriction. A new madtsoiid genus and species, Menarana nosymena, is represented by several associated vertebrae and rib fragments, and part of the basicranium. It was approximately 2.4 m long and appears to have been a powerful, head-first burrower, or at least to have had a burrowing ancestry. Kelyophis hechti, by far the smallest snake in the assemblage (<1 m long), is a new genus and species of primitive nigerophiid based on six isolated vertebral specimens. It was not as specialized for the aquatic lifestyle inferred for other nigerophiids. Although recent molecular phylogeographic studies suggest an early colonization of Madagascar by snakes ancestral to modern Malagasy boids, with subsequent vicariant evolution, the Maevarano Formation assemblage offers no support for this hypothesis. The repeated pattern of extinct archaic lineages being replaced on Madagascar by basal stocks of extant clades (e.g., Anura, Crocodyliformes, Avialae, Mammalia) after the Late Cretaceous is also a plausible scenario for the origin of the extant Malagasy snake fauna.
INTRODUCTION
The early evolution of snakes is poorly documented in the fossil record, with no known occurrences prior to the Cretaceous. With the important exception of a remarkably diverse assemblage (at least nine species representing at least seven families) from the early Late Cretaceous (Cenomanian) of Sudan (CitationWerner and Rage, 1994; CitationRage and Werner, 1999), multi-species assemblages (i.e., more than one species from a single locality or rock unit) of Cretaceous (and even Paleocene) snakes are uncommon. Rare also are occurrences of associated or articulated specimens of early non-marine snakes (a notable exception being Dinilysia; CitationEstes et al., 1970; CitationCaldwell and Albino, 2002; CitationCaldwell and Calvo, 2008). The vast majority of Cretaceous non-marine snake species are represented by isolated vertebrae. Interestingly, Late Cretaceous snakes are far less abundant and speciose on Laurasian than on Gondwanan landmasses, whereas the reverse is true for non-ophidian squamates (i.e., ‘lizards’) (CitationKrause et al., 2003).
This report describes an assemblage of Late Cretaceous (Maastrichtian) snakes from Madagascar that includes at least three species: (1) the previously reported madtsoiid Madtsoia madagascariensis CitationHoffstetter 1961a; (2) a new genus and species of madtsoiid represented by an associated skeleton including a braincase fragment and a partial atlas; and (3) a new genus and species of nigerophiid. This constitutes the first report of the family Nigerophiidae from Madagascar. In addition, owing to the vastly increased sample size since CitationHoffstetter's (1961a) original description of the species, the morphology of the vertebrae and the first-known ribs of Madtsoia madagascariensis are described in detail for the first time.
HISTORY OF STUDY AND GEOLOGICAL CONTEXT
Over 75 years ago, CitationPiveteau (1933) described an isolated, nearly complete vertebra of a large snake discovered by botanist J. Perrier de la Bâthie in Upper Cretaceous strata of what was then known as the Marovoay region of northwestern Madagascar. Owing to the limited material, Piveteau did not name a new taxon but made several relevant comparisons and opined that the specimen represented a member of the Boidae (= Pythonidae of Piveteau), which, at the time, included the genus Madtsoia (then represented only by Madtsoia bai from the Eocene of Argentina; CitationSimpson, 1933). Unfortunately, no precise locality or stratigraphic information was provided and the specimen could not be relocated after being moved from the MNHN in Paris during World War II (CitationHoffstetter, 1961a; confirmed by J.-C. Rage, pers. comm., Feb. 29, 2008). Twenty-eight years later, CitationHoffstetter (1961a) described and illustrated five additional snake vertebrae and a very large zygosphene, all collected by R. Lavocat in 1954 from three areas southeast of the port city of Mahajanga (= Majunga). Hoffstetter concluded that the fossils had been collected from the same general region as Piveteau's specimen, regarded them as all belonging to the same taxon, and referred them to a new species, Madtsoia madagascariensis. He also concurred with Piveteau's conclusions concerning membership of this large snake in the Boidae but assigned Madtsoia to a new subfamily, the Madtsoinae, to which he also allocated Gigantophis from the Paleogene of Egypt (CitationAndrews, 1901, Citation1906). Examination of both Hoffstetter's (Citation1961a:fig. 1) and Lavocat's (Citation1955:fig. 2) maps (see also CitationKrause et al., 2007:fig. 6B) suggests that Perrier de la Bâthie's and Lavocat's specimens of snake vertebrae were all recovered from the Maevarano Formation, even though the rock unit had not yet been formally delimited and named (see CitationRogers et al., 2000).
The Mahajanga Basin Project, conducted jointly by Stony Brook University and the University of Antananarivo, was initiated in 1993, 60 years after CitationPiveteau's (1933) description of the first fossil snake specimen from Madagascar. The reconnaissance expedition and eight field campaigns since (1995, 1996, 1998, 1999, 2001, 2003, 2005, 2007) have focused primarily on the collection of fossil vertebrates and associated contextual data from the Maevarano Formation in the Berivotra Study Area, which lies some 35 km southeast of Mahajanga, but recent reconnaissance (2003, 2005, 2007) has established two additional study areas, the Masiakakoho and Lac Kinkony study areas, west of the Betsiboka River and southwest of Mahajanga (). The Maevarano Formation, which crops out in all three study areas, was named and described by CitationRogers et al. (2000). It has been ascertained to be of Maastrichtian age and to have been deposited in a highly seasonal, semi-arid climate (CitationRogers et al., 2000, Citation2007; CitationRogers and Krause, 2007). The majority of the contained fossils were entombed in massive debris flows (CitationRogers, 2005) as sediments were washed from the crystalline highlands that run down the north-south axis of the island northwestward toward the Mozambique Channel. The vertebrate fauna of the Maevarano Formation includes ray-finned fishes, frogs, turtles, snakes, non-ophidian squamates, crocodyliforms, birds, non-avian dinosaurs, and mammals (most recently reviewed in CitationKrause et al., 2006). The snake specimens described in this report were recovered from the Berivotra and Masiakakoho study areas; none have yet been found in the Lac Kinkony Study Area. Snakes are represented by over 125 specimens, most of them isolated vertebrae and vertebral and rib fragments. One specimen, the holotype of a new genus and species of madtsoiid, consists of associated elements: a sizable braincase fragment, a partial atlas, several complete vertebrae from the mid-trunk and posterior trunk regions, and many vertebral and rib fragments.
METHODS
The specimens described in this report were collected by field crew members of the Mahajanga Basin Project through nine field seasons from 1993 to 2007 via surface collecting, quarrying, and both dry- and wet-screening methods. All specimens were recovered from the Maevarano Formation in the Berivotra and Masiakakoho study areas, Mahajanga Basin, northwestern Madagascar, and prepared in the Stony Brook University Fossil Preparation Laboratory.
Comparisons were made with skeletal material in the collections of the AMNH (including direct comparison with the holotype of Madtsoia bai), CMNH, and MVZ, as well as those of the authors. Other fossils were compared through descriptions and figures in the literature. Vertebral anatomical terminology follows CitationLaDuke (1991), except as modified by CitationHead (2005). However, we continue to follow LaDuke in referring to vertebral regions as divisions of the column (e.g., anterior, mid-, and posterior trunk; cloacal; postcloacal). It must be emphasized, however, that because intracolumnar variation is continuous, a vertebra from, for instance, a posterior position in the anterior trunk region will be difficult to differentiate from one in an anterior position in the mid-trunk region.
The partial basicranium of Menarana nosymena, gen. et sp. nov., (UA 9684-3) was scanned at the High-Resolution X-ray CT (HRXCT) Facility at The University of Texas at Austin and the dataset was rendered in three dimensions using VGStudio MAX 1.2 (Volume Graphics, Heidelberg, Germany). An interactive, Web-deliverable version of the HRXCT data set, as well as animations of 3-D reconstructions and technical information concerning the scans and image processing, can be viewed at http://www.digimorph.org/specimens/Menarana_nosymena; the original full-resolution HRXCT data are available from the authors.
Measurements and Anatomical Abbreviations—All measurements were made with hand-held calipers (Helios) or, in the case of small specimens, with an ocular micrometer in a dissecting microscope. The following measurements (in order of presentation in the tables) were made where possible, following abbreviations of CitationLaDuke (1991): CL = centrum length; NAW = neural arch width; PRW = width across the prezygapophyses; POW = width across the postzygapophyses; PR-PO = length from the anterior edge of one prezygapophyseal facet to the posterior edge of the ipsilateral postzygapophyseal facet; COW = width of the cotyle measured from the outside of the cotylar rim; CNW = condyle width; NSH = vertical height of the neural spine measured from the top of the zygosphene to the highest extremity of the spine; and ZSW = zygosphene width. In addition; ATV = anterior trunk vertebra; MTV = mid-trunk vertebra; PTV = posterior trunk vertebra; CV = cloacal vertebra; and PCV = postcloacal vertebra.
Institutional Abbreviations—AMNH, American Museum of Natural History, New York; CMNH, Carnegie Museum of Natural History, Pittsburgh; FMNH, The Field Museum, Chicago; MVZ, Museum of Vertebrate Zoology, University of California at Berkeley; MNHN, Muséum national d’Histoire naturelle, Paris; QM, Queensland Museum, Brisbane; SAMP, South Australian Museum (Adelaide) Palaeontology; UA, Université d’Antananarivo, Antananarivo, Madagascar.
Taxonomic Abbreviations—In relevant places throughout the text, Madtsoia is abbreviated to Ma. and Menarana is abbreviated to Me. to save space but also to facilitate differentiation between species of the two genera.
SYSTEMATIC PALEONTOLOGY
SQUAMATA CitationOppel, 1811
SERPENTES CitationLinnaeus, 1758
MADTSOIIDAE (CitationHoffstetter, 1961a) CitationMcDowell, 1987
MADTSOIA CitationSimpson, 1933
Type Species—Madtsoia bai CitationSimpson, 1933.
Referred Species—Madtsoia madagascariensis CitationHoffstetter, 1961a and Ma. camposi CitationRage, 1998.
Revised Diagnosis—Distinguished from Alamitophis, Herensugea, Menarana (below), Nanowana, and Patagoniophis by large size and relatively short, broad mid-trunk vertebrae (CL approximately half of PRW). Vertebrae further differ from those of Menarana in having taller neural spines and less depressed neural arches, from those of Gigantophis and Rionegrophis in having less distinct hemal keels, and from those of Wonambi and Yurlunggur in having a single parazygantral foramen on each side. Ribs differ from those of Wonambi and Yurlunggur, which have multiple small foramina in dorsal groove, in having a single large foramen in that position (but a much smaller accessory foramen can be present). They differ from those of Menarana in having a less strongly recessed dorsal facet, in not having the tuber costae drawn out into a crest, and in possessing fewer foramina on anteroventral and posterior surfaces.
Comparisons and Discussion
At the time Madtsoia was named, diagnosed, and described by Simpson in 1933, and then even 28 years later when it was reassessed by CitationHoffstetter (1961a), only one other genus (Gigantophis) of the clade now identified as Madtsoiidae was known. Since 1961, seven additional genera (Alamitophis, Herensugea, Nanowana, Patagoniophis, Rionegrophis, Wonambi, and Yurlunggur) have been named and assigned to the Madtsoiidae and at least two others (Najash [see CitationApesteguía and Zaher, 2006] and Helagras [see CitationHead and Holroyd, 2008]) are questionably allied. Yet, no formal rediagnosis of Madtsoia has been published since that time. As such, and because of the removal of “Madtsoia” laurasiae from the genus (see below) and the considerable addition to knowledge of Ma. madagascariensis based on the new specimens described here, reassessment of vertebral and rib features relative to those of other madtsoiid genera and revision of the diagnosis of Madtsoia are in order, especially because it serves as the type genus of the Madtsoiidae.
The genus Madtsoia, as here defined, consists of three large species: Ma. bai, Ma. camposi, and Ma. madagascariensis, with maximum centrum lengths (CL) of 18–25 mm, and maximum widths across the prezygapophyses (PRW) of 35–65 mm. Three madtsoiid genera (Gigantophis, Wonambi, and Yurlunggur) include species of comparable size. Species of Menarana (defined below) appear to have maximum sizes about one-half to two-thirds those of the large genera (CL = 11–13 mm; PRW = 20–22 mm). Several madtsoiid genera (Alamitophis, Herensugea, Nanowana, and Patagoniophis) have maximum sizes that are much smaller (CL < 8 mm, PRW < 10 mm). Thus, the madtsoiid genera segregate into three distinct size classes. Members of each size class can be distinguished further by differences in vertebral shape: the smaller madtsoiids tend to have relatively elongate vertebrae (length nearly as great as width); Menarana has vertebrae that are depressed overall with extremely low neural spines; and the larger genera have vertebrae that are broader than they are long, and that are never depressed to the degree seen in Menarana.
Vertebrae of the larger madtsoiid genera can be distinguished from one another on the basis of more detailed comparisons. Madtsoia madagascariensis and Wonambi naracoortensis were briefly compared by CitationSmith (1976:43), who stated that, “There is a striking resemblance between Wonambi vertebrae and those of Madstoia [sic] bai…and M. madagascariensis.” However, she did not make detailed comparisons of the two species that would allow differentiation, stating that, “the relationship of Wonambi to Madstoia [sic] or any other boid will remain obscure until the skull [of Madtsoia] is known.” Nevertheless, most paleontologists who work extensively with snakes use vertebrae to differentiate genera and even species. Comparison of vertebrae of Ma. madagascariensis and W. naracoortensis does indeed reveal a strong resemblance in shape. However, the large Australian madtsoiids (Wonambi and Yurlunggur) have a series of small parazygantral foramina, whereas Madtsoia (indeed, most madtsoiids) usually have a single, large foramen recessed in a distinct fossa. Posterior trunk vertebrae of Ma. bai bear paired posterior tubercles on an otherwise broad, low hemal keel that were referred to as ‘paired hypapophyses’ (CitationSimpson, 1933:3, 8); similar structures are seen in Ma. madagascariensis(CitationHoffstetter 1961a) and species of Yurlunggur (CitationScanlon, 1992, Citation1995), but not Ma. camposi, which has a more typical rhombic termination of the hemal keel (CitationRage, 1998). Posterior bifurcation of the keel also occurs in a different form (mostly narrower keels) in Wonambi (CitationSmith, 1976) and other, smaller Australian taxa (CitationScanlon, 1997, Citation2005b). Gigantophis vertebrae have distinctively shaped neural arches (CitationAndrews, 1906:pl. XXVI, figs. 1–3). The laminae are thickened and strongly arched in posterior view (slightly angled in Madtsoia, Wonambi, and Yurlunggur). Anteriorly, the zygosphene also appears hypertrophied, being much broader than the opening of the neural canal. The hypapophysis is low and blunt. Andrews makes no mention of paired tubercles or hypapophyses, and his illustrations do not appear to show any.
The vertebrae of Najash rionegrina are similar to those of madtsoiids in possessing parazygantral foramina, a shallow interzygapophyseal constriction, and large, broad synapophyses that exceed the prezygapophyseal facets laterally, and in lacking accessory processes of the prezygapophyses (CitationApesteguía and Zaher, 2006). Najash is distinct from madtsoiids, but similar to various fossil “anilioids,” in that it lacks a posterior neural arch notch and has hemal keels that are ‘shallow and thin.’ This mosaic of vertebral characters makes Najash vertebrae identifiable, but provides little support for assignment to a higher level taxon.
MADTSOIA MADAGASCARIENSIS CitationHoffstetter, 1961a
(–; )
Holotype Specimen—MNHN MAJ 5, posterior trunk vertebra (CitationHoffstetter, 1961a:fig. 2A).
Type Locality—‘“Gite du Guide,’ North of Berivotra, Madagascar” (CitationRage, 1984:30).
Referred Specimens—Anterior trunk vertebrae: FMNH PR 2545–FMNH PR 2549, FMNH PR 2558, FMNH PR 2569, FMNH PR 2702, UA 9688–UA 9693, UA 9703, UA 9728. Mid-trunk vertebrae: FMNH PR 2550–FMNH PR 2553, MNHN MAJ 9 (CitationHoffstetter, 1961a:fig. 3E), MNHN MAJ 10 (CitationHoffstetter, 1961a:fig. 3F), UA 9695, UA 9697, UA 9698, UA 9745. Posterior trunk vertebrae: FMNH PR 2554, FMNH PR 2555, MNHN MAJ 7 (CitationHoffstetter, 1961a:fig. 2C), UA 9700. Cloacal vertebra: FMNH PR 2556. Postcloacal vertebrae: FMNH PR 2557, UA 9701. Fragmentary vertebrae not assigned to region: FMNH PR 2559–FMNH PR 2568, FMNH PR 2570, FMNH PR 2584, FMNH PR 2585, MNHN MAJ 6 (CitationHoffstetter, 1961a:fig. 2B), MNHN MAJ 8 (CitationHoffstetter, 1961a:fig. 3D), UA 9694, UA 9696, UA 9699, UA 9702, UA 9704–UA 9712, UA 9715–UA 9718, UA 9721, UA 9726, UA 9727, UA 9729–UA 9731, UA 9735, UA 9736, UA 9738–UA 9744, UA 9747, UA 9765, UA 9766, UA 9768, UA 9772. Nearly complete ribs: UA 9746, UA 9763, UA 9764, UA 9775. Proximal rib fragments: FMNH PR 2571, FMNH PR 2582, FMNH PR 2583, UA 9714.
Localities—The first-known specimen of Madtsoia madagascariensis, described by CitationPiveteau (1933), was listed as coming from the region of Marovoay, southeast of Mahajanga (= Majunga). The specimens described by CitationHoffstetter (1961a:fig. 1) were recovered from three areas listed as: (1) north of Berivotra (the holotype, MNHN MAJ 5), (2) south of Berivotra (MNHN MAJ 8–MNHN MAJ 10), and (3) north of the Mahajanga-Ambalabé road, between km 20 and 25 (MNHN MAJ 6, MNHN MAJ 7). The Mahajanga Basin Project, initiated in 1993, has discovered specimens of Ma. madagascariensis in two major areas (): (1) Berivotra Study Area localities MAD93-01, 93-09, 93-14, 93-16, 93-17, 93-18, 93-25, 93-28, 93-30, 93-33, 93-34, 93-35, 93-36, 93-38, 93-73, 93-81, 95-14, 96-01, 96-04, 96-32, 98-08, 98-31, 99-15, 99-39, 01-03, 03-03, 03-04, 03-05, 03-09, 05-64; and (2) Masiakakoho Study Area locality MAD03-23 ().
Age and Distribution—Known only from the Upper Cretaceous (Maastrichtian) Maevarano Formation, Berivotra and Masiakakoho study areas, Mahajanga Basin, northwestern Madagascar.
Revised Diagnosis—Neural spines differ from those of Madtsoia bai and Ma. camposi in being taller and more posteriorly canted. Zygosphenes relatively narrower than in Ma. camposi. Zygapophyses broad and rectangular, similar to those of Ma. bai but broader than those of Ma. camposi. Synapophyses project laterally beyond prezygapophyseal facets, as in Ma. camposi, but not as in Ma. bai, in which synapophyses project far beyond zygapophyses. Ribs differ from those of Ma. bai in lacking a strong anterodorsal process and from those of Ma. camposi in not having the ventral articular facet projecting strongly anteriorly.
Description
An isolated vertebra of this species was described briefly by CitationPiveteau (1933), but no name was applied at that time. CitationHoffstetter (1961a) described six additional specimens (five vertebrae and one zygosphene) and, in addition to naming the species Madtsoia madagascariensis, listed three differences between it and Ma. bai, the only other species of Madtsoia then recognized: (1) the neural spine is taller and its distal portion is inclined posteriorly; (2) in the posterior trunk vertebrae, the hemal keel is more clearly delimited by more marked lateral depressions; and (3) the condyle is more circular in outline and less depressed dorsoventrally. He also described diagnostic characteristics of his new subfamily Madtsoiinae. However, CitationHoffstetter (1961a) provided only a cursory description of the vertebral morphology of Ma. madagascariensis, and the only regions of the vertebral column known to him were the mid- and posterior trunk regions. Based on the specimens recovered as part of the Mahajanga Basin Project, detailed descriptions of vertebrae from the anterior trunk, mid-trunk, posterior trunk, cloacal, and postcloacal regions, as well as parts of seven ribs are provided here.
provides measurements for the specimens of Madtsoia madagascariensis collected by Mahajanga Basin Project teams and allows comparisons of the proportions of vertebrae from different regions of the column. Such comparisons reveal, for example, that the largest complete vertebrae were not the largest specimens in the assemblage, as some fragments (e.g., isolated zygosphenes) were larger than those present on any of the more complete vertebrae. Furthermore, the zygosphene described and illustrated by CitationHoffstetter (1961a:fig. 3D, MNHN MAJ 8) is listed as being 22 mm wide and is therefore larger than the largest of the zygosphenes (FMNH PR 2564; ca. 19.4 mm wide) in the samples collected as part of the Mahajanga Basin Project.
Anterior Trunk Vertebrae—At least 16 specimens represent this vertebral region, previously undescribed in Madtsoia madagascariensis. Several specimens are very well preserved and essentially complete. One of these (FMNH PR 2546; ), from the anterior portion of the anterior trunk region of a large individual, bears a strong hypapophysis. Another specimen (FMNH PR 2548), representing a more posterior segment of the anterior trunk region, has a much reduced hypapophysis and three specimens that are particularly complete and well preserved (FMNH PR 2545, FMNH PR 2547, FMNH PR 2549; ) are from the far posterior portion of this region, resembling mid-trunk vertebrae except for the presence of slightly developed hypapophyses, just anterior to the ventral lip of the condyle. The following description is based primarily on these five specimens.
The centra of anterior trunk vertebrae are narrower than those of mid-trunk vertebrae and the subcentral fossae are less pronounced, especially anteriorly in the region (e.g., FMNH PR 2546). Subcentral foramina are on the sloping portion of the keel in FMNH PR 2546 or in shallow subcentral fossae in more posterior vertebrae. The hypapophysis is robust, elongate, and laterally compressed in FMNH PR 2546, much shorter in FMNH PR 2548, and reduced to a nubbin in FMNH PR 2545, FMNH PR 2547, and FMNH PR 2549. The elongated hypapophysis on FMNH PR 2546, which is paddle-like in lateral view, exhibits a swelling at approximately mid-length of the hypapophysis. This irregular, asymmetrical swelling resembles a bone callus, and may indicate a healed break in the bone. The tip of the hypapophysis is bent slightly toward the left beyond the callus. However, we note that in some madtsoiids, such as Yurlunggur camfieldensis and Riversleigh Yurlunggur spp., bilateral expansions of the hypapophyses are present that may represent serial homologs of ‘paired hypapophyses’ (CitationSimpson, 1933:3, 8) in the mid- and posterior trunk regions and presumably served as sites for muscle attachment. The subcentral ridges are not as distinct as those present on mid-trunk vertebrae, especially anteriorly in the anterior trunk region.
FIGURE 2 Trunk vertebrae of Madtsoia madagascariensis from the Late Cretaceous of Madagascar in l, lateral; a, anterior; p, posterior; d, dorsal; and v, ventral views. A, vertebra from anterior part of anterior trunk region with well-developed hypapophysis, FMNH PR 2546; B, vertebra from posterior part of anterior trunk region, FMNH PR 2549; C, mid-trunk vertebra, FMNH PR 2551; D, vertebra from anterior part of posterior trunk region, FMNH PR 2554; E, vertebra from middle part of posterior trunk region, FMNH PR 2555.
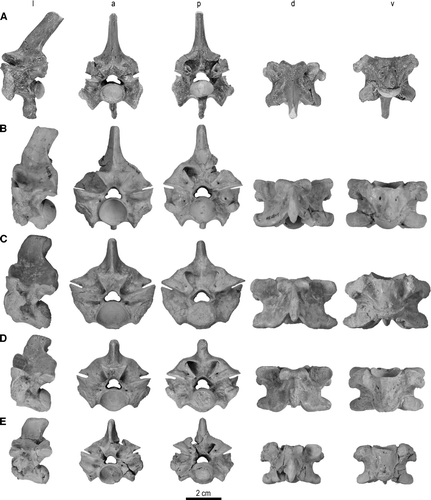
FIGURE 3 Cloacal and postcloacal vertebrae of Madtsoia madagascariensis from the Late Cretaceous of Madagascar in l, lateral; a, anterior; p, posterior; d, dorsal; and v, ventral views. A, cloacal vertebra, FMNH PR 2556; B, postcloacal vertebra, FMNH PR 2557. Articular facets on ventral aspect of postcloacal vertebra for chevron bone enlarged at bottom right.
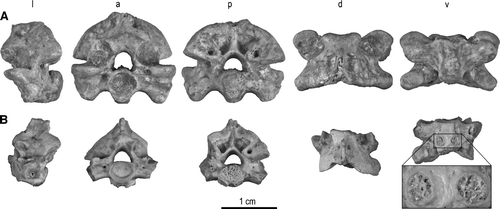
FIGURE 4 Ribs and rib fragments of Madtsoia madagascariensis from the Late Cretaceous of Madagascar. A, anterior; and B, posterior views of UA 9764, nearly complete left rib exhibiting a pathological lesion (indicated by arrows). C, anterior; D, posterior; and I, stereophotographic proximal views of UA 9746, proximal half of right rib (reversed to facilitate comparison). E, anterior; F, posterior; and J, stereophotographic proximal views of FMNH PR 2571, proximal fragment of left rib. G, anterior; and H, posterior views of UA 9763, nearly complete left rib.
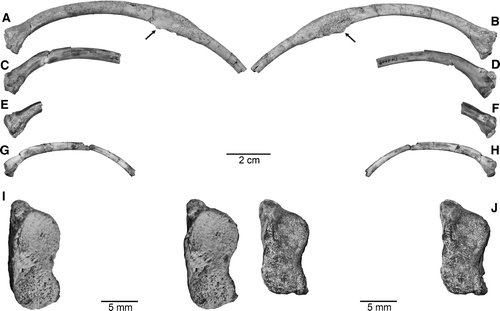
TABLE 1 Measurements of vertebral specimens of Madtsoia madagascariensis. See text for list of abbreviations. ? = vertebral fragment not assigned to region.
The cotyle and condyle are depressed, especially anteriorly in the region, with a slightly recessed ventral cotylar lip, but they are not emarginated ventrally; they differ from the mid-trunk vertebrae in these respects. The neural canal is strongly trifoliate in shape and highly depressed, approximately twice as broad ventrally as high. Paracotylar fossae are present and usually contain one large foramen each, but the number can vary from zero (e.g., FMNH PR 2546—right side, FMNH PR 2548—left side) to two (e.g., UA 9727—both sides).
The zygosphene, although thick, is not massive, but gently convex to flat dorsally. Its lateral margins are not elevated as they are in the mid-trunk region. Its anterior margin is incised by a broad, shallow notch. Zygosphenes from more posterior vertebrae of the anterior trunk approach the massiveness of those of mid-trunk vertebrae. The zygantrum is very large, and similar to those of the mid-trunk vertebrae with two notable exceptions. First, fine subvertical ridges that descend from the laminae in the mid-trunk vertebrae are absent in the more anterior vertebrae of the anterior trunk region (e.g., FMNH PR 2546) and only faintly discernible in the more posterior vertebrae of the region. Second, the roof of the zygantral cavity is distinctly peaked in the vertebra from a far anterior position (FMNH PR 2546), though flattened in the posterior portion of the anterior trunk and all mid-trunk vertebrae.
In FMNH PR 2546, the neural spine is very tall and robust. Its height is slightly greater than twice the height of the laminae above the centrum. Anteriorly, the spine is laterally compressed, but posteriorly it is thickened, creating a triangular section. The spine is canted, extending posteriorly well beyond the level of the postzygapophyses. Postzygosphenal fossae are present, but do not contain foramina. More posterior vertebrae in the anterior trunk series have shorter and anteroposteriorly longer neural spines and can contain up to three small foramina in the postzygosphenal fossae, though the foramina are not all necessarily restricted to the bottom of these fossae (FMNH PR 2545).
The zygapophyseal facets are small, approximately as broad as long. The zygapophyses are not markedly divergent from the centrum, producing a relatively narrow aspect for the vertebra. The synapophyses are large, and well preserved in FMNH PR 2546, in which the diapophyseal portion is separated from the parapophysis by a distinct constriction as a result of a posterior indentation. This indentation becomes more prominent in more posterior vertebrae in the series and is particularly prominent in FMNH PR 2549. The diapophyseal facet is bulbous whereas the parapophysis is relatively flat. The latter extends ventrally well below the lower lip of the cotyle in FMNH PR 2546, the most anterior vertebra in the series, but this disparity is less extreme or even absent (FMNH PR 2545) in more posterior vertebrae of the anterior trunk region.
Mid-trunk Vertebrae–Several complete and nearly complete mid-trunk vertebrae are represented in the Mahajanga Basin Project collection, significantly augmenting the sample available to CitationHoffstetter (1961a). The following description is derived primarily from a typical larger specimen, FMNH PR 2551 ().
The centrum is roughly triangular in ventral view, much broader anteriorly than long. A transversely convex anterior portion is flanked by lateral depressions that contain distinct, paired foramina. The hemal keel is poorly defined anteriorly, but narrows abruptly in its posterior third into a much better defined keel that bears a pair of small, blunt processes posteriorly. The lateral margins of the centrum form prominent subcentral ridges that extend posteromedially from the posteroventral border of the parapophysis, almost to the condyle. The postzygapophyses are transversely broad and elliptical, almost subrectangular in outline.
In anterior view, the cotyle is almost round (very slightly wider than high) and deep, but its ventral lip is recessed posteriorly and emarginated ventrolaterally. The neural canal is relatively small, slightly broader than tall, and roughly triangular; indentations formed by internal ridges along the floor and each of the lateral walls produce a trifoliate outline. Well-developed paracotylar fossae typically contain one or two foramina. Two specimens (FMNH PR 2550 and FMNH PR 2551) have two paracotylar foramina on each side, one larger than the other. Another (FMNH PR 2552) has paired foramina on the left, but a single foramen on the right. Other specimens in which the paracotylar fossae are visible (FMNH PR 2553, UA 9695) have a single foramen on each side. The neural arch laminae rise sharply from front to back and from lateral to medial. The zygosphene is massive and wedge-shaped in anterior view and its facets are angled at approximately 30° from the midline axis. The dorsolateral margins of the zygosphene project upward, due to the large facets, creating a dorsal concavity on each side of the anterior margin of the neural spine.
In posterior view, the condyle is almost round in outline but slightly flattened ventrally; it is directed strongly posterodorsally. The zygantrum is spacious and deep, with substantial zygantral facets that project posteriorly, slightly beyond the margins of the laminae. A deep, broad, V-shaped notch in the posterior margin of the neural arch laminae exposes the zygantrum from above. The anterior face of the zygantral cavity is smooth. A thin, delicate ridge descends ventromedially from the neural arch lamina approximately 30% of the distance to the ventral edge of the cavity on either side of the midline. Directly below these ridges, deep ventral fossae penetrate anteroventrally from the vicinity of the ventral edge of the zygantral facet. The ventrolateral edges of these fossae contain the endozygantral foramina. Parazygantral foramina (one on each side) are also present in well-marked fossae on the posterior face of the neural arch, between the zygantral and postzygapophyseal facets.
In lateral view, the neural spine is prominent, projecting high above the laminae. It is laterally compressed, and elongate, extending from the base of the zygosphene to the posterior edge of the neural arch. The neural spine has a posteriorly curved anterior margin and an overhanging posterior end that gives it a ‘swept-back’ appearance. It overhangs the deeply incised neural arch notch to a considerable degree. At the base of the neural spine, on either side, a pronounced fossa is excavated into the lamina of the neural arch posterior to the zygosphene. One to three small parazygosphenal foramina (CitationHead, 2005) may be found at or near the base of these fossae. Although these fossae and foramina appear to be present in at least one other madtsoiid (Alamitophis argentinus; CitationAlbino, 2000:fig. 2C), they are described specifically here for the first time in Madtsoia madagascariensis. Posteriorly, the neural spine is buttressed by the neural arch laminae, which rise to meet the spine at about three-fourths of its height and about two-thirds of the distance back from the anterior tip. The dorsal edge of the neural spine is laterally compressed. The synapophyses are reniform in shape and massive, their articular surfaces largely eroded, leaving a roughened surface. Indentation of the posterior border of these structures demonstrates that they were each at least partially constricted into a dorsal diapophysis and ventral parapophysis. The parapophysis does not extend below the ventral lip of the cotyle. A single foramen pierces the lateral face of the pedicle.
In dorsal view, the prezygapophyses have large, subrectangular facets whose main axes are oriented laterally. No trace of accessory processes is present. Pre- and postzygapophyses, which contribute greatly to the overall width of the vertebra, are connected by a broad, thick, interzygapophyseal ridge. The zygosphene is broad, but not unusually so, and its anterior margin is shallowly concave (nearly flat).
Posterior Trunk Vertebrae—In addition to the holotype vertebra (MNHN MAJ 5) and MNHN MAJ 7, three vertebrae recovered by Mahajanga Basin Project field crews can be allocated to this region on the basis of their broad and flattened hemal keels, more widely spaced posterior hemal keel tubercles, and the presence of deeper subcentral fossae and paracotylar notches. One of these specimens represents the anterior part of the series (FMNH PR 2554; ), another in the middle of the series (FMNH PR 2555; ), and another, fragmentary specimen (UA 9700), a relatively posterior vertebra in the series. These intra-regional differences are revealed primarily by the increasing depth and distinctness of the subcentral fossae, the related separation of the parapophyses from the cotyle (paracotylar notches), and the increasing breadth of the hemal keel. In general, these vertebrae have narrower zygosphenes, smaller neural canals, and slightly more depressed cotyles and condyles than mid-trunk vertebrae. Where intact, the neural spines are lower, and slightly expanded dorsally with rugose distal sculpturing, which is particularly marked in FMNH PR 2554.
Cloacal Vertebra—FMNH PR 2556 () is assigned to the cloacal region. This is a worn specimen whose extremities are rounded and eroded. The proportions of the vertebra, including its anteroposteriorly shortened aspect and small cotyle and condyle, the presence of strongly arched neural laminae, and reduced zygapophyseal facets would be most unusual for any vertebra other than one from the cloacal region (see CitationLaDuke, 1991). It is assigned to Madtsoia madagascariensis on the basis of its large size and a general correspondence in shape of various morphological attributes (e.g., massive zygosphene that is slightly concave anteriorly, high neural arch) to other material assigned to the species.
The specimen is short anteroposteriorly, giving it a broad aspect when viewed from above or below. The zygapophyses are not very divergent from the centrum. The prezygapophyses are particularly short mediolaterally relative to those on the trunk vertebrae. They also lie in a nearly horizontal plane, and are thus much less inclined than in more anterior regions. The centrum is reduced in size, with a strongly projecting, but ventrally rounded hemal keel occupying most of its ventral face. A true hypapophysis is absent. The condyle is eroded, but its base suggests a small overall size, which also can be inferred from the cotyle. Even though the edges of the cotyle are either broken or heavily worn, it is clear that the size of the cotyle relative to the neural canal is much less than in the trunk vertebrae. The zygosphene is massive and its facets are not as vertically oriented as in more anterior vertebrae. The neural spine, broken off near the base, is positioned posteriorly and is triangular in section. Parazygosphenal fossae are present and there is a foramen in the bottom of at least the right fossa. The paradiapophyseal region is too badly worn to distinguish what type of processes may have been present, though based on other features typical of cloacal vertebrae, it is assumed that they supported lymphapophyses. The neural arch laminae are strongly convex dorsally and thickened in posterior view, and the parazygantral foramina (one on each side) are very large.
Postcloacal Vertebrae—Two postcloacal vertebrae are assigned to Madtsoia madagascariensis. One of these (FMNH PR 2557; ), although exhibiting some damage to its extremities, is clearly from the anterior portion of the postcloacal region. It is distinctive in possessing a transversely narrowed, dorsoventrally thickened zygosphene, and the broken base of a neural spine that would have been moderately tall, based on its section and the angle of ascent of its sides from the base. Beside the neural spine are distinct left and right parazygosphenal fossae, each pierced by a large foramen. The neural arch is vaulted and the intact right postzygapophysis has a large parazygantral foramen (one is also seen in section on the left side). All of these features of FMNH PR 2557, coupled with its size, support assignment to Ma. madagascariensis. Assignment to the postcloacal region is based on the general proportions of the vertebra, and the presence of transverse processes, broken laterally near the base, that are the remnants of postcloacal pleurapophyses.
In addition to the above features, this specimen is particularly noteworthy in having two distinct, rounded articular surfaces on the ventral face of the centrum. The raised edges of the articular surfaces (‘pedicels’ of CitationScanlon and Lee, 2000; CitationLee and Scanlon, 2002; CitationScanlon, 2005a) are produced into a distinct, smooth, circular rim, whereas the centers are rough and pitted, resembling synchondroses. Based on comparisons with non-ophidian squamates (i.e.,’lizards’), and with other madtsoiids known to exhibit similar features (e.g., Wonambi naracoortensis, Alamitophis tingamarra; CitationScanlon and Lee, 2000; CitationScanlon, 1993, Citation2005b), we interpret these well-defined structures as representing articular surfaces for an independent chevron bone. However, in contrast to the far posterior position of the articular pedicels in W. naracoortensis and A. tingamarra, these structures in Madtsoia madagascariensis appear to lie slightly nearer to the middle of the centrum than to its posterior edge (though this is difficult to determine with exact precision because the condyle is eroded away; ).
A second postcloacal vertebra (UA 9701) is missing both postzygapophyses and has a worn condyle and other extremities, but the bases of its pleurapophyses are present. It is assigned to Madtsoia madagascariensis on the basis of its relatively high, laterally compressed neural spine, vaulted neural arch, and relatively large size. The proportions of this vertebra suggest that it is from near the posterior extremity of the postcloacal region.
Ribs—Four nearly complete ribs (missing less than half their shafts) and four proximal rib fragments are assigned to Madtsoia madagascariensis, primarily on the basis of their large size; six of these preserve the entire head, whereas two (FMNH PR 2583, UA 9775) have the ventral articular facet broken away.
Typical of large madtsoiids (e.g., Wonambi naracoortensis; see CitationScanlon and Lee, 2000:fig. 2h), the rib heads have a strong, low, blunt tuber costae, a large, concave, dorsal (diapophyseal) articular facet that is slightly recessed from the relatively flat ventral (parapophyseal) articular facet, and a modest, obtusely pointed anteroventral process. Although the dorsal facet is concave on all specimens, the ventral facet ranges from slightly convex (FMNH PR 2571, UA 9714, UA 9746, UA 9764) to slightly concave (FMNH PR 2582, UA 9763). The dorsal and ventral rib facets are separated from one another by a low, rounded, oblique (oriented from posterodorsal to anteroventral) ridge and, anteriorly, by a gentle notch that gives the proximal view a slightly ‘waisted’ outline. This waisting is particularly noticeable on FMNH PR 2582 and UA 9714, in which the posterior border is also slightly indented.
A prominent dorsal tubercle, with accessory tubercles that descend onto the anterior surface, just distal to the tuber costae, is present on FMNH PR 2571 and UA 9746, but is less distinct on UA 9714, UA 9763, UA 9764, and UA 9775 (this area is at least partially broken away on FMNH PR 2582 and FMNH PR 2583). In addition, UA 9714 has a distinctive crest accentuating its ventral surface in a posterior position, distal to the location of the anteroventral process. This crest rises to form a tubercle near its proximal end, then decreases in height as it runs distally to the broken surface of the neck. This posteroventral crest is absent or poorly developed in FMNH PR 2571, FMNH PR 2582, UA 9746, UA 9763, UA 9764, and UA 9775. In those specimens preserving the dorsal region distal to the head, a prominent foramen, in a shallow depression posterior to the tuber costae, pierces the dorsal surface of the rib; in FMNH PR 2582, UA 9746, and UA 9763, a smaller, accessory foramen lies immediately proximal to this prominent foramen (in FMNH PR 2583 only the smaller foramen is partially preserved, the remainder of the rib being broken away). Two (UA 9763, UA 9764), three (FMNH PR 2571, UA 9714, UA 9747), or even four (FMNH PR 2582) foramina, of variable size and position, are present on the shallowly concave lower anterior face, distal to the ventral facet. Finally, on those specimens preserving this region well enough for observation, one (FMNH PR 2582, UA 9714), two (FMNH PR 2571, UA 9746, UA 9775), or three (UA 9763) foramina are present on the posterior surface, ventral to the midline, in the area near where the head narrows to form the neck. Differences among the eight specimens are likely attributable to differential preservation, individual variation, variability along the length of the vertebral column, and/or even age/size of the individual at death. Size ranges from 9.9 mm (UA 9763) to 16.6 mm (UA 9764) along the longest axis of the rib head (posterodorsal to anteroventral).
The most complete specimen, UA 9764, is of additional interest because it presents an apparent pathological lesion, likely a healed fracture. This specimen has an abrupt swelling just beyond the apparent midpoint of the shaft (i.e., the straightest part of the shaft, distal to the angle, and proximal to a slightly more curved distal region). The swelling has the appearance of a bony callus, but its posterior face is rough and pitted, as though incompletely healed.
Comparisons
Vertebrae–In light of the vast expansion of the known sample of vertebrae of Madtsoia madagascariensis, it is relevant to underscore that this species is clearly a madtsoiid in its large size and the following vertebral features: (1) presence of parazygantral foramina in fossae lateral to each zygantral facet; (2) presence of paracotylar foramina; (3) wide diapophyses; (4) absence of prezygapophyseal processes; (5) hypapophyses limited to anterior trunk region; and (6) hemal keels moderately to well developed on mid- and posterior trunk vertebrae. In addition, as in several other madtsoiids (but not other snakes), the mid- and posterior trunk vertebrae bear short, laterally paired projections on the posterior extremity of the hemal keel.
CitationHoffstetter (1961a) pointed out that the vertebrae of Madtsoia bai differ from those of Ma. madagascariensis in the shape of their neural spines. Those of Ma. bai are more or less vertical in orientation, whereas those of Ma. madagascariensis lean posteriorly. Hoffstetter also stated that, in posterior trunk vertebrae of Ma. madagascariensis, the hemal keel is better defined and the cotyle and condyle are more rounded. Comparisons of serially homologous portions of the present material with the holotype of Ma. bai (which includes only mid- and posterior trunk vertebrae) reveal a host of differences in shape. These include (condition of Ma. bai in parentheses): (1) Madtsoia madagascariensis vertebrae have a high, anteroposteriorly short aspect, with rounded condyles and cotyles and high neural canals (depressed, broad aspect with relatively depressed condyles, cotyles, and neural canals); (2) the zygosphene is massive and wedge-shaped (broad, but not massive, gently convex dorsally); (3) the synapophyses are large and extend laterally, slightly beyond the lateral margin of the prezygapophyses (synapophyses with similar-sized articular surface areas, but much larger because they project far beyond the margin of the prezygapophyses by approximately half the width of the prezygapophyses); (4) posterior margins of the neural arch laminae ascend to approximately three-fourths the height of the neural spine and end about two-thirds of its length back from its anterior edge (the posterior margins of the laminae ascend all the way to the dorsal margin of the neural spine, joining it approximately midway between anterior and posterior edges, giving the extremity of the neural spine a diamond shape from above); and (5) the postzygosphenal fossae are deeply incised and contain small foramina (shallow, no foramina observed).
Vertebrae of Madtsoia madagascariensis differ from those of Ma. camposi CitationRage 1998 in having a higher, anteroposteriorly shorter neural spine, and in lacking a strong, dorsoventrally oriented ridge on the anterior face of the prezygapophyseal buttress (CitationRage, 1998). Rage also indicated that Ma. camposi has a relatively broader and less wedge-like zygosphene than Ma. madagascariensis. Finally, Ma. camposi appears to have much less broadened zygapophyseal facets (CitationRage, 1998:fig. 2). Both Ma. madagascariensis and Ma. bai have distinctly rectangular facets, much broader (mediolaterally) than long (anteroposteriorly), whereas those of Ma. camposi (holotype similar in size to FMNH PR 2551) are roughly square.
A few specimens of snake vertebrae from the Senonian of Niger were mentioned by Citationde Broin et al. (1974) and were illustrated by CitationRage (1981:fig. 2), who assigned them to Madtsoia aff. madagascariensis. Comparisons of Rage's illustrations to the available material of Ma. madagascariensis reveal that, although there are some general similarities, the Niger specimens have a more depressed neural arch, with a broader, lower neural canal; the centrum is more depressed with a broad, more strongly emarginated cotyle; and the hemal keel appears to be better defined and ends in a distinctly angular posterior margin (in Ma. madagascariensis, the posterior margin is more rounded in shape and bears two distinct tubercles). Differences between the specimens from Niger and those of Ma. madagascariensis appear to be at least at the level of species and we therefore recommend that the former be referred to as ?Madtsoia sp. until additional, more diagnostic material can be found, described, and compared.
CitationScanlon and Lee (2000:fig. 2f, g) demonstrated that postcloacal vertebrae of Wonambi naracoortensis possess ‘true’ chevron bones, which are not present in any modern snakes (CitationHoffstetter and Gasc, 1969). Chevron bones are present in non-ophidian lepidosaurs, but are represented in modern and most fossil snakes by the hemapophyses of postcloacal vertebrae, which are fused to the centrum and nearly always paired, but not fused distally. Articular surfaces that suggest the presence of chevron bones in FMNH PR 2557, an anterior postcloacal vertebra of Madtsoia madagascariensis, as well as in Alamitophis tingamarra (CitationScanlon, 1993:fig. 2B; Citation2005b:fig. 6D), support the idea that these structures may be characteristic of Madtsoiidae. If this is true, and if madtsoiids lie outside of ‘crown group snakes’ (Alethinophidia + Scolecophidia; Serpentes sensu CitationLee and Caldwell, 1998), then the presence of paired postcloacal hemapophyses may represent a synapomorphy of Alethinophidia (CitationLee and Scanlon, 2002; CitationScanlon, 2005a; but see CitationRieppel et al., 2002), given that all scolecophidians lack both chevron bones and hemapophyses (CitationList, 1966; CitationHoffstetter and Gasc, 1969). The presence of chevron bones in the pachyophiid Eupodophis descouensi (CitationRage and Escuillié, 2000) may offer further corroboration of this hypothesis, as pachyophiids, like madtsoiids, are often recovered in phylogenetic analyses as basal snakes, lying outside of Scolecophidia + Alethinophidia (e.g., CitationLee et al., 1999; CitationScanlon and Lee, 2000; CitationLee and Scanlon, 2002). However, other analyses have placed these snakes as basal macrostomatans, nested deeply within Alethinophidia (e.g., CitationTchernov et al., 2000; CitationRieppel et al., 2002; CitationApesteguía and Zaher, 2006). Moreover, the structure, position, and relations of the chevron bones in Eupodophis are rather different from those seen in Madtsoia, Wonambi, and Alamitophis, suggesting that these structures in Eupodophis might be autapomorphic rather than plesiomorphic (CitationRieppel and Head, 2004). Thus, the evolution of chevron bones and hemapophyses within snakes remains incompletely understood given the evidence that is currently available.
Ribs—The ribs of Madtsoia madagascariensis share the laterally recessed dorsal articular facet with Ma. bai and Ma. camposi (CitationSimpson, 1933; CitationRage, 1998). Madtsoia madagascariensis differs from Ma. bai in that it lacks a strong anterodorsal process. Madtsoia camposi is distinctive in that the ventral articular facet is thrust anteriorly relative to its position in other madtsoiids (indeed most snakes). Madtsoia camposi apparently shares with Ma. madagascariensis the presence of a ventral crest just distal to the rib head and slightly posterior in position. This crest is preserved in only a few specimens of each species, and appears to be most pronounced in smaller individuals. Although the distribution of foramina has not been reported for Ma. camposi, Ma. bai possesses a large dorsal foramen that is comparable to that of Ma. madagascariensis.
The ribs of Madtsoia madagascariensis resemble those of Wonambi and Yurlunggur in general proportions (CitationScanlon, 1992). However, a large dorsal foramen in Ma. madagascariensis is contained within a fossa, whereas variable numbers of smaller foramina are found in a dorsal groove in Wonambi and Yurlunggur (CitationScanlon, 1992). Although Nanowana, Alamitophis, and Patagoniophis are much smaller than Madtsoia, Nanowana and Alamitophis share the general proportions of the rib head of Ma. madagascariensis (CitationScanlon, 1993). Patagoniophis is more similar to Ma. bai in possessing an expanded anterodorsal process. Madtsoia madagascariensis ribs differ in a number of features from those of Menarana nosymena, described below. Most prominent among these differences is the less strongly recessed dorsal facet. Also, the tuber costae is not drawn out into a crest as it is in Me. nosymena, thus there is a dorsal fossa containing a foramen, rather than a sulcus or groove as in Me. nosymena. Finally, ribs of Menarana possess fewer foramina on their anteroventral and posterior surfaces.
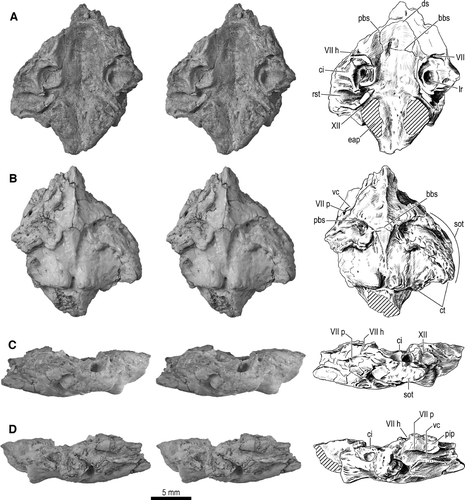
FIGURE 5 Braincase fragment, UA 9684-3 (part of holotype), of Menarana nosymena, gen. et sp. nov., from the Late Cretaceous of Madagascar. Stereophotographs (left and center) and interpretive drawings (right) of A, dorsal; B, ventral; C, left lateral; and D, right lateral views. Abbreviations: bbs, basisphenoid-basioccipital suture; ci, crista interfenestralis; ct, crista tuberalis; ds, dorsum sellae; eap, exoccipital ascending process; lr, lagenar recess; pbs, prootic-basisphenoid suture; pip, inferior process of prootic; rst, recessus scalae tympani; sot, spheno-occipital (basal) tubercle; vc, Vidian canal; VII, facial canal; VII h, foramen for hyomandibular branch of facial nerve; VII p, foramen for palatine branch of facial nerve; and XII, hypoglossal canal.
MENARANA, gen. nov.
Type Species—Menarana nosymena, sp. nov.
Referred Species—Madtsoia laurasiae Rage, 1996.
Diagnosis (modified in part from diagnosis of Madtsoia laurasiae by Rage, 1996a)—Vertebrae differ from those of other large madtsoiids in having lower to obsolete neural spines and more depressed neural arches, particularly in the posterior trunk series, and, with the possible exception of Gigantophis, in having anteroposteriorly expanded prezygapophyseal facets. Further differs from Madtsoia in possessing relatively narrow zygosphenes, diapophyses that do not extend laterally beyond prezygapophyseal facets, hemal keel in posterior trunk undercut laterally by subcentral grooves, keel approaching or exceeding width of condyle and cotyle, and the latter both subtriangular (flattened ventrally and narrowing dorsally). Most comparable to Patagoniophis in possessing low neural spine, depressed and shallowly emarginated neural arch, and hemal keel, defined laterally by grooves, not bifurcated posteriorly but with elongate lateral ridges on its posterior half; distinguished from Patagoniophis by much larger size and proportional differences, such as less elongate centrum. Ribs (based only on Me. nosymena) differ from those of all other madtsoiids in having a strongly recessed dorsal articular facet, leaving a medial pillar that supports the tuber costae posteriorly, and a dorsal crest that encloses a longitudinal sulcus containing a single, prominent foramen.
Etymology—From menarana (Malagasy, meaning ‘snake’). Pronounced may-na-RAH-na.
MENARANA NOSYMENA, gen. et sp. nov.
(–; )
Holotype Specimen—UA 9684, partial skeleton consisting of a large number of articulated or associated complete, nearly complete, and fragmentary vertebrae (including a partial atlas), several fragmentary ribs, and a sizable fragment of the braincase, all presumed to have been derived from the same individual because they share comparable morphology and similar preservational characteristics, represent the same-sized snake, and were collected from the same small area (∼2 m2) at Locality MAD93-14. For descriptive purposes and for tabulation of measurements in , suffixes were added to the specimen number for several individual elements. As such, in the description below, UA 9684-1 is a mid-trunk vertebra, UA 9684-2 is a posterior trunk vertebra, UA 9684-3 is the basicranial fragment, UA 9684-4 is the atlas, and UA 9684-5 is the proximal fragment of a right rib.
Diagnosis—Vertebrae differ from those of Menarana laurasiae in lacking ridge extending dorsomedially from posterodorsal part of diapophysis (interrupting interzygapophyseal ridge and extending to near anterior limit of neural spine), and in possessing shallower neural arch notch into which posterior portion of thicker neural spine projects, mediolaterally narrower zygapophyseal facets, and extremely broad and flat hemal keel on mid- and posterior trunk vertebrae (expanding to width of cotyle anteriorly and with margins drawn out into elongate lateral ridges in posterior half), in which both anterior and posterior ends are undercut laterally by subcentral grooves.
Etymology—From nosy (Malagasy, meaning ‘island’) and mena (Malagasy, meaning ‘red’), in reference to the commonly used nickname for Madagascar, the Red Island. Pronounced know-see-MAY-na.
Type Locality—MAD93-14, Berivotra Study Area, Mahajanga Basin, northwestern Madagascar.
Referred Specimens—Anterior trunk vertebrae: UA 9687 (two associated specimens, designated UA 9687-1 and UA 9687-2 for descriptive purposes). Mid-trunk vertebrae: FMNH PR 2543, FMNH PR 2544, FMNH PR 2703, UA 9686 (juvenile). Posterior trunk vertebra: FMNH PR 2542. Fragmentary vertebrae not assigned to region: UA 9685, UA 9713, UA 9733.
Localities—Berivotra Study Area localities MAD93-14, 93-16, 93-35, 99-31, 05-14; Masiakakoho Study Area localities MAD05-59, 07-37 ().
Age and Distribution—Known only from the Upper Cretaceous (Maastrichtian) Maevarano Formation, Berivotra and Masiakakoho study areas, Mahajanga Basin, northwestern Madagascar.
Description
Braincase Fragment—A single cranial fragment was found in association with the vertebrae and ribs of UA 9684. For descriptive purposes, it is designated UA 9684-3. It is considered to comprise most of the basioccipital and adjacent parts of the paired prootics and opisthotic-exoccipital complexes, as well as the median parabasisphenoid, fused together so that few traces of sutures are retained (). Such fusion of braincase elements, although apparently restricted among extant snakes to small fossorial forms (e.g., Scolecophidia, Uropeltidae; CitationList, 1966; CitationRieppel et al., 2009; CitationRieppel and Zaher, 2002; CitationCundall and Irish, 2008), is known in a large adult (but not in several smaller specimens) of Yurlunggur sp. (CitationScanlon 2006), and is thus consistent with referral of UA9684-3 to Madtsoiidae.
FIGURE 6 Braincase fragment, UA 9684-3 (part of holotype), of Menarana nosymena, gen. et sp. nov., from the Late Cretaceous of Madagascar in dorsolateral view (image obtained from HRXCT dataset). Abbreviations: bbs, basisphenoid-basioccipital suture; ci, crista interfenestralis; ds, dorsum sellae; eap, exoccipital ascending process; lc, lagenar crest; lr, lagenar recess; rst, recessus scalae tympani; VII, facial canal; and XII, hypoglossal canal.
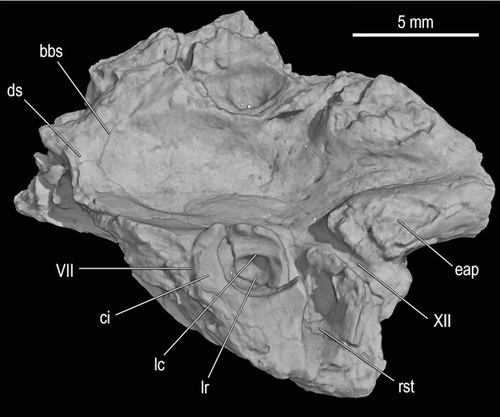
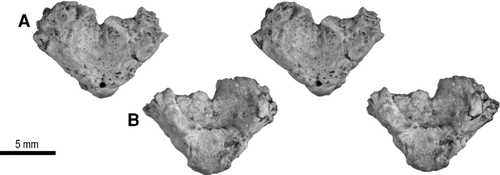
FIGURE 7 Atlas, UA 9684-4 (part of holotype), of Menarana nosymena, gen. et sp. nov., from the Late Cretaceous of Madagascar. Stereophotographs of A, anterior; and B, posterior views.
Remnants of sutural margins can be identified on the ventral (external) surface, but more distinctly on the dorsal (endocranial) surface. Postmortem cracks are also present in this specimen. In some instances it is difficult to differentiate between sutures and cracks, and the sutures are not perfectly symmetrical bilaterally; it is assumed here that some of the cracks were propagated along lines of weakness resulting from sutures or sutural remnants. The dorsal surface reveals an ‘H-shaped’ sutural pattern; the transverse suture across the midline appears to be the contact between basioccipital and parabasisphenoid, and it meets longitudinal sutures (approximately symmetrical, but indistinct posteriorly on the right side, where fusion may be more complete) interpreted as the junctions between the lateral margins of the basioccipital and parabasisphenoid and the medial margins of the prootics. Ventrally, there are deep transverse fissures approaching the midline between ridges representing the posterior margin of the parabasisphenoid and anterolateral crests of the basioccipital (the prootics are presumably exposed ventrally in the lateral part of these fissures, but recessed relative to the other bones), and a thin and interrupted suture across the midline where the sagittal crests of basioccipital and parabasisphenoid meet. No trace of sutures has been detected where the opisthotic-exoccipitals meet either the basioccipital or prootics, but the approximate locations of these boundaries can be inferred by comparison with Yurlunggur, Wonambi, and other squamates.
Posteriorly, the occipital condyle is broken off at an oblique fracture through its neck; this is nearly round in posterior view (slightly flattened dorsally) and no trace of exoccipital-basioccipital sutures is visible on the broken face, so it is unclear whether the exoccipitals met broadly on the dorsal surface of the condyle and neck. Robust ventral tubercles form a ‘collar’ on the neck, separated by a distinct median notch containing a prominent foramen and continuous laterally with the crista tuberalis of the exoccipital (nearly complete on the left side, damaged on the right), the combined crest being strongly concave ventrally. The specimen is broken horizontally just dorsal to the condylar neck, so that only a small ventral segment of the margin of the foramen magnum is preserved.
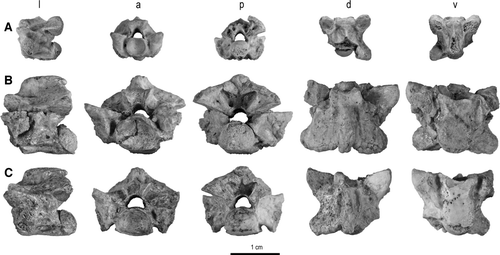
FIGURE 8 Trunk vertebrae of Menarana nosymena, gen. et sp. nov., from the Late Cretaceous of Madagascar in l, lateral; a, anterior; p, posterior; d, dorsal; and v, ventral views. A, anterior trunk vertebra, UA 9687-1 (lateral view reversed); B, mid-trunk vertebra, UA 9684-1 (part of holotype); C, posterior trunk vertebra, UA 9684-2 (part of holotype).
Nearly symmetrical, roughly triangular areas of breakage are seen in dorsal view immediately anterior to the condylar neck on either side of the foramen magnum. Between them is the anteriorly widening posterior part of the braincase floor, where sutures between exoccipitals and basioccipital would be expected (here regarded as fully fused, as in Yurlunggur sp. QMF45111; CitationScanlon, 2006). Six small foramina are present in this area, three on each side of the midline [cf. two and four foramina in Wonambi naracoortensis and Yurlunggur sp., respectively]. The preserved part of the braincase floor forms an elongate, bowl-shaped depression surrounded by broken surfaces, canals, and recesses of the ear region on either side.
The triangular broken areas (sections through ascending arches of exoccipitals) are bounded anterolaterally by canals that extend in a horizontal plane from the endocranial surface to emerge posterolaterally in a concavity dorsal to the crista tuberalis; these are interpreted as foramina for branches of the hypoglossal nerve (XII). (The only other identification to be considered, that of jugular foramina, is suggested by their relatively large size, but ruled out by their ventral position.) Anteriorly adjacent to these openings are a second pair of canals (fully exposed by breakage on the left side, but still partly roofed by bone on the right) that are impressed more deeply (ventrally) into the bone and open more widely in a more lateral position, as a dorsal trough extending (on the left side) to the most lateral part of the crista tuberalis. These canals are identified as the recessus scalae tympani (and thus as being bordered by the basioccipital, exoccipital, and opisthotic, where the latter two elements remain separated by the metotic fissure), and are discussed further below where comparisons are made with other taxa. The recess is overhung anteriorly by the narrow broken end of a bridge-like structure, expanding anteriorly into smoothly concave dorsolateral and dorsomedial surfaces separated by a dorsal ridge; this is the crista interfenestralis (anteroventral, opisthotic part of the opisthotic-exoccipital), bearing the ventral part of the crest defining the fenestra ovalis (occupied in life by the stapedial footplate) and thus separating the (lateral) juxtastapedial recess from the cavum vestibuli. In the floor of the latter is the deep, rounded lagenar recess, and these recesses, each mostly surrounded by vertical crests, are among the most conspicuous features of the specimen in dorsal view. The crista interfenestralis is less conspicuous on the right side as its lateral part is broken away. On the left, it apparently extended to the lateral surface of the braincase as part of the spheno-occipital (basal) tuber, but no distinct traces of sutures are visible between the crista interfenestralis, crista tuberalis, and prootic (in either dorsal or lateral view). Medial to the crista interfenestralis, the anterior wall of the recessus scalae tympani is smoothly continuous with the medially convex wall of the tympanic bulla (sensu CitationOelrich, 1956). Each lagenar recess is partly encircled by a dorsally open groove narrowest posteromedially, then widening anteriorly and ultimately curving back laterally around the medial edge of the recess, and sharply defined from it by an overhanging ridge of bone, the lagenar crest. Anterior to each recess is a transverse parapet of broken bone bordered anteriorly by the floor of a transverse canal, apparently quite unconnected to the cavum vestibuli, and identified here as the canal for the facial nerve (VII), distal to its divergence from the vestibulocochlear nerve (VIII) intracranially. The facial nerve canal is partly preserved for its full width on the left, but somewhat worn, and connects the cranial cavity to the external braincase wall. On the right, the medial part of the canal is broken away but the lateral part is more extensively preserved, including a ventral expansion deep within the bone, which is inferred to be where the palatine and hyomandibular branches of the nerve diverge toward their separate external foramina. The floor of the braincase slopes upward toward the anterior margin of the fragment, representing the posterior slope of the dorsum sellae. HRXCT reveals that a longitudinal groove in the braincase floor, crossing the transverse sutural remnant to the left of the midline, contains a single foramen (transverse slice number 168) that joins a transverse canal within the bone (mainly slice numbers 138–148); however, there is no trace of paired foramina or canals for the abducens (VI) nerves, so the crista sellaris must have been somewhat anterior to the broken edge.
TABLE 2 Measurements of vertebral specimens of Menarana nosymena, gen. et sp. nov. See text for list of abbreviations. ? = vertebral fragment not assigned to region.
In ventral view the specimen is marked by a distinct but smooth sagittal crest, and several more rugose and sculptured transverse crests. The sagittal crest is narrow anteriorly (the boundary of concave ventrolateral areas on the anterior one-third of the fragment) and disappears posteriorly just anterior to the paired ventral tubercles on the condylar neck, but is deepest where it forms a smooth-surfaced, kite-shaped expansion in the middle of the ventral surface; this lies directly between the deep ventrolateral troughs and is crossed by a narrow, sinuous groove that appears to be a remnant of the suture between the parabasisphenoid and basioccipital (as revealed by HRXCT scans, this groove is present only externally, supporting its identification as a fused suture). Extending laterally and somewhat anteriorly from this central ‘boss’ are somewhat sculptured crests formed by the posterior margin of the basisphenoid. More strongly sculptured, thicker, and more sinuous crests also extend posterolaterally from the boss, representing the anterolateral margins of the basioccipital, which are continuous laterally with the basal tubera (complete on left, broken off on right). Posterior to these crests, there is no clear distinction between the basioccipital and exoccipitals, and the paired tubera on the condylar neck are continuous laterally with the crista tuberalis, together extending almost directly laterally to meet the other crests at the basal tuber. The prootics are recessed in ventral view between the crests of the basisphenoid and basioccipital, forming deep transverse troughs as noted above, and these form deep, overhung depressions, pierced by several foramina, at their medial extremities where the three bones are interpreted to have met on each side.
FIGURE 9 Proximal fragment of right rib, UA 9684-5 (part of holotype), of Menarana nosymena, gen. et sp. nov., from the Late Cretaceous of Madagascar in A, anterior; B, posterior, and C, stereophotographic proximal views.
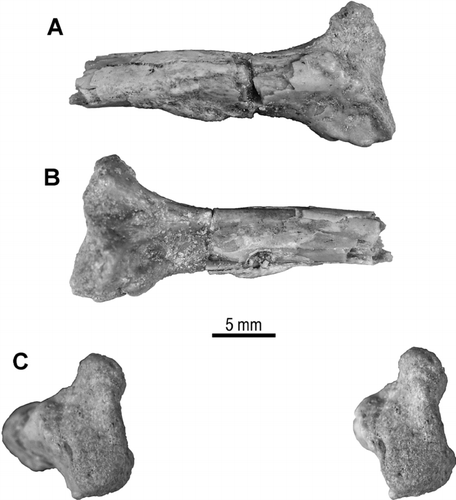
A direct lateral view of the element is difficult to interpret, but 3-D HRXCT renderings at several angles, from ventrolateral to dorsolateral, assist with identification of internal as well as external structures. The fragment is more complete posteriorly on the left side, but anteriorly on the right. The ear region is seen best in left dorsolateral view (), with the deep trough of the recessus scalae tympani diverging from the hypoglossal canal, overhung by the crista interfenestralis, and extending to near the lateral edge of the basal tuber. As far as preserved, there is no sign that the apertura lateralis (occipital recess) was subdivided by a dorsolateral contact between the crista tuberalis and crista interfenestralis (as it is in Wonambi, Yurlunggur, and most modern snakes). However, as in these snakes, and unlike the condition exhibited by Dinilysia and Najash, the fenestra ovalis (as marked by the crest on the crista interfenestralis) was deeply recessed from the lateral skull wall.
Fusion of the elements contributing to the basal tubera appears to be practically complete, so that the margins of the crista tuberalis, crista interfenestralis, basioccipital, and prootic are not discernible laterally; however, the dorsally broken crest forming the anterolateral part of the tuber and bounding the juxtastapedial recess (on both sides) can be identified as part of the crista prootica (forming the anterior part of the crista circumfenestralis). At the dorsolateral margin on each side, a notch represents the foramen for the hyomandibular branch of the facial nerve (as described in dorsal view above), and extending anteriorly from directly below it is a laterally open trough (partly preserved on left, more complete on right) identified as the parabasal (Vidian) canal. The canal is open posteriorly but defined by distinct dorsal and ventral margins that become deeper anteriorly, tending to close laterally (as preserved on right), but both margins are broken; although no suture is preserved, the dorsal and ventral margins of the canal represent parts of the prootic and basisphenoid. Under the overhanging prootic crest on each side (clearly visible ventrolaterally) is a foramen presumably for the palatine branch of the facial nerve, considerably smaller than the hyomandibular foramen posterodorsal to it. The right side preserves a considerable part of the lower anterior process of the prootic, but its anterior and dorsal surfaces are broken and no part of the trigeminal foramen is preserved.
A dorsolateral view of the specimen () allows observation of the medial aspect of the inner braincase wall, including the partly preserved hypoglossal foramen on each side (which would have been entirely within the exoccipital), medial aperture of the recessus scalae tympani (still roofed by bone on right side; at the boundary of the exoccipital and opisthotic with—in most squamates—the basioccipital, all three elements being fused here), and internal foramen of the facial nerve (partly preserved on left). No part of the acoustic foramen is preserved on either side, as the thin wall of the tympanic bulla is broken at too low a level.
The lagenar recess is normally connected to the recessus scalae tympani by the perilymphatic foramen, passing below the posteromedial part of the crista interfenestralis. It was initially unclear whether such passages were obscured by matrix within the recesses, but further preparation and HRXCT scanning shows that this is not the case. The broken posterodorsal margin of the lagenar crest is interrupted on each side (transverse slices 530–540), just medial to the crista interfenestralis, by a semicircular notch that is interpreted as the lower part of the perilymphatic foramen, in a similar position to that of Wonambi (illustrated but not named in CitationScanlon 2005a:fig. 11). There is no indication in micro-CT slices that there was a contact between the crista interfenestralis and crista tuberalis dividing the occipital recess into two lateral openings (i.e., the ‘fenestra pseudorotunda’ appears to be absent).
Atlas—A fragment associated with the skeleton of UA 9684, designated UA 9684-4 (), represents the intercentrum of the atlas and associated lower portions of the two neural arch halves. The size of this element also serves to confirm the association of the basicranial fragment described above with the trunk vertebrae of Menarana nosymena.
The entire structure is a very short, biconcave disc from which the upper parts of the two halves of the neural arch have been broken away. What is interpreted to be the anterior cotyle is evenly concave, narrower, and deeper than what is interpreted to be the posterior cotyle (these relative shapes are consistent with those in comparative specimens of extant snakes). The wall between the two cotyles is complete ventrally but incomplete dorsally, and is marked by a small, ventrally projecting, V-shaped notch. Two symmetrically positioned grooves on the anterior edge appear to mark the anterior portion of the now fused suture between the intercentrum and the neural arch halves. Posteriorly, the intercentrum bears a large articular surface to receive the dens/odontoid process of the axis. The hemal keel is thick and I-shaped, with the top horizontal part of the I situated posteriorly and longer than the bottom horizontal part of the I, which is situated anteriorly. In living snakes, the atlas remains tripartite (or bipartite, as in some uropeltids) throughout ontogeny, without fusion of sutures (CitationHoffstetter and Gasc, 1969).
Anterior Trunk Vertebrae—The only two definitive anterior trunk vertebrae, UA 9687-1 and UA 9687-2, are damaged. UA 9687-1 () is the most complete specimen and forms the primary basis for the following description; it is missing the neural spine and hypapophysis, and the posterior portion of the neural arch is damaged, though the right postzygapophysis is intact. UA 9687-2 is comprised of the centrum as well as the left prezygapophysis.
In ventral view, the centrum is short and broad, with strong subcentral ridges that converge toward the posterior end. The hypapophysis is broken off near its base, which is triangular in shape, with the sharp apex being directed anteriorly. The postzygapophyseal facet, preserved only on the right side, is rounded.
In anterior view, the cotyle is round and the neural canal triangular. The zygosphene is wedge-shaped with gently convex dorsal and slightly concave anterior borders. The zygapophyses do not extend very far lateral to the neural arch, and have nearly horizontal facets. Two paracotylar foramina are present on the right (one much larger than the other), and three small foramina are present on the left. The prezygapophyseal buttress is massive. There is no indication of any type of accessory processes.
From above, the zygosphene is narrow and the prezygapophyses are rounded. The posterior part of the neural arch is broken away, except for the right postzygapophysis. The interzygapophyseal ridge is well developed.
In lateral view, there is a single lateral foramen on each side. The synapophyses are heavily eroded, but their outline shows that they were large and rounded. The neural spine is missing.
In posterior view, the condyle is rounded and the neural canal is triangular and vaulted. There is a large parazygantral foramen above the intact right postzygapophysis, beside the zygantral facet. In general, the vertebra is taller, relative to its width, than those in the mid-trunk and posterior trunk regions.
Mid-trunk Vertebrae—A single mid-trunk vertebra, UA 9684-1 (part of holotype), was selected to serve as the primary specimen on which to base this description of mid-trunk vertebral morphology ().
In ventral view, the vertebra is broad with a wide, flattened hemal keel that occupies most of the ventral surface. The lateral edge of this keel region is pierced by a single subcentral foramen on each side. These foramina are closer to the anterior than to the posterior end. Lateral to the keel region, there are raised shelves that represent the lateral portion of the ventral face of the centrum. These raised areas are confluent with paracotylar notches and correspond to weakly developed subcentral paralymphatic fossae, indicating a position in the posterior portion of the mid-trunk region.
The anterior face is depressed, with a broad, low neural canal (though some dorsoventral crushing exaggerates the lowness) and a cotyle that is wider than high. The cotyle is also recessed and relatively emarginate below. The paracotylar fossae are distinct and each is flanked by a more or less vertically oriented keel that protrudes slightly forward from the prezygapophyseal buttress. On the anterior face of the prezygapophyseal buttress, just below the facet and lateral to the keel, there is a minute tubercle that points anteriorly. This tubercle differs in size, position, and orientation from typical prezyapophyseal accessory processes seen in most alethinophidian snakes. There is no obvious sign of a foramen in the paracotylar fossa on the right side and the fossa is damaged on the left. The zygosphene is narrow (just slightly wider than the neural canal) and wedge-shaped with a concave dorsal surface along its most anterior border. Its facets are oriented approximately 20° from the vertical. The prezygapophyses project laterally to a modest extent. Their facets are oriented at a low angle (approximately 20°) to the horizontal plane.
In posterior view, the condyle is subspherical (wider than high) and directed posterodorsally. The zygantrum is deep but not wide. Its anterior face is convex and bears endozygantral foramina (visible on at least the left side) within laterally positioned fossae. The neural arch pedicles are low, bringing the postzygapophyses into close proximity with the condyle. The posterior edge of the neural arch has a narrow, shallow notch that is occupied largely by the posterior edge of the neural spine. The posterior surface of the postzygapophysis bears a single foramen at the bottom of a shallow fossa.
In lateral view, the neural spine is barely raised above the level of the posterior margin of the neural arch laminae. The anterior end of the spine drops abruptly to the base of the zygosphene. Breakage in the interzygapophyseal ridge area of this specimen may obscure some detail. The subcentral ridges are strong and sharply angular anteriorly, just behind the synapophyses, but they curve medially and merge with the body of the centrum before reaching the condyle. The synapophyses of UA 9684-1 are eroded and their features cannot be determined.
From above, the vertebra appears broad and flattened. The neural spine, rugose along its dorsal aspect, is narrowly pointed anteriorly, but broadens into a lozenge-shaped structure, widest where the neural arch joins it near its posterior end. The posterior tip of the neural spine is blunt and wide and occupies most of the small posterior neural arch notch. Beside the base of the neural spine are distinct parazygosphenal fossae. These are delimited laterally by low ridges that proceed posteriorly from the lateral margins of the zygosphene. No parazygosphenal foramina were detected within or near the parazygosphenal fossae. The interzygapophyseal ridges are broad and thick. The zygosphene is narrow from above and concave anteriorly. The prezygapophyses are reniform in shape from above and project anterolaterally.
Most of the vertebrae identified as from the mid-trunk region of this species are similar to UA 9684-1. These include several additional complete and fragmentary specimens from UA 9684, as well as FMNH PR 2703 and FMNH PR 2543 (both nearly complete and well-preserved specimens), UA 9686 (a nearly complete immature vertebra), and FMNH PR 2544 (parts of two vertebrae, one a centrum and the other a neural arch). The width of the hemal keel and its distinctness from the ventral face of the centrum vary such that some specimens bear broadened, more flattened keels, similar to those in vertebrae identified as being from the posterior trunk region. These specimens are instead assigned to the mid-trunk region because their subcentral paralymphatic fossae and paracotylar notches are not as strongly developed as those of posterior trunk specimens. In some fragmentary vertebral centra of UA 9684, the synapophyses are well preserved. They include a rounded diapophyseal facet that projects laterally and slightly posteriorly, which is distinct from a ventral parapophyseal facet that is laterally flattened and projects slightly below the adjacent edge of the centrum (but not lower than the hemal keel). Although parazygosphenal foramina were not found in the parazygosphenal fossae of any specimens of Menarana nosymena, several vertebrae possess foramina on the base of the neural spine just above the fossa. These are interpreted as homologous to the parazygosphenal foramina identified in Madtsoia madagascariensis, but occupying a slightly different position. The foramina in the paracotylar fossae can be present on both sides (FMNH PR 2543, FMNH PR 2544), present on only one side (FMNH PR 2703), or absent on both sides (UA 9684-1, UA 9686). Similarly, distinct endozygantral foramina can be observed deep within the lateral fossae of the zygantra on some specimens, but not on others.
One vertebra, UA 9686, is assigned to this species on the basis of similar overall morphology with differences as would be expected in an immature specimen. Specific characters that support this assignment include the broad, depressed neural arch, broad hemal keel, low neural spine, and interzygapophyseal ridges that are broad and sharp. Features that support identification as a juvenile include the fact that the cotyle and condyle are largely composed of rough, unfinished endochondral bone surfaces. Also, the edge of the cotyle is particularly thin and is damaged in several places. Unusual features of this specimen include a zygosphene that is very narrow, less than the width of the cotyle. The neural spine is slightly taller than in other observed specimens, suggesting an anterior position within the mid-trunk series. The latter two features could also be due to its young age.
Posterior Trunk Vertebrae—Vertebrae from this region, represented by several specimens of UA 9684, but also FMNH PR 2542, are not noticeably more depressed, but are slightly narrower in aspect than the mid-trunk vertebrae. In addition, the zygapophyses do not extend as far from the neural arch and the hemal keels are broader and particularly flattened ventrally, and are more strongly differentiated from the centrum by deeply incised subcentral paralymphatic fossae. In the best-preserved posterior trunk vertebra of UA 9684, designated UA 9684-2 (), these fossae are incised so deeply as to undercut the lateral edge of the hemal keel, producing a distinctive lateral lip, especially on the left side. The paracotylar notches are deeper on this specimen as well. The neural spine, however, is not lower than is typical in other vertebrae examined; indeed, it is almost identical in size and shape as on the mid-trunk vertebra described above (UA 9684-1).
Ribs—Roughly 100 rib fragments, including approximately 20 rib heads (most of them poorly preserved), were recovered in association with the partial skeleton of UA 9684 (one of these is designated as UA 9684-5 and illustrated in ). The heads of these ribs have distinct dorsal (diapophyseal) and ventral (parapophyseal) facets of approximately equal size. Thus, the articular surfaces are about twice as high as they are wide. They also have well-developed tubera costae that are oriented approximately parallel to the long axes of the articular surfaces. These are produced into strong ridges that run distally along the posterodorsal border of the shaft for a considerable distance until they finally merge onto the rib shafts. The dorsal surface of each rib just distal to the dorsal articular facet is marked by a strong groove or sulcus that contains a prominent foramen. The ridge of the tuber costae forms the posterior border of this sulcus. The anterior aspect of the diapophyseal articular facet is strongly recessed distally from the level of the parapophyseal facet. However, the tuber costae is supported by a strong column of bone that extends dorsally from the more proximal parapophyseal facet. The diapophyseal articular surface extends onto the anterior surface of this column, producing a concave shape anteriorly, while maintaining a dorsoventrally convex aspect. The ventral articular facet is relatively flat and featureless, but is bounded by both anteroventral and posteroventral processes, the former more robust than the latter. The anterior face of the rib head has a ventrally situated fossa distal to the ventral facet and below the cylindrical extension of the shaft. This contains one or two foramina. The posteroventral process may be extended as a crest along the posteroventral border of the head and neck. A large foramen is present on the posterior surface at the point where the head narrows to form the neck of the rib on all specimens where this region is preserved.
Comparisons
Braincase—Relevant taxa for comparison include not only Wonambi and Yurlunggur (madtsoiids in which the basioccipital and adjacent elements are known—CitationBarrie, 1990; CitationScanlon and Lee, 2000; CitationRieppel et al., 2002; CitationScanlon 2003, 2005a, 2006), but also Najash (a braincase referred to N. rionegrina lacking the basioccipital but retaining adjacent bones—CitationApesteguía and Zaher, 2006), Dinilysia (a close outgroup to madtsoiids, the braincase of which is known by several specimens and closely resembles that of Najash as well as Australian forms—CitationCaldwell and Albino, 2002; CitationApesteguía and Zaher, 2006; CitationScanlon, 2006; CitationCaldwell and Calvo, 2008), basal modern snakes, and varanoid and mosasauroid ‘lizards’.
As noted already, the fusion of the basioccipital with all adjacent elements (parabasisphenoid, exoccipital-opisthotics, and prootics) is highly unusual, but a similar condition is known in one large specimen of Yurlunggur sp. (CitationScanlon 2006). This can be interpreted as a shared derived character of Madtsoiidae and can be predicted to occur in other members of the group, if only in late stages of ontogeny (near maximum adult size). Late ontogenetic fusions occur in some colubroid snakes, but this seems always to involve superficial overgrowth of sutures by discrete exostoses. Fractured surfaces that cross suture lines in the Yurlunggur specimen and in HRXCT slices in Menarana reveal that in both, the fusion of bones (indicated by uniform bone texture) is complete internally before the external and intracranial suture lines begin to disappear. On the other hand, whereas there is no positive sign of fusion of braincase elements in the comparably large Wonambi (CitationScanlon, 2005a), we note that only the basioccipital-parabasisphenoid suture was not disarticulated either before burial or during preparation, and might be co-ossified in SAMP30178. This suture remains unfused in the Yurlunggur specimen, but the order of fusions might vary between taxa.
Yurlunggur, Wonambi, and Menarana all exhibit mid-ventral keels on the basioccipital and parabasisphenoid, a common condition in Macrostomata (possibly convergently), whereas there are only paired crests, and the bones are concave across the midline in Dinilysia and the skull referred to Najash, as in ‘lizards.’ In both Wonambi and (most distinctly) Menarana, but not in Yurlunggur, the crests expand into a kite-shaped boss at the suture.
Menarana resembles Dinilysia in having the basal tubera extending far posteriorly, whereas in Wonambi they project mainly laterally, and in Yurlunggur they are oriented similarly to those of Menarana but are relatively shorter. Menarana also has an extremely thick ‘collar’ on the condylar process of the basioccipital, formed by large paired tubercles flanking a midline groove containing a foramen posterior to the sagittal keel, and continuous laterally with the almost transverse crista tuberalis. A similar ‘collar’ is present in various extant booid taxa (e.g., Python, Antaresia, Boa, and Eunectes), all of which bear distinct tubercles similar to those of Me. nosymena. This feature seems to be more frequently expressed in large individuals, hence it may be more an allometric function of size than a character indicating phylogenetic affinity. Dissection of a large Epicrates cenchria (unnumbered specimen in collection of first author) revealed that this ‘collar’ provides an attachment site for the atlanto-occipital ligament. In Yurlunggur there are also distinct but flatter tubercles posterior to the keel, and a midline foramen, but the ‘collar’ extends along the margins of the basioccipital toward the furthest anterolateral part of the crista tuberalis. Wonambi has weakly developed paired tubercles and a small foramen at the posterior end of the keel, and thin crests extending along the sides of the keel and diverging anterolaterally to intersect the thick anterolateral crests on the basioccipital. Dinilysia is most similar to Menarana in the orientation of these structures but crests are less prominent ventrally. Similar features and variation occur within macrostomatan groups, and the very strong development of these crests in Menarana implies relatively high loads at the craniovertebral joint, consistent with head-first burrowing behavior (see “Paleobiology and Paleoecology”).
The more posterior transverse ridges near the condylar process in the basicranial fragment of Menarana are absent in Wonambi, but are well developed in several examined specimens of Python, Antaresia, Boa, and Eunectes, all of which also bear distinct tubercles similar to those of Me. nosymena. In most alethinophidian snakes, a single basioccipital crest provides insertion points for the M. rectus capitis anterior, pars ventralis (medially), and the medial head of the M. rectus capitis anterior, pars dorsalis (lateral extremity) (CitationPregill, 1977).
Vertebrae—The morphology of these specimens agrees closely with that detailed by CitationRage (1996a, 1999) in his diagnosis of Madtsoia laurasiae, a species known only from vertebral specimens. The following diagnostic characters are shared between these two taxa: (1) neural spine very low, barely exceeding height of posterior border of neural arch; (2) neural arch depressed compared to that of other large madtsoiids; (3) zygosphene not as thick as in other large madtsoiids; and (4) diapophyses do not extend laterally beyond prezygapophyses. These similarities between Menarana nosymena and Me. laurasiae suggest a strong taxonomic affinity and that the two species may tentatively be regarded as sister taxa. However, vertebral morphology is notoriously labile among snakes and the possibility of a convergent adaptive response under similar selective regimes cannot be excluded. Nonetheless, in the absence of contradictory evidence, Madtsoia laurasiae is here transferred to the genus Menarana.
Despite their close similarities, vertebrae of Menarana nosymena differ from those of Me. laurasiae (see CitationRage, 1996a:fig. 1; 1999:fig. 10) in a number of features: (1) Me. nosymena lacks the raised process that extends posterodorsally from the diapophyses, described as diagnostic of Me. laurasiae (CitationRage, 1996a, 1999); (2) the zygapophyses are not as broad as those in Me. laurasiae; (3) the posterior neural arch notch is very shallow in Me. nosymena and largely filled by the posterior end of the neural spine, whereas the notch is deeply incised in the holotype of Me. laurasiae; and (4) the neural spines are very thick in Me. nosymena, especially posteriorly, and they project into the posterior neural arch notch, whereas in Me. laurasiae the spine appears thinner and has no posterior projection. Some of the Malagasy specimens have well-defined, narrow hemal keels, as described by CitationRage (1996a, 1999) for Me. laurasiae, but most have low, broad, flat hemal keels. In this respect, they agree with Rage's (1999) description of posterior trunk vertebrae. The posterior trunk vertebrae are marked by strongly developed subcentral fossae and paracotylar notches, which reinforces their assignment to this region of the column.
FIGURE 10 Trunk vertebrae of Kelyophis hechti, gen. et sp. nov., from the Late Cretaceous of Madagascar in l, lateral; a, anterior; p, posterior; d, dorsal; and v, ventral views. A, anterior trunk vertebra, FMNH PR 2539 (lateral view reversed); B, mid-trunk vertebra, UA 9682 (holotype).
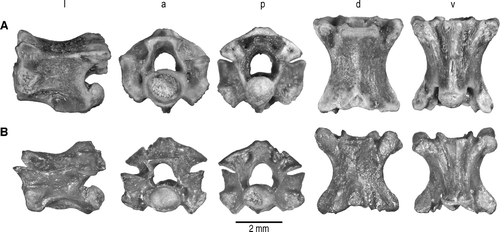
Finally, the following features of the Malagasy specimens are not mentioned by CitationRage (1996a, 1999) in his description of Menarana laurasiae and are not determinable from his illustrations: (1) parazygosphenal fossae are present, though not as deeply incised as those of Madtsoia madagascariensis, described above; and (2) the prezygapophyses have a minute, but distinct tubercle on the anterior face, near the lateral extremity, just below the facet, and facing directly anteriorly. The latter character appears to be a barely formed ‘accessory process,’ but its homology to that of other alethinophidians is doubtful. It does not agree in position with the accessory processes of boas and pythons, caenophidians, or other examined macrostomatans, in which the process is placed at the anterolateral corner of the prezygapophyses and is more laterally than anteriorly oriented. The absence of accessory processes is otherwise characteristic of the Madtsoiidae.
TABLE 3 Measurements of vertebral specimens of Kelyophis hechti, gen. et sp. nov. See text for list of abbreviations.
Ribs—The ribs of Menarana nosymena differ from those of Madtsoia madagascariensis in several features. The diapophyseal articular facet is much more strongly recessed distally than that of Ma. madagascariensis, producing a more strongly concave articular surface that is obliquely oriented in a cranial direction, and appears less prominent overall. The tuber costae of Me. nosymena is relatively larger than that of Ma. madagascariensis, and its size is accentuated by the recession of the diapophyseal surface from the posterior column that links it to the parapophyseal facet. The dorsal surface of the rib head of Me. nosymena lacks the pronounced tubercle seen in specimens of Ma. madagascariensis, and has in its place a strong crest. This crest encloses a distinct longitudinal sulcus that contains the dorsal rib foramen in Me. nosymena. The dorsal foramen is not found within a sulcus in Ma. madagascariensis. The anteroventral and posteroventral crests are not as distinct as those of Ma. madagascariensis (as seen in UA 9714), and the ventral anterior fossa usually includes only one foramen, occasionally two, whereas in Ma. madagascariensis, the known specimens exhibit three such foramina.
Other authors have identified several differences among the ribs of madtsoiid snakes. These include the prominence of the dorsal (diapophyseal) articular facet, its position relative to the ventral (parapophyseal) facet, and whether it bears an anterodorsal process; the size and orientation of the tuber costae; and the presence of a dorsal groove and the number of foramina that it contains. The ribs of Madtsoia bai differ significantly from those of either Malagasy species in that they possess a marked anterodorsal process and the tuber costae is strongly tilted posteriorly. Madtsoia bai does possess a dorsal groove.
NIGEROPHIIDAE CitationRage, 1975
KELYOPHIS, gen. nov.
Type Species–Kelyophis hechti, gen. et. sp. nov.
Diagnosis—As for the type and only species.
Etymology—From kely (Malagasy), meaning ‘small,’ and ophis (Greek), meaning ‘serpent’; in reference to the small size of this snake. Pronounced kay-lee-O-phis.
KELYOPHIS HECHTI, gen. et sp. nov.
(; )
Holotype Specimen—UA 9682, a single, nearly complete mid-trunk vertebra from a juvenile individual.
Diagnosis—Nigerophiid with synapophyses positioned ventrally, but less so than in Nigerophis and Indophis. Centrum downswept posteriorly, as in former two genera, but also distinctly narrowed posteriorly, unlike Nigerophis and Indophis. Postzygapophyses elevated above level of condyle, but not as strongly as in Nigerophis and Indophis. Neural spines lower than those of Indophis, but of comparable height to those of Nigerophis. Further differs from other nigerophiid genera in having more robust vertebrae with stronger interzygapophyseal ridges, subcentral ridges, and prezygapophyseal buttresses.
Etymology—Named for the late Dr. Max K. Hecht, graduate advisor of the senior author, for his contributions to knowledge of reptilian evolution and for suggesting this study to the senior author.
Type Locality—MAD93-01, Berivotra Study Area, Mahajanga Basin, northwestern Madagascar.
Referred Specimens—Anterior trunk vertebrae: FMNH PR 2539 (juvenile), FMNH PR 2540, UA 9683. Mid-trunk vertebra: FMNH PR 2541 (juvenile). Fragmentary vertebra not assigned to region: UA 9725.
Localities—Berivotra Study Area localities MAD93-01, 93-35, and 93-37; Masiakakoho Study Area localities MAD03-15 and 05-59 ().
Age and Distribution—Known only from the Upper Cretaceous (Maastrichtian) Maevarano Formation, Berivotra and Masiakakoho study areas, Mahajanga Basin, northwestern Madagascar.
Description
Six vertebral specimens are assigned to this new taxon, three of which (FMNH PR 2539 and FMNH PR 2540, both from the anterior trunk region, and UA 9682, from the mid-trunk region) are relatively complete and well preserved; they serve as the primary basis for the following description. The other three specimens, UA 9683 (partial centrum from anterior trunk series), FMNH PR 2541 (fragmentary and poorly preserved mid-trunk centrum and partial neural arch), and UA 9725 (centrum not assigned to vertebral region) contribute little, other than the fact that UA 9683 is the largest known specimen of Kelyophis hechti (). UA 9682, as well as FMNH PR 2539 and FMNH PR 2541, are identified as representing juvenile individuals based on their small size as well as by preservation characteristics employed by CitationLaDuke (1991). Specifically, the perichondral walls of the vertebra are thin, but opaque. This is particularly notable near the edges of damaged surfaces. Furthermore, a remnant of the early endochondral ossification center of the centrum is clearly visible (much lighter color) in the center of the cotyle.
Anterior Trunk Vertebrae—Two specimens clearly belong to the anterior trunk series and form the basis for the following description. FMNH PR 2540 represents an adult individual, whereas FMNH PR 2539 () is much smaller and represents a juvenile ().
In ventral view, the centrum is elongate, narrow, and has strong subcentral ridges. The hemal keel is broken posteriorly on FMNH PR 2540, but clearly did not contact the condyle; this is less clearly the case in FMNH PR 2539, which, although more complete, exhibits some effects of postmortem erosion. Anteriorly, the hemal keel gives rise to a broad, flattened, triangular plate that merges into the lower lip of the cotyle. This flattened platform bears a slight mid-ventral ridge. The eroded parapophyses project ventrally to (FMNH PR 2539) or beyond (FMNH PR 2540) the ventral border of the centrum.
In anterior view, the cotyle is circular in outline. Deep paracotylar fossae possess a single foramen on each side. The synapophyses are eroded, but are located in a relatively ventral position. The prezygapophyseal buttress above the diapophysis is strong, but does not produce an anterolateral keel, as it does in Nigerophis. The prezygapophyseal facets are somewhat elevated, and tilted dorsally at their lateral ends. Their distal tips are broken away on both specimens. The neural canal is roughly triangular in shape, broader ventrally than dorsally. The zygosphene (broken off on the left side of FMNH PR 2540 but complete in FMNH PR 2539) is moderately thin and convex dorsally; as for vertebrae in the mid-trunk region, it is roughly the same width as the cotyle.
In posterior view, the condyle is small and round. The posterior end of the neural arch is largely broken away in FMNH PR 2540, including the zygantrum, but enough of the left postzygapophysis is present to see a parazygantral foramen in a shallow fossa. The neural arch is complete and well preserved in FMNH PR 2539 and the parazygantral foramina are clearly present.
In dorsal view, the neural arch is relatively long and narrow. The laminae meet at a slightly peaked mid-sagittal ridge (less pronounced in FMNH PR 2539 than in FMNH PR 2540). The posterior portion of the neural arch is broken away in FMNH PR 2540, but the relatively complete neural arch of FMNH PR 2539 demonstrates that the neural spine was, at most, poorly developed and strongly restricted to the posteriormost end of the neural arch. The prezygapophyseal facets are teardrop-shaped and directed anterolaterally. The zygosphene is narrow and, as best seen in FMNH PR 2539, its anterior margin is shallowly concave. The relatively shallow interzygapophyseal constriction is narrowest anterior to mid-vertebra.
In lateral view, the neural arch laminae and pedicles meet at a sharp angle, producing a strong interzygapophyseal ridge. The body of the centrum is downswept posteriorly, whereas the neural arch is slightly upswept. There is a distinct lateral foramen on the pedicle, just above the subcentral ridge on each side of FMNH PR 2540. One or more smaller, much less distinct foramina lie between this primary foramen and the interzygapophyseal ridge. Two or more lateral foramina also occur on the laminae of FMNH PR 2539. The parapophysis apparently had the same posterior connection to the subcentral ridges seen in the holotype. The prezygapophyseal buttress and synapophysis are more robustly developed in FMNH PR 2540 than in the juvenile specimens. The hemal keel of FMNH PR 2540 is broken off posteriorly and that of FMNH PR 2539 is eroded but, in both cases, the remnants suggest that it was produced into a low hypapophysis, in which case these specimens are derived from the posterior portion of the anterior trunk series.
Mid-trunk Vertebrae—UA 9682, the holotype, is a nearly complete vertebra from the posterior portion of the mid-trunk region (). It is small, with a centrum length (lower lip of cotyle to extremity of condyle) of 3.0 mm and a width across the postzygapophyses of 3.5 mm. We regard this specimen to represent a juvenile individual but proportions of various parts of the vertebra suggest that it was approaching an adult shape. For example, the neural canal, although relatively large, is narrow. Furthermore, the zygosphene is relatively narrow, the zygapophyses are relatively large, and the centrum is elongate and narrow in ventral view, as in adult snakes. The specimen is identified as a mid-trunk vertebra because it lacks a hypapophysis and its subcentral paralymphatic notches and fossae are only weakly developed.
The centrum of the vertebra is elongate and narrow in ventral view, widening moderately at the synapophyses. The lateral border of the centrum is smoothly rounded posteriorly, but gives rise to a moderately developed subcentral ridge anteriorly. This ridge is confluent with a posterior extension of the parapophysis. The hemal keel is distinct, and strongly projecting ventrally from the centrum, especially at its posterior extremity. Although there is a chip of bone missing from the middle of the hemal keel, the posterior end is intact and indicates the absence of a hypapophysis. The hemal keel widens anteriorly to produce a triangular platform that forms the ventral lip of the cotyle. The ventral surface of the centrum is slightly indented adjacent to the margin of the hemal keel, and there is a single, small foramen on each side, at the deepest part of the indentation. A slight notch occurs between the parapophyses and the lower lip of the cotyle. Following the positioning criteria of CitationLaDuke (1991), this vertebra is from the posterior portion of the mid-trunk region. The postzygapophyseal facets are slightly elongate ovals (slightly truncated on the right by breakage) that are directed obliquely posterolaterally (more posteriorly than laterally).
In anterior view, the cotyle is depressed with only faint evidence of ventrolateral emargination of the lip. Dorsally, the edge of the lip is broken away. Distinct paracotylar fossae on each side contain a single, large foramen. The neural canal is high, narrow, arched dorsally, and surmounted by a thin, narrow zygosphene. The anterior border of the zygosphene is concave in dorsal view, with anteriorly directed lateral extensions produced by the zygosphenal facets. The zygosphene is subequal in width to the cotyle. The synapophyses are divided into parapophyseal and diapophyseal regions by a weak posterior indentation (only the left synapophysis has an intact articular surface).
In dorsal view, the prezygapophyseal facets are elongate, oval surfaces that are directed obliquely anterolaterally (more anteriorly than laterally). They bear no trace of accessory processes, and the diapophyses extend further laterally than the prezygapophyses. The interzygapophyseal ridge is distinct, and moderately developed. The relatively shallow interzygapophyseal constriction is narrowest anterior to mid-vertebra. The neural arch has a smoothly rounded dorsolateral surface. The neural spine, though eroded dorsally, is a very low, short, posteriorly restricted tubercle. The base of the neural spine is roughly triangular in section, the apex being directed anteriorly. The posterior neural arch notch is very shallow.
In posterior view, the neural arch is depressed, but dorsally positioned, leaving a larger-than-typical gap between the postzygapophyses and the condyle. The zygantral facets extend slightly beyond the posterior margin of the neural arch laminae. Lateral to the zygantral facets are shallow fossae with distinct parazygantral foramina. The condyle is relatively small and depressed. A significant portion of its surface (mostly on the left side) is broken away, but the overall shape is clear.
In lateral view, the neural spine is restricted to the posterior part of the neural arch. Although the top of the spine is slightly eroded, it does not appear to have been more than a low tubercle. The diapophysis is larger than the parapophysis and bulbously convex. The parapophysis lacks a parapophyseal process, but has a distinct posterior extension that is connected to the anterior extremity of the subcentral ridge. The interzygapophyseal ridge is distinct, but only moderately developed. There is a minute foramen in the middle of the pedicle of the neural arch.
Comparisons
In addition to its small size, Kelyophis is assigned to the Nigerophiidae on the basis of its elongate vertebral centrum, tubercular shape of the neural spine and its restriction to the posterior portion of the neural arch, reduced notch of the neural arch, absence of true accessory processes on the prezygapophyses, ventral deflection of the posterior portion of the centrum, elevation of the zygapophyseal facets above the centrum, and the somewhat ventrally positioned and ventrolaterally directed synapophyses (CitationRage and Werner, 1999). Parazygantral and paracotylar foramina are absent in most nigerophiids (Nessovophis, Nigerophis, Nubianophis, and Woutersophis) but are present in Kelyophis and some specimens of Indophis sahnii. As for many basal snakes, members (including questionable members) of the family Nigerophiidae are characterized by the absence of hypapophyses in mid- and posterior trunk vertebrae (CitationRage, 1975, 1980; CitationRage and Prasad, 1992; CitationWerner and Rage, 1994; CitationPrasad and Rage, 1995; CitationAverianov, 1997; CitationRage and Werner, 1999; CitationRage et al., 2003, Citation2004). CitationRage and Werner (1999) refined the diagnosis of the Nigerophiidae and underscored the tentativeness of the assignment of Indophis to this family. Yet, as currently defined, it is the clade to which Kelyophis should be assigned.
Kelyophis hechti differs from Nigerophis and Nubianophis in possessing stronger subcentral and interzygapophyseal ridges, and centra that are broader anteriorly than posteriorly. In these regards, the Malagasy specimens more closely resemble those of Indophis sahnii. In addition, Indophis and Kelyophis both possess parazygantral foramina, a characteristic generally associated with Madtsoiidae. Indophis, Nessovophis, and Nigerophis all possess prominent vertical ridges on the anterolateral aspect of the prezygapophyseal buttresses that project beyond the prezygapophyseal facet. These are lacking in Kelyophis (condition not known in Nubianophis). Nessovophis is unusual in possessing a centrum that is triangular in cross section, but a similar section of the centrum of Nigerophis is subtriangular (CitationRage, 1975). Published figures of I. sahnii (CitationRage and Prasad, 1992:figs. 1–5; CitationPrasad and Rage, 1995:fig. 15; CitationRage et al., 2004:fig. 3F–H) indicate that it differs from Kelyophis in that it has a higher neural spine, more dorsally deflected posterior neural arch, more ventrally positioned synapophyses, and a narrower, higher aspect overall. Thus the two forms are different enough to warrant distinction at the generic level. Moreover, both FMNH PR 2540 and UA 9683, vertebrae of mature K. hechti, are considerably larger than any Indophis specimens. Some of the differences noted, such as the robustness of the prezygapophyseal buttress and synapophyses in the present material, may thus be due to allometric changes with increased size. Based on their overall similarity, if Indophis is ultimately removed from the Nigerophiidae, then Kelyophis will probably be removed as well.
INDETERMINATE SPECIMENS
There are a number of specimens that cannot be assigned, primarily owing to their incompleteness, to any of the species listed above, even though they may, indeed, pertain to them.
The following specimens, listed by locality, possess one or more features of the Madtsoiidae and are referable to that family: Locality MAD93-35: FMNH PR 2572—nearly complete but eroded vertebra. Locality MAD93-38: FMNH PR 2573—fragmentary vertebra. Locality MAD93-73: FMNH PR 2577—complete vertebra.
The following specimens are clearly parts of snake vertebrae but cannot be assigned confidently even to familial level: Locality MAD93-01: FMNH PR 2574—vertebral centrum. Locality MAD93-06: UA 9719—vertebral fragment. Locality MAD93-19: UA 9720—zygosphene. Locality MAD93-27: FMNH PR 2575—vertebral centrum. Locality MAD93-35: FMNH PR 2579—vertebral centrum. Locality MAD93-40: FMNH PR 2576—vertebral fragment. Locality MAD93-44: UA 9722—vertebral fragment. Locality MAD93-81: UA 9723—zygosphene, UA 9724—vertebral centrum, UA 9732—vertebral condyle. Locality MAD93-86: FMNH PR 2578—vertebral centrum. Locality MAD05-59: FMNH PR 2580—neural arch.
DISCUSSION
Diversity and Abundance
New species of extant non-marine Malagasy snakes are being discovered and described at a rapid rate (an average of more than one species per year for the last 25 years [CitationCadle, 2003]). There are now over 85 extant non-marine species of snakes known from Madagascar (and the nearby islands of the Comoros and La Réunion) (CitationRaxworthy, 2003). Of these, three are boids, 74 are colubrids, and 11 are typhlopids. All except one species of typhlopid (Ramphotyphlops braminus) are considered endemic.
Not surprisingly, the assemblage of snakes recovered as a result of the Mahajanga Basin Project reveals a much lower diversity on Madagascar during the Late Cretaceous. This most likely reflects reality, owing to a lack of diversification, but is also undoubtedly influenced strongly by relatively poor sampling. Nonetheless, the Mahajanga Basin Project collections demonstrate that Late Cretaceous snake species diversity on the island was greater than previously reported (CitationPiveteau, 1933; CitationHoffstetter, 1961a; CitationRage, 1984), with the addition of a new genus and species of madtsoiid, Menarana nosymena, and a new genus and species of nigerophiid, Kelyophis hechti. Furthermore, relatively abundant and well-preserved new material of a previously known form, Madtsoia madagascariensis, provides a vastly improved assessment of variability in vertebral anatomy and the first information on rib anatomy. Based on numbers of specimens (94 from over 30 localities), Ma. madagascariensis appears to be much more abundant in the assemblage than the other two species but, again, this could well represent a sampling artifact in that Ma. madagascariensis was much larger (and thus fossils of it are easier to find) than either Me. nosymena (nine specimens from six localities) or K. hechti (six specimens from five localities).
Phylogeny
The primary objective of this report is to identify and describe the two madtsoiid taxa and the one nigerophiid taxon that occur in the Maevarano Formation assemblage. Comprehensive and robust phylogenetic assessment of generic or species-level relationships within the families Madtsoiidae and Nigerophiidae have not been published but are beyond the scope of this paper; as such, phylogenetic placement of Madtsoia madagascariensis and Menarana nosymena within Madtsoiidae and Kelyophis hechti within Nigerophiidae is not rigorously evaluated. A particular hindrance for assessing madtsoiid relationships relates to the currently very poor knowledge of serial variation in the vertebrae of madtsoiid taxa that are reasonably well represented by fossil material. Scanlon (in prep.) is currently conducting such an assessment for the Australian madtsoiid Yurlunggur, now represented by several skeletons, and will employ those data, coupled with the character states gleaned from the Malagasy madtsoiids detailed in this paper, in a more comprehensive analysis of madtsoiid relationships. Assessment of the phylogenetic relationships of Nigerophiidae is plagued by a more severe problem: the fact that the contained taxa are each represented by only a few vertebrae. The wisdom of attempting a phylogenetic assessment, such as that conducted by CitationAverianov (1997) for nigerophiids (and palaeophiids), by employing characters derived strictly from isolated vertebrae has been questioned by CitationRage et al. (2003:699).
Biogeography
Madtsoiidae—Madtsoiids were widespread on Gondwana during the Late Cretaceous and Paleogene, known from all major landmasses except Antarctica (; ). They disappear from the fossil record in the mid-Paleogene except in Australia, where they survived into the Pleistocene. Removal of Menarana laurasiae from the genus Madtsoia leaves only three valid species in the genus: Ma. bai from the early Eocene (and possibly late Paleocene) of Argentina, Ma. camposi from the middle Paleocene of Brazil, and Ma. madagascariensis from the Late Cretaceous of Madagascar. Phylogenetic and therefore biogeographic ties between various clades of latest Cretaceous vertebrates of Madagascar with those of South America (and the Indian subcontinent) are now well documented (e.g., see CitationKrause et al. [2006] for review of evidence from crocodyliforms, non-avian dinosaurs, and mammals; and CitationEvans et al. [2008] for evidence from frogs). It is tempting to suggest that the genus Madtsoia provides yet another line of independent evidence of such a special connection (i.e., to the exclusion of others). However, the fact that there are many other snake fossils assigned to Madtsoia (or ?Madtsoia) or to the Madtsoiidae (or ?Madtsoiidae) from the Late Cretaceous of Argentina, Niger, Sudan, Romania, France, and India, as well as from the Paleogene of Argentina, Bolivia, Morocco, India, and Australia (see ; ), reveals a potentially much broader distribution and more complicated pattern. This underscores the need for a comprehensive taxonomic review and phylogenetic assessment of the Madtsoiidae before any firm biogeographic conclusions can be drawn.
FIGURE 11 Temporal and geographic distribution of madtsoiid (localities indicated by solid symbols, numbers) and nigerophiid (open symbols, letters) snakes. See and for more detailed information.
TABLE 4 Temporal and geographic distribution of madtsoiid and questionably madtsoiid snakes (arranged alphabetically by genus and species, and then by more uncertain family-level attributions). : = Early; = Middle; = Late.
TABLE 5 Temporal and geographic distribution of nigerophiid and questionably nigerophiid snakes (arranged alphabetically).
Nonetheless, the disjunct distribution of the two known species of Menarana, Me. laurasiae from the Campanian of Spain (CitationRage, 1996a, 1999) and Me. nosymena from the Maastrichtian of Madagascar, invites consideration. Specimens of madtsoiids, or questionable madtsoiids, have also been reported from the Campanian of France (CitationSigé et al., 1997) and the Maastrichtian of Romania (CitationFolie and Codrea, 2005). Other typically Gondwanan vertebrate taxa are known from these and other Campanian/Maastrichtian localities in southern Europe as well (CitationPereda-Superbiola, 2009), but there are no pre-Campanian Late Cretaceous records of madtsoiids from Europe. Gheerbrant and Rage (2006:236) opined that the “European madtsoiids were immigrants from Africa that reached Europe probably during the Late Cretaceous” (see also CitationRage, 1995, 1996a, 1999; CitationRage et al., 2008). When during the Late Cretaceous is, however, a critical question and CitationPereda-Superbiola (2009) notes that nothing excludes a dispersal event during the Early Cretaceous. In this regard, the composition of the snake fauna from the Late Cretaceous of Africa is obviously relevant. There are no Campanian/Maastrichtian records of madtsoiids from Africa at all. Late Cretaceous African madtsoiids are known only from the early Senonian of Niger (Citationde Broin et al., 1974; CitationRage, 1981; Madtsoia aff. madagascariensis—re-identified in this paper as ?Madtsoia sp.) and the Cenomanian of Sudan (CitationRage and Werner, 1999; Madtsoiidae indet.). These two pre-Campanian records, therefore, are too indeterminate to shed light on the issue.
In light of this poor fossil record, there are several possibilities to explain the presence of Menarana (and other shared faunal elements) in the Campanian/Maastrichtian of Madagascar and southern Europe, including the following.
-
A pan-Gondwanan (including southern Europe) or nearly pan-Gondwanan (for the issue at hand, Australia is more or less peripheral to the argument) Early Cretaceous distribution of which the Campanian/Maastrichtian records of Menarana in Madagascar and southern Europe are relictual. During the Late Cretaceous, Europe was a complex archipelago (CitationDercourt et al., 2000) and, in a recent assessment of the biogeographic affinities of Late Cretaceous European tetrapods, CitationPereda-Superbiola (2009:65) concluded that “isolation from other landmasses may have facilitated survival of relict taxa in Europe until Campanian-Maastrichitan times.” This scenario, if it pertains to Menarana, requires that Menarana evolved before South America separated from Africa at or near the Early/Late Cretaceous boundary. This can be confirmed, in part, through the discovery of Menarana in pre-Late Cretaceous horizons on any Gondwanan landmass. The absence of such records, especially from the relatively well-sampled South American record, is currently the only evidence against this scenario.
-
Africa (only) as an intermediate landmass, with connections to Madagascar via a Late Cretaceous land bridge or via sweepstakes dispersal across the marine barrier formed by the Mozambique Channel, with a minimal distance of 430 km. Discovery of Menarana in the Cretaceous of Africa (and non-discovery from South America) would at least be consistent with this scenario. CitationGheerbrant and Rage (2006) argued that the presence of Madtsoia aff. madagascariensis in the pre-Campanian Late Cretaceous of Niger and M. madagascariensis in the Maastrichtian of Madagascar indicates that there was dispersal between the two landmasses, purportedly along a land bridge formed by the Davie Ridge (CitationTaquet, 1982; CitationRage, 1988; see also CitationMcCall, 1997). Re-identification of the Niger madtsoiid as ?Madtsoia sp. de-emphasizes the need to invoke a land bridge. In any case, there is no evidence that one existed. The Davie Ridge is a north-south linear feature on the floor of the Mozambique Channel, paralleling the east coast of Tanzania and Mozambique, that represents the strike-slip fault along which Madagascar moved southward some 1000 km until it reached its current position relative to Africa roughly 120 Ma, when it re-sutured with the African plate. There is no geological evidence for a continuous, emergent land bridge along the Davie Ridge during the Late Cretaceous and Early Tertiary and, indeed, there is considerable faunal evidence, albeit largely negative, against it (Krause et al., 1997a, Citation1999; CitationRabinowitz and Woods, 2006). Whether or not Menarana dispersed across the Mozambique Channel without the aid of a land bridge is impossible to test in light of the current fossil record.
-
A northern dispersal route between Madagascar and Europe that involved the Seychelles Plateau, the Indian subcontinent, and mainland Asia (CitationRage, 1996b, 2003). This seems highly unlikely for several reasons. First, it assumes that “the absence of numerous taxa in Africa… represent true absences” and that “the role of Africa as an intervening landmass… is ruled out” (CitationRage, 2003:661). The generally very poor record of Late Cretaceous fossil vertebrates from mainland Africa, particularly from Campanian/Maastrichtian horizons, as detailed above, does not permit these assumptions. Second, physical connection between the passive margin of the Indian subcontinent and the active margin of Asia likely did not occur until at least 55 Ma (e.g., CitationAli and Aitchison, 2008; CitationGarzanti, 2008). Even if it occurred at the very end of the Cretaceous, as CitationRage (2003) suggests, it is too late to account for the presence of Menarana in the Campanian of Spain if the dispersal was from the Indian subcontinent to Eurasia and also too late to have provided a connection to Madagascar if the dispersal was from Eurasia to the Indian subcontinent since the India-Seychelles block separated from Madagascar at roughly 88 Ma (CitationStorey et al., 1995, Citation1997). Third, there is not a single record of Menarana from well-sampled Cretaceous horizons in either the Indian subcontinent or mainland Asia.
Clearly, a convincing explanation to account for the disjunct distribution of species of Menarana in Campanian/Maastrichtian horizons of Madagascar and southern Europe is still lacking but, of the scenarios considered, the first seems the most likely.
Nigerophiidae—Nigerophiids are much less well known than madtsoiids in terms of both species diversity and temporal and geographic distribution (; ). Their earliest known occurrence is in the Cenomanian of Africa (Nubianophis, Sudan—CitationRage and Werner, 1999), where they survived into at least the Paleocene (Nigerophis, Niger—CitationRage, 1975). Referral of taxa recovered from the Maastrichtian of the Indian subcontinent (Indophis, India—CitationRage and Prasad, 1992; CitationPrasad and Rage, 1995; CitationRage et al., 2004), the Eocene of western Asia (“Nessovophis,” Kazakhstan—CitationAverianov, 1997; CitationRage et al., 2003), and the Eocene of Europe (Wouterophis, Belgium—CitationRage, 1980) to the Nigerophiidae is tentative. The addition of Kelyophis from the Maastrichtian of Madagascar, especially in the absence of an hypothesis of relationships among nigerophiids, does little to elucidate the biogeographic history of this poorly known clade other than to add another element for future consideration when a rigorous assessment of Nigerophiidae is undertaken; again, such an assessment is beyond the scope of this study.
Origins of the Modern Malagasy Snake Fauna—The occurrence of snake remains in the Upper Cretaceous Maevarano Formation currently provides the only direct fossil evidence of potential relevance to elucidate the biogeographic history of the diverse radiation of extant snakes on Madagascar. The fossil snake assemblage recovered from the Mahajanga Basin includes only madtsoiids and nigerophiids and is therefore archaic in aspect; none of the extant Malagasy families (Boidae, “Colubridae,” Typhlopidae) are represented.
The biogeography of the Malagasy herpetofauna has historically been a subject of considerable interest (e.g., CitationMertens, 1972; CitationRage, 1996b; CitationVences et al., 2001, Citation2003; CitationRaxworthy et al., 2002; CitationNagy et al., 2003; CitationNoonan and Chippindale, 2006a, Citation2006b). Based on molecular phylogenetic evidence, Noonan and Chippindale (Citation2006a, Citation2006b) recently suggested that several Malagasy reptilian groups (including boid snakes) originated as vicariant derivatives of more cosmopolitan Gondwanan lineages when Madagascar became isolated from the other southern landmasses. This claim is based on similar estimated timing of divergence of the Malagasy groups, with Boidae originating approximately 75 Ma, Pelomedusidae 80 Ma, and Iguanidae 67 Ma, and general congruence of these events with the separation of Madagascar from the Indian subcontinent (ca. 88 Ma—CitationStorey et al., 1995, Citation1997) and Antarctica (as recent as ca. 80 Ma—CitationHay et al., 1999). The present study, based on a sample of fossils from an admittedly small area and a thin time horizon in the Mahajanga Basin of northwestern Madagascar, provides no support for the hypothesis that boid snakes were present on the island at the time that it rifted from the other Gondwanan plates. Furthermore, the fossil record from other parts of Gondwana has yet to yield any boid fossils that antedate the purported time of separation of Madagascar from other southern landmasses. At present, the Maevarano Formation snake fauna presents a pattern that is congruent with that shown by many other Malagasy taxa in the fauna (with the possible exception of the podocnemidid Erymnochelys—see CitationGaffney and Forster, 2003): the species present in the latest Cretaceous deposits of the Mahajanga Basin belong to archaic Mesozoic lineages that were eliminated and replaced by basal stocks of the modern fauna sometime after the close of the Cretaceous (CitationKrause et al., 2006; CitationYoder and Nowak, 2006).
Paleobiology and Paleoecology
Our ability to infer aspects of the paleobiology and paleoecology of the Maevarano Formation snake assemblage is limited for three principal reasons. First, distributional data do not provide meaningful insight into differences among the three described species, given that two of them have been found at several localities (Madtsoia madagascariensis and Menarana nosymena at MAD93-16, Ma. madagascariensis and Kelyophis hechti at MAD93-01, and Me. nosymena and K. hechti at MAD05-59) and all three co-occur at MAD93-35. Thus, the single conclusion that can be drawn from these data is that all three species had the ability to survive in the highly seasonal, semi-arid climate deduced from sedimentological and taphonomic analyses of the Maevarano Formation (CitationRogers et al., 2000, Citation2007; CitationRogers and Krause, 2007).
Second, the phylogenetic placement of these taxa remains uncertain. Nigerophiids are believed by some to be closely related to acrochordids (CitationRage, 1984, Citation1987; CitationMcDowell, 1987), but they have not been included in any large-scale cladistic analyses of snake interrelationships, presumably because their morphology remains known only very incompletely. In contrast, madtsoiids have been included in several phylogenetic analyses, but the results of these studies have varied significantly; some have recovered them as basal snakes, lying outside of the clade containing Scolecophidia + Alethinophidia (CitationScanlon and Lee, 2000; CitationLee and Scanlon, 2002; CitationScanlon, 2006), whereas others have concluded that they are more derived alethinophidians, nested within Macrostomata (CitationRieppel et al., 2002). Collectively, these points of uncertainty effectively preclude rigorous historical analysis of the natural histories of these three species.
Finally, as is the case for most fossil snakes, especially terrestrial ones, the Maevarano taxa are known predominantly from vertebral specimens. Although some studies have attempted to correlate aspects of snake vertebral morphology with habitat, locomotor mode, diet, or method of prey capture (e.g., CitationJohnson, 1955; CitationHoffstetter and Gasc, 1969; CitationRuben, 1977; CitationLillywhite et al., 2000), few detailed analyses have been undertaken in this regard. Moreover, CitationMoon (1999) recently demonstrated that considerable caution must be exercised when attempting to make functional inferences about snakes based solely on vertebral morphology. Consequently, much of what follows should be regarded as somewhat speculative until such time as more rigorous analyses of the functional morphology of the vertebrae of extant snakes can be completed.
Madtsoia madagascariensis —Although the detailed descriptions above serve to greatly expand our knowledge of the anatomy of Madtsoia madagascariensis, they provide frustratingly few clues about its paleobiology, given our rudimentary current understanding of the functional morphology of snake vertebrae. Indeed, in many respects, the vertebrae of Ma. madagascariensis strongly resemble those of many terrestrial generalists among extant snakes, including in particular a variety of boids and pythonids (e.g., CitationGasc, 1974), which collectively exhibit a wide range of life history strategies. Many such snakes exhibit strong arboreal or semi-aquatic tendencies, and the dietary diversity they exhibit is extraordinarily high, including a wide array of mammals, birds, crocodilians, and lepidosaurs (e.g., CitationShine, 1991; CitationGreene, 1997). However, whereas discrete anatomical characters may not (yet) provide significant insight into the paleobiology of Ma. madagascariensis, one more general feature of its vertebrae—size—may be more informative in this regard, as it serves as a useful proxy for estimating the overall body size of this species.
Body size is an extremely important factor in determining a number of life history traits in snakes, including feeding, locomotion, and habitat usage (e.g., CitationGreene, 1997). However, estimating snake body size solely from measurements of individual vertebrae presents a number of potential difficulties, not the least of which is that vertebral number varies widely, ranging from just over 100 to more than 550 (e.g., CitationRochebrune, 1881; CitationAlexander and Gans, 1966; CitationPolly et al., 2001; upper end of range approximated indirectly based on scale counts provided by CitationGow [1977] and CitationHahn and Wallach [1998]). Nevertheless, a recent study by CitationMcCartney et al. (2008) demonstrated that nearly all standard morphometric measurements of snake vertebrae are highly correlated with measurements of total length. Furthermore, on the basis of this somewhat unexpected finding, these authors proposed a method for estimating the size of fossil snakes from isolated vertebrae and provided equations for doing so based on regressions of vertebral size against total length, calculated across a phylogenetically and morphologically diverse sample of extant snakes. Using this method, along with morphometric data from one of the largest and best preserved mid-trunk vertebrae of Madtsoia madagascariensis (FMNH PR 2553; ), we estimate the total length of this species to be approximately 5.1 m. Moreover, the width of such mid-trunk vertebrae and the length and curvature of the most completely preserved ribs (e.g., UA 9764) suggest a mid-body diameter of approximately 15 cm. Taken together, these two estimates are suggestive of a relatively heavy-bodied snake, with an overall body mass of at least 50 kg. However, large adults of Ma. madagascariensis may have reached significantly greater proportions than this; the largest vertebral specimen known from this species (MNHN MAJ 8; see CitationHoffstetter, 1961a:fig. 3D) is an isolated zygosphene that is over 50% wider than that of FMNH PR 2553, suggesting that this species may have at least occasionally reached lengths of nearly 8 m.
By analogy with modern snakes, the estimated proportions of Madtsoia madagascariensis indicate a relatively slow-moving snake that would likely have relied predominantly on a rectilinear mechanism of locomotion (e.g., CitationMosauer, 1932; CitationBogert, 1947; CitationGray, 1968; CitationGans, 1974). Consistent with this hypothesis is the complete absence of accessory prezygapophyseal processes, which tend to be greatly enlarged anterolaterally in taxa that rely primarily on a lateral undulatory mechanism of locomotion, such as most colubroids (excluding viperids) and scolecophidians (CitationJohnson, 1955). Moreover, the overall shape of the vertebrae in Ma. madagascariensis differs greatly from that seen in rapidly moving locomotor specialists, typically characterized by relatively narrow, elongate vertebrae (CitationJohnson, 1955).
The large size of Madtsoia madagascariensis also suggests it was a sit-and-wait ambush predator rather than an active forager. Unfortunately, with no knowledge of its cranial morphology, it is difficult to estimate a size range for prey that it may have exploited, and thus the type of prey that it may have consumed. Nevertheless, it can be safely assumed that this species faced the same fundamental physiological challenge that all snakes do in feeding: it had to provide adequate nourishment for a very long body while retaining a relatively small head, a most serious challenge indeed for a gape-limited predator that must swallow prey whole, and a problem with a limited number of solutions (CitationGans, 1961; CitationGreene, 1997). One such solution is to eat very large numbers of relatively small prey (microphagy). However, this strategy has evolved only rarely within Serpentes; it is restricted primarily to blindsnakes (Scolecophidia), which feed almost exclusively on ant brood and termites (e.g., CitationShine and Webb, 1990; CitationWebb and Shine, 1993; CitationWebb et al., 2000; CitationKley, 2003a, Citation2003b, Citation2003c). It is significant to note that few microphagous snakes even approach 1 m in length, and most are in fact significantly smaller than this. The vast majority of all other modern snakes—nearly 90% of approximately 3000 recognized species, including all those that approach or exceed 5 m in length—are macrophagous, feeding infrequently on relatively large prey. Therefore, it is reasonable to assume that Ma. madagascariensis was also macrophagous. Furthermore, and again by analogy with modern giant snakes (e.g., CitationShine et al., 1998), it is also likely that this species would have exhibited an ontogenetic shift in diet, such that relatively small vertebrates would have been deleted from the diets of large adult individuals (a very common phenomenon among extant snakes and one fully predicted by optimal foraging theory; CitationArnold, 1993). Thus, although juvenile Ma. madagascariensis may have fed on a relatively wide array of small vertebrates, adults probably preyed on a narrower range of larger taxa. Possible adult prey among the known fauna of the Maevarano Formation (see CitationKrause et al., 2006, for a recent review) would have included medium-sized crocodyliforms (e.g., adult Simosuchus clarki, subadult Mahajungasuchus insignis) as well as small theropod dinosaurs (e.g., adult Masiakasaurus knopfleri, subadult Majungasaurus crenatissimus). Such large and potentially injurious prey would have almost certainly necessitated a highly efficient mechanism of prey subjugation. Given the lack of any evidence suggesting the presence of a venom delivery system in madtsoiids (e.g., CitationScanlon, 2005a, 2006), and the relative inefficiency of some mechanisms of prey subjugation, such as simple biting and/or pinioning used by many modern snakes when feeding on relatively harmless prey (e.g., CitationLoop and Bailey, 1972; CitationWillard, 1977; CitationGreenwald, 1978; Citationde Queiroz, 1984), we consider it most likely that Ma. madagascariensis was a constrictor. However, we emphasize that this inference is based entirely on a process of elimination from the collective constellation of prey subjugation mechanisms exhibited by living snakes (for a recent review, see CitationCundall and Greene, 2000); constriction appears to be first and foremost a behavioral innovation, and osteological correlates of this behavior have yet to be identified conclusively (CitationGreene and Burghardt, 1978).
Menarana nosymena —Although known much less completely than Madtsoia madagascariensis, Menarana nosymena differs from the former species in exhibiting a number of clearly adaptive morphological traits that collectively offer considerable insight into its paleobiology.
Perhaps the most remarkable aspect of the morphology of Menarana nosymena is the extensive degree to which individual elements of the braincase (viz., parabasisphenoid, basioccipital, exoccipitals, prootics) have become fused together. Among the more than 25,000 recognized species of extant tetrapods, such a pattern of extensive basicranial fusion is seen only among the most highly specialized limb-reduced, head-first burrowers, such as in the ‘os basale’ of caecilians (e.g., CitationWiedersheim, 1879; CitationTaylor, 1969; CitationWake and Hanken, 1982), the ‘otic-occipital complex’ of amphisbaenians (e.g., CitationZangerl, 1944; CitationMaisano et al., 2006; CitationMontero and Gans, 2008), and the ‘otico-occipital complex’ of derived uropeltid snakes (CitationRieppel and Zaher, 2002; CitationBaumeister, 1908; CitationCundall and Irish, 2008). The repeated convergent evolution of this morphological phenomenon in these three highly fossorial clades, together with its general absence among other tetrapods, suggests strongly that either Me. nosymena was itself a very powerful head-first burrower, or that it evolved from ancestors that were. Discovery of additional cranial material, in particular elements of the snout, would likely help to refine this interpretation. However, it is interesting to note in this context that adult Yurlunggur also exhibit fusion among several elements of the posterior braincase (though fewer than in Menarana), yet clearly retain a rather unspecialized snout that would appear to be very poorly suited for head-first burrowing (CitationScanlon, 2006). Moreover, the very large size of this taxon alone (estimated to be approximately 5 m; CitationScanlon, 2006) would appear to be enough to make burrowing through compact soils impossible (see below). Thus, the presence of these basicranial fusions in large adult Yurlunggur likely represents the retention of an ancestral trait, rather than direct evidence of adaptive modifications within this particular taxon.
The morphology of the trunk vertebrae of Menarana nosymena is also consistent with the hypothesis of a burrowing lifestyle or ancestry. Most telling in this respect are the very low neural spines and the rather depressed overall appearance of the vertebrae, features that are nearly ubiquitous among extant snakes that exhibit strong fossorial tendencies (e.g., scolecophidians, Anilius, Cylindrophis, uropeltids, Xenopeltis, Loxocemus) (CitationHoffstetter and Gasc, 1969; CitationGasc, 1974). Somewhat more difficult to interpret is the morphology of the atlas, in which the neural arches are fused completely with the intercentrum. However, in light of other available evidence, it is tempting to consider this also an adaptation for burrowing, as this might serve to maintain the integrity of the anterior atlantal cotyle (and thus the craniovertebral joint as a whole) under the extreme loading regimes that would be expected during tunnel construction, as it would prevent the three constituent components of the cotyle from being forced apart. Indeed, modifications of the atlas-axis complex are relatively common among head-first limbless burrowers (CitationGans, 1958; CitationWilliams, 1959; CitationTaylor, 1977; CitationWake, 1980). However, among the most specialized head-first burrowing squamates, most notably amphisbaenians and uropeltid snakes, modifications to the atlas most commonly involve reduction rather than fortification. Specifically, there is a tendency toward reduction or loss of the atlantal intercentrum, as well as reduction in size of the articular facets on the pedicles of the atlantal neural arches, which together greatly reduce the overall contribution of the atlas to the craniovertebral joint (CitationZangerl, 1945; CitationWilliams, 1959). The result of these modifications is ultimately very similar to that of atlantal fusion in Menarana: the occipital condyle is received predominantly by a single (rather than tripartite) element—the odontoid process in amphisbaenians, and the axial cotyle in uropeltids (members of the latter clade lack an odontoid process).
Despite the numerous morphological features discussed above that Menarana nosymena shares with many extant forms of limb-reduced, head-first burrowing amphibians and reptiles, it must be emphasized that few such taxa exceed even 1 m in total length, and the vast majority are significantly smaller than this. Perhaps even more significant, most are less than 1 cm in diameter, and nearly all are less than 2.5 cm in diameter. The latter is particularly important because it has been suggested that the force required to push an object through a given substrate increases with the cross-sectional area (and thus the square of the diameter) of that object (CitationGans, 1960). That is, it would be predicted that as the diameter of a burrowing snake's head increases, the force that would be required to effectively burrow through compact soil would increase very rapidly, according to a quadratic function rather than a linear one. This implies, for example, that if a snake having a head diameter of 1 cm required 25 N of force to burrow through a given substrate, one with a head diameter of 3 cm would have to generate a much greater 225 N of pushing force to burrow through the same medium. Given this relationship, and the inherent physiological and mechanical limitations common to all vertebrate skeletal muscles with respect to their capacity to generate force, it remains somewhat questionable whether a head-first burrowing mechanism could have been effective, or even possible, in a limbless animal of the size of Me. nosymena, which we estimate to have been approximately 2.4 m in total length (using the equations of CitationMcCartney et al., 2008) and in excess of 7 cm in mid-body diameter. Nevertheless, available anatomical evidence points strongly toward this species having at least a burrowing ancestry, if not a burrowing lifestyle itself, as inferred for some other madtsoiids (e.g., Herensugea caristiorum; CitationRage, 1999).
The jaw apparatus of Menarana nosymena, like that of Madtsoia madagascariensis, remains completely unknown, making inferences about its diet and feeding difficult at best. However, once again body size provides the most important clues in this respect, as it significantly narrows the list of potential prey. By analogy with modern alethinophidian snakes, the estimated proportions of Me. nosymena suggest a maximum prey size for this species of well under 5 kg, and possibly as small as 1–2 kg. Thus, we can eliminate from the list of potential prey all of the larger known fauna of the Maevarano Formation, including adults of all non-avian dinosaurs and most crocodyliforms (the one known exception being the very small Araripesuchus tsangatsangana; CitationTurner, 2006). More likely forms of potential prey include much smaller ground-dwelling or fossorial vertebrates, possibly including other snakes, non-ophidian squamates (‘lizards’) or small mammals.
Kelyophis hechti —Kelyophis hechti, the Malagasy nigerophiid, was probably less than 1 m long and thus much smaller than the madtsoiids, Madtsoia madagascariensis and Menarana nosymena. It appears to have been more generalized than other nigerophiids, such as Nigerophis, Nubianophis, and Indophis, which have been interpreted to have been very highly specialized aquatic snakes on the basis of their ventrally positioned synapophyses, their ‘peculiar’ prezygapophyseal buttresses, and the high, narrow shape of their mid-trunk, posterior trunk, and postcloacal vertebrae (CitationRage and Prasad, 1992; CitationPrasad and Rage, 1995; CitationRage and Werner, 1999). Based on its somewhat shorter vertebrae with less ventrally shifted synapophyses, Kelyophis was apparently less well adapted to an aquatic lifestyle. However, given the extremely limited nature of the available material representing this species, few additional inferences can be made about its paleobiology.
ACKNOWLEDGMENTS
We gratefully acknowledge field teams of the Mahajanga Basin Project for the collection of specimens described in this report; A. Rasoamiaramanana of the Université d’Antananarivo, B. Andriamihaja and his staff of the Madagascar Institute pour la Conservation des Environnements Tropicaux, and the villagers of Berivotra for logistical support in the field; J. Groenke and V. Heisey for preparation of fossils; M. Colbert for scanning the partial basicranium of the holotype of Menarana nosymena; J. Maisano for processing of μCT images; R. Bonett, M. Norell, and D. Wake for access to comparative material; S. Burch, R. Jacobs, J. Sertich, and W. Simpson for assistance with curation; M. Stewart for photography; L. Betti-Nash for drawing or arranging the figures; J.-C. Rage and M. Godinot for curatorial information concerning MNHN specimens of Madtsoia madagascariensis; and R. Jacobs for assistance in compiling distributional data. We also thank J. McCartney, J. Pruetz, and J. Sertich for reviewing early drafts, or parts of drafts, of the manuscript, and the anonymous JVP reviewers for their helpful comments on the submitted draft. This research was funded by grants from the National Science Foundation (DEB-9224396, EAR-9418816, EAR-9706302, DEB-9904045, EAR-0106477, EAR-0116517, EAR-0446488) and the National Geographic Society (1999, 2001, 2004) to DWK.
Notes
*Estimated because of slight breakage or erosion.
*Estimated because of slight breakage or erosion.
*Estimated because of slight breakage or erosion.
*Revised age from CitationSeiffert (2006).
LITERATURE CITED
- Albino , A. M. 1986 . “ Nuevos Boidae Madtsoiinae en el Cretacico tardio de Patagonia (Formacion Los Alamitos, Rio Negro, Argentina) ” . In Simposio Evolucion de los Vertebrados Mesozoicos , Edited by: Bonaparte , J. F. 15 – 21 . Mendoza : Actas IV Congreso Argentino de Paleontología y Bioestratigrfía .
- Albino , A. M. 1993 . Snakes from the Paleocene and Eocene of Patagonia, Argentina: paleoecology and coevolution with mammals . Historical Biology , 7 : 51 – 69 .
- Albino , A. M. 1994 . Una nueva serpiente (Reptilia) en el Cretácico superior de Patagonia, Argentina . Pesquisas , 21 : 58 – 63 .
- Albino , A. M. 2000 . New record of snakes from the Cretaceous of Patagonia (Argentina) . Geodiversitas , 22 : 247 – 253 .
- Albino , A. M. 2007 . “ Lepidosauria; pp ” . In Patagonian Mesozoic Reptiles , Edited by: Gasparini , Z. , Salgado , L. and Coria , R. A. 87 – 115 . Indiana : Indiana University Press, Bloomington & Indianapolis .
- Alexander , A. A. and Gans , C. 1966 . The pattern of dermal-vertebral correlation in snakes and amphisbaenians . Zoologische Mededelingen , 41 : 171 – 190 .
- Ali , J. R. and Aitchison , J. C. 2008 . Gondwana to Asia: plate tectonics, paleogeography and the biological connectivity of the Indian sub-continent from the Middle Jurassic through latest Eocene (166–35 Ma) . Earth-Science Reviews , 88 : 145 – 166 .
- Andrews , C. W. 1901 . Preliminary note on some recently discovered extinct vertebrates from Egypt (Part II) . Geological Magazine , 8 : 434 – 444 .
- Andrews , C. W. 1906 . A descriptive catalogue of the Tertiary Vertebrata of the Fayûm, Egypt , 306 – 312 . London. Ophidia : British Museum .
- Apesteguía , S. and Zaher , H. 2006 . A Cretaceous terrestrial snake with robust hindlimbs and a sacrum . Nature , 440 : 1037 – 1040 .
- Arnold , S. J. 1993 . “ Foraging theory and prey-size–predator-size relations in snakes; pp ” . In Snakes: Ecology and Behavior , Edited by: Seigel , R. A. and Collins , J. T. 87 – 115 . New York, New York : McGraw-Hill .
- Averianov , A. O. 1997 . Paleogene sea snakes from the eastern part of Tethys . Russian Journal of Herpetology , 4 : 128 – 142 .
- Barrie , D. J. 1990 . Skull elements and additional remains of the Pleistocene boid snake Wonambi naracoortensis . Memoirs of the Queensland Museum , 28 : 139 – 151 .
- Baumeister , L. 1908 . “ Beiträge zur Anatomie und Physiologie der Rhinophiden. Integument, Drüsen der Mundhöhle, Augen und Skeletsystem ” . In Zoologische Jahrbücher, Abteilung für Anatomie und Ontogenie der Tiere Vol. 26 , 423 – 526 .
- Bogert , C. M. 1947 . Rectilinear locomotion in snakes . Copeia , 1947 : 253 – 254 .
- Cadle , J. E. 2003 . “ Colubridae, snakes; pp ” . In The Natural History of Madagascar , Edited by: Goodman , S. M. and Benstead , J. P. 997 – 1004 . Chicago, Illinois : University of Chicago Press .
- Caldwell , M. W. and Albino , A. M. 2002 . Exceptionally preserved skeletons of the Cretaceous snake Dinilysia patagonica Woodward, 1901 . Journal of Vertebrate Paleontology , 22 : 861 – 866 .
- Caldwell , M. W. and Calvo , J. 2008 . Details of a new skull and articulated cervical column of Dinilysia patagonica Woodward, 1901 . Journal of Vertebrate Paleontology , 28 : 349 – 362 .
- Cundall , D. and Greene , H. W. 2000 . “ Feeding in snakes ” . In Feeding: Form, Function, and Evolution in Tetrapod Vertebrates , Edited by: Schwenk , K. 293 – 333 . San Diego, California : Academic Press .
- Cundall , D. and Irish , F. 2008 . “ The snake skull ” . In Biology of the Reptilia, Volume 20, Morphology H (The Skull of Lepidosauria) , Edited by: Gans , C. , Gaunt , A. S and Adler , K. 349 – 692 . Ithaca, New York : Society for the Study of Amphibians and Reptiles .
- de Broin , F. , Buffetaut , E. , Koeniguer , J. C. , Rage , J.-C. , Taquet , P. , Vergnaud-Grazzini , C. and Wenz , S. 1974 . La faune de Vertébrés continentaux du gisement d’In Beceten (Sénonien du Niger) . Comptes rendus de l’Académie des Sciences, Paris , 279 : 469 – 472 .
- de Queiroz , A . 1984 . Effects of prey type on the prey-handling behavior of the bullsnake, Pituophis melanoleucus . Journal of Herpetology , 18 : 333 – 336 .
- Dercourt , J. , Gaetani , M. , Vrielynick , B. , Barrier , E. , Biju-Duval , B. , Brunet , M.-F. , Cadet , J.-P. , Crasquin , S. and Sandulescu , M. 2000 . “ Atlas Peri-Tethys ” . In Palaeogeographical maps , 269 Paris, 24 maps : CCGM/CGMW .
- Estes , R. , Frazzetta , T. H. and Williams , E. E. 1970 . “ Studies on the fossil snake Dinilysia patagonica Woodward. Part 1 ” . In Cranial morphology. Bulletin of the Comparative Zoology , Vol. 140 , 25 – 74 . Harvard University .
- Evans , S. E. , Jones , M. E. H. and Krause , D. W. 2008 . A giant frog with South American affinities from the Late Cretaceous of Madagascar . Proceedings of the National Academy of Sciences of the United States of America , 105 : 2951 – 2956 .
- Folie , A. and Codrea , V. 2005 . New lissamphibians and squamates from the Maastrichtian of Haţeg Basin, Romania . Acta Palaeontologica Polonica , 50 : 57 – 71 .
- Gaffney , E. S. and Forster , C. A. 2003 . Side-necked turtle lower jaws (Podocnemididae, Bothremydidae) from the Late Cretaceous Maevarano Formation of Madagascar . American Museum Novitates , 3397 : 1 – 13 .
- Gans , C . 1958 . Modifications of the head joint in acrodont amphisbaenids and their functional implication [abstract] . Anatomical Record , 132 : 441
- Gans , C. 1960 . Studies on amphisbaenids (Amphisbaenia, Reptilia). 1. A taxonomic revision of the Trogonophinae, and a functional interpretation of the amphisbaenid adaptive pattern . Bulletin of the American Museum of Natural History , 119 : 129 – 204 .
- Gans , C. 1961 . The feeding mechanism of snakes and its possible evolution . American Zoologist , 1 : 217 – 227 .
- Gans , C. 1974 . Biomechanics: An Approach to Vertebrate Biology , 261 Ann Arbor, Michigan : University of Michigan Press .
- Garzanti , E. 2008 . Comment on “When and where did India and Asia collide?” by Jonathan C. Aitchison, Jason R. Ali, and Aileen M. Davis . Journal of Geophysical Research , 113 : B04411 doi:10.1029/2007JB005276
- Gasc , J.-P. 1974 . “ L’interprétation fonctionelle de l’appareil musculo-squelettique de l’axe vertébral chez les serpents (Reptilia) ” . In Mémoires du Muséum National d’Histoire Naturelle, Paris, Série A, Zoologie Vol. 83 , 1 – 182 .
- Gayet , M. , Rage , J.-C. and Rana , R. S. 1985 . “ Nouvelles ichthyofaune et herpétofaune de Gitti Khadan, le plus ancient gisement connu du Deccan (Crétacé/Paléocène) à microvertébrés. Implications paléogéographiques ” . In Paléogéographie de l’Inde, du Tibet et du Sud-Est Asiatique , Edited by: Jaeger , J.-J. , Rage , J.-C. and Buffetaut , E. 55 – 65 . Mémoire de la Societé géologique de France 147 .
- Gheerbrant , E. and Rage , J.-C. 2006 . Paleobiogeography of Africa: How distinct from Gondwana and Laurasia? . Palaeogeography, Palaeoclimatology, Palaeoecology , 241 : 224 – 246 .
- Gheerbrant , E. , Cappetta , H. , Feist , M. , Jaeger , J.-J. , Sudre , J. , Vianey-Liaud , M. and Sigé , B. 1993 . La succession des faunes de vertebrés d’âge paléocène supérieur et éocène inférieur dans le basin d’Ouarzazate, Maroc. Contexte géologique, portée biostratigraphique et paléogéographique . Newsletters in Stratigraphy , 28 : 33 – 58 .
- Gomez , R. O. and Baez , A. M. 2006 . A new madtsoiid snake (Squamata, Ophidia) from the Upper Cretaceous of Patagonia , San Juan, , Argentina : XXII Jornadas Argentinas de Paleontología de Vertebrados . (2006):21
- González Riga , B. J. 1999 . Hallazgo de vertebrados fósiles en la Formación Loncoche, Cretácico Superior de la provincia de Mendoza, Argentina . Ameghiniana , 36 : 401 – 410 .
- Gow , G. F. 1977 . A new species of Python from Arnhem Land . Australian Zoologists , 19 : 133 – 139 .
- Gray , J. 1968 . “ Animal Locomotion ” . 479 New York, New York : W.W. Norton & Company .
- Greene , H. W. 1997 . Snakes: The Evolution of Mystery in Nature , 351 Berkeley, California : University of California Press .
- Greene , H. W. and Burghardt , G. M. 1978 . Behavior and phylogeny: constriction in ancient and modern snakes . Science , 200 : 74 – 76 .
- Greenwald , O. E. 1978 . Kinematics and time relations of prey capture by gopher snakes . Copeia , 1978 : 263 – 268 .
- Hahn , D. E. and Wallach , V. 1998 . Comments on the systematics of Old World Leptotyphlops (Serpentes: Leptotyphlopidae), with description of a new species . Hamadryad , 23 : 50 – 62 .
- Hay , W. W. , DeConto , R. M. , Wold , C. N. , Wilson , K. M. , Voigt , S. , Schulz , M. , Wold , A. R. , Dullo , W. C. , Ronov , A. B. , Balukhovsky , A. N. and Söding , E. 1999 . “ Alternative global Cretaceous paleogeography ” . In Evolution of the Cretaceous Ocean-Climate System , Edited by: Barrera , E. and Johnson , C. C. 1 – 47 . Geological Society of America . Special Paper 33
- Head , J. J. 2005 . Snakes of the Siwalik Group (Miocene of Pakistan): systematics and relationship to environmental change . Palaeontologia Electronica , 8.1.18A : 1 – 33 .
- Head , J. and Holroyd , P. 2008 . Assembly and biogeography of North American Paleogene snake faunas based on an expanded fossil record . Journal of Vertebrate Paleontology , 28 ( 3, Supplement ) : 90A
- Hoffstetter , R. 1959 . Un serpent terrestre dans le Cretace inférieur du Sahara . Bulletin de la Societé géologique de France , 7 : 897 – 902 .
- Hoffstetter , R. 1961a . Nouveaux restes d’un serpent boïdé (Madtsoia madagascariensis nov. sp.) dans le Crétacé supérieur de Madagascar , Vol. 33 , 152 – 160 . Paris : Bulletin du Muséum national d’Histoire naturelle .
- Hoffstetter , R. 1961b . Nouvelles récoltes de serpents fossiles dans l’Éocène supérieur du désert Libyque , Vol. 33 , 326 – 331 . Paris : Bulletin du Muséum national d’Histoire naturelle .
- Hoffstetter , R. and Gasc , J.-P. 1969 . “ Vertebrae and ribs of modern reptiles; pp ” . In Biology of the Reptilia , Edited by: Gans , C. , Bellairs , A. d’A. and Parsons , T. S. Vol. 1 , 201 – 310 . London : Morphology A. Academic Press .
- Johnson , R. G. 1955 . The adaptive and phylogenetic significance of vertebral form in snakes . Evolution , 9 : 367 – 388 .
- Kley , N. J. 2003a . “ Early blindsnakes (Anomalepididae) ” . In Grzimek's Animal Life Encyclopedia , 2nd edition , Edited by: Hutchins , M. , Murphy , J. B. and Schlager , N. 369 – 372 . Farmington Hills, Michigan : Gale Group .
- Kley , N. J. 2003b . “ Slender blindsnakes (Leptotyphlopidae) ” . In Grzimek's Animal Life Encyclopedia , 2nd edition , Edited by: Hutchins , M. , Murphy , J. B. and Schlager , N. 373 – 377 . Michigan : Gale Group, Farmington Hills .
- Kley , N. J. 2003c . “ Blindsnakes (Typhlopidae) ” . In Grzimek's Animal Life Encyclopedia , 2nd edition , Edited by: Hutchins , M. , Murphy , J. B. and Schlager , N. 379 – 385 . Farmington Hills, Michigan : Gale Group .
- Krause , D. W. , Evans , S. E. and Gao , K.-Q. 2003 . First definitive evidence of Mesozoic lizards from Madagascar . Journal of Vertebrate Paleontology , 23 : 842 – 856 .
- Krause , D. W. , Hartman , J. H. and Wells , N. A. 1997 . “ Late Cretaceous vertebrates from Madagascar: Implications for biotic change in deep time ” . In Natural Change and Human Impact in Madagascar , Edited by: Goodman , S. D. and Patterson , B. D. 3 – 43 . Washington, D.C : Smithsonian Institution Press .
- Krause , D. W. , Sampson , S. D. , Carrano , M. T. and O’Connor , P. M. 2007 . Overview of the history of discovery, taxonomy, phylogeny, and biogeography of Majungasaurus crenatissimus (Theropoda: Abelisauridae) from the Late Cretaceous of Madagascar , 1 – 20 . Society of Vertebrate Paleontology Memoir 8 .
- Krause , D. W. , O’Connor , P. M. , Curry Rogers , K. , Sampson , S. D. , Buckley , G. A. and Rogers , R. R. 2006 . “ Late Cretaceous terrestrial vertebrates from Madagascar: implications for Latin American biogeography ” . In 51st Annual Systematics Symposium, Missouri Botanical Garden, Latin American Biogeography—Causes and Effects , 178 – 208 . Latin American Biogeography—Causes and Effects. Annals of the Missouri Botanical Garden 93 .
- Krause , D. W. , Rogers , R. R. , Forster , C. A. , Hartman , J. H. , Buckley , G. A. and Sampson , S. D. 1999 . The Late Cretaceous vertebrate fauna of Madagascar: implications for Gondwanan paleobiogeography . GSA Today , 9 : 1 – 7 .
- LaDuke , T. C. 1991 . The fossil snakes of Pit 91, Rancho La Brea, California . Contributions in Science, Natural History Museum of Los Angeles County , 424 : 1 – 28 .
- Lavocat , R. 1955 . Etude des gisements de Dinosauriens de la region de Majunga (Madagascar) . Travaux du Bureau Géologique , 69 : 1 – 19 .
- Lee , M. S. Y. and Caldwell , M. W. 1998 . Anatomy and relationships of Pachyrhachis problematicus, a primitive snake with hindlimbs . Philosophical Transactions of the Royal Society of London, Series , B353 : 1521 – 1552 .
- Lee , M. S. Y. and Scanlon , J. D. 2002 . Snake phylogeny based on osteology, soft anatomy and behaviour . Biological Reviews , 77 : 333 – 402 .
- Lee , M. S. Y. , Caldwell , M. W. and Scanlon , J. D. 1999 . A second primitive marine snake: Pachyophis woodwardi from the Cretaceous of Bosnia-Herzegovina . Journal of Zoology, London , 248 : 509 – 520 .
- Lillywhite , H. B. , LaFrentz , J. R. , Lin , Y. C. and Tu , M. C. 2000 . The cantilever ability of snakes . Journal of Herpetology , 34 : 523 – 528 .
- Linnaeus , C. 1758 . Systema naturæ per regna tria naturæ, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Tomus I , 824 Laurentii Salvii, Holmiæ : Editio decima, reformata .
- List , J. C. 1966 . Comparative osteology of the snake families Typhlopidae and Leptotyphlopidae . Illinois Biological Monographs , 36 : 1 – 112 .
- Loop , M. S. and Bailey , L. G. 1972 . The effect of relative prey size on the ingestion behavior of rodent-eating snakes . Phychonomic Science , 28 : 167 – 169 .
- Mackness , B. S. and Scanlon , J. D. 1999 . The first Pliocene record of the madtsoiid snake genus Yurlunggur Scanlon, 1992 from Queensland . Memoirs of the Queensland Museum , 43 : 783 – 785 .
- Maisano , J. A. , Kearney , M. and Rowe , T. 2006 . Cranial anatomy of the spade-headed amphisbaenian Diplometopon zarudnyi (Squamata, Amphisbaenia) based on high-resolution X-ray computed tomography . Journal of Morphology , 267 : 70 – 102 .
- Martinelli , A. G. and Forasiepi , A. M. 2004 . Late Cretaceous vertebrates from Bajo de Santa Rosa (Allen Formation), Río Negro province, Argentina, with the description of a new sauropod dinosaur (Titanosauridae) . Revista del Museo Argentino de Ciencias Naturales , 6 : 257 – 305 .
- McCall , R. A. 1997 . Implications of recent geological investigations of the Mozambique Channel for the mammalian colonization of Madagascar . Proceedings of the Royal Society of London B , 264 : 663 – 665 .
- McCartney , J. A. , Kley , N. J. and O’Leary , M. A. 2008 . Body size of the giant Eocene snake Palaeophis colossaeus (Serpentes: Palaeophiidae) estimated from recently collected material from Mali . Journal of Vertebrate Paleontology , 28 ( 3, Supplement ) : 114A
- McDowell , S. B. 1987 . “ Systematics ” . In Snakes: Ecology and Evolutionary History , Edited by: Siegel , R. A. , Collins , J. T. and Novak , S. S. 3 – 50 . New York : McGraw-Hill Publications Company .
- Megirian , D. , Murray , P. , Schwartz , L. and von der Borch , C. 2004 . Late Oligocene Kangaroo Well Local Fauna from the Ulta Limestone (new name), and climate of the Miocene oscillation across Central Australia . Australian Journal of Earth Sciences , 51 : 701 – 741 .
- Mertens , R. 1972 . Madagaskars Herpetofauna und die Kontinentaldrift. Studien über die Reptilienfauna Madagaskars VI . Zool. Med. , 46 : 91 – 98 .
- Montero , R. and Gans , C. 2008 . “ An atlas of amphisbaenian skull anatomy ” . In Biology of the Reptilia, Volume 21, Morphology I (The Skull and Appendicular Locomotor Apparatus of Lepidosauria) , Edited by: Gans , C. , Gaunt , A. S. and Adler , K. 621 – 738 . Ithaca, New York : Society for the Study of Amphibians and Reptiles .
- Moon , B. R. 1999 . Testing an inference of function from structure: snake vertebrae do the twist . Journal of Morphology , 241 : 217 – 225 .
- Mosauer , W. 1932 . On the locomotion of snakes . Science , 76 : 583 – 585 .
- Nagy , Z. T. , Joger , U. , Wink , M. , Glaw , F. and Vences , M. 2003 . Multiple colonization of Madagascar and Socotra by colubrid snakes: evidence from nuclear and mitochondrial gene phylogenies . Proceedings of the Royal Society of London B , 270 : 2613 – 2621 .
- Noonan , B. P. and Chippindale , P. T. 2006a . Dispersal and vicariance: The complex evolutionary history of boid snakes . Molecular Phylogenetics and Evolution , 40 : 347 – 358 .
- Noonan , B. P. and Chippindale , P. T. 2006b . Vicariant origin of Malagasy reptiles supports Late Cretaceous Antarctic landbridge . American Naturalist , 168 : 730 – 741 .
- Oelrich , T. M. 1956 . “ The anatomy of the head of Ctenosaura pectinata (Iguanidae) ” . In Miscellaneous Publications, Museum of Zoology, University of Michigan Vol. 94 , 1 – 122 .
- Oppel , M. 1811 . Die Ordnungen, Familien, und Gatungen der Reptilien, als Prodrom einer Naturgeschichte derselben , 87 Munchen : Joseph Lindauer .
- Pereda-Superbiola , X. 2009 . Biogeographical affinities of Late Cretaceous continental tetrapods of Europe: a review . Bulletin de la Société géologique de France , 180 : 57 – 71 .
- Piveteau , J. 1933 . Un ophidien du Crétacé supérieur de Madagascar . Bulletin de la Société géologique de France (5) , 3 : 597 – 602 .
- Polly , P. D. , Head , J. J. and Cohn , M. J. 2001 . “ Testing modularity and dissociation: the evolution of regional proportions in snakes ” . In Beyond Heterochrony: The Evolution of Development , Edited by: Zelditch , M. L. 305 – 335 . New York, New York : John Wiley & Sons .
- Prasad , G. V. R. and Rage , J.-C. 1995 . Amphibians and squamates from the Maastrichtian of Naskal, India . Cretaceous Research , 16 : 95 – 107 .
- Pregill , G. K. 1977 . Axial myology of the racer Coluber constrictor with emphasis on the neck region . Transactions of the San Diego Society of Natural History , 18 : 185 – 206 .
- Rabinowitz , P. D. and Woods , S. 2006 . The Africa-Madagascar connection and mammalian migrations . Journal of African Earth Sciences , 44 : 270 – 276 .
- Rage , J.-C. 1975 . Un serpent du Paléocène du Niger. Etude préliminaire sur l’origine des Caenophidiens (Reptilia, Serpentes) . Comptes Rendus de l’Académie des Sciences, Paris (D) , 281 : 515 – 518 .
- Rage , J.-C. 1980 . Un serpent marin nouveau de l’Éocene de Belgique. Le problème des serpents marine du Paléogène . Comptes Rendus de l’Académie des Seances, Paris (D) , 291 : 469 – 471 .
- Rage , J.-C. 1981 . Les continents péri-atlantiques au Crétacé supérieur: migrations des faunes continentales et problèmes paléogéographiques . Cretaceous Research , 2 : 65 – 84 .
- Rage , J.-C. 1984 . “ Serpentes ” . In Handbuch der Paläoherpetologie, part 11 , 80 Stuttgart : Gustav Fischer Verlag .
- Rage , J.-C. 1987 . “ Fossil history ” . In Snakes: Ecology and Evolutionary Biology , Edited by: Seigel , R. A. , Collins , J. T. and Novak , S. S. 51 – 76 . New York, New York : McGraw-Hill .
- Rage , J.-C. 1988 . “ Gondwana, Tethys, and terrestrial vertebrates during the Mesozoic and Cenozoic ” . In Gondwana and Tethys , Edited by: Audley-Charles , M. G. and Hallam , A. 255 – 273 . Geological Society Special Publication 37 .
- Rage , J.-C. 1991 . “ Squamate reptiles from the early Paleocene of the Tiupampa area (Santa Lucia Formation), Bolivia ” . In Fosiles y Facies de Bolivia—Volume 1 Vertebrados , Edited by: Suarez-Soruco , R. 503 – 508 . Revista Técnica de YPFB 12 .
- Rage , J.-C. 1995 . La Tethys et les dispersions transtethysiennes par voie terrestre . Biogeographica , 71 : 109 – 126 .
- Rage , J.-C. 1996a . Les Madtsoiidae (Reptilia, Serpentes) du Crétacé supérieur d’Europe: témoins gondwaniens d’une dispersion transtéthysienne . Comptes Rendus de l’Académie des Sciences Paris (2) , 322 : 603 – 608 .
- Rage , J.-C. 1996b . “ Le peuplement animal de Madagascar: une composante venue de Laurasie est-elle envisageable? ” . In Biogéographie de Madagascar , Edited by: Lourenço , W. R. 27 – 35 . Paris : ORSTOM .
- Rage , J.-C. 1998 . Fossil snakes from the Palaeocene of São José de Itaborai, Brazil. Part 1 . Madtsoiidae, Aniliidae. Palaeovertebrata, Montpellier , 27 : 109 – 144 .
- Rage , J.-C. 1999 . Squamates (Reptilia) from the Upper Cretaceous of Laño (Basque Country, Spain). Estudios del Museo Ciencias Naturales . de Alava , 14 ( 1 ) : 121 – 133 .
- Rage , J.-C. 2003 . Relationships of the Malagasy fauna during the Late Cretaceous: Northern or Southern routes? . Acta Palaeontologica Polonica , 48 : 661 – 662 .
- Rage , J.-C. and Escuillié , F. 2000 . “ Un nouveau serpent bipède du Cénomanien (Crétacé). Implications phylétiques ” . In Comptes Rendus de l’Academie des Sciences, Paris, Sciences de la Terre et des planètes Vol. 330 , 513 – 520 .
- Rage , J.-C. and Prasad , G. V. R. 1992 . New snakes from the Late Cretaceous (Maastrichtian) of Naskal, India . Neues Jahrbuch für Geologie und Paläontologie Abhandlungen , 187 : 83 – 97 .
- Rage , J.-C. and Werner , C. 1999 . Mid-Cretaceous (Cenomanian) Snakes from Wadi Abu Hashim, Sudan: the earliest snake assemblage . Palaeontologia Africana , 35 : 85 – 110 .
- Rage , J.-C. , Prasad , G. V. R. and Bajpai , S. 2004 . Additional snakes from uppermost Cretaceous (Maastrichtian) of India . Cretaceous Research , 25 : 425 – 434 .
- Rage , J.-C. , Bajpai , S. , Thewissen , J. G. M. and Tiwari , B. N. 2003 . Early Eocene snakes from Kutch, Western India, with a review of the Palaeophiidae . Geodiversitas , 25 : 695 – 716 .
- Rage , J.-C. , Folie , A. , Rana , R. S. , Singh , H. , Rose , K. D. and Smith , T. 2008 . A diverse snake fauna from the early Eocene of Vastan Lignite Mine, Gujarat, India . Acta Palaeontologica Polonica , 53 : 391 – 403 .
- Raxworthy , C. J. 2003 . “ Introduction to the reptiles ” . In The Natural History of Madagascar , Edited by: Goodman , S. M. and Benstead , J. P. 934 – 949 . Chicago, Illinois : University of Chicago Press .
- Raxworthy , C. J. , Forstner , M. R. J. and Nussbaum , R. A. 2002 . Chameleon radiation by oceanic dispersal . Nature , 415 : 784 – 787 .
- Rieppel , O. and Head , J. J. 2004 . New specimens of the fossil snake genus Eupodophis Rage & Escuillié, from Cenomanian (Late Cretaceous) of Lebanon . Memorie della Società Italiana di Scienze Naturali e del Museo Civico di Storia Naturale di Milano , 32 ( 2 ) : 1 – 26 .
- Rieppel , O. and Zaher , H. 2002 . The skull of the Uropeltinae (Reptilia, Serpentes), with special reference to the otico-occipital region . Bulletin of the Natural History Museum (Zoology) , 68 : 123 – 130 .
- Rieppel , O. , Kley , N. J. and Maisano , J. A. 2009 . Morphology of the skull of the white-nosed blindsnake, Liotyphlops albirostris (Scolecophidia: Anomalepididae), as revealed by high-resolution X-ray computed tomography . Journal of Morphology , 270 : 536 – 557 .
- Rieppel , O. , Kluge , A. G. and Zaher , H. 2002 . Testing the phylogenetic relationships of the Pleistocene snake Wonambi naracoortensis Smith . Journal of Vertebrate Paleontology , 22 : 812 – 829 .
- de Rochebrune , A.-T. 1881 . Mémoire sur les Vertèbres des Ophidiens . Journal de L’Anatomie et de la Physiologie , 17 : 185 – 229 .
- Rogers , R. R. 2005 . Fine-grained debris flows and extraordinary vertebrate burials in the Late Cretaceous of Madagascar . Geology , 33 : 297 – 300 .
- Rogers , R. R. and Krause , D. W. 2007 . Tracking an ancient killer . Scientific American , 296 : 42 – 51 .
- Rogers , R. R. , Hartman , J. H. and Krause , D. W. 2000 . Stratigraphic analysis of Upper Cretaceous Rocks in the Mahajanga Basin, northwestern Madagascar: implications for ancient and modern faunas . Journal of Geology , 108 : 275 – 301 .
- Rogers , R. R. , Krause , D. W. , Curry , K. Rogers , Rasoamiaramanana , A. H. and Rahantarisoa , L. 2007 . Paleoenvironment and paleoecology of Majungasaurus crenatissimus (Theropoda: Abelisauridae) from the Late Cretaceous of Madagascar . Society of Vertebrate Paleontology Memoir , 8 : 21 – 31 .
- Ruben , J. A. 1977 . Some correlates of cranial and cervical morphology with predatory modes in snakes . Journal of Morphology , 152 : 89 – 100 .
- Scanlon , J. D. 1992 . A new large madtsoiid snake from the Miocene of the Northern Territory . The Beagle, Records of the Northern Territory Museum of Arts and Sciences , 9 : 49 – 60 .
- Scanlon , J. D. 1993 . Madtsoiid snakes from the Eocene Tingamarra fauna of eastern Queensland , Vol. 3 , 3 – 8 . Kaupia : Darmstädter Beiträge zur Naturgeschichte .
- Scanlon , J. D. 1995 . First records from Wellington Caves, New South Wales, of the extinct madtsoiid snake Wonambi naracoortensis Smith, 1976 . Proceedings of the Linnean Society of New South Wales , 115 : 233 – 238 .
- Scanlon , J. D. 1997 . Nanowana gen. nov., small madtsoiid snakes from the Miocene of Riversleigh; sympatric species with divergently specialized dentition . Memoirs of the Queensland Museum , 41 : 393 – 412 .
- Scanlon , J. D. 2003 . The basicranial morphology of madtsoiid snakes (Squamata, Ophidia) and the earliest Alethinophidia (Serpentes) . Journal of Vertebrate Paleontology , 23 : 971 – 976 .
- Scanlon , J. D. 2004 . First known axis vertebra of a madtsoiid snake (Yurlunggur camfieldensis) and remarks on the neck of snakes . The Beagle: Records of the Museums and Art Galleries of the Northern Territory , 20 : 207 – 215 .
- Scanlon , J. D. 2005a . Cranial morphology of the Plio-Pleistocene giant madtsoiid snake Wonambi naracoortensis . Acta Palaeontologica Polonica , 50 : 139 – 180 .
- Scanlon , J. D. 2005b . Australia's oldest known snakes: Patagoniophis, Alamitophis, and cf. Madtsoia (Squamata: Madtsoiidae) from the Eocene of Queensland . Memoirs of the Queensland Museum , 51 : 215 – 235 .
- Scanlon , J. D. 2006 . Skull of the large non-macrostomatan snake Yurlunggur from the Australian Oligo-Miocene . Nature , 439 : 839 – 842 .
- Scanlon , J. D. and Lee , M. S. Y. 2000 . The Pleistocene serpent Wonambi and the early evolution of snakes . Nature , 403 : 416 – 420 .
- Seiffert , E. 2006 . Revised age estimates for the later Paleogene mammal faunas of Egypt and Oman . Proceedings of the National Academy of Science , 103 : 5000 – 5005 .
- Shine , R. 1991 . Australian Snakes: A Natural History , 223 Balgowlah, New South Wales : Reed Books .
- Shine , R. and Webb , J. K. 1990 . Natural history of Australian typhlopid snakes . Journal of Herpetology , 24 : 357 – 363 .
- Shine , R. , Harlow , P. S. , Keogh , J. S. and Boeadi . 1998 . The influence of sex and body size on food habits of a giant tropical snake, Python reticulatus . Functional Ecology , 12 : 248 – 258 .
- Sigé , B. , Buscalioni , A. D. , Dugaud , S. , Gayet , M. , Orth , B. , Rage , J.-C. and Sanz , J. L. 1997 . Etat des données sur le gisement Crétacé supérieur continental de Champ-Garimond (Gard, Sud de la France) . Münchner Geowissenschaftliche Abhandlungen A , 34 : 111 – 130 .
- Simpson , G. G. 1933 . A new fossil snake from the Notostylops beds of Patagonia . Bulletin of the American Museum of Natural History , 67 : 1 – 22 .
- Simpson , G. G. 1935 . Early and middle Tertiary geology of the Gaiman region, Chubut, Argentina . American Museum Novitates , 775 : 1 – 29 .
- Smith , M. J. 1976 . Small fossil vertebrates from Victoria Cave, Naracoorte, South Australia. IV. Reptiles . Transactions of the Royal Society of South Australia , 100 : 39 – 51 .
- Storey , M. , Mahoney , J. J. and Saunders , A. D. 1997 . Cretaceous basalts in Madagascar and the transition between plume and continental lithosphere mantle sources . Geophysical Monograph , 100 : 95 – 122 .
- Storey , M. , Mahoney , J. J. , Saunders , A. D. , Duncan , R. A. , Kelley , S. P. and Coffin , M. F. 1995 . Timing of hot spot-related volcanism and the breakup of Madagascar and India . Science , 267 : 852 – 855 .
- Taquet , P. 1982 . “ Une connexion continentale entre Afrique et Madagascar au Crétacé supérieur: données géologiques et paléontologiques ” . In Phylogénie et Paléobiogéographie , Edited by: Buffetaut , E. , Janvier , P. , Rage , J. C. and Tassy , P. 385 – 391 . Geobios : Livre jubilaire en l’honneur de R. Hoffstetter . Mémoire Spécial 6
- Taylor , E. H. 1969 . Skulls of Gymnophiona and their significance in the taxonomy of the group . University of Kansas Science Bulletin , 48 : 585 – 687 .
- Taylor , E. H. 1977 . Comparative anatomy of caecilian anterior vertebrae . University of Kansas Science Bulletin , 51 : 219 – 231 .
- Tchernov , E. , Rieppel , O. , Zaher , H. , Polcyn , M. J. and Jacobs , L. L. 2000 . A fossil snake with limbs . Science , 287 : 2010 – 2012 .
- Turner , A. H. 2006 . Osteology and phylogeny of a new species of Araripesuchus (Crocodyliformes: Mesoeucrocodylia) from the Late Cretaceous of Madagascar. . Historical Biology , 18 : 255 – 369 .
- Vences , M. , Glaw , F. , Kosuch , J. , Bohme , W. and Veith , M. 2001 . Phylogeny of South American and Malagasy boine snakes: molecular evidence for the validity of Sanzinia and Acrantophis and biogeographic implications . Copeia , 2001 : 1151 – 1154 .
- Vences , M. , Vieites , D. R. , Glaw , F. , Brinkmann , H. , Kosuch , J. , Veith , M. and Meyer , A. 2003 . Multiple overseas dispersal in amphibians . Proceedings of the Royal Society of London, B , 270 : 2435 – 2442 .
- Wake , M. H. 1980 . Morphometrics of the skeleton of Dermophis mexicanus (Amphibia: Gymnophiona). Part I. The vertebrae, with comparisons to other species . Journal of Morphology , 165 : 117 – 130 .
- Wake , M. H. and Hanken , J. 1982 . Development of the skull of Dermophis mexicanus (Amphibia: Gymnophiona), with comments on skull kinesis and amphibian relationships . Journal of Morphology , 173 : 203 – 223 .
- Webb , J. K. and Shine , R. 1993 . Dietary habits of Australian blindsnakes (Typhlopidae) . Copeia , 1993 : 762 – 770 .
- Webb , J. K. , Shine , R. , Branch , W. R. and Harlow , P. S. 2000 . Life-history strategies in basal snakes: reproduction and dietary habits of the African thread snake Leptotyphlops scutifrons (Serpentes: Leptotyphlopidae) . Journal of Zoology, London , 250 : 321 – 327 .
- Werner , C. and Rage , J-C. 1994 . Mid-Cretaceous snakes from Sudan. A preliminary report on an unexpectedly diverse snake fauna . Comptes Rendus de l’Académie des Sciences, Paris (2) , 319 : 247 – 252 .
- Wiedersheim , R. 1879 . Die Anatomie der Gymnophionen , 101 Jena, , Germany : Gustav Fischer .
- Willard , D. E. 1977 . Constricting methods of snakes . Copeia , 1977 : 379 – 382 .
- Williams , E. E. 1959 . The occipito-vertebral joint in the burrowing snakes of the family Uropeltidae . Breviora , 106 : 1 – 10 .
- Yoder , A. D. and Nowak , M. D. 2006 . Has vicariance or dispersal been the predominant biogeographic force in Madagascar? Only time will tell . Annual Reviews of Ecology, Evolution and Systematics , 37 : 405 – 431 .
- Zangerl , R. 1944 . Contributions to the osteology of the skull of the Amphisbaenidae . American Midland Naturalist , 31 : 417 – 454 .
- Zangerl , R. 1945 . Contributions to the osteology of the post-cranial skeleton of the Amphisbaenidae . American Midland Naturalist , 33 : 764 – 780 .