ABSTRACT
The dodo Raphus cucullatus Linnaeus, Citation1758, an extinct and flightless, giant pigeon endemic to Mauritius, has fascinated people since its discovery, yet has remained surprisingly poorly known. Until the mid-19th century, almost all that was known about the dodo was based on illustrations and written accounts by 17th century mariners, often of questionable accuracy. Furthermore, only a few fragmentary remains of dodos collected prior to the bird's extinction exist. Our understanding of the dodo's anatomy was substantially enhanced by the discovery in 1865 of subfossil bones in a marsh called the Mare aux Songes, situated in southeastern Mauritius. However, no contextual information was recorded during early excavation efforts, and the majority of excavated material comprised larger dodo bones, almost all of which were unassociated. Here we present a modern interdisciplinary analysis of the Mare aux Songes, a 4200-year-old multitaxic vertebrate concentration Lagerstätte. Our analysis of the deposits at this site provides the first detailed overview of the ecosystem inhabited by the dodo. The interplay of climatic and geological conditions led to the exceptional preservation of the animal and associated plant remains at the Mare aux Songes and provides a window into the past ecosystem of Mauritius. This interdisciplinary research approach provides an ecological framework for the dodo, complementing insights on its anatomy derived from the only associated dodo skeletons known, both of which were collected by Etienne Thirioux and are the primary subject of this memoir.
Citation for this article: Rijsdijk, K. F., J. P. Hume, P. G. B. de Louw, H. J. M. Meijer, A. Janoo, E. J. de Boer, L. Steel, J. de Vos, L. G. van der Sluis, H. Hooghiemstra, F. B. V. Florens, C. Baider, T. J. J. Vernimmen, P. Baas, A. H. van Heteren, V. Rupear, G. Beebeejaun, A. Grihault, J. van der Plicht, M. Besselink, J. K. Lubeek, M. Jansen, S. J. Kluiving, H. Hollund, B. Shapiro, M. Collins, M. Buckley, R. M. Jayasena, N. Porch, R. Floore, F. Bunnik, A. Biedlingmaier, J. Leavitt, G. Monfette, A. Kimelblatt, A. Randall, P. Floore, and L. P. A. M. Claessens. 2015. A review of the dodo and its ecosystem: insights from a vertebrate concentration Lagerstätte in Mauritius; pp. 3–20 in L. P. A. M. Claessens, H. J. M. Meijer, J. P. Hume, and K. F. Rijsdijk (eds.), Anatomy of the Dodo (Raphus cucullatus L., 1758): An Osteological Study of the Thirioux Specimens. Society of Vertebrate Paleontology Memoir 15. Journal of Vertebrate Paleontology 35(6, Supplement).
INTRODUCTION
The dodo Raphus cucullatus Linnaeus, Citation1758 (), a giant, flightless pigeon endemic to the Mascarene island of Mauritius, became extinct just three centuries ago—a blink of an eye in terms of geological time—yet the historical record prior to the discovery of subfossil skeletal material of this vanished species comprises just a few scraps of skin, a small number of bones, and a handful of inadequate pictures and accounts (see Hume, Citation2006; Parish, Citation2013). Strickland and Melville (Citation1848:5–6) presented a most fitting summary in their now classic monograph on the dodo, highlighting the complications that study of a species so recently lost to the world could entail:
In the case of the Didinæ, it is unfortunately no easy matter to collect satisfactory information as to their structure, habits, and affinities. We possess only the rude descriptions of unscientific voyagers, three or four oil paintings, and a few scattered osseous fragments, which have survived the neglect of two hundred years. The paleontologist has, in many cases, far better data for determining the zoological characters of a species which perished myriads of years ago, than those presented by a group of birds, several species of which were living in the reign of Charles the First.
FIGURE 1. Scaled illustration by J.P.H. of the desiccated dodo head held at the Oxford University Museum of Natural History. A, right lateral view of skull, covered with desiccated skin; B, desiccated skin covering from the left side of the face, dissected from the specimen in 1847; C, left lateral view of the skull and mandible with skin covering removed (shown in B); D, left scleral ossicles in lateral (left) and medial (right) view. Scale bar equals 50 mm.

This monograph, the third of its kind, complements two earlier monographic works on the subject, those of Strickland and Melville (Citation1848) mentioned above and Owen (Citation1866a). All previous osteological work was based upon unassociated, composite skeletons, combining bones from many individuals and both sexes. Therefore, precise reconstructions based on associated skeletal elements of the dodo's physique, locomotion, and physiology were not possible (Hume, Citation2005; Meijer et al., Citation2012; Hume et al., Citation2014a; Claessens et al., 2015a). In this memoir (Claessens et al., 2015b), we describe the osteology of two nearly complete, associated skeletons of the dodo that were collected around 1900 by French-born amateur naturalist Louis Etienne Thirioux in caves or, more likely, boulder scree in the valleys surrounding the mountains of central Mauritius (Hume, Citation2005; Claessens and Hume, 2015). These skeletons are presently housed at the Mauritius Institute in Port Louis and at the Natural Science Museum in Durban, South Africa, but have not been studied in detail previously.
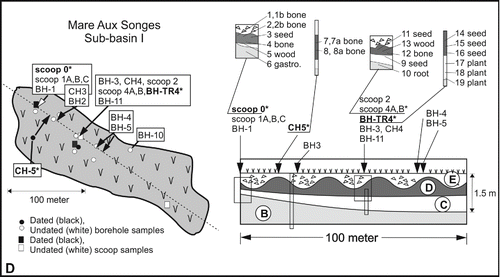
The bulk of the dodo subfossil material for pre-2005 studies on dodo anatomy were retrieved from an exceptionally rich vertebrate concentration Lagerstätte preserving multiple taxa called the Mare aux Songes, a marsh situated in a rocky valley near the southeastern coast of Mauritius (; Rijsdijk et al., Citation2009; Hume et al., Citation2014a). The 1.8-ha Lagerstätte situated in sub-basin I, the major sub-basin of the valley complex (), comprises an up to 0.5 m thick bonebed containing more than 20 vertebrate species, plant remains, terrestrial and freshwater mollusk and insect subfossils, and a suite of microfossils. Remarkably, the vertebrate subfossils in sub-basin I accumulated in less than a century ca. 4200 years ago, suggesting mass mortality events led to its formation (; Rijsdijk et al., Citation2009). Paleoecological research has shown that the vertebrate mass mortality was triggered by a series of extreme climatic drought events that affected a large part of the southwestern Indian Ocean region (De Boer et al., Citation2014, Citation2015). A unique combination of local geomorphic and hydrotaphonomic factors, coupled with eustatic sea level rise, resulted in excellent preservation of the subfossil material, which provided data for interdisciplinary research on the taphonomy and ecology of the Mare aux Songes bonebed (Hume, Citation2005; Rijsdijk et al., Citation2009, Citation2011; Meijer et al., Citation2012; Hume et al., Citation2014a).
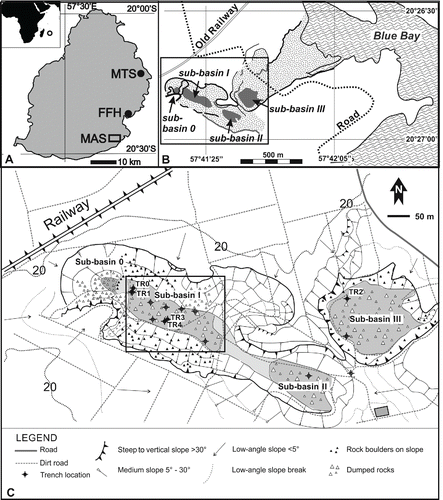
FIGURE 2. A, location of Mauritius in the southwestern Indian Ocean and locations of Mare aux Songes (MAS), Fort Frederik Hendrik (FFH), and Lake Tatos (MTS); B, map of Mare aux Songes and the sub-basins near the coast. Rectangular frame shows extent of inset C; C, geomorphological map of the Mare aux Songes area showing positions of all sub-basins (0, I, II, III) and locations of trenches TR1 (TR0), TR2, and TR3 (TR4). Rectangular frame shows extent of inset D; D, left panel showing extent of marsh at sub-basin I. The dashed line represents the longitudinal cross-section with positions of dated and undated samples from cores, scoops, and trenches. Right panel showing the longitudinal cross-section through the marsh with locations of radiocarbon dated samples (see ). B is carbonate sands, C is lake marl and gyttja, D is fossil layer, and E is dumped basalt boulder layer.
This bonebed has produced evidence enabling a high-resolution study of the ecosystem of the dodo, whereas our new analysis of the Thirioux dodo skeletons allows for the first osteological studies of associated remains of single individuals (see Claessens et al., 2015a). We further discuss the excavation history of the Mare aux Songes bonebed since its discovery in 1865, the subsequent work from 2005 to 2011, and explain how the bonebed was formed and how it was modified by environmental processes. We present clarification of how this insular ecosystem functioned under changing environmental conditions. Finally, we reflect on the environmental and human-induced stresses that the dodo and contemporary species must have endured on the island of Mauritius and what we can learn from the dodo on the potential resilience of insular vertebrates.
HISTORICAL BACKGROUND
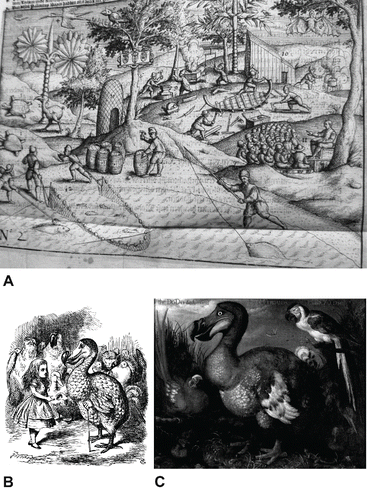
The isolated Mascarene Islands, comprising Mauritius, Reunion, and Rodrigues, are volcanic in origin and situated in the southwestern Indian Ocean. Mauritius lies 829 km east of Madagascar, the nearest large landmass (). Arab traders probably discovered the Mascarene Islands as early as the 13th century (Hume, Citation2013), followed by the Portuguese in the early 16th century, but neither the Arabs nor Portuguese settled there, as far as we know (North-Coombes, Citation1994). A Dutch trading fleet under Vice Admiral Wybrandt van Warwijck, en route to the Far East, claimed Mauritius for The Netherlands in September 1598, naming the island after stadtholder Prince Maurits of Orange Nassau (Moree, Citation1998, Citation2001). Subsequently, it was used as a port of call for provisioning and refurbishing ships. In 1602, Mauritius was administered by the newly founded Dutch East India Company (Vereenigde Oostindische Compagnie, VOC), whose primary aim was to monopolize the spice trade. The VOC kept journals describing their voyages, and these not only became important source material for future voyages, but also material for authors. The dodo was mentioned for the first time in 1599 in a small publication entitled ‘A True Report,’ which also gave an account of the voyage; only the English printing survives today (Van Neck, 1599; Anonymous, Citation1601; Hume, Citation2006). As more information became available from returning mariners, the publications were expanded, and the first published depiction of the dodo appeared in 1600, followed by a second edition in 1601 (; Anonymous, 1601). The importance of Mauritius as a ship refurbishment station was soon realized, and the island became an important stopover for outward and homeward bound VOC fleets. With the increase of European competitors in the Indian Ocean, the VOC established a permanent settlement in 1638, constructing Fort Frederik Hendrik beside the southeastern harbor, the present-day Vieux Grand Port (). This period of occupation, which saw the introduction of slaves from Madagascar and cutting down of ebony trees, ended in 1658, when the VOC abandoned the island. In 1652, the Cape of Good Hope was developed as an excellent port of call, which left Mauritius as a costly and superfluous establishment (Sleigh, Citation1993). However, with continued threats from rival English and French trading fleets, the Dutch reestablished a settlement on Mauritius in 1664, before abandoning the island completely in 1710 (Moree, Citation1998). During the second period of Dutch occupation, the population grew to 251, consisting of a few VOC servants, mostly farmers (freeburghers, some of them second generation) and slaves. The establishment managed to overcome the initial hardships of survival and depended more or less successfully on husbandry (Sleigh, Citation1993, Citation2000), while the felling of ebony trees and clearing for cattle farming was reestablished. The introduced invasive species must have had a great impact on the island's ecosystems, ultimately resulting in the extinction of the dodo and several other endemic species (Floore and Schrire, Citation1997; Griffiths and Florens, Citation2006; Cheke and Hume, Citation2008; Peters et al., Citation2009; Floore and Jayasena, Citation2010). During this period, live dodos and other Mauritian birds were shipped to Europe, India, and Japan (Cheke and Hume, Citation2008; Winters and Hume, Citation2014; Hume and Winters, Citation2015).
FIGURE 3. A, the earliest illustration of a dodo based on contemporary descriptions from the ‘Het Tvveede Boeck’ (1601) in a largely mythicized ecological context. Photograph by J.P.H.; B, the dodo in Lewis Carroll's ‘Alice in Wonderland’ (1865), illustrated by John Tenniel, was based on the dodo by Roelandt Savery; C, painting of the dodo by Roelandt Savery executed in ca. 1626 and held at the NHMUK, London. Photograph by J.P.H.
The first faunal and floral studies of Mauritius were made during the French occupation (1715–1810), long after the dodo had disappeared; therefore, our understanding of the bird and its ecology was largely restricted to 17th century mariners' accounts and illustrations. Very few specimens arrived in European museum collections, and most of those succumbed to insect damage (Hume, Citation2006). Such was the paucity of physical evidence that the very existence of the dodo was doubted by some scientists (Hume, 2006); however, John Duncan, curator at the Ashmolean Museum, described a desiccated dodo head () and foot held at the museum (Duncan, Citation1828). John Theodore Reinhardt, a Danish professor, examined a second dodo skull at the Copenhagen Museum and concluded that it was a giant pigeon (Reinhardt, Citation1842). This notion was initially met with ridicule until the monograph of Strickland and Melville (Citation1848) was published, which confirmed the dodo's columbid affinities. Strickland and Melville had the skin of the Oxford skull and foot dissected in order to study the cranium and tarsometatarsus (Hume et al., Citation2006), and they also figured a desiccated foot once held at the then British Museum (Natural History) (now the Natural History Museum, London).
Further interest in the dodo was created after the publication of Charles Darwin's ‘Origin of Species’ in 1859. Alfred Newton, a natural historian based at the University Museum of Zoology, Cambridge, U.K., was especially interested in the dodo and had just applied to become first professor of comparative anatomy and zoology at the university (Hume et al., Citation2009). Newton regularly corresponded with Darwin and became one of the first disciples of Darwin's theory (Hume et al., Citation2014b). Alfred's brother Edward, who was assistant Colonial Secretary on Mauritius, also had a great interest in natural history and was ideally located to report the discovery of any dodo fossil material (Hume et al., Citation2009, Citation2014b). Richard Owen, superintendent at the British Museum (Natural History), who became a stern adversary of Darwin's theory, was also keen to receive dodo fossil material. He made a request to the Bishop of Mauritius, Vincent Ryan, to notify him should any dodo remains be found. Owen had also written a testimonial in favor of Alfred's request to become professor and hinted that his support carried more weight than any other (Hume et al., Citation2009).
Around the same time, Charles Dodgson (better known by his pen name, Lewis Carroll) included the dodo in his best-selling novel ‘Alice's Adventures in Wonderland,’ published in 1865 (). The dodo was immortalized in the book by the illustrator John Tenniel, who based his image on an iconic painting of a dodo by a Flemish artist, Roelandt Savery (Fuller, Citation2002) (). The book became an international bestseller and was available across the entire British Empire, adding to the fame of the dodo.
The publication of ‘Alice's Adventures in Wonderland’ coincided with a spectacular discovery of subfossil dodo bones at Mare aux Songes in Mauritius in 1865 (Clark, Citation1866; Hume et al., Citation2009). Harry Higginson, a railway engineer, chanced upon the site when imported laborers were digging for peat and stockpiling bones and informed a local schoolteacher and amateur natural historian, George Clark (see Hume et al., Citation2009; Hume, Citation2012). Clark monopolized the site and sent a first consignment of bones to Richard Owen in September 1865. However, under instruction from Edward Newton, Clark organized another shipment of bones for Alfred, who was intending to sell them by auction the following year. Owen was tipped-off about the consignment by Captain Mylius, Clark's brother-in-law, and Owen intercepted the bones. He arranged a new deal with Clark via Mylius and promptly retained all of the material. Alfred was obviously furious, which in part may have been because of the loss of financial gain, and was going to make a formal complaint, but Owen blatantly blackmailed Alfred from taking any further action by threatening his application to become professor at Cambridge. Alfred had to relinquish his claim on the dodo and also had to withdraw a manuscript describing the dodo's anatomy. Owen wasted no time in monopolizing the discovery, giving public lectures in January 1866, before publishing his first monograph on the dodo in October of that year (Owen, Citation1866a; Hume et al., Citation2009). Crucially, this monograph and Owen's reconstructed skeleton were based on unassociated dodo bones from dozens of individuals of unknown age and sex, which were retrieved from the small (0.33 ha) sub-basin 0 situated adjacent to sub-basin I of the Mare aux Songes (Hume et al., Citation2014a; ). Regardless, this work served as a key reference on the morphology, physiology, and biology of the dodo for subsequent workers over the next 150 years (Livezey, Citation1993; Cheke and Hume, Citation2008; Hume et al., Citation2014a).
Such was the interest in the dodo in 1865 that virtually all other subfossil material was ignored; only one new species of parrot, Lophopsittacus mauritianus, was described from the Mare aux Songes based on a single mandible (Owen, Citation1866b), a specimen that was inadvertently included amongst the dodo remains sent to Owen. The following year, the French comparative anatomist, Alphonse Milne-Edwards, received rallid specimens from the Mare aux Songes from Edward Newton (Milne-Edwards, Citation1867), from which he described a new coot, Fulica newtonii Milne-Edwards, Citation1867. Albert Günther, a reptile specialist at the British Museum (Natural History), received some giant tortoise remains in the early 1870s from which he described two species (see Günther, Citation1877). The Mare aux Songes was reexplored in 1889 under the guidance of Théodore Sauzier, when six new species of bird were described from subfossil bones (Newton and Gadow, Citation1893).
The family of the amateur naturalist Paul Carié inherited the Mare aux Songes site in 1902 (Hume et al., Citation2009), so Carié was able to collect more material. He obtained further dodo bones and new species of reptile, all of which were deposited at the Muséum National d'Histoire Naturelle (MNHN), Paris (Hume, Citation2012). The last excavations of sub-basin 0 took place in the 1930s, but no previous work included detailed contextual descriptions of the faunal and floral diversity and the geological context of the site (Hume et al., Citation2014a). The infilling of the sub-basins of the Mare aux Songes in 1943 to combat malaria prevented any further excavations, after which the site was largely forgotten (Van Wissen, Citation1995).
THE REDISCOVERY OF MARE AUX SONGES
The rediscovery of the Mare aux Songes fossil site in 2005 was partly due to an investigation by a University of Tokyo research team in 1995, in collaboration with the owners of the then Mon Trésor, Mon Desert (MTMD) Sugar Estate (now Omnicane) and Mauritius Sugar Industry Research Institute (MSIRI). The Japanese team used coring equipment to penetrate the dumped boulder layer capping sub-basin I, in search of the bonebed discovered by Clark in 1865 (Grihault, Citation2005; ) and were successful in finding bone fragments, including dodo. However, due to a series of unfortunate events, when both of the principal organizers of the excavations died within a short time of each other (see Hume et al., Citation2014a), the results were not scientifically published; again the site remained neglected.
In 2005, K.F.R. and F.B. were invited to Mauritius by archaeologist P.F., project leader of the archaeological excavations at Fort Frederik Hendrik at Vieux Grand Port, to reconstruct the landscape as it was prior to human settlement, based on Quaternary geological and palynological reconstructions (Floore and Schrire, Citation1997; Floore and Jayasena, Citation2010; ). The primary aim of the archaeological project, which began in 1997, was to locate the first human settlement on Mauritius and compare findings with documentary records. The fort's archaeological record proved extremely productive and not only provided 17th century information on the building structures and human population, but also revealed the nature and impact of the human colonization (Peters et al., Citation2009). During archaeological excavations at Fort Frederik Hendrik from 1995 until 2006, 10,000 bones were found of animals slaughtered during the Dutch occupation from 1638 to 1710 (P. Floore, pers. comm., 2006; Peters et al., Citation2009). The remains of native animals included dugong Dugong dugon (Statius Müller, Citation1776), some birds, and giant tortoises, but dodos were absent (Floore and Jayasena, Citation2010). The anthropogenic deposits of Fort Frederik Hendrik, consisting of clayey soils, proved unsuitable for the conservation of pollen needed for the environmental reconstruction of the 17th century habitation. In consequence, a program was set up in 2005 to identify marsh and peat deposits in an area of approximately 10 km around the excavation site to provide a palynological context. One of these selected spots was the Mare aux Songes (Nauta, Citation2006).
To collect pollen samples from the Mare aux Songes for palynological studies, it was attempted in 2005 to penetrate the dumped boulders at the marsh, but without success. In turn, MTMD offered the above-mentioned cores for description, and these were instrumental in obtaining permission to reexcavate the Mare aux Songes by means of a mechanical digger. Trench TR0 excavated in sub-basin I proved extremely rich in bone and plant remains and led to the rediscovery of the vertebrate concentration Lagerstätte (Nicholls, 2005; Rijsdijk et al., Citation2009; ). The bone material, comprising bird, mammal, reptile, and fish, was set in a matrix of peat, intermixed with subfossil wood stems, leaves, macroscopic seeds and fungi, along with insect and terrestrial and freshwater mollusk remains. Most importantly, the richness of the Mare aux Songes subfossil locality gave an unprecedented insight into the paleoecology of Mauritius, long before the island was discovered by humans. As a result of the discoveries, the Dodo Research Programme was initiated, in order to address the following questions: (1) How did the Lagerstätte form; (2) How did the vertebrates accumulate at the site, and (3) What can we learn from the Lagerstätte about the ecology and biology of the biota of Mauritius before human arrival? The methods to answer these questions effectively followed those outlined by Behrensmeyer and Kidwell (Citation1985).
The site was excavated for six successive years. In the first year (Expedition I), the aim was to assess the fossil richness of the sub-basins. A succession of 10 exploratory cores were taken, which established that only sub-basins I and III contained vertebrate remains (). Basin 0 was not sampled because its location remained unknown at that time (Hume et al., Citation2014a). In addition, piezometers (pressure sensors) were installed in each of the three sub-basins, at 1 and 3 m below surface levels, to monitor the groundwater table and to analyze water samples (Rijsdijk et al., Citation2009, Citation2011). Mechanical digging machines excavated three trenches (TR): TR1 at the margin of basin I, TR2 in sub-basin III, and TR3 in the middle of sub-basin I (). Bulk samples were sieved from all trenches (Rijsdijk et al., Citation2009). The first excavation was also crucial in establishing a methodology for undertaking a scientific excavation below the water table (Hume et al., Citation2014a).
The subsequent expeditions focused on excavating in situ by creating a dry trench (TR4) in sub-basin I, close to TR3. TR4 was so rich in vertebrate bones that it took two expeditions (Expedition II: 2007, Expedition III: 2008) to process the bone material, with more than 100 × 100-liter sacks being used for the excavated sediment. It took another four sessions (Expeditions IV–VI; 2009–2011) to finish the in situ excavations in TR4, which included the recording of three-dimensional (3D) orientations of individual bones (Rijsdijk et al., unpubl. data). A total of 27 samples were taken for radiocarbon dating (; ). The Mare aux Songes fossil locality is now completely protected within a fenced boundary at Omnicane, and members of the Dodo Research Programme are still processing the finds.
TABLE 1. Radiocarbon dates from sub-basin 1.
THE SETTING OF MARE AUX SONGES
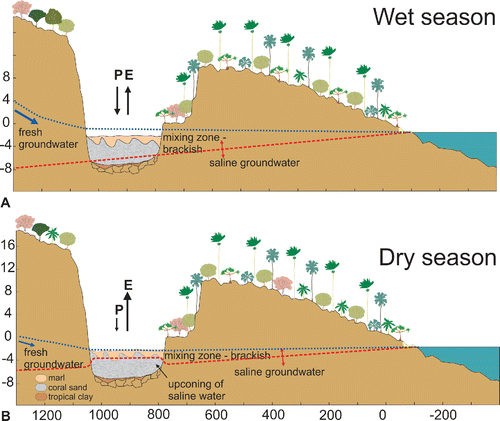
Mare aux Songes is a rocky valley formed <100 kyr ago, possibly as a combination of lava tunnel collapse and phreatic-magmatic explosive activity (De Boer et al., 2105). The valley comprises four sub-basins, three of which, sub-basins 0, I, and III, contain subfossil bones (). The mean groundwater level is approximately 0.5 m below present land surface level. The basins are hydrologically connected with the ocean through permeable basalts (), and as a result, fresh groundwater overlies a saline water wedge that increases in thickness towards the ocean, less than 2 km away. As a consequence, the groundwater levels fluctuate in phase with the tide, resulting in the saline groundwater wedge increasing the salinity of the water in the sub-basins. Present water salinity increases from sub-basins I to III, from low- (90 mS/m) to high- (580 mS/m) brackish conditions, respectively (Rijsdijk et al., Citation2009). The freshwater input at Mare aux Songes is through upwelling groundwater that is mainly derived from net rainfall from the Mauritius uplands. The precipitation at Mauritius is influenced by the Southern Hemisphere monsoon system, and the precipitation is highest from November to April. The coastal lowlands (<50 m elevation) under influence of dry cool easterly trade winds experience a dry season from May to October (Senapathi et al., Citation2010). Mean annual precipitation varies with altitude, with mean annual rainfall of more than 4000 mm in the Mauritius uplands (>300 m) and less than 1300 mm in the eastern coastal lowlands where the Mare aux Songes is situated. In this region, mean annual evapotranspiration exceeds mean annual precipitation, resulting in water deficit most of the year (Padya, Citation1989). Two years of continuous measurements of groundwater fluctuations indicate that the water tables are sensitive to the balance of net rainfall and evaporation within the Mare aux Songes catchment area (, B). Furthermore, groundwater tables vary with the seasons: during the wet season water tables are ca. 300 mm higher and during the dry season 200 mm lower than the mean groundwater level (Rijsdijk et al., Citation2011).
FIGURE 4. Panels showing a transverse cross-section through the Mare aux Songes. The dashed dark gray line shows the saline water wedge that intruded in the permeable basalts making up the rock valley and its shoulders. The black arrows show how this groundwater table fluctuates with the tide. The overlying freshwater lens is depicted as light gray dotted line. The line that crosses the valley indicates the lake level; the line that crosses the rock represents the groundwater table. P = precipitation, E = evaporation. Horizontal axis in meters from the coast. Vertical axis in meters above mean ocean level. A, upper panel. During the wet season, E does not exceed P and the saline wedge remains stationary below the freshwater lens; B, lower panel. During an extreme drought event and during the dry season, E exceeds P and the input of freshwater is decreased, so consequently the saline water wedge level cones upwards, salinizing the shrinking freshwater lake.
STRATIGRAPHY
Sedimentological analyses of five mechanical digger scoop samples and seven cores in sub-basin I () demonstrated that the bonebed (unit D in ) at the Mare aux Songes is a continuous lenticular bed, spanning 1.8 ha, that is 0.4 m thick at its margins and achieves its greatest thickness (ca. 0.75 m) in the center of the marsh (Rijsdijk et al., Citation2009, Citation2011). However, the continuity of the bonebed is disrupted by basaltic rocks (unit E) that were dumped in 1943 and have sunk into the peaty bonebed (unit D) (; Rijsdijk et al., Citation2009, Citation2011; De Boer et al., Citation2015). In the center of the marsh, at the base of the peaty bonebed, the peat is rich in wood stems and rootlets. The bonebed is underlain by a basal gyttja–lake marl bed (unit C). Both gyttja and lake marl are organic sediments formed by degraded remains of plants and microscopic animals in a lake setting, whereby marl is particularly rich in calcium-carbonate due to the upwelling carbonate-rich groundwater. Both the bonebed (unit D) and the gyttja–lake marl beds (unit C) thin at the margins of the paleolake (TR0, TR1), where they are up to 0.4 and 0.2 m thick, respectively, but both units thicken towards the center of the paleolake (TR3, TR4), where they become up to 0.75 and 1.15 m thick, respectively (De Boer et al., Citation2015). Near the edges of the paleolake the bonebed forms a wood- and bone-supported mass (). Even the matrix of this bone mass comprises smaller bone fragments (<5 mm), mixed with plant debris and peat (Rijsdijk et al., Citation2009, Citation2011). Towards the center of the sub-basin, as the bonebed (unit D) and underlying organic beds (unit C) increase in thickness, a corresponding increase in peat matrix leads to the bonebed being matrix-supported. In this region, bones form local clusters or are dispersed in the peat matrix (). Here the peaty bonebed grades upwards into a ca. 30 cm thick, horizontally laminated, dense peat layer interstratified with a distinct ca. 5 cm thick seed layer. The dark-brown peat forming the matrix of the bonebed comprises a compacted mass of nonsticky, decomposed plant debris rich in small seeds (<1 mm). The presence of one or two discontinuous laminae of coral sand within the bonebed reflects a subaerial influx of coral sands in sub-basin I from the nearby beach sands, likely blown into the sub-basin during cyclones.
FIGURE 5. A, top left panel shows a transverse cross-section through the Mare aux Songes, depicting the stratigraphy. Top right panel is an idealized succession of sediment layers at the former center of the paleolake (after De Boer et al., Citation2015); B, sediments exposed in 100 cm wide excavator scoop at TR1 at the former lake margin of sub-basin I: bone-supported bonebed, mixed with wood stems, and seeds; C, sediments exposed in 50 cm wide excavator scoop at TR4 in the middle of sub-basin II matrix-supported bonebed, with peat as matrix; the lower rectangular fossils are tree and root remains. Photographs by R.M.J.
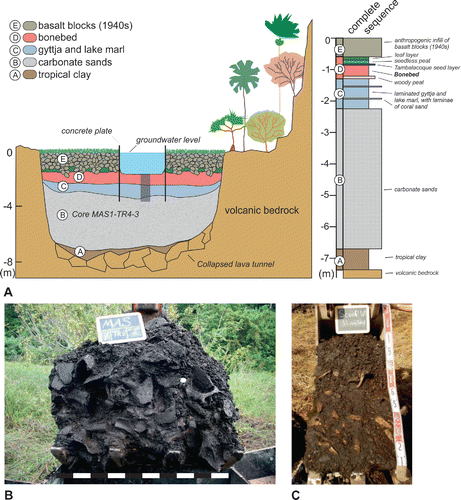
Hydrological data, diatoms, and pollen provide evidence of frequent lake margin floor exposures during the drought events 4200 years ago, which, in turn, explains the coarseness of the matrix of the bonebed at the former lake edge (Rijsdijk et al., Citation2009, Citation2011). The decomposed peaty materials were washed out by wind and wave action at the lake shore, resulting in a concentrated bonebed. In contrast, tranquil conditions occurred in the deeper parts of the lake, which accounted for an accumulation of finer organic peat debris (<1 mm), leading to a thicker matrix-supported bonebed with bones locally dispersed in peat. The basal woody peat bed forming the base of the bonebed (unit D) is up to 30 cm thick and contains various tree stems and branches that lie at a low angle relative to the former lake floor. These floral remains, which include rootlets and stems (Vernimmen et al., unpubl. data, see below), represent a classic sea-level-driven drowning of terrestrial arboreal vegetation when the lake deepened during Holocene sea level rises ca. 5000 years ago (Rijsdijk et al., Citation2011; De Boer et al., Citation2014, Citation2015). The underlying laminated black organogenic sediment (gyttja) and gray lake marl unit (, unit C; see also ) represent an alkaline groundwater-fed shallow lake and marsh environment. Unit C overlies a unit of aeolian carbonate sands (unit B) up to 8 m thick, deposited during the Pleistocene, and the sequence is concluded by up to 2 m of clayey soils (unit A) overlying the bedrock (; Rijsdijk et al., Citation2011).
Samples for radiocarbon dating (n = 27), of which 23 were successful, were obtained from the bonebed and just below it at three localities (TR0, CH5, and TR4) in sub-basin I (; ). These included bones (n = 7), a mollusk, and plant material (n = 12). These radiocarbon dates indicate that the bonebed is nearly isochronic and was formed approximately 4200 years ago (see below). The calibrated radiocarbon dates overlap due to plateau effects, as also demonstrated by multiple dates on single samples (n = 4) (). A Bayesian based calibrated age-depth model indicates that the bonebed, including the underlying gyttja and lake marl layer, formed within three centuries between 4370 and 4070 cal BP (; De Boer et al., Citation2015).
MASS MORTALITY AND CAUSE OF DEATH
On a surface of less than 15 m2 at five excavation sites in the Mare aux Songes, ca. 10,000 bones were excavated, of which ca. 300 were from dodo. Assuming that a uniform distribution and density of animal remains were deposited in the marsh, it can be deduced that many thousands of vertebrates, including dodos, must have died in an area of only 1.8 ha, approximately 4200 years ago. The vertebrates represented in the deposit are dominated by two extinct species of giant tortoise (Cylindraspis), which are found alongside at least six unidentifiable passerine species, two species of macrochiropteran fruit bats (Pteropus), the dodo, and other birds; approximately 22 vertebrate species have been identified (Rijsdijk et al., Citation2011).
The formation of the multitaxic bonebed at Mare aux Songes came as a result of a coincidental interplay of environmental conditions. Sea level rise pushed upwards the groundwater table which led to the formation of a freshwater lake 4500 years ago (), and the generally dry conditions prevailing in the lowlands attracted a rich fauna to the lake. Ongoing sea level rise led to a deepening of the lake, with water depths of up to 1 m reached 4200 years ago (Rijsdijk et al., Citation2009, Citation2011). After this period, extreme droughts associated with a global climatic regime shift that was characterized by a monsoonal collapse (De Boer et al., Citation2014; see below) led to a critical lowering of groundwater and subsequent up-coning of the saline water wedge, especially during the dry seasons (). During dry episodes, the freshwater lake began to contract, concentrating the living vertebrates around the shrinking water body and on the exposed soft lake surface.
At the same time, up-coning of the saline water wedge increased the salinity of the groundwater at sub-basin I, which likely became undrinkable for animals. Further evidence is derived from the presence of fungal spores of Sporomiella and diatoms, indicative of the presence of concentrated vertebrate excrement (nutrients) inducive to hypereutrophic water conditions, blooming of cyanobacteria, and poisoned water (De Boer et al., Citation2015; see below). These circumstances facilitated blooms of toxic cyanonbacteria, as is evidenced by spikes of cyanobacterial pigments within the bone layer. Repetitive seasonal contamination and poisoning of the freshwater led to the accumulation of vertebrate remains at the Mare aux Songes. An anomalously high nitrogen isotope ratio measured in bone collagen from a giant tortoise subfossil from Mare aux Songes may be attributed to a urea-based physiological response to retain water during periods of drought, thus leading to nitrogen isotope enrichment in the bone tissue (Van der Sluis et al., Citation2014).
Interdisciplinary analysis of deposits in a nearby coastal lake, Mare Tatos, 26 km NNE of Mare aux Songes (), shows that anomalous decadal to centennial drought events occurred between 4350 and 4130 cal BP (De Boer et al., Citation2014, Citation2015). This period, also referred to as the ‘4.2 ka megadrought,’ has been recorded in other sites around the Indian Ocean and is associated with civilization collapses in Egypt, Pakistan, Mesopotamia, and eastern Africa (Gasse, Citation2000; Thompson et al., Citation2002; Marchant and Hooghiemstra, Citation2004; Staubwasser and Weiss, 2006; MacDonald, Citation2011). At both Mare Tatos and Mare aux Songes, the megadrought is also reflected in increased concentration of microcharcoal that indicates increased frequencies of natural fires. The megadrought was triggered by a global climatic regime shift that involved a monsoonal collapse that ultimately led to the current configuration and increased activity of the El Niño southern oscillation, a phenomenon that has prevailed for the last 4000 years (see De Boer et al., Citation2014). A high-resolution multiproxy analysis of a continuous core at TR4 in the Mare aux Songes (), supported by a Bayesian-based calibrated age-depth model (Blaauw and Christen, 2011), suggests that the megadrought was interrupted by two wetter periods. The bonebed postdates the second wet period and was formed during the third dry period from 4190 to ca. 4130 cal yr BP (De Boer et al., Citation2015). After the third drought, the lake at Mare aux Songes became permanently refilled and the lake floor was permanently immersed.
Ultimately, anomalous drought events were instrumental in the death of thousands of vertebrates through a combination of poisoned water conditions, drowning, and miring, within a period of less than 100 years.
TAPHONOMIC ANALYSIS
The majority of the skeletal material from the bonebed in sub-basin I is disarticulated and disassociated, but between 2005 and 2011 two partial associations were recorded: the pelvis and some leg bones of a dodo (Rijsdijk et al., Citation2009) and a carapace of the giant tortoise Cylindraspis inepta (Günther, 1877) containing humeri and femora (Hume, Citation2014; Hume et al., unpubl. data). Most bones are chaotically mixed, with no clear preferential orientations (, B; Rijsdijk et al., unpubl. data), but in general, the bones lie subhorizontal or at low angle dips (<25°). The deposit is dominated by larger and heavier long bones, whereas smaller and more pneumatic bones are relatively rare (Hume, Citation2005; Meijer et al., Citation2012). This is likely due to bioturbation, desiccation resulting in the flotation of carcasses, scavenging, and weathering (Hume, Citation2005; Meijer et al., Citation2012; Hume et al., Citation2014a), especially during the periodic wet seasonal recharging of the Mare aux Songes lake. The subfossil bones are macroscopically well preserved, apart from overall brown staining by plant tannins and macroscopic mechanical fractures (Meijer et al., Citation2012; Hume et al., Citation2014a; ).These fractures were likely created by the dumping of basalt boulders in 1943 and, to a lesser degree, by our sampling technique using mechanical diggers (Meijer et al., Citation2012). The exceptional preservation of the bone material is attributed to suitable hydrological conditions, with groundwater rich in CaCO3 as a result of upwelling through carbonate sands that underlie the bonebed. This created alkalic conditions in the peat which prevented geochemical dissolution of bone tissue; this factor was conducive to exceptional bone preservation (Rijsdijk et al., Citation2009; Van der Sluis et al., Citation2014).
FIGURE 6. A, view from above of an in situ dodo pelvis in the Mare aux Songes bonebed. Photograph by Mikel R. Rijsdijk; B, mapped view of the in situ dodo pelvis, long bone (dark gray, red in online PDF), and tortoise long bone and carapace fragments (light gray, pink in online PDF). X-axis and Y-axis are coordinates in cm; three-digit numbers next to bones are depths of bone center relative to mean sea level in (m).
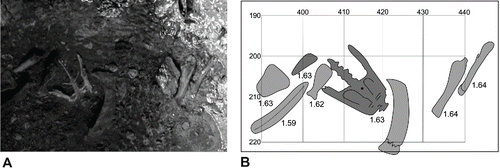
FIGURE 7. A, range of color variation in dodo tibiotarsi from the Mare aux Songes sub-basin I (Hume et al., Citation2014a:). Photograph by Arike Gill; left bone specimen rectangular cut is due to aDNA sampling; B, dodo left femur with polydirectional scratches (inset) interpreted as bioturbation derived from giant tortoise trampling (see Meijer et al., Citation2012:); inset close-up of polydirectional scratches (after Meijer et al., Citation2012:). Photographs by H.J.M.M; C, microcracks in bone sample MaS-tr0-05 (×100). Photograph by L.G.V.; D, pyrite clusters in bone sample MaS-tr1-04 (×40). Photograph by L.G.V.; E, color variation in dodo tibiotarsi from sub-basin 0 (after Hume et al., Citation2014a:). Bones held at the NHMUK, London. Note the extensive root marks on far left specimen, and the pitted surface of the specimen on the far right. Photograph by J.P.H. Scale bars equal 10 mm (A, E).
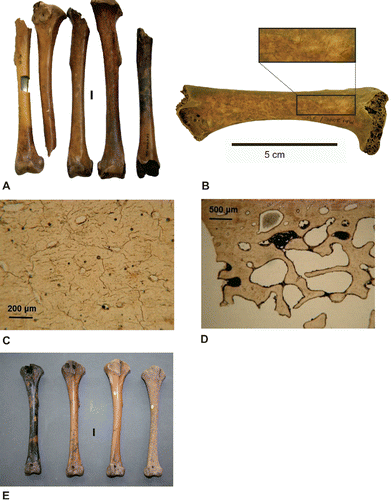
The surfaces of most bones are covered with micropitting and scratching, attributed to trampling predominantly by the abundant tortoises wallowing through the bone-rich sediments (Meijer et al., Citation2012; ). Microscopically, the bones show virtually no bioerosion, confirming their near-permanent submergence in anoxic groundwater since 4200 years ago, thus inhibiting microbial decay. However, the bones do show signs of chemical degradation at a microscopic level in the form of etching, infiltration by iron compounds, and microfracturing (). The anoxic, iron- and organic-rich burial environment (including carcasses) provided conditions for the formation of pyrite grains in bone pores (). Some grains have oxidized to form iron oxide hydroxides and sulfuric acid, causing chemical degradation of the bone. This testifies to alternating oxic and anoxic conditions at Mare aux Songes, most likely caused by fluctuating groundwater levels during the megadrought ca. 4200 years ago (Van der Sluis et al., Citation2014).
In spite of chemical degradation, subfossil bone collagen was of suitable preservation for radiocarbon dating and stable isotope analyses, so that the diet of the dodo and both giant tortoise species could be interpreted. Both dodo and tortoises were predominantly herbivorous and mainly fed on C3 plants and their products, e.g., seeds and fruits (Rijsdijk et al., Citation2009; Van der Sluis et al., Citation2014). Ancient mitochondrial DNA was characterized from the dodo head in the Oxford University Museum of Natural History (Shapiro et al., Citation2002), but the sampled bone material did not originate from the Holocene site of Mare aux Songes. Although bone collagen is preserved in Mare aux Songes bones, attempts to extract aDNA from dodo bones collected in sub-basin I have been unsuccessful (Rijsdijk et al., Citation2009). However, Austin and Arnold (Citation2001) obtained aDNA from two tortoise bones from the younger sub-basin 0 deposit. More recently, new aDNA extraction and preparation technologies have enabled the recovery of short fragments of aDNA from extremely poorly preserved subfossil remains (Dabney et al., Citation2013). Using these methods, aDNA was extracted from 12 dodo bones in July 2014 at a specialized ancient DNA facility at University of California Santa Cruz (Santa Cruz, California), and, following genomic library preparation (Meyer and Kircher, Citation2010), approximately one million fragments of DNA were sequenced. The resulting data were mapped to an assembled mitochondrial genome sequence from the Nicobar pigeon Caloenas nicobarica (Linnaeus, Citation1758), which is the closest living relative to the dodo (Shapiro et al., Citation2002). Three of the 12 Mare aux Songes sub-basin I extracts yielded between one and six sequence fragments that mapped to the Nicobar pigeon mitochondrial genome, all of which were less than 45 base pairs in length. These results show that although aDNA is present in the dodo bones from Mare aux Songes sub-basin I, most of it is in very poor condition compared with that preserved in the Oxford dodo (Shapiro et al., Citation2002). These results are consistent with thermal age estimates from the site (Rijsdijk et al., Citation2009). In addition, most aDNA recovered from Mare aux Songes bones was not from the dodo, but instead closely matches sequences of microbes and plants, most likely reflecting postmortem colonization of these bones by other organisms.
Clark's (Citation1866) original 1865 excavation site at sub-basin 0 has proved to be a different depositional environment from that in sub-basin I, which explains the remarkable difference in bone taphonomy and aDNA preservation from the sites (Hume, 2003; Hume et al., Citation2014a; ). Although the bones in sub-basin I were only exposed subaerially during droughts 4200 years ago, for most of the time (>4000 years) they remained under anoxic, water-logged conditions; the bones in the higher-positioned sub-basin 0 were exposed more frequently due to a more shallow burial in the marsh on the lake shore (ibid). Sub-basin 0 formed 2500 years ago when sea levels reached present-day levels (Zinke et al., Citation2003; Camoin et al., Citation2004). This setting explains the wide range of taphofacies of the bones from this deposit, with various degrees of pedological alterations, discolorations, and biochemical erosion of the bones; in addition, its younger age also may explain the better preservation of aDNA (Hume, Citation2005; Hume et al., Citation2014a; ). In contrast, the more stable conditions in sub-basin I explain the narrow range of taphofacies of the dodo bones (n > 250) and tortoise bones (n > 10,000) preserved there (Meijer et al., Citation2012; ).
The almost complete disarticulation and disassociation of subfossil remains in the bonebeds at the Mare aux Songes resulted in all subsequent dodo osteological work being based on incomplete, composite skeletal material (Owen, Citation1866a), made up of individuals of unknown age and sex (Livezey, Citation1993).
TOWARDS AN ECOLOGICAL RECONSTRUCTION
The abundant admixture of plant debris in the bonebed, and the presence of plant microfossils and invertebrates, provides an unprecedented opportunity to reconstruct the paleoenvironment at Mare aux Songes. The near-coastal setting of the Mare aux Songes rock basin explains the gradients from saline to freshwater and from dry lowland to wet basins; these ecotones led to high plant diversity. The abundance of macro- and microscopic plant remains such as seeds, fruits, branches, stems, and tree roots reflects the richness of the flora at the time, as confirmed by macroscopic and microscopic analyses (). The bonebed contains high densities of seeds (of 10–30 mm), dominated by tambalacoque, Sideroxylon grandiflorum (Sapotaceae), bois d'olive, Cassine orientalis (Celastraceae), and several species of screw pine, Pandanus (Pandanaceae). The presence of S. grandiflorum is interesting, because this species was considered to be a montane endemic and never reported from the coastal lowland areas (Baider and Florens, Citation2006; Florens et al., Citation2012). Wet forest species recorded at Mare aux Songes are confined to high rainfall sites today and are typical of a wet canopy forest plant community, especially Eugenia elliptica (Myrtaceae), Antirhea borbonica (Rubiaceae), and Canarium paniculatum (Burseraceae) (Florens et al., Citation2012). The finer sieve fractions (0.25–2 mm) of a 500-ml soil sample yielded >1000 seeds and other recognizable parts of plants. Microscopic analysis revealed that besides trees and shrubs, these could belong to smaller, non-woody plants. The seed assemblage of the bonebed confirms that the Mare aux Songes and surrounding area supported a wet forest plant community, which persisted in an otherwise dry coastal zone characterized by substantially drier vegetation, including characteristic species like Foetidia mauritiana (Lecythidaceae) or Terminalia bentzoe (Combretaceae).
FIGURE 8. A, well-preserved subfossil wood remains from sub-basin I; B, transverse section of Ficus; C, growth ring border in Cassine orientale. Photographs by T.J.J.V. Scale bars in cm (A) and equal 500 μm (B, C).
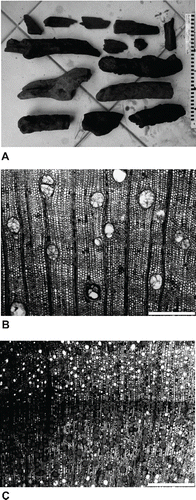
The peaty bonebed in sub-basin I contains stems, roots, and branches ranging in thickness from 10 to 300 mm (). The local presence of at least 28 different taxa of dicot trees, shrubs, and one epiphyte has been revealed by microscopic analysis of ca. 350 samples of subfossil wood (Vernimmen et al., unpubl. data). Due to the predominantly wet, anoxic burial conditions, most anatomical characters of wood have been well preserved (, C), enabling in some cases identification to species level. Several monocot stems were also found, and at least one type of palm was identified. The total wood assemblage includes taxa with either a broad ecological range or specific ecological preferences. These range from dry to wet forest and lowland to upland settings, which suggests that species composition at this low elevation site was diverse and very different from the present impoverished lowland forest on Mauritius (Vernimmen et al., unpubl. data). The fruiting bodies of a mushroom (species indeterminate), spores of other fungi, and a moss are also present in the deposit. These were presumably growing on the trunks of trees. Interestingly, many of the subfossil wood stems from a number of plant taxa show evidence of growth rings when cut in transverse section, which is indicative of seasonal differences in water availability (Vernimmen et al., unpubl. data; ).
The gyttja underlying the bonebed, the peat forming the matrix of the bonebed, and the peat capping the bonebed all contain pollen, spores, diatoms, and a suite of other microfossils. Continuous cores have been obtained from the bonebed and organogenic layers underlying it (). Palynological studies of sediment cores indicate the presence of palm woodland and semidry coastal forest associations (De Boer et al., Citation2014, Citation2015). Palm woodland is represented by Latania, Dictyosperma, Acanthophoenix, and Pandanus; semidry forest by Ficus, Eugenia, Sapotaceae, Terminalia bentzoë, Diospyros, Tabernaemontana, and Cassine orientalis, small trees of Gardenia type, Ixora, Zanthoxylum, Antidesma, Foetidia, and Hilsenbergia type, and shrubs of Dodonaea and Dombeya. Taxa characteristic of palm woodland are better represented by pollen than by the analysis of fossil dicot wood and other plant macrofossils (). We speculate that the eastern coast of the Mare aux Songes basins were predominantly affected by trade winds blowing landwards, delivering palm woodland pollen from the vegetation east of Mare aux Songes, whereas the wood fossils and larger seeds in the Mare aux Songes basins dominated by semidry forest taxa are all deposited from local standing vegetation. Although speculative, this may indicate that the vegetation around Mare aux Songes and further landward was dominated by semidry forest, whereas palm woodland occurred as a strip of vegetation between the lowlands and the coast (De Boer et al., 2015). The palm woodland may have provided an open vegetation community relatively easily accessible to non-volant vertebrates. The semidry forest must have been much denser, however, with a high trunk density that is attributed to the influence of frequent cyclones (Vaughan and Wiehe, Citation1937; Florens, Citation2008). This cyclone-resistant vegetation was described by the early colonists as dense and hard to penetrate (Moree, Citation1998; Cheke and Hume, Citation2008).
FIGURE 9. A, opening of a Russian core tip by E.J.B. and H.H. after sampling in TR4; B, opened Russian core shows laminated organogenic (gyttja) and lake marl sediments underlying the bonebed; C, simplified integrated pollen diagram of Mare aux Songes (after De Boer et al., Citation2015). Photographs by K.F.R.
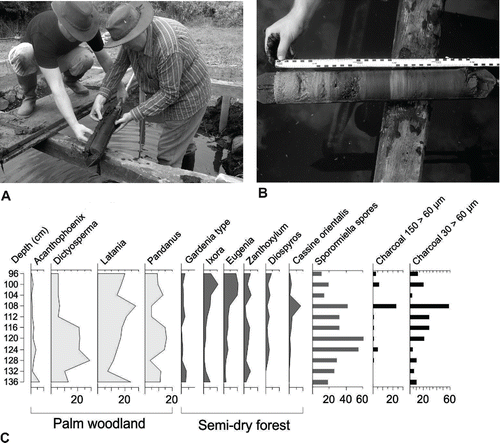
The pollen record revealed a remarkable lack of small shrubs and grasses in the Mauritian coastal forest understorey vegetation until human advent (De Boer et al., Citation2014; ). Shrub and grass communities, increased biomass, and increased fire frequencies are generally associated with reduced or absent grazing regimes after megafaunal extinctions (Vaughan and Wiehe, Citation1937; Burney et al., Citation2003; De Boer et al., Citation2015). Seeds and fruits from unlignified plants, as well as wood samples from shrub-like species are, however, identified. It is plausible, based on studies on the reintroduction program of giant tortoises on Île aux Aigrettes (Griffiths, Citation2014), that high concentrations of giant tortoises kept the biomass in the understorey to a minimum by grazing and browsing. Other indications that larger vertebrate fauna had an important role in the lowland forest ecosystems are based on (a) the presence of plant defense mechanisms such as spines on juvenile Tectiphiala palms; (b) the occurrence of heterophylly in several plant genera, where the juvenile leaves tend to be inconspicuous relative to the leaves of adult plants, to avoid being eaten (Cheke and Hume, Citation2008); and (c) the presence of many plant species producing cauliflorous fruits close to the ground, hence accessible to large flightless frugivorous vertebrates that would have served as seed disseminators (Florens, Citation2008). Given the former high abundance of giant tortoises on Mauritius (Cheke and Hume, Citation2008), they must have played a significant role in seed dispersal and in maintaining habitat heterogeneity through disturbances by grazing and browsing (Griffiths, Citation2014).
Because coprophilous fungal spores generally have a limited aerial dispersal range (Wood and Wilmshurst, Citation2012), the coprophilous fungal spores in the sediment cores of coastal sites suggest that herbivores lived around these sites (De Boer et al., Citation2014, Citation2015; ). The noted absence of fungal spores in the upland records suggests lower concentrations of these animals in montane forest (De Boer et al., 2013a, 2013b). Although large terrestrial vertebrates, e.g., dodos and tortoises, occurred in the uplands, heterophylly is very rare in the remaining forest patches, perhaps indicating that the main biotope for the populations of larger vertebrates was the coastal lowlands (Florens, Citation2002; Cheke and Hume, Citation2008; De Boer et al., Citation2014, 2015). The presence of insect remains in the Mare aux Songes, including now extinct bird and giant tortoise specialist dung beetles (Scarabaeinae), which appear to have been confined to the coastal lowlands, further supports the notion that it was this ecotype that was the most vertebrate diverse prior to human arrival (Hume, Citation2009, Citation2012; Porch, unpubl. data).
RESILIENCE AND EXTINCTION: A CONCLUSION
The bonebed of sub-basin I at Mare aux Songes () provides a window of less than a century documenting the response of a coastal ecosystem to an extreme climatic event 4200 years ago. Although many thousands of vertebrates died within an area of 1.8 ha as a result of this pre-human-contact catastrophic event, the dodo and other vertebrates survived until the arrival of humans over 3500 years later.
The exact date of extinction of the dodo continues to be debated (Roberts and Solow, Citation2003; Hume et al., Citation2004; Mlíkovský, Citation2004; Cheke, Citation2006; Cheke and Hume, Citation2008; Jackson, Citation2014), but what is certain is that during the second half of the 17th century, the dodo became extinct. After initially disappearing from the coastal lowlands, the last remnants of the dodo population were driven into remote areas of the island, where reproduction finally dropped to zero. Hunting of dodos by humans was probably negligible because the human population never reached more than a few hundred during Dutch occupation on an island 1865 km2 in size, and only around 5% of the forests on the east coast had been cleared by the time of the dodo's extinction (Moree, Citation1998; Floore and Jayasena, Citation2010; Winters and Hume, Citation2015). Furthermore, the interior of Mauritius was virtually impenetrable and unexplored (Hume and Winters, Citation2015). Moreover, historic reports indicate that the dodo was neither a prime food source nor hunting goal, and a refuse layer of thousands of animal remains found at Fort Frederik Hendrik and dated to the last quarter of the 17th century failed to find any evidence for slaughtering of dodos (Cheke and Hume, Citation2008; Peters et al., 2011). It is more likely that introduced species such as Javan deer, Rusa timorensis (Blainville, Citation1822), goat, Capra hircus (Linnaeus, Citation1758), pig, Sus scrofa Linnaeus, Citation1758, crab-eating macaque, Macaca fascicularis Raffles, Citation1821, and black rat, Rattus rattus (Linnaeus, Citation1758) were responsible for the dodo's extinction by destroying the understory vegetation, competing for food sources, and, in the case of the pig, macaque, and black rat, preying on dodo eggs and chicks (Cheke and Hume, Citation2008; Hume, Citation2013). The worst invasive species was probably the black rat. Radiocarbon dates show that this aggressive and adaptable rodent had reached Mauritius as early as the 14th century (Hume, Citation2013) and was already a scourge when the Dutch attempted to introduce agriculture in the early 1600s (Cheke and Hume, Citation2008). Following the introduction of other invasive animals in the early 1600s, the dodo met its demise approximately 80 years after its discovery by Europeans.
Current research indicates that the dodo was a resilient species that had survived many hundreds of thousands of years of volcanic and climatic extreme events on the island of Mauritius (Rijsdijk et al., Citation2011). However, the dodo and many other contemporaneous species were unable to survive the multitude of anthropogenic changes that were to beset the island after human colonization (Griffiths and Florens, Citation2006; Cheke and Hume, Citation2008; Florens, Citation2013). The rediscovery of the bonebed at Mare aux Songes has provided robust data to reconstruct the world of the dodo and assess its functioning in an ecosystem that was affected by environmental change, and ultimately, by human impact.
IMPORTANCE OF THE THIRIOUX DODOS
The results of the multidisciplinary investigation of the Mare aux Songes concentration Lagerstätte that resulted in the first scientific reconstructions of the dodo skeleton (Owen, Citation1866a, 1872; Newton and Gadow, Citation1893) underscore the importance of the Thirioux dodo finds. The Thirioux dodos comprise one almost complete, associated skeleton from a single individual (Port Louis specimen) and one partial composite skeleton (Durban specimen). Although the exact provenance of the Thirioux finds remains unknown (Claessens and Hume, 2015), these exceptional specimens provide important new information on dodo anatomy. These include the relative skeletal proportions from a single bird, and, collectively, they provide information on the anatomy of a near-complete skeleton, including multiple skeletal elements that were hitherto unknown or undescribed. Whereas the present memoir on the osteology of the dodo (Claessens et al., 2015b), based on the Thirioux specimens, opens up new lines of research on its morphology, physiology, and paleobiology, the Mare aux Songes provides complimentary contextual ecological data and fundamental insights into the functioning of an insular ecosystem and its sensitivity to both environmental and human factors.
Submitted December 17, 2014; revisions received October 9, 2015; accepted October 21, 2015.
Memoirs Editor: Randy Irmis.
ACKNOWLEDGMENTS
We thank co-discoverers E. Lenting and C. Foo Kune for their invaluable support. We further thank M.-L. Foo Kune, P. La Hausse de la Louvière, S. Saumtally, O. Griffiths, A. Gill, R. Prŷs-Jones, G. Middleton, P. Moree, I. Prins, M.R. Rijsdijk, M. van der Meer, M. Schilthuizen, V. Tatayah, the volunteers of the Mauritian Wildlife Fund, the staff and technicians of the Natural History Museum of Mauritius, and the staff and technicians of OMNICANE Mon Tresor Team for their invaluable support. J.P.H. thanks R. Allain and C. Sagne (MNHN), S. Chapman (NHMUK), M. Brooke and M. Lowe (UMZC), and M. Nowak-Kemp (OUMNH) for access to materials in their care. Field work and research was funded and supported by Omnicane, TNO—the Geological Survey of The Netherlands, the Treub Foundation for Research in the Tropics, World Wildlife Fund—The Netherlands, Deltares, Stichting Dodo Research, The Hague, Mauritius Museums Council, Hollandia Archaeology, Taylor Smith Group (Mauritius), Air Mauritius, Mauritius Sugar Industry Research Institute, Royal Society of Arts & Sciences of Mauritius, Mauritius Wildlife Foundation, and the Naturalis Biodiversity Center. Support for J.P.H. was provided by the Percy Sladen Centenary Fund, DIF, and Special Funds (Natural History Museum, London). E.J.d.B.'s work was carried out with the financial support from The Netherlands Organisation for Scientific Research (project number ALW 819.01.009). Support for L.C. was provided by the National Science Foundation (Aves 3D project, DBI 0743327) and a College of the Holy Cross Research and Publication grant. H.J.M.M. received support from the Treub Foundation for Research in the Tropics, the Spanish Ministerio de Economía y Competitividad (CGL2011-28681), and the Generalitat de Catalunya (BP-B-00174 to H.J.M.M.). T.J.J.V. was funded by The Netherlands Organisation for Scientific Research (project number ALW 819.01.008) and SYNTHESYS Synthesis of Systematic Resources (project numbers BE-TAF-2974, GB-TAF-4289 and GBTAF-5235). We appreciate the constructive and critical feedback by T. Worthy, G. Dyke, and J. Parish.
LITERATURE CITED
- Anonymous. 1601. Het Tvveede Boeck. Journal oft Dagh-register / inhoudende een warachtig verhael ende historische vertellinghe van de Reyse / gedaen door de acht schepen van Amstelredame / gheseylt in den Maert Martij 1598 onder 't beleydt van den Admirael Jacob Cornelisz. Neck ende Wybrandt van Warwijck als vice admirael–van hare zeylagie ende gedenwaerdighe zaken ende geschiedenissen haer op de voortz-reys bejeghent. Cornelis Claesz., Amsterdam, 50 pp.
- Austin, J. J., and E. N. Arnold. 2001. Ancient mitochondrial DNA and morphology elucidate an extinct island radiation of Indian Ocean giant tortoises (Cylindraspis). Proceedings of the Royal Society of London B 268:2515–2523.
- Baider, C., and F. B. V. Florens. 2006. Current decline of the ‘Dodo-tree’: a case of broken-down interactions with extinct species or the result of new interactions with alien invaders?; pp 199–214 in W. Laurance and C. Peres (eds.), Emerging Threats to Tropical Forests. University of Chicago Press, Chicago, Illinois.
- Behrensmeyer, A. K., and S. M. Kidwell. 1985. Taphonomy's contributions to paleobiology. Paleobiology 11:105–119.
- Blainville, H. M. D. de. 1822. Sur les caractères distinctifs des espèces de cerfs. Journal de Physique, de Chimie, d'Histoire Naturelle et des Arts, Paris 94:254–285.
- Burney D. A., G. S. Robinson, and L. P. Burney. 2003. Sporormiella and the late Holocene extinctions in Madagascar. Proceedings of the National Academy of Science of the United States of America 100:10800–10805.
- Camoin, G. F., L. F. Montaggioni, and C. J. R. Braithwaite. 2004. Late glacial to post glacial sea levels in the western Indian Ocean. Marine Geology 206:119–146.
- Cheke, A. S. 2006. Establishing extinction dates—the curious case of the Dodo Raphus cucullatus and the Red Hen Aphanapteryx bonasia. Ibis 148:155–158.
- Cheke, A. S., and J. P. Hume. 2008. Lost Land of the Dodo. Yale University Press, New Haven, Connecticut, 464 pp.
- Claessens, L. P. A. M., and J. P. Hume. 2015. The provenance and history of the Thirioux dodos; pp. 21–28 in L. P. A. M. Claessens, H. J. M. Meijer, J. P. Hume, and K. F. Rijsdijk (eds.), Anatomy of the Dodo (Raphus cucullatus L., 1758): An Osteological Study of the Thirioux Specimens. Society of Vertebrate Paleontology Memoir 15. Journal of Vertebrate Paleontology 35(6, Supplement).
- Claessens, L. P. A. M., H. J. M. Meijer, and J. P. Hume. 2015a. The morphology of the Thirioux dodos; pp. 29–187 in L. P. A. M. Claessens, H. J. M. Meijer, J. P. Hume, and K. F. Rijsdijk (eds.), Anatomy of the Dodo (Raphus cucullatus L., 1758): An Osteological Study of the Thirioux Specimens. Society of Vertebrate Paleontology Memoir 15. Journal of Vertebrate Paleontology 35(6, Supplement).
- Claessens, L. P. A. M., H. J. M. Meijer, J. P. Hume, and K. F. Rijsdijk (eds.). 2015b. Anatomy of the Dodo (Raphus cucullatus L., 1758): An Osteological Study of the Thirioux Specimens. Society of Vertebrate Paleontology Memoir 15. Journal of Vertebrate Paleontology 35(6, Supplement), 188 pp.
- Clark, G. 1866. Account of the late discovery of Dodos' remains in the island of Mauritius. Ibis 2:141–146.
- Dabney, J., M. Knapp, I. Glocke, M.-T. Gansauge, A. Weihmann, B. Nickel, C. Valdiosera, N. García, S. Pääbo, J.-L. Arsuaga, and M. Meyer. 2013. Complete mitochondrial genome sequence of a middle Pleistocene cave bear reconstructed from ultrashort DNA fragments. Proceedings of the National Academy of Sciences of the United States of America 110:15758–15763.
- De Boer, E. J., M. I. Vélez, K. F. Rijsdijk, P. G. B. de Louw, T. J. Vernimmen, P. M. Visser, R. Tjallingii, and H. Hooghiemstra. 2015. A deadly cocktail: how a drought around 4200 cal yr BP caused mass mortality events at the infamous ‘dodo swamp’ in Mauritius. The Holocene 25:758–777.
- De Boer, E. J., R. Tjallingii, M. I. Vélez, K. F. Rijsdijk, A. Vlug, G. J. Reichart, P. G. B. de Louw, F. B. V. Florens, C. Baider, and H. Hooghiemstra. 2014. Climate variability in the SW Indian Ocean from an 8000-yr long multi-proxy record in the Mauritian lowlands shows a middle to late Holocene shift from negative IOD-state to ENSO-state. Quaternary Science Reviews 86:175–189.
- Duncan, J. S. 1828. A summary review of the authorities on which naturalists are justified in believing that the Dodo, Didus ineptus Linn., was a bird existing in the Isle of France, or the neighbouring islands until a recent period. Zoological Journal 3:554–567.
- Floore, P. M., and R. M. Jayasena. 2010. In want of everything? Archaeological perceptions of a Dutch outstation on Mauritius (1638–1710). Post-Medieval Archeology 44:320–340.
- Floore, P. M., and C. Schrire. 1997. Reports on the Archaeological Survey of the Ruins of Vieux Grand Port, 2–30 July 1997. Preliminary Report. Friends of the Environment, Mauritius.
- Florens, F. B. V. 2002. Osteological finds on Trois Mamelles mountain extends the known ecological range of the extinct endemic Mauritian tortoise Cylindraspis sp. Phelsuma 10:56–58.
- Florens, F. B. V. 2008. Ecologie des forêts tropicales de l'île Maurice et impact des espèces introduites envahissantes. Ph.D. dissertation. Université de La Réunion, Réunion, France, 226 pp.
- Florens, F. B. V. 2013. Conservation in Mauritius and Rodrigues: challenges and achievements from two ecologically devastated oceanic islands; pp. 40–50 in N. Sodhi, L. Gibson, and P. Raven (eds.), Conservation Biology: Voices from the Tropics. Wiley-Blackwell, Hoboken, New Jersey.
- Florens, F. B. V., C. Baider, G. M. N. Martin, and D. Strasberg. 2012. Surviving 370 years of human impact: what remains of tree diversity and structure of the lowland wet forests of oceanic island Mauritius? Biodiversity and Conservation 21:2139–2167.
- Fuller, E. 2002. Dodo: A Brief History. Universe Books, New York, 180 pp.
- Gasse, F. 2000. Hydrological changes in the African tropics since the Last Glacial Maximum. Quaternary Science Reviews 19:189–211.
- Grihault, A. 2005. Dodo, the Bird Behind the Legend. IPC, Cassis, Mauritius, 169 pp.
- Griffiths, C. 2014. Rewilding in the western Indian Ocean; pp. 325–349 in J. Gerlach (ed.), Western Indian Ocean Tortoises. Siri Scientific Press, Manchester, U.K.
- Griffiths, O. L., and F. B. V. Florens. 2006. Non-marine Molluscs of the Mascarene Islands. Bioculture Press, Mauritius, 185 pp.
- Günther, A. C. L. G. 1877. The Gigantic Land-Tortoises (Living and Extinct) in the Collection of the British Museum. Taylor and Francis, London, 96 pp.
- Hogg, A. G., Q. Hua, P. G. Blackwell, M. Niu, C. E. Buck, T. P. Guilderson, T. J. Heaton, J. G. Palmer, P. J. Reimer, R. W. Reimer, C. S. M. Turney, and S. R. H. Zimmerman. 2013. SHCal13 Southern Hemisphere Calibration, 0–50.000 years cal BP. Radiocarbon 55:1889–1903.
- Hume, J. P. 2005. Contrasting taphofacies in ocean island settings: the fossil record of Mascarene vertebrates; pp. 129–144 in J. A. Alcover and P. Bove (eds.), Proceedings of the International Symposium “Insular Vertebrate Evolution: the Palaeontological Approach, Palma de Malloraca, Balearic Islands, Spain, 16–19 September 2003. Monografies de la Societat d'Història Natural de les Balears 12.
- Hume, J. P. 2006. The history of the dodo Raphus cucullatus and the penguin of Mauritius. Historical Biology 2:65–89.
- Hume, J. P. 2009. Dodo; pp. 228–232 in R. G. Gillespie and D. A. Clague (eds.), Encyclopedia of Islands. University of California Press, Berkeley, California.
- Hume, J. P. 2012. The Dodo: from extinction to the fossil record. Geology Today 28:147–151.
- Hume, J. P. 2013. A synopsis of the pre-human avifauna of the Mascarene Islands; pp. 195–237 in U. B. Göhlich and A. Kroh (eds.), Proceeding of the 8th International Meeting of the Society of Avian Paleontology and Evolution. Naturhistorisches Museum, Vienna, Austria, 11–16 June 2012.
- Hume, J. P. 2014. Fossil discoveries on Mauritius and Rodrigues; pp. 203–228 in J. Gerlach (ed.), Western Indian Ocean Tortoises. Siri Scientific Press, Manchester, U.K.
- Hume, J. P., and L. Steel. 2013. Fight Club: a unique weapon in the wing of the solitaire, Pezophaps solitaria (Aves: Columbidae), an extinct flightless bird from Rodrigues, Mascarene Islands. Biological Journal of the Linnean Society 110:32–44.
- Hume, J. P., and R. Winters. 2015. Captive birds on Dutch Mauritius: bad-tempered parrots, warty pigeons and notes on other native animals. Historical Biology 27:258–264.
- Hume, J. P., A. S. Cheke, and A. McOran-Campbell. 2009. How Owen ‘stole’ the Dodo: academic rivalry and disputed rights to a newly-discovered subfossil deposit in nineteenth century Mauritius. Historical Biology 21:1–18.
- Hume, J. P., A. Datta, and D. M. Martill. 2006. Unpublished drawings of the Dodo Raphus cucullatus and a note on Dodo skin relics. Bulletin of the British Ornithologists' Club 126A:49–54.
- Hume, J. P., P. G. B. de Louw, and K. F. Rijsdijk. 2014a. Rediscovery of a lost Lagerstätten: a comparative analysis of the historical and recent Mare aux Songes dodo excavations on Mauritius. Historical Biology 27:1127–1140.
- Hume, J. P., D. M. Martill, and C. Dewdney. 2004. Dutch diaries and the demise of the dodo. Nature 429:6992.
- Hume, J. P., L. Steel, A. A. André, and A. Meunier. 2014b. In the footsteps of the bone collectors: nineteenth-century cave exploration on Rodrigues Island, Indian Ocean. Historical Biology 27:265–286.
- Jackson, A. 2014. Added credence for a late Dodo extinction date. Historical Biology 26:699–701.
- Linnaeus, C. 1758. Systema Naturae per Tegna Tria Naturae, Secundum Classes, Ordines, Genera, Species, cum Characteribus, Differentiis, Synonymis, Locis. Tenth Edition. Laurentii Salvii, Stockholm, 824 pp.
- Livezey, B. C. 1993. An ecomorphological review of the dodo (Raphus cucullatus) and solitaire (Pezophaps solitaria), flightless Columbiformes of the Mascarene Islands. Journal of Zoology 230:247–292.
- MacDonald, G. 2011. Potential influence of the Pacific Ocean on the Indian summer monsoon and Harappan decline. Quaternary International 229:140–148.
- Marchant, R. A., and H. Hooghiemstra. 2004. Rapid environmental change in tropical Africa and Latin America about 4000 years before present: a review. Earth Science Reviews 66:217–260.
- Meijer, H. J. M., G. Gill, P. G. B. de Louw, L. W. van den Hoek Ostende, J. P. Hume, and K. F. Rijsdijk. 2012. Dodo remains from an in situ context from Mare aux Songes, Mauritius. Naturwissenschaften 99:177–84.
- Meyer, M., and M. Kircher. 2010. Illumina sequencing library preparation for highly multiplexed target capture and sequencing. Cold Spring Harbor Protocols: pdb-prot5448.
- Milne-Edwards, A. 1867. Mémoire sur une espéce éteinte du genre Fulica. Annales des Sciences Naturelles Zoologie et Paléontologie 5:195–220.
- Mlíkovský, J. 2004. Extinction of the Dodo Raphus cucullatus (Aves: Raphidae): dating reconsidered. Journal of the National Museum, Natural History Series 173:111–112.
- Moree, P. 1998. A Concise History of Dutch Mauritius 1598–1710. A Fruitful and Healthy Land. Kegan Paul International, London, 127 pp.
- Moree, P. 2001. Dodos and Galjoenen. De Reis van het schip Gelderland naar Oost-Indië, 1601–1603. Walburg Pers, Zutphen, The Netherlands, 348 pp.
- Nauta, A. 2006. Mauritius voor de Europese invasie; Dodo-botjes, lavatunnels en schatkaarten, Nieuwsbrief van het KNGMG, ALW en KTFG 31:1–3.
- Newton, E., and H. Gadow. 1893. On additional bones of the dodo and other extinct birds from Mauritius obtained by Mr Theodore Sauzier. Transactions of the Zoological Society of London 13:281–302.
- Nicholls, H. 2006. Digging for dodo. Nature 443:138–140.
- North-Coombes, A. 1994. La Découverte des Mascareignes. Mauritius Printing Specialists Ltd., xx, Mauritius, 185 pp.
- Owen, R. 1866a. On the osteology of the dodo (Didus ineptus, Linn.); pp. 21–55 in R. Owen and W. J. Broderip (eds.), Memoir on the Dodo (Didus ineptus), with an Historical Introduction. Taylor and Francis, London.
- Owen, R. 1866b. Evidence of a species, perhaps extinct, of large parrot (Psittacus mauritianus, Owen), contemporary with the Dodo, in the island of Mauritius. Ibis 8:168–171.
- Owen, R. 1872. On the Dodo (Part II). Notes on the Articulated Skeleton of the Dodo (Didus ineptus, Linn.) in the British Museum. Transactions of the Zoological Society of London 7:513–525.
- Padya, B. M. 1989. Weather and Climate of Mauritius (Geography of Mauritius Series). Mahatma Gandhi Institute Press, Moka, Mauritius, 283 pp.
- Parish, J. C. 2013. The Dodo and the Solitaire: A Natural History. Indiana University Press, Bloomington, Indiana, 406 pp.
- Peters, N., W. van Neer, S. Debruyne, and S. Peters. 2009. Late 17th-century AD faunal remains from the Dutch “Fort Frederik Hendrik” at Mauritius (Indian Ocean). Archaeofauna 18:159–184.
- Raffles, T. S. 1821. Descriptive catalogue of a zoological collection, made on account of the Honourable East India Company, in the Island of Sumatra and its vicinity, under the direction of Sir Thomas Stamford Raffles, Lieutenant-Governor of Fort Marlborough. Transactions of the Linnean Society of London 13:246–247.
- Reinhardt, J. T. 1842. pp. 71–72 in H. Kroyer (ed.), Nøjere Oplysning om det I Kiøbenhavn fundne Drontehoved. Vol. 4. Naturhistorisk Tidskrift, København (Denmark).
- Rijsdijk, K. F., J. Zinke, P. G. B. de Louw, J. P. Hume, J. van der Plicht, H. Hooghiemstra, H. J. M. Meijer, H. B. Vonhof, N. Porch, F. B. V. Florens, C. Baider, B. van Geel, J. Brinkkemper, T. Vernimmen, and A. Janoo. 2011. Mid-Holocene (4.2 kyr BP) mass mortalities in Mauritius (Mascarenes): insular vertebrates resilient to climatic extremes but vulnerable to human impact. The Holocene 21:1179–1194.
- Rijsdijk, K. F., J. P. Hume, F. Bunnik, F. B. V. Florens, C. Baider, B. Shapiro, J. van der Plicht, A. Janoo, O. Griffiths, L. W. van den Hoek Ostende, H. Cremer, T. Vernimmen, P. G. B. de Louw, A. Bholah, S. Saumtally, N. Porch, J. Haile, M. Buckley, M. Collins, and E. Gittenberger. 2009. Mid-Holocene vertebrate bone concentration-Lagerstätte on oceanic island Mauritius provides a window into the ecosystem of the dodo (Raphus cucullatus). Quaternary Science Reviews 28:14–24.
- Roberts, D. L., and A. R. Solow. 2003. Flightless birds: when did the dodo become extinct? Nature 426:245.
- Shapiro, B., D. Sibthorpe, A. Rambaut, J. Austin, G. M. Wragg, O. R. P. Bininda-Emonds, P. L. M. Lee, and A. Cooper. 2002. Flight of the dodo. Science 295:1683–1683.
- Senapathi, D., F. Underwood, E. Black, M. A. Nicoll, and K. Norris. 2010. Evidence for long-term regional changes in precipitation on the East Coast Mountains in Mauritius. International Journal of Climatology 30:1164–1177.
- Sleigh, D. 1993. pp. 639–678 in Die Buitenposte: VOC-Buitenposte onder Kaapse bestuur 1652–1795, Haum Uitgewers, Pretoria.
- Sleigh, D. 2000. The economy of Mauritius during the Second Dutch Occupation (1664–1710); pp. 51–56 in S. J. T. Evers and V. Y. Hookomsing (eds.), Globalisation and the South-West Indian Ocean. International Institute for Asian Studies, Leiden, The Netherlands, and University of Mauritius, Reduit, Mauritius.
- Statius Müller, P. L. 1776. Des Ritters Carl von Linné Königlich Schwedischen Leibarztes &c. &c. vollständigen Natursystems Supplements- und Register-Band über alle sechs Theile oder Classen des Thierreichs. Mit einer ausführlichen Erklärung. Nebst drey Kupfertafeln. Tome 1. Raspe, Nürnberg, Germany, 384 pp.
- Staubwasser, M., and H. Weiss. 2006. Holocene climate and cultural evolution in late prehistoric–early historic West Asia. Quaternary Research, 66:372–387.
- Strickland, H. E., and A. G. Melville. 1848. The Dodo and Its Kindred. Reeve, Benham and Reeve, London, 141 pp.
- Thompson, L. G., E. Mosley-Thompson, M. E. Davis, K. A. Henderson, H. H. Brecher, V. S. Zagorodnov, T. A. Mashiotta, P.-N. Lin, V. N. Mikhalenko, D. R. Hardy, and J. Beer. 2002. Kilimanjaro ice core records: evidence of Holocene climate change in tropical Africa. Science 298:589–593.
- Van der Sluis, L. G., H. I. Hollund, M. Buckley, P. G. B. de Louw, K. F. Rijsdijk, and H. Kars. 2014. Combining histology, stable isotope analysis and ZooMS collagen fingerprinting to investigate the taphonomic history and dietary behaviour of extinct giant tortoises from the Mare aux Songes deposit on Mauritius, Palaeogeography, Palaeoclimatology, Palaeoecology 15:80–91.
- Van Neck, J. 1599. A True Report of the gainefull, prosperous and speedy voyage to Java in the East Indies, performed by a fleete of eight ships of Amsterdam: Which set forth from Texell in Holland, the first of Maie 1598 Stilo Neue. Whereof foure returned againe the 19. July Anno 1599. In less than 15. Months, the other foure went forward from Java for the Moluccas. London.
- Van Wissen, B. 1995. Dodo: Raphus cucullatus (Didus ineptus). ISP/Zoölogisch Museum, Amsterdam, 102 pp.
- Vaughan, R. E., and P. O. Wiehe. 1937. Studies on the vegetation of Mauritius I. A preliminary survey of the plant communities. Journal of Ecology 25:289–343.
- Winters, R., and J. P. Hume. 2014. The dodo, the deer and a 1647 voyage to Japan. Historical Biology 27:258–264.
- Wood, J. R., and J. M. Wilmshurst. 2012. Wetland soil moisture complicates the use of Sporormiella to trace past herbivore populations. Journal of Quaternary Science 27:254–259.
- Zinke, J., J. J. G. Reijmer, B. A. Thomassin, W. Chr. Dullo, P. M. Grootes, and H. Erlekeuser. 2003. Postglacial flooding history of Mayotte Lagoon (Comoro Archipelago, southwest Indian Ocean). Marine Geology 194:181–196.