ABSTRACT
In this paper we re-examine the taxonomy and systematic position of the Eocene stingrays from Bolca Lagerstätte which are traditionally assigned to Urolophus crassicaudatus (Blainville). The analysis of their tooth morphology supports an assignment to the Eocene stingray genus Arechia Cappetta, a taxon known from isolated teeth from the Ypresian-Lutetian of northern and western Africa. The teeth of the Bolca specimens differ from the type species A. arambourgi in some characters (i.e., labial face with concave profile just below the crest, convex lower down; lingual face slightly more developed than the labial, with convex profile in its upper part and a concave profile in its mid-lower region) that justify the recognition of a second species within the genus, i.e., A. crassicaudata. This taxon also shows a unique combination of features (e.g., pectoral disc large and rhomboid; tail short, 44–52% of total length; ca. 238 vertebral centra; distal segment of propterygia located between mouth and antorbital cartilage; mesopterygium single, not fused to radials; 100–117 pectoral radials; 15–17 pelvic-fin radials; elongated caudal fin of aplesodic type) that supports its sister-group relationship with the living urolophids Urolophus and Trygonoptera. Arechia was a typical inhabitant of the near coastal and warm habitats that characterize the Monte Postale paleoenvironment.
SUPPLEMENTAL DATA—Supplemental materials are available for this article for free at www.tandfonline.com/UJVP
INTRODUCTION
The Ypresian fossil Lagerstätte of Bolca, in northeastern Italy, is one of the few Paleogene deposits where fossils of cartilaginous fishes (Chondrichthyes) are represented by complete and articulated skeletal remains (Marramà et al., Citation2018a). In the last few years, several studies provided new insights into systematics, relationships, and paleobiology of sharks and rays from the Pesciara and Monte Postale sites of Bolca (Fanti et al., Citation2016, Citation2019; Marramà et al., Citation2018a, Citation2018b, Citation2018c, Citation2019a, Citation2019b, Citation2019c, Citation2019d). The batoids, in particular, are rather abundant and represented by electric rays, guitarfishes, thornback rays, and stingrays, with these latter representing the most diverse lineage of chondrichthyans of Bolca. One of the stingray taxa, described by Blainville (Citation1818) as Trygonobatus crassicaudatus, was later assigned to the stingaree genus Urolophus Müller and Henle, Citation1837 by Jaekel (Citation1894) in his comprehensive review of the elasmobranch fishes from Bolca. Since then, this taxonomic status has been widely accepted but a revisionary study of these fossils has not been provided up to now. According to Cappetta (Citation2012), however, the size and morphology of the teeth of ‘Urolophus’ crassicaudatus are quite different from those of the living Urolophus species and more similar to those of Arechia Cappetta, Citation1983, an extinct stingray genus described for isolated teeth from the Ypresian basin of Ouled Abdoun in Morocco (Cappetta, Citation1983), and occurring from the Ypresian to the Lutetian of northern and western Africa (Cappetta, Citation1987, Citation2012; Cappetta and Traverse, Citation1988; Noubhani and Cappetta, Citation1997; Tabuce et al., Citation2005; Sambou et al., Citation2017).
The taxonomic history of ‘Urolophus’ crassicaudatus is very complex. After being poorly described and placed in the genus Trygonobatus by Blainville (Citation1818), without figures or reference to any type specimen, the taxon was subsequently transferred without further description or illustrations to Trygon oblongus by Agassiz (Citation1833–1844) and to Trygon brevicauda by Heckel (Citation1851). A specimen housed in the Natural History Museum of Vienna (NHMW 1853.0027.0005) was later described as Urolophus princeps by Heckel (Citation1853), whereas Molin (Citation1861) named another specimen housed in the Museo di Geologia e Paleontologia dell’Università di Padova (MGP-PD 8875C/76C) as Taeniura knerii. Subsequently, Jaekel (Citation1894) concluded that all the taxa listed above should be regarded as junior synonyms of Tygonobatus crassicaudatus Blainville, Citation1818, and subsequently transferred the species to Urolophus. Unfortunately, Blainville (Citation1818) never indicated the type specimen and that indicated by Blot (Citation1980) as the holotype of ‘Urolophus’ crassicaudatus in the collections of the Museum National d’Histoire Naturelle of Paris cannot be regarded as such. In fact, Eastman (Citation1904, Citation1905) and more recently Marramà et al. (Citation2019c) recognized that there is no stingray specimen in this institution that can be considered as the type reported by Blainville (Citation1818). For this reason, the original holotype of Trygonobatus crassicaudatus must be considered lost and a neotype must be selected for this species.
In this paper, we provide a detailed description of the skeletal anatomy of the fossil material from Bolca originally assigned to Urolophus crassicaudatus and re-evaluate its systematic position in the context of our current understanding of the stingray interrelationships.
GEOLOGICAL SETTING
The historical literature suggests that the specimens examined in this study were collected from the fossiliferous layers of the Monte Postale, which is located about 2 km north-east of the village of Bolca (Verona Province, northeast Italy), in the eastern part of the Lessini Mountains, southern Alps, about 300 m from the better known Pesciara site. The Monte Postale sedimentary succession includes the Cretaceous Scaglia Rossa Formation up to Ypresian fossiliferous limestone; in the uppermost part, the latter contain abundant larger benthic foraminiferans of the genus Alveolina, and marine and brackish mollusks, of almost the same age and similar sedimentological features as the Pesciara site, mostly comprising finely laminated micritic limestones with fish and plant remains. Papazzoni et al. (Citation2017) investigated the stratigraphic relationships between the two fossiliferous deposits, suggesting that the uppermost productive sequence of Monte Postale should correlate with that of the Pesciara site, although the fossiliferous laminites of Pesciara appear to be slightly younger than those of Monte Postale. Based on large benthic foraminifera and calcareous nannoplankton, the uppermost strata of the Monte Postale site were assigned to Shallow Benthic Zone 11 by Papazzoni et al. (Citation2017) and correspond to the late Cuisian (late Ypresian), around 50 Ma. Evidence of a coralgal rim, lagoonal deposits, and a fore-reef system was hypothesized for the Monte Postale paleobiotope (Vescogni et al., Citation2016), and this interpretation is supported by recent paleoecological and taphonomic studies of the Monte Postale fish assemblage (Marramà et al., Citation2016).
MATERIALS AND METHODS
The present study is based on six articulated specimens traditionally assigned to Urolophus crassicaudatus, from the Monte Postale site of the Bolca Lagerstätte. The material is currently housed in the collections of the Museo Civico di Storia Naturale di Verona, Museo di Geologia e Paleontologia dell’Università degli Studi di Padova, and Naturhistorisches Museum Wien. Some of the specimens were examined under ultraviolet light in order to distinguish the preserved skeletal and soft tissues from grout or pigments. Measurements were taken to the nearest 0.1 mm. Osteological terminology primarily follows Carvalho et al. (Citation2004) and Aschliman et al. (Citation2012a). Tooth terminology follows Cappetta (Citation2012).
The phylogenetic analysis is based on the morphological data set of Marramà et al. (Citation2019c), which in turn was based on the matrix of Carvalho et al. (Citation2004), with inputs mainly from Claeson et al. (Citation2010) and Aschliman et al. (Citation2012a) (Supplemental Data 1). The data matrix resulted in 102 characters coded for 33 taxa (Supplemental Data 2). The matrix was compiled in Mesquite v.3.03 (Maddison and Maddison, Citation2008) and the phylogenetic analysis was performed with Tree Analysis using New Technology (TNT v.1.5) employing the branch-and-bound method with 1,000 replicates, 10 trees saved per replication, and collapsing trees after search (Goloboff et al., Citation2008). Additionally, the data matrix also was analysed using the PAUP*4.0166a software to crosscheck the results retrieved from the TNT analysis. ‘MaxTrees’ was set to 30,000 and the TBR branch-swapping algorithm was employed. All the characters are unordered and given equal weight in the main analysis. Tree length, Bremer support, consistency and retention indices were calculated for the single tree retrieved using TNT.
Institutional Abbreviations—MCSNV, Museo Civico di Storia Naturale di Verona, Verona, Italy; MGP-PD, Museo di Geologia e Paleontologia, Università di Padova; NHMW, Naturhistorisches Museum Wien, Wien, Austria.
Anatomical Abbreviations—bh, basihyal; cb, ceratobranchials; df, dorsal fontanelle; DW, disc width; hb, hypobranchials; hyo, hyomandibula; mc, Meckel’s cartilage; mes, mesopterygium; met, metapterygium; mp, medial plate; nc, nasal capsules; pq, palatoquadrate; pro, propterygium; ps, pseudohyoid; sca, scapulocoracoid; syn2, thoracolumbar synarcual; TL, total length.
SYSTEMATIC PALEONTOLOGY
Class CHONDRICHTHYES Huxley, Citation1880
Superorder BATOMORPHII Cappetta, Citation1980
Order MYLIOBATIFORMES Compagno, Citation1973
Family UROLOPHIDAE Müller and Henle, Citation1841
Genus ARECHIA Cappetta, Citation1983
Type Species—Arechia arambourgi Cappetta, Citation1983.
Included Species—Arechia arambourgi Cappetta, Citation1983; Arechia crassicaudata (Blainville, Citation1818).
Amended Diagnosis—Small teeth up to 2.8 mm wide, and broader than long; crown high, not cuspidate; enameloid surface smooth; edge of the labial and lingual visors convex, sometimes with a weak central concavity or notch; high, sharp, lingually displaced transverse cutting crest that does not reach the blunt lateral angles and having a constriction at its origin; root lower than crown and slightly expanded mesiodistally; basal face of the lobes slightly convex and joining feebly at the labial face of the root that is oblique and straight; root lobes divergent and divided by a broad and deep furrow; root with a large central foramen and paracentral foramina; scattered foramina on the labial face of the root.
Remarks—The stingray genus Arechia was introduced by Cappetta (Citation1983) after the revision of Raja praealba Arambourg, Citation1952, a taxon represented by isolated teeth from the Ypresian basin of Ouled Abdoun, Morocco. Teeth considered by Arambourg (Citation1952) as belonging to males of R. praealba were assigned to the new genus Merabatis by Cappetta (Citation1983), whereas those considered to be of females were included in the new taxon Arechia arambourgi. Since both taxa were based on isolated teeth only, Cappetta (Citation1983) did not define their exact systematic position within batoids, although he recognized their ‘dasyatoid’ resemblances and placement within the stingray order Myliobatiformes. Arechia was diagnosed by Cappetta (Citation1983, Citation2012) by a series of dental characters that are unquestionably present also in the species from Bolca described herein, supporting its placement within this genus.
Considering that the main purpose of the International Code of Zoological Nomenclature (ICZN, Citation1999) is to guarantee the nomenclatural stability, and considering the synonymy list below, we retain the specific name proposed by Blainville (Citation1818) and suggest the new combination Arechia crassicaudata (Blainville, Citation1818).
ARECHIA CRASSICAUDATA (Blainville, Citation1818)
(–)
Trygonobatus crassicaudatus Blainville, Citation1818:337.
Trygonobatus crassicaudatus de Blainville; Bronn, Citation1831:8.
Trygon oblongus Agassiz, Citation1833–Citation1844:vol. 1:44 (nomen nudum; no description or illustration).
Trygon oblongus Agassiz; Agassiz, Citation1835a:297.
Trygon oblongus Agassiz; Agassiz, Citation1835b:14.
Trygon oblongus Agassiz; De Zigno, Citation1874:181.
Trygon brevicauda Heckel, Citation1851:324 (nomen nudum; no description or illustration).
Urolophus princeps Heckel, Citation1853:124 (nomen dubium; insufficient description; no illustration).
Taeniura knerii Molin, Citation1861:581 (nomen dubium; insufficient description; no illustration).
Urolophus princeps Heck.; Kner and Steindacher, Citation1863:32, pl. 6, fig. 2.
Urolophus princeps, Heckel; De Zigno, Citation1874:183.
Taeniura knerii, Molin; De Zigno, Citation1874:182.
Urolophus princeps, Heckel; Woodward, Citation1889:154.
Urolophus crassicauda de Blainville sp.; Jaekel, Citation1894:148, pl. 5.
Urolophus crassicaudatus (Blainville); Eastman, Citation1904:24.
Urolophus crassicaudatus (de Blainville); Eastman, Citation1905:9.
Urolophus crassicaudatus (de Blainville); D’Erasmo, Citation1922:22.
Urolophus crassicaudatus (de Blainville); Blot, Citation1980:345.
Urolophus crassicaudatus Eastman; Frickhinger, Citation1991:216.
‘Urolophus’ crassicaudatus; Carvalho, Maisey, and Grande, Citation2004:11
‘Urolophus’ sp.; Carvalho, Maisey, and Grande, Citation2004:11.
‘Urolophus’ crassicaudatus; Cappetta, Citation2012:425, fig. 416s.
‘Urolophus’ crassicaudatus (Blainville, Citation1818); Carnevale, Bannikov, Marramà, Tyler, and Zorzin, Citation2014:41.
‘Urolophus’ sp.; Carnevale, Bannikov, Marramà, Tyler, and Zorzin, Citation2014:41.
‘Urolophus’ crassicaudatus; Marramà, Carnevale, Engelbrecht, Claeson, Zorzin, Fornasiero, and Kriwet, Citation2018a:287, fig. 9c, d (non fig. 9a, b).
FIGURE 1. Arechia crassicaudata (Blainville, Citation1818) MCSNV IG.VR.27607, neotype, from the Monte Postale site of the Bolca Lagerstätte. Photographs under A, visible and B, ultraviolet light. Scale bars equal 100 mm.
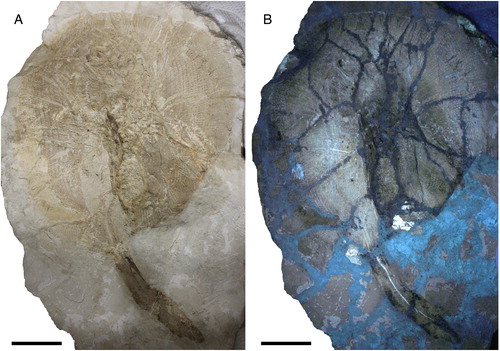
FIGURE 2. Arechia crassicaudata (Blainville, Citation1818) from the Monte Postale site of the Bolca Lagerstätte. A, B, MCSNV VII.B.82/83, part and counterpart; C, D, MGP-PD 8875C/76C, part and counterpart, named by Molin (Citation1861) as Taeniura knerii. Scale bars equal 100 mm.

FIGURE 3. Arechia crassicaudata (Blainville, Citation1818) from the Monte Postale site of the Bolca Lagerstätte. A, B, MCSNV T.317/318, part and counterpart; C, MCSNV VII.B.84/85, part and counterpart; D, NHMW 1853-0027-0005. Scale bars equal 100 mm.

FIGURE 4. Arechia crassicaudata (Blainville, Citation1818) from the Monte Postale site of the Bolca Lagerstätte. A, B, MGP-PD 8875C/76C, part and counterpart, close-up of the head and pectoral girdle. C, reconstruction based on both slabs with pectoral radials omitted. Scale bars equal 50 mm.
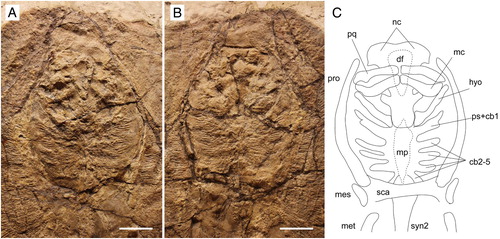
FIGURE 5. Arechia crassicaudata (Blainville, Citation1818) from the Monte Postale site of the Bolca Lagerstätte. A, MCSNV IG.VR.27607, close-up of head and pectoral girdle; B, reconstruction with pectoral radials omitted. Scale bars equal 50 mm.
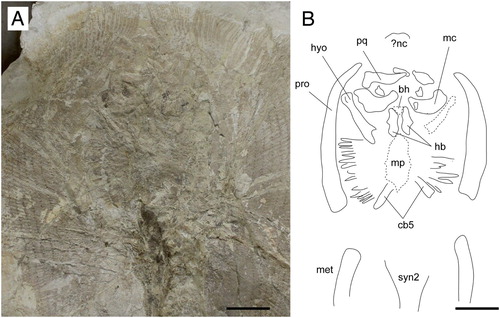
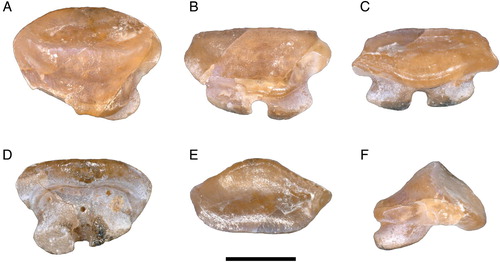
FIGURE 6. A single tooth extracted from the neotype of Arechia crassicaudata (Blainville, Citation1818), MCSNV IG.VR.27607, in A, linguo-occlusal, B, lingual, C, labio-basal, D, basal, E, labial, and F, profile views. Scale bar equals 1 mm.
Neotype—MCSNV IG.VR.27607, nearly complete, articulated skeleton, 1103.4 mm TL, 685.4 mm DW ().
Referred Material—MCSNV VII.B.82/83, a nearly complete articulated skeleton in part and counterpart, 1028.0 mm TL, 637.4 mm DW (); MGP-PD 8875C/76C, a partially complete articulated skeleton lacking the tip of the tail, in part and counterpart, 567.1 mm DW; named by Molin (Citation1861) as Taeniura knerii (); MCSNV T.317/318, an incomplete, partially articulated skeleton, in part and counterpart, 955.5 mm TL, 530.0 mm DW, erroneously indicated by Cappetta (Citation2012) as the holotype of ‘Urolophus’ crassicaudatus (); MCSNV VII.B.84/85, a nearly complete articulated skeleton, in part and counterpart, 949.3 mm TL, 604.1 mm DW (); NHMW 1853.0027.0005, incomplete skeleton, with pieces of slab erroneously assembled together; described by Heckel (Citation1853) as Urolophus princeps ().
Type Locality and Horizon—Monte Postale site, Bolca Konservat-Lagerstätte, Italy; early Eocene, late Ypresian, middle Cuisian, SBZ 11, Alveolina dainelli Zone; 50.7–48.9 Ma (see Papazzoni et al., Citation2017).
Amended Diagnosis—A species of Arechia that differs from A. arambourgi in the following dental characters: crown with two transverse cutting crests separated by a depression; in profile view, the labial crown face is concave just below the main crest, but convex further basally; lingual crown face slightly more developed than the labial one, with convex profile in its upper part and concave profile in its mid-lower region; edge of the lingual visor slightly more convex than the labial one. Furthermore, Arechia can be diagnosed by the following skeletal features: pectoral disc rhomboidal, slightly longer than wide (about 1.1 times); tail short, 44–52% TL; basihyal not fragmented and separated from the first hypobranchials; approximately 238 vertebral centra; distal segment of the propterygium located between mouth and antorbital cartilage; mesopterygium single, with external margins straight and not fused to radials; 100–117 pectoral radials; 15–17 pelvic-fin radials; aplesodic caudal fin of 20–22% TL.
Description
The description of the cranial and postcranial morphology of Arechia crassicaudata (Blainville, Citation1818) is based on six articulated skeletons characterized by different degrees of completeness (–). Although the overall outline and body morphology of the specimens is still recognizable, most of the cranial and girdle elements are disarticulated, fragmented, and displaced from their original position, due to the inadequate preservation that commonly characterizes the specimens from the Monte Postale site (Marramà et al., Citation2016). Counts and measurements are listed in Supplemental Data 1 (Table S1). The specimens are of similar size, with the largest one being characterized by 69 cm disc width and 110 cm in total length. The disc is rhomboidal, not wing-like, with the maximum width in the anterior third of disc length. The disc length is slightly longer than the disc width (about 1.1 times), with the disc width being 56–64% of the total length. The tail is short, thicker in its proximal part, measuring ca. 44–52% of total length.
Neurocranium—The neurocranium is most clearly preserved in MGP-PD 8875C/76C. It is anteroposteriorly elongated, with the greatest width at the level of the nasal capsules (). The rostral cartilage is absent as is the case in all adult stingrays (e.g., Miyake et al., Citation1992). The nasal capsules appear broad, ovoid in shape and laterally expanded with a rounded and biconvex anterior margin. Small preorbital processes protrude from the posterolateral aspect of the nasal capsules (). The supraorbital process is difficult to detect. The postorbital processes are long, shelf-like, and anterolaterally directed. The otic capsules provide articulation surfaces for the proximal portion of the hyomandibulae. Although the specimen MGP-PD 8875C/76C mostly exposes its ventral side, it is possible to observe the outline of the dorsal fontanelle on the neurocranium (). The dorsal fontanelle is triangular in outline, anteroposteriorly elongated, and covers about 50–60% of the neurocranial length. However, the fontanelle does not exhibit any median constriction, which usually represents a remnant of the epiphyseal bar that separates an anterior precerebral from a posterior fronto-parietal fontanelle in some stingrays (Miyake, Citation1988; Carvalho et al., Citation2004). Antorbital cartilages are not preserved in any specimen.
Jaws—The upper and lower jaws are massive and robust, extend laterally, and occupy almost the entire space between the propterygia (, ). The antimeres are separated at the symphysis, and the occlusal width is greater than the diastema width.
The palatoquadrate appears to be labiolingually compressed, slightly narrower than Meckel’s cartilage, and relatively straight on its dorsal flange. The anterior processes of the Meckel’s cartilage are difficult to detect. There is no evidence of the lateral projections of the lower jaws (‘wing-like processes’ of Carvalho et al., Citation2004) which are typical for myliobatids.
Hyoid and Gill Arches—The hyomandibulae appear slender and straight, not arched, and narrow at about their midlength (, ). The hyomandibulae project anterolaterally, reaching the mesial wall of the propterygia just posterior to the postero-ventral corner of the lower jaw. The distal end of the hyomandibulae articulates with the lower jaw through a stout and strong terminal portion in MCSNV IG.VR.27607, whereas their proximal portion at the articulation with the neurocranium is enlarged. The presence of the secondary hyomandibular cartilages that are characteristic of Urolophus are difficult to detect, due to poor preservation. The ventral gill arches of A. crassicaudata are poorly preserved. However, the outline of the central medial plate (derived from the fusion of the basibranchial copula and the basibranchial components) can be seen at least in MGP-PD 8875C-76C and MCSNV IG.VR.27607 (, ). The medial plate appears anteroposteriorly elongated. Its posterior distal tip seems to taper into small median projections. The basihyal appears unfragmented and is clearly separate from the first hypobranchials, which are stout and robust in MCSNV IG.VR.27607 (). There are five pairs of ceratobranchials articulating with the lateral margins of the medial plate. The first appears fused to the pseudohyoid whereas the condition of the last two ceratobranchials is difficult to determine. The fifth ceratobranchial pair articulates distally with the anterior margin of the scapulocoracoid. Filamentous branchial rays are associated with the ceratobranchials although the number of elements associated with each ceratobranchial is difficult to ascertain.
Synarcuals and Vertebral Column—The anterior (cervicothoracic) synarcual is difficult to discern whereas the outline of the posterior (thoracolumbar) synarcual cartilage can be detected (, ). The thoracolumbar synarcual is triangular in shape, articulates anteriorly with the anterior synarcual, and tapers posteriorly with its posterior margin ending approximately mid-way between the scapulocoracoid and pelvic girdle. The vertebral column of Arechia crassicaudata consists of ca. 238 vertebral centra, counted on the best preserved specimen MCSNV IG.VR.27607. However, it is difficult to diagnose the exact number of vertebrae because they are often damaged or lost. There are about 20 trunk (monospondylous) centra, counted from the first distinguishable centrum to the anterior margin of the puboischiadic bar. About 218 are diplospondylous (of which 148 are from the anterior margin of the puboischiadic bar to the caudal-fin origin, and 65–70 caudal). The vertebral centra are small, subrectangular in shape and anteroposteriorly short. Some neural spines are visible in the abdominal cavity in MCSNV T.317/318 (). They are long, laterally compressed, and postero-obliquely oriented in relation to the centra. Ventral arches are difficult to distinguish in the examined material. Ribs are absent.
Pectoral Fins and Girdle—The scapulocoracoid is poorly preserved in all the specimens examined. The coracoid bar appears as a single straight and robust transverse cartilage, located ventral to the cervicothoracic synarcual, mid-way along the pectoral disc (). The suprascapulae are not preserved, but as in all stingrays, they were probably fused to the median crest of the cervicothoracic synarcual (Miyake, Citation1988; Lovejoy, Citation1996; Aschliman et al., Citation2012a).
The propterygium is long, arched, tapering distally and extending to the anterior disc margin. Although the distal segment of the propterygium is difficult to recognize, it is likely that it was located between the mouth and the antorbital cartilage, resembling the condition observed in the stingray genera Urolophus, Urotrygon, Urobatis, and Plesiobatis (Carvalho et al., Citation2004; Aschliman et al., Citation2012a). The proximal portion of the propterygium is larger than the distal one. The mesopterygium is a single, small, and subtriangular bone, whose external margins are more or less straight and not fused to the radials (Supplemental Data 1, Fig. S1A, B). This contrasts with the unique condition seen in Urolophus and Trygonoptera in which the external margins of mesopterygia are sinuous, appearing to be fused with the articulating radial elements (Carvalho et al., Citation2004). The metapterygia are slightly shorter than the propterygium, arched and tapered posteriorly, ending slightly behind the anterior margin of the puboischiadic bar. There are 100–117 pectoral radials of which 47–52 are propterygial, 12–15 mesopterygial, and 40–50 metapterygial. Each radial comprises at least 23–25 segments each of which bifurcate twice. The radials of Arechia are calcified in chain-like patterns, forming the so-called ‘catenated calcification’ typical of batoids with undulatory swimming mode, including most of the benthic stingrays (Schaefer and Summers, Citation2005).
Pelvic Girdle and Fins—The pelvic region is poorly preserved in all the specimens examined. However, the pelvic fins are clearly single-lobed, protruding slightly beyond the disc, and their length equals about 15–16% of total length. The puboischiadic bar can be partly recognized in MCSNV IG.VR.27607 but is poorly preserved (). The puboischiadic bar width is estimated about 9% of the total body length (or 14% of disc width) as measured across the pelvic-fin bases. The bar is clearly enlarged at its distal corners but obturator foramina are difficult to recognize. Each basipterygium appears to support 15–17 pelvic-fin radials. No claspers were recognized in any of the specimens examined.
Dorsal and Caudal Fins—Dorsal fins can be present or absent in extant urolophids. The Arechia specimens described herein do not show any structures (i.e., radials) anterior to the serrated sting that would suggest the presence of a dorsal fin. However, it is unclear whether this is a real anatomical feature or the consequence of taphonomic processes. There is no cartilaginous rod stiffening the distal part of the vertebral column in the tail. As in living urolophids, most of the specimens of Arechia show an elongated and lobe-like caudal fin measuring ca. 20–22% of the total length and supported by distinct radial cartilages (about 50–60 in each dorsal and caudal lobe) (Supplemental Data 1, Fig. S1C–E). Radial cartilages do not reach the margins of the caudal fin, which is therefore of the aplesodic type.
Dentition—The overall tooth morphology of the specimens examined is consistent with that of the genus Arechia Cappetta, Citation1983. The dentition exhibits a gradient monognathic heterodonty, with lateral teeth becoming smaller and enlarged labiolingually. The single tooth extracted from MCSNV IG.VR.27607 is about 2 mm in crown width, broader than long with a slightly convex labial contour and a very strongly convex lingual outline (). The crown is high and not cuspidate. The enameloid surface is completely smooth. The edge of the labial visor is rather broad and convex. The edge of the lingual visor is convex, with a central notch. There is a high, sharp, and lingually displaced transverse cutting crest that does not reach the lateral angles, which are blunt. A second transverse cutting crest occurs labially but it is lower and blunter than the lingual one, resembling the condition observed in Urolophus, Trygonoptera, Urobatis, and Himantura (Herman et al., Citation2000). Although not specifically mentioned, it seems that this character can be recognized also in the type species A. arambourgi (see Cappetta, Citation1983, Citation2012). At the labial surface, a weak depression can be observed in between the two transverse cutting crests. In profile view, the labial face has a concave profile just below the crest that becomes convex further basally. The lingual face is slightly more developed than the labial one; it has a convex profile in its upper part and a concave profile in its mid-lower region. The root is lower than the crown and slightly expanded mesiodistally. There are two root lobes whose basal face is subtriangular, slightly convex, and feebly joins the labial face of the oblique and straight root. The root lobes are divided by a broad and deep furrow with a large central foramen and one or two paracentral foramina. Other foramina open also on the labial face of the root, just below its junction with the crown.
Squamation and Stings—The skin of modern urolophid genera Urolophus and Trygonoptera is completely devoid of dermal denticles and thorns, whereas Spinilophus possesses some sparse denticles and thorns (Last et al., Citation2016). All the examined specimens of Arechia mostly lack dermal denticles and thorns. However, some of them show very small and scattered structures having star shape on the disc margin and tail that might be interpreted as dermal denticles or remains of the original pigmentation (Supplemental Data 1, Fig. S2A, B). However it is hard to draw firm conclusions given the poor preservation of the specimens. A single, elongate, and dorsoventrally flattened serrated caudal sting occurs in the majority of the specimens (Supplemental Data 1, Fig. S2C–F). The sting origin is located at about mid way down the length of the tail, posterior to the pelvic fins and just anterior to the caudal-fin origin, at ca. 70–77% of the total body length. The best-preserved stings are about 9% of the body length. Four to six irregular grooves run parallel to the main axis on the dorsal side of the sting. The serrations on the sting are small, hook shaped, and directed transversely to the caudal axis of the sting. The number of serrations per side is difficult to determine due to the lack of the distal tip of the stings. No specific characters of the sting, useful to discriminate Arechia from other urolophids, can be recognized.
Stomach Contents—Two specimens of A. crassicaudata show abdominal gut content consisting of partially digested bony fishes which are completely preserved in the abdominal cavity between the pectoral and pelvic girdles, on one side of the vertebral column, in a position comparable to that occupied by the gut-intestine tract in extant stingrays (Supplemental Data 1, Fig. S3). The stomach content of MCSNV VII.B.84/85 consists of a single vertebral column with associated arches, and disarticulated dorsal and anal fins. Conversely, the bones of the head and tail of the bony fish appear scattered around the vertebral column, showing little evidence of digestion thereby suggesting that the consumption occurred shortly before the death of the stingray. Despite the degree of disarticulation of the skeleton, the overall morphology of the body axis of the fish as well as the presence of considerably large dorsal-fin spines showing canals running parallel to the main spine axis are consistent with those of the extinct squirrelfish Eoholocentrum macrocephalum (Blainville, Citation1818), family Holocentridae (see Sorbini and Tirapelle, Citation1974).
The neotype of A. crassicaudata, MCSNV IG.VR.27607, shows two or three small fishes inside the abdomen. Due to the considerable degree of disarticulation, it was not possible to establish the precise taxonomic identity of the bony fishes, although the general morphology of the vertebral column appears to be consistent with those of small percomorphs.
A third specimen, MGP-PD 8875C/76C, shows a possible cololite, or intestinal fossil content still in situ in the abdominal cavity (Supplemental Data 1, Fig. S3C). The cololite is relatively short and uncoiled with a whitish coloration. It is not possible to distinguish what kind of food the cololite is composed of, with the exception of a small chela of a decapod crustacean.
The presence of crustacean and fish remains as stomach/gut content of three out of six individuals of Arechia, suggests that the food preferences of this taxon were quite similar to those of extant urolophids, which feed mainly on polychaete worms, crustaceans, and small bony fishes (Last and Compagno, Citation1999; Last et al., Citation2016a).
Phylogenetic Analysis
The analysis conducted in TNT resulted in a single parsimonious tree with a length of 215 steps, a consistency index (CI) of 0.64, and a retention index (RI) of 0.80 (), and a topology that is consistent with those depicted in previous studies on Bolca stingrays (e.g., Marramà et al., Citation2019c). The Myliobatiformes are recovered as a monophyletic group (Bremer value 4) supported herein by ten characters: basihyal as a single element, but separated from the first hypobranchials (ch. 19[1]); presence of a median projection of the basibranchial medial plate (ch. 22[1]); presence of levator and depressor rostri muscles (ch. 66[1]), serrated tail stings (ch. 67[1]); thorns absent (ch. 69[1]); rostral cartilage vestigial or absent (ch. 73[1]); postorbital process very broad and shelf-like (ch. 74[1]); jugal arch absent (ch. 75[1]); presence of ball and socket articulation between scapular process and synarcual (ch. 78[1]); presence of a thoracolumbar synarcual (ch. 79[1]). As in the molecular analysis of Aschliman et al. (Citation2012b), the sixgill stingray Hexatrygon is inferred to be the sister taxon to all other stingrays, in contrast with molecular phylogenies interpreting this taxon as sister to Gymnura (Bertozzi et al., Citation2016) or to the urolophids (Naylor et al., Citation2012a, Citation2012b).
FIGURE 7. The single parsimonious tree retrieved using the branch-and-bound search in TNT showing the hypothetical phylogenetic relationships of Arechia Cappetta, Citation1983 within the Myliobatiformes. Character number above and state below each node. Extinct taxa are marked with a dagger.
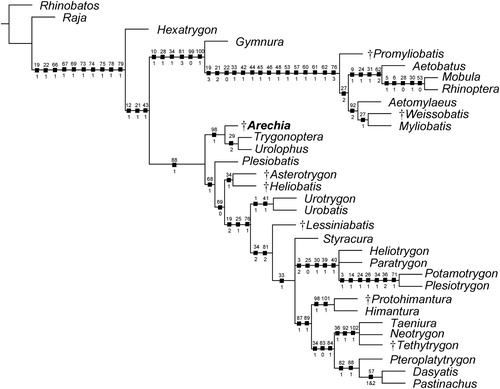
The remaining stingrays are dichotomously grouped into two large clades (Myliobatoidea and Dasyatoidea) whose nature can be possibly linked to the different body shape, calcifications of radials, swimming mode, and life style (Schaefer and Summers, Citation2005; Hall et al., Citation2018).
The monophyly of the clade comprising all benthic stingrays (i.e., Dasyatoidea) is weakly supported (Bremer value 1) by a single character, the spiracularis split into lateral and medial bundles, with the medial bundle inserting on the posterior surface of Meckel’s cartilage and the lateral bundle inserting onto the dorsal edge of the hyomandibula (ch. 88[1]). The dasyatoids include taxa having a rhomboid or ovoid pectoral disc, and a ‘catenated’ calcification pattern of radials, which possibly reflect their undulatory swimming mode and benthic habits (Schaefer and Summers, Citation2005).
Arechia is herein inferred to be a basal member of the dasyatoid stingrays, sister to the living urolophids Urolophus and Trygonoptera. This relationship is supported by a single ambiguous character: the presence of a second transverse keel on teeth (ch. 98[1]). Urolophus and Trygonoptera are united by a single character: an external margin of the mesopterygia which is fused to radial elements (ch. 29[2]). This character appears absent in Arechia in which the external margins of the mesopterygia are straight and not fused to the radials. The presence of the enlarged optic nerve foramen (ch. 8[1]) supports the sister-group relationship between Urolophus and Trygonoptera in previous phylogenetic analyses (e.g., Carvalho et al., Citation2004; Marramà et al., Citation2019a, Citation2019b, Citation2019c). However, this character does not support the group in this analysis, possibly because its presence is difficult to determine in Arechia. The monophyly of the Urolophidae was also recovered by previous morphological (e.g., Carvalho et al., Citation2004; Claeson et al., Citation2010) and molecular analyses (e.g., Naylor et al., Citation2012b; Bertozzi et al., Citation2016), whereas the family appears to be paraphyletic according to Naylor et al. (Citation2012a) with Trygonoptera being sister to Hexatrygon, and Urolophus sister to Plesiobatis. The systematic position of Arechia also is supported when the data matrix is analyzed with PAUP* 4.0, which yielded 18 equally parsimonious trees with a tree length of 216 steps, a CI of 0.63, and a RI of 0.80.
Among the other dasyatoid stingrays, Plesiobatis, the Eocene stingrays from the Green River Formation, the urotrygonids and Lessiniabatis form successive sister taxa to all potamotrygonids and dasyatids. Asterotrygon and Heliobatis form a monophyletic clade that is weakly supported (Bremer value 1) by a single ambiguous character (caudal fin reduced to tail folds; ch. 34[1]) that seems to have been achieved independently from the dasyatids (Marramà et al., Citation2019b). Urotrygonid stingrays (Urotrygon and Urobatis) are monophyletic as detected in Aschliman et al. (Citation2012a) and Bertozzi et al. (Citation2016). The enigmatic extinct stingray Lessiniabatis from Bolca is sister to a polytomy that includes potamotrygonids and dasyatids. Although Styracura is certainly closely related to the freshwater potamotrygonids as suggested by morphological, molecular, and chrono/geographic evidence (Lovejoy, Citation1996; Lovejoy et al., Citation1998; Carvalho et al., Citation2004, Citation2016; Aschliman et al., Citation2012b; Naylor et al., Citation2012a, Citation2012b; Bertozzi et al., Citation2016), our phylogeny did not recognize Styracura as a genuine member of the Potamotrygonidae, possibly because Styracura lacks some characters of the lateral line, and pectoral and pelvic girdle skeleton, which are typically found in freshwater potamotrygonids (Carvalho et al., Citation2016).
Finally, our analysis weakly supports (Bremer value 1) a clade that includes Gymnura as sister to all pelagic stingrays with six synapomorphies: short orbital region with more anteriorly placed supraorbital and postorbital processes (ch. 10[1]); lateral expansion of the radials in pectoral region (ch. 28[1]); caudal fin absent (ch. 34[2]); first segment of propterygium adjacent to anterior margin of antorbital cartilage or anterior to margin of nasal capsule (ch. 81[3]); ‘crustal’ calcification pattern of radials (ch. 99[0]); and wing-like pectoral disc, with fins greatly expanded (ch. 100[1]). However, our hypothesis contrasts with recent molecular analyses in resurrecting the Gymnura plus ‘Myliobatidae’ clade, whose relationship is weakly supported possibly because of the limited set of taxa and ambiguous character states (see Aschliman, Citation2014). In fact, more robust molecular analyses resolved Gymnura as sister of Urolophus (Aschliman et al., Citation2012b), Plesiobatis (Naylor et al., Citation2012b), Hexatrygon (Bertozzi et al., Citation2016), or placed it much closer to the base of all myliobatiformes (Last et al., Citation2016).
Although the tree loses resolution after bootstrap analysis, some clades are still retrieved (e.g., freshwater potamotrygonids, Gymnura plus ‘myliobatids’, urotrygonids), including the sister-group relationship of Arechia with the extant urolophids (Supplemental Data 1, Fig. S4).
DISCUSSION
Comparative Remarks
The Urolophidae, also known as stingarees or round stingrays, are a myliobatiform family represented by 28 living species arranged in three genera (Urolophus, Trygonoptera, and Spinilophus; Last et al., Citation2016). They are small to medium-sized stingrays (up to 90 cm TL) characterized by an oval, circular or rhomboid pectoral disc and a short tail with an elongate lobe-like caudal fin. A dorsal fin can be present in some species. The skin is completely devoid of denticles and thorns in Urolophus and Trygonoptera, but usually one or more serrated stings are present on the tail, well behind the pelvic fins (Last and Compagno, Citation1999; Last et al., Citation2016). The monophyly of the family Urolophidae appears to be supported in morphology-based phylogenetic analyses by the presence of mesopterygia fused to radial elements, an enlarged foramen for the optic nerve in the neurocranium, and a second transverse tooth keel (e.g., Carvalho et al., Citation2004; Claeson et al., Citation2010; Marramà et al., Citation2019a, Citation2019b, Citation2019c).
In our study, the presence of the thoracolumbar synarcual, a serrated sting, and the absence of rostral cartilage support the inclusion of Arechia within the batoid order Myliobatiformes. The presence of a rhomboidal pectoral disc, a short tail with an elongate lobe-like caudal fin, skin mostly devoid of denticles and thorns, and second transverse tooth keel support the inclusion of Arechia within the Urolophidae and its sister-group relationship with Urolophus plus Trygonoptera in our phylogeny.
A combination of several characters argues against the placement of Arechia within the other stingray lineages. For example, the presence of a complete caudal fin excludes its assignment to the Dasyatidae, Potamotrygonidae, ‘myliobatoids,’ or to the Eocene stingrays from the Green River Formation, because these taxa have replaced the caudal fin with tail folds or a cartilaginous stiffening rod in the terminal part of the tail (e.g., Carvalho et al., Citation2004). The absence of some traits characterizing pelagic/benthopelagic stingrays (e.g., wing-like pectoral disc, crustal calcification of radials, crushing dental plates, basihyal absent) rules out a close relationship with Arechia. Although the overall body plan of Arechia and living urolophids is similar to that of the Urotrygonidae, the presence of a single basihyal in Arechia excludes a close relationship with Urobatis and Urotrygon (basihyal absent or fragmented; Carvalho et al., Citation2004). Furthermore, the unique tooth crown morphology and the absence of any tooth enameloid ornamentation in the Bolca specimens excludes their alignment with other Eocene stingray taxa such as Aturobatis, Coupatezia, Heterotorpedo, Hypolophodon, Jacquhermania, Merabatis, Meridiania, and Ouledia (see Cappetta, Citation2012).
Finally, Arechia can be distinguished from other members of the family Urolophidae (Urolophus, Trygonoptera, and Spinilophus) based on size (110 vs. 90 cm) and by several different body proportions and meristic counts (Supplemental Data 1, Table S2). Arechia crassicaudata differs from the type species A. arambourgi in some tooth characters: in profile view, a labial crown face with a concavity just below the main crest, but being convex further down (nearly straight in A. arambourgi); lingual crown face slightly more developed than the labial one, with a convex profile in its upper part and concave profile in its mid-lower region (nearly straight in A. arambourgi); edge of the lingual visor being slightly more convex than the labial one (equally convex in A. arambourgi).
Fossil Record and Paleoecology of Urolophids
The paleoecological role of the urolophids from the Monte Postale site of the Bolca Lagerstätte has been poorly investigated so far. Living representatives of the Urolophidae are temperate to tropical marine batoids mostly occurring on continental and insular shelves of the Indo-Australian Archipelago and northwestern Pacific, although some species can occur in estuaries or slopes up to 420 m depth (Last and Compagno, Citation1999; Last et al., Citation2016). They are usually slow-swimming bottom-dwellers, occurring on soft mud, and feeding on polychaete worms, crustaceans, and small benthic fishes (Last and Compagno, Citation1999; Last et al., Citation2016). Quantitative paleoecological and taphonomic analyses of the fish assemblage of Monte Postale site suggest that the fossiliferous sediments were deposited close to an emerged coastal area, possibly characterized by the presence of mangroves and seagrass, in a coral reef context (Marramà et al., Citation2016; Vescogni et al., Citation2016). From this perspective, the presence of Arechia inhabiting the warm shallow-water habitats of the Monte Postale paleobiotope is therefore consistent with the ecological and environmental preferences of modern urolophids.
The fossil record of urolophids is poor when compared with the other stingray lineages, possibly because their isolated teeth are often mis-assigned to the wastebasket genus Dasyatis. Beside Arechia crassicaudata other occurrences of the genus are reported from the Ypresian to Lutetian of northern and western Africa (Cappetta, Citation1983, Citation1987, Citation2012; Cappetta and Traverse, Citation1988; Noubhani and Cappetta, Citation1997; Tabuce et al., Citation2005; Sambou et al., Citation2017, Citation2020), and possibly Mississippi, U.S.A., the Netherlands, and France (Bor, Citation1985; Cappetta, Citation2012). Urolophus was reported by Hasse (Citation1882) in the Lutetian of Belgium, and by Noetling (Citation1885) in the Lutetian of Russia, on the basis of vertebral centra. Isolated teeth of Urolophus from the Oligocene of Germany were described by Freess (Citation1991). However, all the occurrences of Urolophus need to be verified according to Cappetta (Citation2012). Indeterminate urolophid fossil teeth were also reported from the Lutetian of Morocco (Tabuce et al., Citation2005). Teeth of Urolophus halleri were reported from Piacentian of California (Fitch, Citation1964) although this taxon is today regarded as a species of Urobatis (family Urotrygonidae).
Molecular analyses suggested that the clade containing Urolophus and Trygonoptera possibly diverged from gymnurids around 75 Ma ago (Aschliman et al., Citation2012b) or from plesiobatids around the K-Pg boundary (Bertozzi et al., Citation2016). Since Arechia possibly represents the oldest urolophid (50 Ma), the divergence time estimates of the family appear to be quite consistent with its stratigraphic occurrence in the fossil record, or at least, a small gap exists (15–25 Ma). Moreover, it is plausible to assume a Tethyan origin for the family Urolophidae based on the oldest fossils occurring in the Tethyan realm, as also suggested for other batoid lineages.
CONCLUSIONS
The systematic revision of the fossil stingrays from Bolca traditionally identified as Urolophus crassicaudatus (Blainville, Citation1818) confirmed previous assumptions that these articulated fossil specimens cannot be attributed to the genus Urolophus. Considering that the general dental characters of the specimens from Bolca are consistent with those of Arechia these individuals represent the first skeletal record of this Eocene stingray genus and the only articulated fossil remains of the Urolophidae known so far.
Supplemental Material
Download Zip (67 MB)ACKNOWLEDGMENTS
We thank R. Zorzin (MCSNV), M. Fornero and L. Del Favero (MGP-PD), and U. Göhlich (NHMW) for providing facilities and access to fossil material under their care. We also thank two anonymous reviewers and the editors for the valuable comments and suggestions that improved the quality of the manuscript. Financial support was provided by the Austrian Science Fund (FWF) [M2368-B25 to G.M.] and the Università degli Studi di Torino [ex-60% 2019, and 2020 grants to G.C. and G.M.].
LITERATURE CITED
- Agassiz, L. 1833–1844. Recherches sur les Poissons Fossiles. Petitpierre, Neuchâtel, 1420 pp.
- Agassiz, L. 1835a. Kritische revision der in der Ittiolitologia Veronese abgebildeten fossilen Fishes. Neues Jahrbuch für Mineralogie, Geognosie, Geologie und PetrefaktenKunde 1835:290–316.
- Agassiz, L. 1835b. Revue Critique des Poissons Fossiles Figurés dans l’Ittiolitologia Veronese. Petitpierre et Prince, Neuchâtel, 44 pp.
- Aschliman, N. C. 2014. Interrelationships of the durophagous stingrays (Batoidea: Myliobatidae). Environmental Biology of Fishes 97:967–979. doi: 10.1007/s10641-014-0261-8
- Aschliman, N. C., K. M. Claeson, and J. D. McEachran. 2012a. Phylogeny of Batoidea; pp. 57–96 in J. F. Carrier, J. A. Musick, and M. R. Heithaus (eds.), Biology of Sharks and Their Relatives, second edition. CRC Press, Boca Raton, Florida.
- Aschliman, N. C., M. Nishida, M. Miya, J. G. Inoue, K. M. Rosana, and G. J. P. Naylor. 2012b. Body plan convergence in the evolution of skates and rays (Chondrichthyes: Batoidea). Molecular Phylogenetics and Evolution 63:28–42. doi: 10.1016/j.ympev.2011.12.012
- Arambourg, C. 1952. Les vertébrés fossiles des gisements de phosphates (Maroc-Algeérie-Tunisie). Notes et Mémoires du Service Géologique du Maroc 92:1–372.
- Bertozzi, T., M. S. Y. Lee, and S. C. Donnellan. 2016. Stingray diversification across the end-Cretaceous extinctions. Memoirs of Museum Victoria 74:379–390. doi: 10.24199/j.mmv.2016.74.26
- Blainville, H. D. 1818. Sur les ichthyolites ou les poissons fossiles. Nouveau Dictionnaire d’Histoire Naturelle 27:310–391.
- Blot, J. 1980. La faune ichthyologique des gisements du Monte Bolca (Province de Verone, Italie). Catalogue systématique présentant l’état actuel des 160 recherches concernant cette faune. Bulletin du Muséum National d’Histoire Naturelle Paris 2:339–396.
- Bor, T. J. 1985. Elasmobranch teeth (Vertebrata, Pisces) from the Dongen Formation (Eocene) in the Netherlands. Mededelingen Van De Werkgroep Voor Tertiaire En Kwartaire Geologie 22:73–122.
- Bronn, H. G. 1831. Italiens Tertiär-Gebilde und Deren Organische Einschlüsse. Neue Akademische Buschandlung von Karl Groos, Heidelberg, 176 pp.
- Cappetta, H. 1980. Les selaciens du Cretace superieur du Liban. II: batoides. Palaeontographica, Abteilung A 168:149–229.
- Cappetta, H. 1983. Additions à la faune de sélaciens fossils du Maroc. 2: Révision de Raja praealba Arambourg, 1952, espèce de l’Yprésien des Ouled Abdoun. Tertiary Research 5:1–8.
- Cappetta, H. 1987. Handbook of Paleoichthyology, Volume 3B: Chondrichthyes II. Gustav Fischer Verlag, Stuttgart, 193 pp.
- Cappetta, H. 2012. Handbook of Paleoichthyology - Chondrichthyes - Mesozoic and Cenozoic Elasmobranchii: Teeth. F. Pfeil-Verlag, München, 512 pp.
- Cappetta, H., and M. Traverse. 1988. Une riche faune de sélaciens dans le bassin a phosphate de Kpogamé-Hahotoé (Éocène moyen du Togo): note préliminaire et précisions sur la structure et l'âge du gisement. Geobios 21:359–365. doi: 10.1016/S0016-6995(88)80058-5
- Carnevale, G., A. F. Bannikov, G. Marramà, J. C. Tyler, and R. Zorzin. 2014. The Pesciara-Monte Postale Fossil-Lagerstätte: 2. Fishes and other vertebrates; pp. 37–63 in C. A. Papazzoni, L. Giusberti, G. Carnevale, G. Roghi, D. Bassi, and R. Zorzin (eds.), The Bolca Fossil-Lagerstätte: A Window into the Eocene World. Società Paleontologica Italiana, Modena, Italy.
- Carvalho, M. R., T. S. Loboda, and J. P. Silva. 2016. A new subfamily, Styracurinae, and new genus, Styracura, for Himantura schmardae (Werner, 1904) and Himantura pacifica (Beebe & Tee-Van, 1941) (Chondrichthyes: Myliobatiformes). Zootaxa 4175:201–221. doi: 10.11646/zootaxa.4175.3.1
- Carvalho, M. R., J. C. Maisey, and L. Grande. 2004. Freshwater stingrays of the Green River formation of Wyoming (Early Eocene), with the description of a new genus and species and an analysis of its phylogenetic relationships (Chondrichthyes: Myliobatiformes). Bulletin of the American Museum of Natural History 284:1–136. doi: 10.1206/0003-0090(2004)284<0001:FSOTGR>2.0.CO;2
- Claeson, K. M., M. A. O’Leary, E. M. Roberts, F. Sissoko, M. Bouaré, L. Tapanila, D. Goodwin, and M. D. Gottfried. 2010. First Mesozoic record of the stingray Myliobatis wurnoensis from Mali and a phylogenetic analysis of Myliobatidae incorporating dental characters. Acta Palaeontologica Polonica 55:655–674. doi: 10.4202/app.2009.1117
- Compagno, L. J. V. 1973. Interrelationships of living elasmobranchs; pp. 15–61 in P. H. Greenwood, R. S. Miles, and C. Patterson (eds.), Interrelationships of Fishes. Academic Press, New York.
- D’Erasmo, G. 1922. Catalogo dei pesci fossili delle Tre Venezie. Memorie dell’Istituto di Geologia della Regia Università di Padova 6:1–181.
- De Zigno, A. 1874. Catalogo Ragionato dei Pesci Fossili del Calcare Eoceno di M. Bolca e M. Postale. Stabilimento Tipografia Grimaldo e C., Venice, 215 pp.
- Eastman, C. R. 1904. Description of Bolca fishes. Bulletin of the Museum of Comparative Zoology 46:1–36.
- Eastman, C. R. 1905. Les types de Poissons fossiles du Monte Bolca au Muséum d’Histoire Naturelle de Paris. Mémoires de la Société géologique de France 34:1–33.
- Fanti, F., G. Mazzuferi, and G. Marramà. 2019. Egg preservation in an Eocene stingray (Myliobatiformes, Dasyatidae) from Italy. Journal of Vertebrate Paleontology 39:e1578967. doi: 10.1080/02724634.2019.1578967
- Fanti, F., D. Minelli, G. Larocca Conte, and T. Miyashita. 2016. An exceptionally preserved Eocene shark and the rise of modern predatory-prey interaction in the coral reef food web. Zoological Letters 2:246. doi: 10.1186/s40851-016-0045-4
- Fitch, J. E. 1964. The fish fauna of the Playa del Rey locality, a southern California marine Pleistocene deposit. Los Angeles County Museum Contributions in Science 82:1–35.
- Freess, W. B. 1991. Elasmobranchii und Teleostei des Sternberger Gesteins (Oberoligozän). Archiv für Geschiebekunde 1:131–216.
- Frickhinger, K. A. 1991. Fossilien Atlas: Fische. Mergus, Melle, 1088 pp.
- Goloboff, P. A., J. S. Farris, and K. C. Nixon. 2008. TNT, a free program for phylogenetic analysis. Cladistics 24:774–786. doi: 10.1111/j.1096-0031.2008.00217.x
- Hall, K. C., P. J. Hundt, J. D. Swenson, A. P. Summers, and K. D. Crow. 2018. The evolution of underwater flight: the redistribution of pectoral fin rays, in manta rays and their relatives (Myliobatidae). Journal of Morphology 279:1155–1170. doi: 10.1002/jmor.20837
- Hasse, C. 1882. Das natürliche System der Elasmobranchier auf Grundlage des Baues und der Entwicklung ihrer Wirbelsäule. Eine morphologische und paläontologische Studie. II. Besonderer Theil. Gustav Fischer Verlag, Jena, 284 pp.
- Heckel, M. J. 1851. Bericht einer auf Kosten der kais. Akademie der Wissenschaften durch Oberösterreich nach Salzburg, München, Innsbruck, Botzen, Verona, Padua, Venedig und Triest unternommenen Reise. Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften. Mathematisch–Naturwissenschaftliche Klasse 7:281–333.
- Heckel, M. J. 1853. Bericht über die vom Herrn Cavalière Achille de Zigno hier angelangte Sammlung fossiler Fische. Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften. Mathematisch-Naturwissenschaftliche Klasse 11:122–138.
- Herman, J., M. Hovestadt-Euler, D. C. Hovestadt, and M. Stehmann. 2000. Contributions to the study of the comparative morphology of teeth and other relevant ichthyodorulites in living supra-specific taxa of Chondrichthyan fishes. Part B: Batomorphii 4c: Order Rajiformes - Suborder Myliobatoidei - Superfamily Dasyatoidea - Family Dasyatidae - Subfamily Dasyatinae - Genus: Urobatis, Subfamily Potamotrygoninae Genus: Paratrygon, Superfamily Plesiobatoidea - Family Plesiobatidae - Genus: Plesiobatis, Superfamily Myliobatoidea - Family Myliobatidae - Subfamily Myliobatinae - Genera: Aetobatus, Aetomylaeus, Myliobatis and Pteromylaeus, Subfamily Rhinopterinae - Genus: Rhinoptera and Subfamily Mobulinae - Genera: Manta and Mobula. Addendum 1 to 4a: erratum to Genus Pteroplatytrygon. Bulletin de l’Institut Royal des Sciences Naturelles de Belgique Biologie 70:5–67.
- Huxley, T. H. 1880. On the application of the laws of evolution to the arrangement of the Vertebrata, and more particularly of the Mammalia. Proceedings of the Zoological Society London 43:649–662.
- International Commission on Zoological Nomenclature. 1999. International Code of Zoological Nomenclature, fourth edition. International Trust for Zoological Nomenclature, London, xxix + 306 pp.
- Jaekel, O. 1894. Die eocänen Selachier vom Monte Bolca: ein Beitrag zur Morphogenie der Wirbelthiere. J. Springer, Berlin, 176 pp.
- Kner, R., and F. Steindachner. 1863. Neue Beiträge zur Kenntnis der fossilen Fische Österreichs. Denkschriften der Kaiserlichen Akademie der Wissenschaften, Mathematisch–Naturwissenschaftliche Classe 21:17–36.
- Last, P. R., and L. J. V. Compagno. 1999. Urolophidae; pp. 1469–1476 in K. E. Carpenter and V. H. Niem (eds.), FAO Species Identification Guide for Fishery Purposes. The Living Marine Resources of the Western Central Pacific. Volume 3. Batoid Fishes, Chimaeras and Bony Fishes Part 1 (Elopidae to Linophrynidae). FAO, Rome.
- Last, P. R., W. White, M. R. Carvalho, B. Séret, M. Stehmann, and G. J. P. Naylor. 2016. Rays of the World. CSIRO Publishing, Clayton North, 790 pp.
- Lovejoy, N. R. 1996. Systematics of myliobatoid elasmobranchs: with emphasis on the phylogeny and historical biogeography of Neotropical freshwater stingrays (Potamotrygonidae: Rajiformes). Zoological Journal of the Linnean Society 117:207–257. doi: 10.1111/j.1096-3642.1996.tb02189.x
- Lovejoy, N. R., E. Birminghan, and A. P. Martin. 1998. South American rays came in with the sea. Nature 396:421–422. doi: 10.1038/24757
- Maddison, W. P., and D. R. Maddison. 2008. Mesquite: A Modular System for Evolutionary Analysis. Version 3.03. Available at www.mesquiteproject.org. Accessed April 15, 2018.
- Marramà, G., G. Carnevale, and J. Kriwet. 2018c. New observations on the anatomy and paleobiology of the Eocene requiem shark †Eogaleus bolcensis (Carcharhiniformes, Carcharhinidae) from Bolca Lagerstätte, Italy. Comptes Rendus Palevol 17:443–459. doi: 10.1016/j.crpv.2018.04.005
- Marramà, G., G. Carnevale, G. J. P. Naylor, and J. Kriwet. 2019a. Mosaic of plesiomorphic and derived characters in an Eocene myliobatiform batomorph (Chondrichthyes, Elasmobranchii) from Italy defines a new, basal body plan in pelagic stingrays. Zoological Letters 5:13. doi: 10.1186/s40851-019-0128-0
- Marramà, G., G. Carnevale, G. J. P. Naylor, and J. Kriwet. 2019b. Reappraisal of the Eocene whiptail stingrays (Myliobatiformes, Dasyatidae) of the Bolca Lagerstätte, Italy. Zoologica Scripta 48:168–184. doi: 10.1111/zsc.12330
- Marramà, G., K. M. Claeson, G. Carnevale, and J. Kriwet. 2018b. Revision of Eocene electric rays (Torpediniformes, Batomorphii) from the Bolca Konservat-Lagerstätte, Italy, reveals the first fossil embryo in situ in marine batoids and provides new insights into the origin of trophic novelties in coral reef fishes. Journal of Systematic Palaeontology 16:1189–1219. doi: 10.1080/14772019.2017.1371257
- Marramà, G., A. Engelbrecht, G. Carnevale, and J. Kriwet. 2019d. Eocene sand tiger sharks (Lamniformes, Odontaspididae) from the Bolca Konservat-Lagerstätte, Italy: palaeobiology, palaeobiogeography and evolutionary significance, Historical Biology 31:102–116. doi: 10.1080/08912963.2017.1341503
- Marramà, G., A. F. Bannikov, J. C. Tyler, R. Zorzin, and G. Carnevale. 2016. Controlled excavations in the Pesciara and Monte Postale sites provide new insights about the paleoecology and taphonomy of the fish assemblages of the Eocene Bolca Konservat-Lagerstätte, Italy. Palaeogeography, Palaeoclimatology, Palaeoecology 454:228–245. doi: 10.1016/j.palaeo.2016.04.021
- Marramà, G., G. Carnevale, L. Giusberti, G. J. P. Naylor, and J. Kriwet. 2019c. A bizarre Eocene dasyatoid batomorph (Elasmobranchii, Myliobatiformes) from the Bolca Lagerstätte (Italy) reveals a new, extinct body plan for stingrays. Scientific Reports 9:14087. doi: 10.1038/s41598-019-50544-y
- Marramà, G., G. Carnevale, A. Engelbrecht, K. M. Claeson, R. Zorzin, M. Fornasiero, and J. Kriwet. 2018a. A synoptic review of the Eocene (Ypresian) cartilaginous fishes (Chondrichthyes: Holocephali, Elasmobranchii) of the Bolca Konservat-Lagerstätte, Italy. Paläontologische Zeitschrift 92:283–313. doi: 10.1007/s12542-017-0387-z
- Miyake, T. 1988. The systematics of the stingray genus Urotrygon with comments on the interrelationships within Urolophidae (Chondrichthyes: Myliobatiformes). Ph.D. dissertation, Texas A&M University, College Station, Texas.
- Miyake, T., J. D. McEachran, P. J. Walton, and B. K. Hall. 1992. Development and morphology of rostral cartilages in batoid fishes (Chondrichthyes: Batoidea), with comments on homology within vertebrates. Biological Journal of the Linnean Society 46:259–298. doi: 10.1111/j.1095-8312.1992.tb00864.x
- Müller, J., and F. G. J. Henle. 1837. Gattungen der Haifische und Rochen nach einer von ihm mit Hrn. Henle unternommenen gemeinschaftlichen Arbeit über die Naturgeschichte der Knorpelfische. Bericht Akademie der Wissenschaften zu Berlin 1837:111–118.
- Müller, J., and F. G. J. Henle. 1841. Systematische Beschreibung der Plagiostomen. Veit Berlin, 200 pp.
- Molin, R. 1861. De Rajidis tribus bolcanis. Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften. Mathematisch-Naturwissenschaftliche Klasse 42:576–582.
- Naylor, G. J. P., J. N. Caira, K. Jensen, A. M. Rosana, N. Straube, and C. Lakner. 2012a. Elasmobranch phylogeny: a mitochondrial estimate based on 595 species; pp. 31–56 in J. C. Carrier, J. A. Musick, and M. R. Heithaus (eds.), Biology of Sharks and Their Relatives, second edition. CRC Press, Boca Raton.
- Naylor, G. J. P., J. N. Caira, K. Jensen, A. M. Rosana, W. T. White, and P. R. Last. 2012b. A DNA sequence-based approach to the identification of shark and rays species and its implication of global elasmobranch diversity and parasitology. Bulletin of the American Museum of Natural History 367:1–262. doi: 10.1206/754.1
- Noetling, F. 1885. Die Fauna des samländischen Tertiärs. Abhandlungen zur geologischen Specialkarte von Preussen und den Thüringischen Staaten 6:1–218.
- Noubhani, A., and H. Cappetta. 1997. Les Orectolobiformes, Carcharhiniformes et Myliobatiformes (Elasmobranchii, Neoselachii) des Bassins à phosphate du Maroc (Maastrichtien-Lutétien basal). Systématique, biostratigraphie, évolution et dynamique des faunes. Palaeo Ichthyologica 8:1–327.
- Papazzoni, C. A., E. Fornaciari, L. Giusberti, A. Vescogni, and B. Fornaciari. 2017. Integrating shallow benthic and calcareous nannofossil zones: the lower Eocene of the Monte Postale section (northern Italy). Palaios 32:6–17. doi: 10.2110/palo.2016.014
- Sambou, B. S., R. Sarr, L. Hautier, H. Cappetta, and S. Adnet. 2017. The selachian fauna (sharks and rays) of the phosphate series of Ndendouri-Ouali Diala (Matam, Western Senegal): dating and paleoenvironmental interests. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 283:205–219. doi: 10.1127/njgpa/2017/0637
- Sambou, B. S., L. Hautier, R. Sarr, R. Tabuce, F. Lihoreau, M. Thiam, R. Lebrun, J. E. Martin, H. Cappetta, and S. Adnet. 2020. Contribution to the reappraisal of the mid Paleogene ichtyofauna of Western Africa with three new enigmatical elasmobranchs from Thanetian–Lutetian of Senegal. Annales de Paléontologie 106:102400. doi: 10.1016/j.annpal.2020.102400
- Schaefer, J. T., and A. P. Summers. 2005. Batoid wing skeletal structure: novel morphologies, mechanical implications, and phylogenetic patterns. Journal of Morphology 264:298–313. doi: 10.1002/jmor.10331
- Sorbini, L., and R. Tirapelle. 1974. Gli Holocentridae di Monte Bolca. I: Eoholocentrum, nov. gen., Eoholocentrum macrocephalum (De Blainville) (Pisces-Actinopterygii). Studi e Ricerche sui Giacimenti Terziari di Bolca 2:205–232.
- Tabuce, R., S. Adnet, H. Cappetta, A. Noubhani, and F. Quillevere. 2005. Aznag (bassin d’Ouarzazate, Maroc), nouvelle localité à sélaciens et mammifères de l’Eocène moyen (Lutétien) d’Afrique. Bulletin de la Société géologique de France 176:381–400. doi: 10.2113/176.4.381
- Vescogni, A., F. R. Bosellini, C. A. Papazzoni, L. Giusberti, G. Roghi, E. Fornaciari, S. Dominici, and R. Zorzin. 2016. Coralgal buildups associated with the Bolca Fossil-Lagerstätten: new evidence from the Ypresian of Monte Postale (NE Italy). Facies 62:21. doi: 10.1007/s10347-016-0472-x
- Woodward, A. S. 1889. Catalogue of the Fossil Fishes in the British Museum. Part. I. Elasmobranchii. British Museum, London, 474 pp.
