ABSTRACT
A new hard-shelled sea turtle (Pan-Cheloniidae) with vestigial soft tissues from the lower Eocene (Ypresian) Fur Formation of Denmark is described and illustrated. The fossil (DK 807) comprises a partial, yet fully articulated carapace (estimated original length ∼50 cm) where the individual bones mostly are preserved in three dimensions, together with an intact sacrum, a consecutive series of articulated caudal vertebrae, a complete pelvic girdle, and both hind limbs. Primitive characters in the pelvis and limbs, along with free ribs that contact the posterior peripherals suggest affinity with the extinct pan-cheloniid Eochelone; however, because of the incomplete nature of the fossil, DK 807 is kept in open nomenclature. Associated with the skeletal elements are soft-tissue residues that include remnant epidermal scutes and a nearly complete outline of a rear paddle. The flipper-shaped halo likely represents traces of skin preserved as a dark bedding-parallel film. Its wrinkled and striated surface texture attests to an originally scaleless configuration comparable to the soft integument of living adult dermochelyid (leatherback) turtles, and unlike that of extant cheloniids. Scratches, scars and indentations on the bony carapace likely represent incompletely healed bite marks inflicted by a crocodylian or another large-sized seagoing tetrapod.
INTRODUCTION
Turtles (Testudines) are a diverse group of reptiles that inhabit a wide range of terrestrial, freshwater and marine environments. Today’s sea turtles in particular have an almost cosmopolitan distribution, and form a monophyletic group that includes two families: the hard-shelled Cheloniidae and leather-shelled Dermochelyidae (Joyce et al., Citation2021). Both these clades originated in the Early Cretaceous (Cadena and Parham, Citation2015; Evers and Benson, Citation2019; Gentry et al., Citation2019), and have left behind a rich fossil record that provides evidence for multiple adaptive radiations during the Cenozoic (Zangerl, Citation1980; Weems and Brown, Citation2017). However, despite this vast geological archive, there are hitherto only few published reports of skin preservation in testudines (e.g., Fielding et al., Citation2005; Tong et al., Citation2006; Li et al., Citation2014; Lindgren et al., Citation2014, Citation2017).
A partial but semi-articulated sea turtle was recently unearthed from a carbonate concretion belonging to the Eocene Fur Formation on the Island of Mors, northern Jutland, Denmark. Combined acid and mechanical preparations revealed preserved soft-tissue remains, a discovery justifying the fossil being declared a Danekræ in 2013 (i.e., a Danish national treasure; Rasmussen et al., Citation2016). This specimen—which hereafter is referred to by its accession number, DK 807—is the latest in a series of fossil turtles that have been recovered from the marine diatomaceous strata of the Fur Formation. Taxa recognized thus far have all been assigned to Cheloniidae and Dermochelyidae (Nielsen, Citation1959; Karl and Madsen, Citation2012), although a nearly complete freshwater turtle also has been collected from these same deposits (RGD, pers. obs.).
FIGURE 1. A, map of the Limfjord region of northern Jutland, Denmark, showing the geographic extension of Fur Formation strata (depicted in dark gray) and the approximate location of the Ejerslev Quarry in which DK 807 was found. Modified from Pederson and Buchardt (Citation1996:). B, stratigraphic column of the upper Ølst Formation and Fur Formation with the approximate horizon yielding DK 807 indicated by a sea turtle silhouette. Modified from Rasmussen et al. (Citation2016:).
The Fur Formation is generally interpreted as representing an offshore oceanic accumulation (Bonde, Citation1979; Pedersen and Surlyk, Citation1983; Rasmussen et al., Citation2016); however, the fine-grained detrital sediments contain a fossil biota that derives from both terrestrial and coastal marine environments (Bonde et al., Citation2008; Rasmussen et al., Citation2016) to suggest a more complex depositional setting. Furthermore, favorable burial conditions and a mild geothermal history have facilitated not only the preservation of faunal components that otherwise are rare in the fossil record, such as snakes (Kristensen et al., Citation2012), insects (Henriksen, Citation1922; Larsson, Citation1975; Lindgren et al., Citation2019) and birds (Lindow and Dyke, Citation2006, Citation2007; Bonde et al., Citation2008; Waterhouse et al., Citation2008; Dyke and Lindow, Citation2009, Bourdon et al., Citation2016), but also allowed the retention of a broad array of soft-tissue structures (e.g., Bonde et al., Citation2008; Rasmussen et al., Citation2016). Some of these extraordinary fossils even contain traces of endogenous biomolecules, including eumelanin pigment (Lindgren et al., Citation2012, Citation2017, Citation2019; Gren et al., Citation2017), heme (a hemoglobin-derived porphyrin) and proteinaceous compounds of presumed keratinous origin (Lindgren et al., Citation2017).
The aim of the present contribution is to formally describe DK 807. In addition, we discuss taxonomic and taphonomic aspects of this unique fossil, as well as a potential predator-prey interaction in the archaic North Sea Basin.
GEOLOGICAL SETTING
The Fur Formation is an approximately 60-meter-thick, tephra-bearing diatomite located in the Limfjord region of northern Jutland, Denmark (Pedersen and Surlyk, Citation1983). The marine deposits are exposed in coastal cliff sections, together with a few inland quarries on the islands of Mors and Fur (). Strata of the Fur Formation are locally known as ‘Mo-clay’, and mined for a variety of reasons, including the manufacturing of absorbent materials (Rasmussen et al., Citation2016).
The Fur Formation is of Ypresian (earliest Eocene) age (i.e., between 56.0–54.6 Ma) based on 39Ar/40Ar dating of interbedded volcanic ash layers (Stokke et al., Citation2020 and references therein). Stratigraphically, the rock unit overlays the Ølst Formation, and is in turn disconformably succeeded by the Røsnæs Formation (Pedersen and Surlyk, Citation1983). The Fur Formation is sub-divided into the lower Knudeklint Member and upper Silstrup Member (Pedersen and Surlyk, Citation1983). Sedimentologically, the depositional succession has been characterized as a clayey marine diatomite with nearly 200 interbedded basaltic volcanic ash layers (Pedersen and Surlyk, Citation1983; Rasmussen et al., Citation2016). The deposits consist mostly (∼45–65%) of diatom frustules (Mitlehner, Citation1996), with input of clay minerals (∼30–45%) and volcanic ash (∼10%) (Pedersen et al., Citation2004). Carbonate concretions are common in some stratigraphic intervals (Dyke and Lindow, Citation2009), including in ash layer +31 in which DK 807 was found ().
Combined sedimentological and fossil evidence suggests that the Limfjord region once was part of a restricted marine basin with water depths ranging from about 100 to 500 m (Rasmussen et al., Citation2016). Local upwelling and periodic volcanic eruptions from the nearby North Atlantic Igneous Province promoted a continuous flow of detrital minerals, dust and diatom frustules some distance out at sea, and these fine-grained detrital particles accumulated below the storm wave-base under mostly poorly oxygenated bottom conditions (Pedersen and Surlyk, Citation1983).
MATERIALS AND METHODS
DK 807 was collected in 2013 from the Ejerslev mine on the Island of Mors () and is currently exhibited at Museum Mors, Mo-clay Museum, in Denmark. Initially, the fossil was prepared out of the host rock using a combination of mechanical tools and citric acid; adhering soft tissues were subsequently exposed with an air scribe. Following these procedures, DK 807 was digitally reproduced (Supplementary 3D Model 1) employing a portable 3D scanner (Artec 3D Space Spider), with data processing utilizing the Artec Studio 14 imaging software package. Additional virtual manipulations were performed in MeshLab. The fossil and comparative materials were photographed using a Nikon D3500 camera with a standard 18–55 mm lens kit equipped with an UV polarizing filter. Close-up images were taken using a +10 macro filter. While the systematics follows those of Joyce (Citation2007) and Joyce et al. (Citation2021), the osteological terminology has primarily been adopted from Zangerl (Citation1969) and Wyneken (Citation2001), with specific information on flipper elements obtained from Sánchez-Villagra et al. (Citation2007).
Institutional Abbreviations: DK, Danekræ, Danish national treasure registration, (formally maintained by NHMD), Denmark; FUM, Museum Salling, Fur Museum, Fur, Denmark; IRSNB, Institut Royal des Sciences Naturelles de Belgique, Brussels, Belgium; MHM, Museum Mors, Mo-clay Museum, Nykøbing Mors, Denmark; NHMD, Natural History Museum of Denmark, Copenhagen, Denmark
SYSTEMATIC PALEONTOLOGY
TESTUDINES Linnaeus, Citation1758
CRYPTODIRA Cope, Citation1868
CHELONIOIDEA Baur, Citation1893
PAN-CHELONIIDAE Joyce et al., Citation2004
PAN-CHELONIIDAE INDET.
DESCRIPTION
General Description
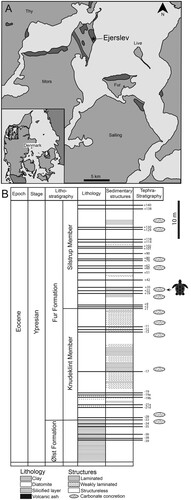
DK 807 comprises the following skeletal elements: (1) right posterior portion of the carapace; (2) right xiphiplastron; (3) pelvic girdle; (4) both hind limbs; (5) sacrum; and (6) a consecutive series consisting of 17 articulated and two isolated caudal vertebrae (). Associated with these remains is dark-colored matter representing remnant scutes, as well as skin webbing in between the phalanges and surrounding the left tibia and fibula (, ). In addition to DK 807, a small number of other fossils are preserved in the concretion housing the turtle (). The most noteworthy of these include three insects and dark-colored matter of unknown origin ().
FIGURE 2. DK-807, Pan-Cheloniidae indet. A, photographic and B, diagrammatic representation of the fossil when viewed from above. Abbreviations: carp, carapace; ca. ver, caudal vertebrae; dig, digit; fem, femur; fib, fibula; ili, ilium; lpp, lateral pubic process; mt, metatarsal; pub, pubis; sac, sacrum; tib, tibia; xip, xiphiplastron.
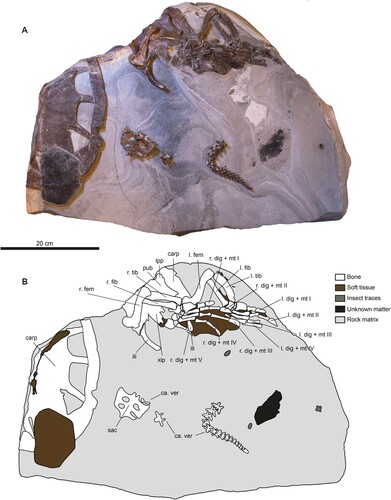
FIGURE 3. DK 807, Pan-Cheloniidae indet., carapace. A, photographic and B, diagrammatic representation of the external surface (hypothetical skeletal element boundaries underneath the scute tissue are denoted by dotted lines). Abbreviations: co, costal; n, neural; per, peripheral; py, pygal; spy, suprapygal.
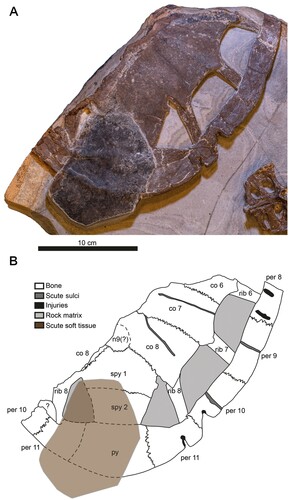
FIGURE 4. Details of the carapace of DK 807, Pan-Cheloniidae indet. A, photograph of a carbonaceous scute. B, 3D rendering of the posterior portion of the carapace. The digitally reconstructed carapace is tilted to enhance features otherwise obscured by the scute. C, potential organic traces on the anterior external surface of the carapace (arrowhead). D, inferred bite traces (arrowheads) on the left side of the bony carapace. E, putative bite marks (arrowheads) on the posterior surface of the bony carapace.

FIGURE 5. DK 807, Pan-Cheloniidae indet., pelvic girdle and hind limbs. A, photographic and B, diagrammatic representation of the ventral and lateral surfaces of the pelvis and limbs. Skeletal elements shaded in dark gray indicate association with the left limb, whereas light gray denote right limb elements. C, xiphiplastron straddling the ischium; note pronounced metischial processes (denoted by white stars). White arrowheads indicate projections from the caudal end of the xiphiplastron. Potential tissue remains between the xiphiplatron and metatarsal 5 are marked by black arrowheads. D, dark-colored tissue traces in the space between the tibia (right side of the image) and the fibula (left side of the image) indicated by black arrowheads. Note large tibial pit (white star). E, overview of flipper webbing. F, close-up of flipper trace between phalanges III and IV, illustrating the dark-colored striations that are present in between the skeletal elements. Black arrowhead denotes folded tissue. Abbreviations: ace, acetabulum; acm, astragalocalcaneum; carp, carapace; d, distal tarsal; fem, femur; fib, fibula; ili, ilium; lpp, lateral pubic process; m, metatarsal; met, metischial process; pub, pubis; tib, tibia; tib pit, tibial pit; xip, xiphiplastron. Phalanges and their associated metatarsals are labeled by roman numerals.
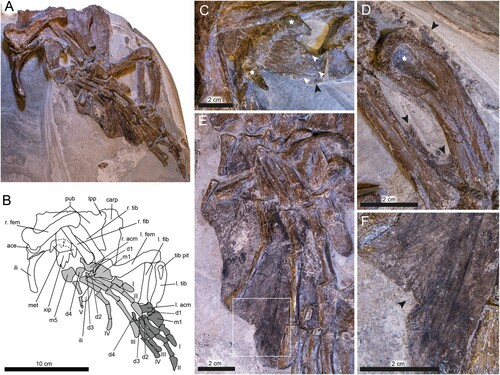
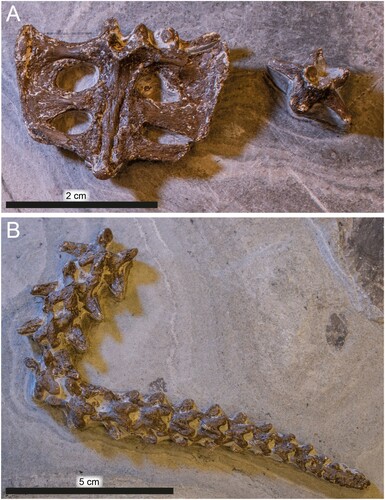
FIGURE 6. DK 807, Pan-Cheloniidae indet., detached elements. A, sacrum and an isolated caudal vertebra. B, articulated string of caudal vertebra.
Notwithstanding minor flattening of the carapace and girdle elements from diagenetic compaction, the individual bones of DK 807 generally are in a pristine condition, although some have experienced minor displacement prior to final burial.
Shell
The shell is represented by an articulated, dorsally exposed segment comprising the right posterior side of the bony carapace (), together with the right xiphiplastron (). In addition, parts of the left side of the carapace are located underneath the pelvic girdle (). The main portion of the carapace measures approximately 30 cm in diameter, with an estimated original length of about 50 cm along its longitudinal mid-line. When intact, the shell likely was cordiform or oval in shape with a tapering caudal end, similar to that of extant cheloniids. There is no evidence of a central keel, the external surface of the bones lacks distinct ornamentations.
The individual elements of the carapace largely remain articulated. The right side of the main section preserves costals 6 to 8, peripherals 8 to 11, neural 9, suprapygals 1 and 2, and one pygal, whereas the less intact left side comprises costal 8 and peripherals 10 to 11 (). In addition, three thoracic ribs are present on the right side of the carapace and one on the left side. These ribs connect costals 6, 7 and 8 with peripherals 8, 9 and 11, respectively; the single rib on the left side joins a fragmentary costal 8 with peripheral 11. All ribs are straight, unlike the condition in most other pan-cheloniids where at least the last rib usually is slightly curved. Fontanelles are prominent, and either sub-triangular (posteriormost one) or sub-rectangular in outline; openings of the latter category are longer than wide. Parts of the caudal termination of the carapace are obscured by a remnant scute (–); however, from a virtual 3D reconstruction (, Supplementary 3D Model 1), it is possible to determine the outline of several underlying bones, including a pygal, suprapygal 2 and left peripheral 11.
The costals are incomplete; however, all three elements have concave lateral margins and additionally do not contact the peripherals (). Costal 6 is about 5.5 cm at its widest point, but is broken both medially and anteriorly, rendering it difficult to assess its original shape. Costal 7 has a sub-rectangular form, and, as preserved, measures 7.2 cm in length. Costal 8 (8.2 × 4.6 cm) also has a sub-rectangular outline and contacts both suprapygal 1 and neural 9. Ribs emerge underneath the posterolateral corners of these elements. Sulci are present and appear to converge medially (). The single costal element on the left side of the carapace is represented only by a fragment.
A single neural, presumably the 9th, is located immediately adjacent to costal 8. Preserved sutures indicate a broadly circular shape.
Whereas peripherals 8 to 10 are relatively long and narrow, peripheral 11 is comparably stout. Peripheral 8 (3.6 × 2.2 cm) is incomplete but presumably originally had a rectangular shape. Peripheral 9 is the longest element of the shell margin (6.8 × 2.9 cm); it has a sub-rectangular outline but with a weak concavity at its center housing a shallow sulcus. The succeeding peripheral 10 is similar in shape, but somewhat shorter (6.1 × 2.5 cm), and with only a very superficial sulcus. The last peripheral (11) is relatively shorter and wider (5.4 × 3.8 cm) than the proceeding elements, and has a broadly rhomboidal shape. Of the two peripherals on the left side of the shell, one (peripheral 10) is incomplete and the other one (peripheral 11) obscured by a displaced scute. An unidentified element (labeled ‘?’ in ) shaped as a flat-topped bulge, occurs adjacent to the suture between peripherals 10 and 11.
Collectively, the suprapygals form a posteriorly tapering structure (). Suprapygal 2 articulates with the pygal, although this junction partially is obscured by the dislocated scute.
The outline of the pygal can only be determined from the virtual 3D-reconstruction (); the element appears to be roughly box-shaped with an evenly rounded and un-notched posterior edge.
Additional carapace material occurs underneath the right portion of the pelvic girdle (). These skeletal remains appear to represent the ventral side of at least two costals (6? and 7?) preserved in partial articulation.
A single (right) xiphiplastron is present in DK 807. Following deposition of the cadaver, this bone was shifted from its original anatomical position and came to rest on top of the ischium, thereby obscuring most of the ischiatic symphysis (). The xiphiplastron is longer than wide, tapers posteriorly, and ends in three finger-like extensions (, denoted by white arrowheads).
Pelvic Girdle
The pelvic girdle is nearly complete and most of the individual bones remain in articulation (, ). However, with respect to the carapace, the girdle has rotated 180°, and thus is accessible in ventral view. The overall width of the pelvis is 12.2 cm and it is 15.6 cm long. All girdle elements are preserved; these include both pubes that are retained in life-position, a pair of ilia, and ischia that are partly obscured by the right femur and xiphiplastron ().
The right ilium (5.6 × 1.0 cm) is narrow and substantially longer than wide, with a moderately medially curved shaft. The left ilium appears to have been separated from the pelvis following compressional flattening, and shifted proximally to a position underneath the right flipper, thus appearing as a narrow shaft of bone that lies perpendicular to the metatarsus of phalanx IV ().
The pubes are wide, broad and thin, and measure about 12.2 cm in width and 4.5 cm in length. In transverse view, these elements are moderately curved ventrolaterally. The medial contact of these bones is mostly obscured by the right limb, thereby preventing access to both the symphyses and fenestrae. Whereas the right lateral pubic process is missing, the left one is partially preserved (). This protrusion is rounded and longer than wide.
The ischia are mostly obscured by the xiphiplastron, although a portion of the left ischium is visible near the acetabulum articulation. Metischial processes straddle the lateral sides of the xiphiplastron, and a faint outline of the ischial elements can be seen forming a symphysis (). The metischial processes are robust, elongate and project ventrally from the ischia to form horn-like structures (, marked by white stars).
Hind Limbs
Both hind limbs are present (, ). Whereas the right paddle is accessible in dorsal view and rests on top of the pelvic girdle, the left one is seen in ventral view posterior to the pelvis. While the right limb is relatively intact, the left one is missing at least one tarsal bone, parts of digits III and IV, as well as digit V. The limb elements are preserved in articulation, with the right femur being partially retained within the acetabulum, and the fibula/tibia emplacement occurring almost in life position. However, some of the other elements, including the pedes, have undergone mild compressional flattening. The elongate pes of the right paddle (i.e., metatarsus and phalanges) have detached from the tibia/fibula complex and shifted laterally. The left limb has slid downwards out of its acetabulum, although the individual bones remain mostly in articulation.
Whereas the right femur is accessible in oblique posterior view, the left one is preserved in anterior aspect (). Both elements are approximately 9 cm long, and equipped with slender, sigmoidal shafts that have a slight laterodistal torque (). The femoral head is oriented almost perpendicular to the shaft and is anteroposteriorly compressed. In the right femur, the major trochanter has been crushed, thereby exposing the inner cancellous bone (). Moreover, the minor trochanter is hidden within the acetabulum. In the right femur, the tibial condyle is incomplete and the fibular condyle gone (). In the left femur, the minor trochanter is incomplete. A shallow V-shaped notch extends ventrally from this protrusion (see Supplementary 3D model); however, the majority of this structure (along with the major trochanter) is obscured by the metatarsals of the right paddle. While the tibial condyle is preserved as a posteriorly projecting ridge, the fibular condyle remains embedded in the sedimentary matrix ().
The taphonomically compressed epipodials are only slightly shorter than the propodials (about 78% of femoral length). The tibiae are long and narrow with an expanded proximal end. Whereas the anterior margin of the shaft is only gently bent, the posterior one shows a steeper inclination. The proximal surface is wide and robust with a broad, teardrop-shaped pit located at its center (). The bone tapers distally and ends in a relatively flat articular surface. The fibulae are columnar elements in which the epiphyses are only slightly expanded and the articular surfaces gently rounded (note, though, that the anterior portion of the distal end of the right fibula has broken off).
The tarsi and metatarsi are dorsoventrally flattened (). The astragalus and calcaneum have fused into a single irregular ossification (astragalocalcaneum). Whereas the first distal tarsus (d1) is gently curved with a discoid outline (), the second (d2) and third distal tarsi (d3) are small, compact and almost spherical. The fourth distal tarsus (d4) is dorsoventrally flattened and equipped with multiple facets. Metatarsus I is broad and rectangular, and metatarsi II–IV are columnar with concave proximal and rather flattened distal ends. The proximal face of metatarsal 5 is also flat; from here, the shaft narrows somewhat and then widens into a fan-like structure ().
The phalangeal formula is 2-3-3-3-3 (digits I–V) (). Both phalanges of digit I and the first two of digit II are short and columnar, with expanded proximal and distal ends (). The distal termination of the first phalanx of digit II is shaped as a shallow concavity (). The proximal ends of the first phalanx of digits I and II also are developed into shallow depressions (), indicating movable articulations. In digits III and IV, the second phalanx is the longest. In digit V, the first phalanx is considerably larger than the second one (). Relative to the condition in extant sea turtles, the ungual phalanx is robust in digits I–IV; however, this element is much reduced in digit V ().
Sacrum
The sacrum is intact but detached from the rest of the specimen (, ). This skeletal unit is broader than long (about 7.0 × 5.6 cm) and accessible in ventral view. The sacrum comprises three fused vertebrae of sub-equal dimensions (all measuring about 1.4 × 0.7 cm). The first two pairs of sacral ribs are broadly expanded distally to form blade-like extensions that protrude laterally from the vertebral centra (). The third sacral rib pair deviate slightly from the two succeeding ones in that the costae are bent slightly forward (). They are also smaller and fused to the second pair of ribs only on the right side. These rib mergings border three oval foramina in addition to an incomplete opening in the left side of the complex ().
Vertebral Column
In addition to the sacrum, the backbone is represented by two isolated vertebrae (, ) and a consecutive section comprising 17 articulated caudals that are visible in dorsal aspect (). In similarity with other pan-cheloniids, all vertebrae are procoelous. Pre- and post-zygapophyses are well-developed and remain mostly in articulation (). Transverse processes are prominent and slightly bent posteriorly, but decrease in size toward the tip of the caudal segment (). The last two caudals are fused into a single ossification.
Soft Tissues
Remnant soft tissues are extensive in DK 807, and preserved as thin, bedding-parallel films of dark matter. A sub-hexagonal scute, measuring about 10.9 × 8.3 cm, covers part of the posterior segment of the bony carapace ( and ). This epidermal appendage is grossly similar in both shape and size to vertebral scutes of living cheloniids, to suggest minor displacement during burial. A second, more incomplete scute is represented by a narrow strip of brownish material that covers parts of costals 6 and 7 (). Based on its location (and assuming no post-depositional dislocation), the dark-colored residue likely represents the fragmented remains of a pleural scute. Additional, dark-colored traces extend from the inferred vertebral scute to an area in the level with suprapygal 1 and costal 8.
Soft-tissues of the hind limbs mainly occur as a dark halo around the pes of the right paddle, with some additional residues surrounding the left flipper bones (, ). The remains on the right limb are confined to spaces in between the metatarsals and phalanges, creating a webbed structure surrounding digits II–V. The preserved material is sheet-like, and distinct from the underlying diatomaceous sedimentary matrix. The film is mostly brownish in color, with patches of glossy black material located at the proximal end of the flipper (between digits III and IV), as well as in the distal portion of the fin between digits IV and V. Dark striae are visible in the film, with the most prominent ones occurring in an area between digits III and IV (). These striations are virtually straight and mostly run parallel to the digits, although some of them bifurcate (). They appear to be comprised of folded sheets of the dark-colored matter that have undergone secondary compression to create two-dimensional ‘silhouettes’ of wrinkles. Some of the preserved soft-tissue matter even appears to have folded onto itself, revealing a small section of the flipper surface that originally faced downwards (). Notably, there is no evidence of scales in this soft-tissue residue. Overall, the outline of the dark film closely resembles the shape of rear paddles of modern sea turtles.
Additional soft-tissue structures comprise: (1) a patchy, glossy light-brown film on the bedding plane surface between phalange I and II of the left limb; (2) patches of a dark film between the tibia and fibula of the left limb (—black arrowheads); (3) amorphous brownish matter surrounding the anterior portion of the tibia (); and (4) dark material in spaces between the posterior part of the xiphiplastron and metatarsal V of the left paddle ().
Puncture Marks and Scratches
Anomalous structures in the form of punctures, furrows and indentations are present on the bony carapace. Of these, two distinct marks occur on the lateral side of the shell (), with two additional ones in the shell margin () and a fifth on top of the last fontanelle (). The anterior lateral notch (measuring 2.3 cm at its widest part) comprises a large triangular gap at the posterior end of peripheral 10, and extends well into peripheral 11. Another puncture mark is located 1.5 cm behind the first one, and it is somewhat smaller (0.5 cm at its widest part), more restricted and with an almost keyhole-like profile in dorsal aspect. In both marginal notches, deep circular depressions (∼4 mm in diameter) can be found inside the medial edges (, indicated by arrowheads on the right). The fontanelle indentation (measuring 0.7 cm in maximum width) is shallow and triangular in outline (, left arrowhead). Additional damage is apparent elsewhere in this specimen, including a 6 mm long ovoid laceration (, bottom arrowhead) and deep linear furrows on peripheral 8 (, top arrowhead).
Comparisons and Remarks
Based on the flattened tarsals, elongate phalanges, paddle-shaped limbs, and pointed caudal termination of the carapace (see Evers and Benson, Citation2019), DK 807 can be confidently assigned to Chelonioidea. Moreover, affiliation with Protostegidae can be excluded because this family went extinct at the end of the Cretaceous (Lehman and Tomlinson, Citation2004; Evers et al., Citation2019). Likewise, attribution to Dermochelyidae is highly unlikely because: (1) dermal ossicles are lacking; (2) the costals are plate-like; and (3) epidermal scutes cover the bony carapace (Wood et al., Citation1996; Albright et al., Citation2003). Consequently, we consider DK 807 to be a pan-cheloniid based on the flattened tarsals, elongate phalanges, paddle-shaped limbs and pointed caudal termination of the carapace.
Previously described hard-shelled sea turtles from the Fur Formation include two articulated skeletons (MHM K1 and MHM K2) assigned to Tasbacka Nessov, Citation1987 by Karl and Madsen (Citation2012), and a reasonably complete carapace of a juvenile Puppigerus Cope, Citation1870 (FUM N 15877; Karl and Madsen, Citation2012). Unfortunately, comparisons with these other Danish specimens are hampered either by ontogenetic differences (MHM-K2 and FUM-N 15877 both represent skeletally immature individuals) or a lack of overlapping skeletal elements.
On a broader geographic scale, a number of marine turtle species roamed the world’s oceans and epicontinental waterways during the Eocene (e.g., Lapparent de Broin, Citation2001; Tong and Hirayama, Citation2008; Tong et al., Citation2012; Grant-Mackie et al., Citation2011; Weems and Brown, Citation2017). Of these, DK 807 shares affinity with either Eochelone, Puppigerus, or Argillochelys based on the following combination of character states: (1) a pronounced metischial process on the ischia; (2) a conspicuous tibial pit; (3) presence of femoral trochanters that appear to be separated by a notch; and (4) a free peripheral between the 7th and 8th rib (Moody, Citation1968; Parham and Pyeson, Citation2010; Lapparent de Broin et al., Citation2018; Evers et al., Citation2019). These turtle genera had an almost cosmopolitan distribution during the Eocene (Grant-Mackie et al., Citation2011), although the majority of described forms lived in what is now Europe, North Africa and North America (Lapparent de Broin, Citation2001; Tong and Hirayama, Citation2008; Tong et al., Citation2012; Weems, Citation2014; Zvonok et al., Citation2019). Whereas fossils of Puppigerus and Argillochelys have been documented in deposits of Ypresian age (Moody, Citation1997), Eochelone thus far has not been reported from strata older than the Lutetian (Moody, Citation1997; Zvonok et al., Citation2019).
Although Puppigerus has been previously described from the Fur Formation (Karl and Madsen, Citation2012), the xiphiplastra of this genus are wide and hence unlike the narrow elements seen in DK 807 (Zvonok et al., Citation2019). In addition, Puppigerus have carapace fontanelles that seemingly closed early during ontogeny (), a condition that contrasts markedly with the conspicuous shell openings seen in the relatively large-sized DK 807. Although distinct fontanelles are considered a feature of immaturity in some turtles (Wyneken, Citation2001; Karl and Madsen, Citation2012; Zvonok et al., Citation2019), the thickness of the costal bones, fused pygal and pelvic elements, as well as estimated total length of the carapace (∼50 cm), all suggest that DK 807 was at least sub-adult when it died (see Wyneken, Citation2001). Moreover, skeletally mature Puppigerus have carapace lengths that typically range between 38 and 40 cm (e.g., specimens IRSNB R 0004, 0078 and 0081; Figure 7), which is considerably smaller than DK 807.
FIGURE 7. Comparison of carapace material from Eocene stem-cheloniids housed in the collections at IRSNB. A, Eochelone brabantica (IRSNB R 0061); B, Puppigerus camperi (IRSNB R 0004); C, Eochelone brabantica (IRSNB R 0339); D, Puppigerus camperi (IRSNB R 0078).
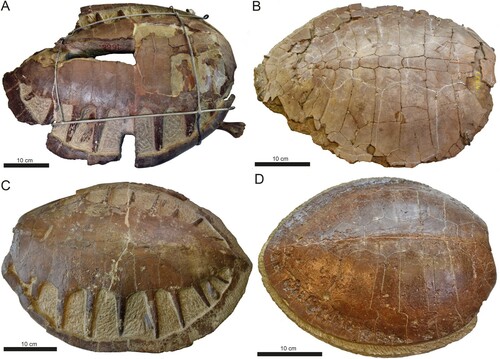
Without access to cranial material, it is not possible to distinguish DK 807 from the other two genera; i.e., Eochelone and Argillochelys, as these share similar postcranial characters (Parham and Pyeson, Citation2010; Weems and Brown, Citation2017; Lapparent de Broin et al., Citation2018). Accordingly, DK 807 is here retained in open nomenclature pending the discovery of more complete skeletal material from the Fur Formation. However, we do note that the estimated original carapace length of DK 807 is greater than that of all previously described presumed adult Argillochelys (see Lapparent de Broin et al., Citation2018), but within the size range (50–73 cm) of specimens attributable to Eochelone (e.g., IRSNB R 0001, 0061, 0339 and 0340; Figure 7). Also, notwithstanding some deformation from burial compaction, the preserved caudal termination of the carapace in DK 807 appears to be more pointed than seen in previously described species of Eochelone, including E. brabantica Dollo, Citation1903 () and E. voltregana Lapparent de Broin, Murelaga, Pérex-García, Farrés, and Altimiras, Citation2018 (see Lapparent de Broin et al., Citation2018:figs. 3, 7).
DISCUSSION
Taphonomy and Soft-Tissue Preservation
The burial conditions contributing to the exceptional preservation of Fur Formation fossils have been addressed in a number of publications (Dyke and Lindow, Citation2009; Lindgren et al., Citation2012, Citation2014, Citation2015, Citation2017, Citation2019; McNamara et al., Citation2013). Generally, fossils come in one of two ways: either as flattened remains in the diatomite or as three-dimensional bodies in the interbedded carbonate concretions (Dyke and Lindow, Citation2009). Regarding the nodules, Pedersen and Buchardt (Citation1996) demonstrated that these are biogenic in origin, with stable carbon ratios consistent with syn-depositional carbonate precipitation mediated by marine bacteria. Elsewhere (Berner, Citation1968), it has further been shown that bacterially induced decay can generate reactions that promote the deposition of calcium salts; these might then be replaced by calcite over relatively short time spans.
We propose that when the body of DK 807 settled on the seafloor, the carcass became partially dismembered as the result of scavenging, rupture of the body wall from the escape of decompositional gases and/or actions of weak benthic currents (as has previously been documented in the Fur Formation; see Pedersen and Surlyk, Citation1983). Partial burial of the carcass in the bottom mud exposed the shell and hind limbs to clay minerals, which could have facilitated fixation of the organics in the integument (Drouin et al., Citation2010; Forchielli et al., Citation2014; Wilson and Butterfield, Citation2014). The decaying carcass then served as nucleation sites for sulfate-reducing anaerobic bacteria which produced the carbonate concretion, thereby eventually encasing the remains (Pederson and Buchardt, Citation1996). The rapid formation of the concretion, along with stagnant, poorly oxygenated bottom waters, protected the cadaver from further decay by inhibiting decomposition and preventing scavenging. Moreover, the concretion sheltered the fossil from compressional effects of overburden pressure during diagenesis. Finally, the low temperature and pressure regimes experienced by the sediments of the Fur Formation resulted in a mild diagenesis (McNamara et al., Citation2013) that enhanced long-term conservation of stabilized components of the soft tissues.
Among the more noticeable aspects of the soft tissues in DK 807 is the absence of apparent scales in the flippers combined with the presence of prominent scutes on the carapace. This is in stark contrast to the condition in modern cheloniids, which have heavily keratinized scales covering most parts of their bodies (Rodríguez et al., Citation2018). It is difficult to determine whether similar scaly patterns originally were present in DK 807, although the striations in the flipper trace suggest that the tissues were soft and pliable at least at the time of fossilization. Scaleless skin occurs in some extant turtles, including soft-shelled members of the family Trionychidae, adult individuals of the leatherback turtle, Dermochelys coriacea, and the pig-nosed turtle, Carettochelys insculpta (Rodríguez et al., Citation2018). The limb integument of these turtles superficially resembles the fossilized ‘skin’ in DK 807, especially the webbed paddles of pig-nosed turtles (Delfino et al., Citation2009: fig. 1).
An alternative explanation to the apparent absence of scales in DK 807 could be that it represents a taphonomic artifact. The black, glossy film seen as patches in the flipper residue () could be traces of these elusive scales, a scenario in which they have decayed to a point beyond confident recognition. In one of these patches, present between digits V and IV, a faint outline of a scale-like structure may be seen (). However, considering the heterogeneous coloration in this area and lack of other similar structures, this feature may instead be the result of uneven rock splitting. Also, it cannot be excluded that this patch does not represent underlying fat or muscle tissues. Regardless, these alternative scenarios are difficult to reconcile with the fact that at least one epidermal scute on the carapace retains structural fidelity (). Further in-depth investigations are required to clarify the nature of the soft-tissue traces in DK 807, and will be the focus of a forthcoming publication.
Bite Marks
Lacerations and punctures associated with spongy bone tissue growth in the carapace () most likely were caused by a traumatic injury that the sea turtle survived long enough to partially heal. Punctures within these indentations are consistent with an incision caused by the bite of a large predator that latched onto DK 807 and sheared its bone in a posterior direction as the turtle tried to evade its attacker, or conversely, as this animal pulled back in an attempt to manipulate its prey.
Narrowing down the identity of the predator that injured DK 807 is difficult. The wounds are inconsistent with those inflicted by sharks, as such bite marks typically occur as shallow scratches arranged in a sub-parallel fashion, and occasionally include traces of serrations (see Schwimmer et al., Citation1997; Shimada and Hooks, Citation2004; Milàn et al., Citation2011; Myrvold et al., Citation2018). A crocodyliform, on the other hand, is a more plausible candidate because of the distinctive ovoid to semi-circular marks (compare Milàn et al., Citation2010; Drumheller and Brochu, Citation2014). Additionally, these scars are roughly similar in size and shape, suggesting that they were inflicted by an animal with a relatively homodont tooth arrangement, such as a crocodilian (Drumheller and Brochu, Citation2014).
Chelonivory by crocodylomorphs is commonly inferred for damage on turtle fossils (e.g., Ericson, Citation1984; Schwimmer, Citation2010; Milàn et al., Citation2011; Rothschild et al., Citation2013; Myrvold et al., Citation2018; Cadena et al., Citation2020), with some of these individuals being reported to have survived the attack with missing sections of the shell and healed callouses (de Valais et al., Citation2020). Such behaviors also are supported by frequent observations of antagonistic interactions between these two reptile groups in modern habitats (Hirth et al., Citation1993; Sutherland and Sutherland, Citation2003; Milàn et al., Citation2010; Whiting and Whiting, Citation2011). However, it should be pointed out that although an interaction between DK 807 and an unknown crocodyliform is plausible, additional lines of evidence (e.g., embedded teeth) are needed for a confident identification of the aggressor. Nevertheless, the inferred predator-prey interaction observed in DK 807 adds to the growing record of predatory attacks on fossil sea turtles in what is now Denmark (see, e.g., Milàn et al., Citation2011; Myrvold et al., Citation2018).
Supplemental Material
Download Zip (52.5 MB)ACKNOWLEDGMENTS
We would like to thank F. Osbæck for taking pictures of DK 807 during the preparation of the fossil. We would also like to express our gratitude to D. Lawver for providing information on turtle anatomy, M. Mostadius for giving us access to testudines housed in the zoological collections at Lund Biological Museum, J.C. Sagebiel for access to pan-cheloniids in the collections at the University of Texas at Austin’s Vertebrate Paleontology Laboratory, B.E.K. Lindow for information on the Danish pan-cheloniid collection at NHMD, B.P. Schultz and R.L. Sylvestersen for access to the fossil turtle material at FUM, and last but not least, A. Folie and C. Cousin for access to the pan-cheloniid collection at IRSNB. We are also grateful for the assistance from G. Bianco at the 3D Laboratory, Department of Biology, Lund University. The authors are thankful to editor T. Lyson for the handling of the manuscript, and reviewers E. Cadena, E. Vlachos, and S. Evers for their constructive comments that greatly improved the quality of this paper. Financial support was provided through a Grant for Distinguished Young Researchers to J.L. (Swedish Research Council, award No. 642-2014-3773).
LITERATURE CITED
- Albright, L. B., III, M. O. Woodburne, J. A. Case, and A. S. Chaney. 2003. A leatherback sea turtle from the Eocene of Antarctica: implications for antiquity of gigantothermy in Dermochelyidae. Journal of Vertebrate Paleontology 23:945–949.
- Baur, G. 1893. Notes on the classification of the Cryptodira. American Naturalist 27:672–675.
- Berner, R.A. 1968. Calcium carbonate concretions formed by the decomposition of organic matter. Science 159:195–197.
- Bonde, N. 1979. Palaeoenvironment in the ‘North Sea’ as indicated by the fish bearing Mo-Clay deposit (Paleocene/Eocene), Denmark. Mededelingen van de werkgroep voor tertiaire en kwartaire geologie 16:3–16.
- Bonde, N., S. Andersen., N. Hald, and S. L. Jakobsen. 2008. Danekræ- Danmarks bedste fossiler. Gyldendal Fakta, Copenhagen, Denmark, 72–159pp.
- Bourdon, E., A. V. Krisoffersen, and N. Bonde. 2016. A roller–like bird (Coracii) from the Early Eocene of Denmark. Scientific Reports 6:34050.
- Cadena, E. A., and J. F. Parham. 2015. Oldest known marine turtle? A new protostegid from the lower Cretaceous of Colombia. PaleoBios 32:1–42.
- Cadena, E. A., T. M. Scheyer, J. D. Carrillo-Briceño, R. Sánchez, A. Vanegas, M. Pardo, D.M. Hansen, and M.R. Sánchez-Villagra. 2020. The anatomy, paleobiology, and evolutionary relationship of the largest extinct side-necked turtle. Science Advances 6:eaay4593.
- Cope, E. D. 1868. On the origin of genera. Proceedings of the Academy of the Natural Sciences of Philadelphia 20:242–300.
- Cope, E. D. 1870. Synopsis of the extinct Batrachia, Reptilia and Aves of North America. Part II. Transactions of the American Philosophical Society, New Series 14:105–235.
- de Valais, S., I. Díaz-Martínez, P. Citton, I. Maniel, and M. de la Fuente. 2020. A predation attempt in a late Cretaceous pleurodire turtle from Patagonia. Cretaceous Research 107:104290.
- Delfino, M., U. Fritz, and M. R. Sánchez-Villagra. 2009. Evolutionary and developmental aspects of phalangeal formula variation in pig-nose and soft-shelled turtles (Carettochelyidae and Trionychidae. Organisms Diversity and Evolution 10:69–79
- Dollo, L. 1903. Eochelone brabantica, tortue marine nouvelle du Bruxellien (Eocène moyen) de la Belgique et l’évolution des chéloniens marins. Bulletins de l’Académie Royale des Sciences de Belgique 8:792–801.
- Drouin, S., M. Boussafir, J. Robert, P. Alberic., A. Durand. 2010. Carboxylic acid sorption on synthetic clays in sea water: In vitro experiments and implications for organo-clay behaviour under marine conditions. Organic Geochemistry 41:192–199.
- Drumheller, S. K., and C. A. Brochu. 2014. A diagnosis of Alligator mississippiensis bite marks with comparisons to existing crocodylian datasets. Ichons 21:131–146.
- Dyke, G., and B. Lindow. 2009. Taphonomy and abundance of birds from the lower Eocene Fur Formation of Denmark. Geological Journal 44:365–373.
- Ericson, B. R., 1984, Chelonivorous habits of the Paleocene crocodile Leidysuchus formidabilis: Scientific Publications of the Science Museum of Minnesota, New Series 5:1–9.
- Evers, S. W., and R. B. J. Benson. 2019. A new phylogenetic hypothesis of turtles with implications for the timing and number of evolutionary transitions to marine lifestyles in the group. Palaeontology 62:99–134.
- Evers, S.W., P. M. Barrett, R. B. J. Benson. 2019. Anatomy of Rhinochelys pulchriceps (Protostegidae) and marine adaptation during the early evolution of chelonids. Peer J 7:e6811.
- Fielding, S., D. M. Martill, and D. Naish. 2005. Solnhofen-style soft-tissue preservation in a new species of turtle from the Crato Formation (early Cretaceous, Aptian) of north-east Brazil. Palaeontology 48:1301–1310.
- Forchielli, A., M. Steiner, J. Kasbohm, S. Hu and H. Keupp. 2014. Taphonomic traits of clay-hosted early Cambrian Burgess Shale-type fossil Lagerstätten in South China. Palaeogeography, Palaeoclimatology, Palaeoecology 398:59–85.
- Gentry, A.D., J.A. Ebersole, and C.R. Kiernan. 2019. Asmodochelys parhami, a new fossil marine turtle from the Campanian Demopolis Chalk and the stratigraphic congruence of competing marine turtle phylogenies. Royal Society Open Science 6:191950
- Grant-Mackie, J. A., J. Hill, and B. J. Gill. 2011. Two Eocene chelonioid turtles from Northland, New Zealand. New Zealand Journal of Geology and Geophysics 54:181–194.
- Gren, J. A., P. Sjövall, M. E. Eriksson, R. L. Sylvestersen, F. Marone, K. G. V. Sigfridsson Clauss, G. J. Taylor, S. Carlson, P. Uvdal, and J. Lindgren. 2017. Molecular and microstructural inventory of an isolated fossil bird feather from the Eocene Fur Formation of Denmark. Palaeontology 60:73–90.
- Henriksen, K. L. 1922. Eocene insects from Denmark. Danmarks Geologiske Undersøgelse 2:1–36.
- Hirth, H. F., J. Kasu, and T. Mala. 1993. Observations on a leatherback turtle Dermochelys coriacea nesting population near Piguwa, Papua New Guinea. Biological Conservation 65:77–82.
- Joyce W. G. 2007. Phylogenetic relationships of Mesozoic turtles. Bulletin of the Peabody Museum of Natural History 4:3–102.
- Joyce W. G., J. F. Parham, and J. A. Gauthier. 2004. Developing a protocol for the conversion of rank-based taxon names to phylogenetically defined clade names, as exemplified by turtles. Journal of Paleontology 78:989–1013.
- Joyce, W. G., J. Anquetin, E. Cadena, J. Claude, I. G. Danilov, S. W. Evers, G. S. Ferreira, A. D. Gentry, G. L. Georgalis, T. R. Lyson, A. Pérez-García, M. Rabi, J. Sterli. N. S. Vitek, and J. F. Parham. 2021. A nomenclature for fossil and living turtles using phylogenetically defined clade names. Swiss Journal of Palaeontology 140:5.
- Karl, H., and H. Madsen. 2012. Tasbacka danica n. sp., a new Eocene marine turtle of Denmark (Testudines: Chelonioidea). Studia Palaeocheloniologica 9:193–204.
- Kristensen, H. V., G. Cuny, A. R. Rasmussen, and H. Madsen. 2012. Earliest record of the fossil snake Palaeophis from the Paleocene/Eocene boundary in Denmark. Bulletin de la Société Géologique de France 183:623–627.
- Lapparent de Broin, F. de. 2001. The European turtle fauna from the Triassic to the Present. Dumerilia 4:155–217.
- Lapparent de Broin, F. de., X. Murelaga, A. Pérex-García, F. Farrés, and J. Altimiras. 2018. The turtles from the upper Eocene, Osona County (Ebro Basin, Catalonia, Spain): new material and its faunistic and environmental context. Fossil Record 21:237–284.
- Larsson, S. G. 1975. Palaeobiology and mode of burial of the insects of the lower Eocene Mo-clay of Denmark. Bulletin of the Geological Society of Denmark 24:193–209.
- Lehman, T. M., and S. L. Tomlinson. 2004. Terlinguachelys fischbecki, a new genus and species of sea turtle (Chelonioidea: Protostegidae) from the upper Cretaceous of Texas. Journal of Paleontology 78:1163–1178.
- Li, Q., J. A. Clarke, K. Gao, C. Zhou, Q. Meng, D. Li, L. D’Alba, and M. D. Shawkey. 2014. Melanosome evolution indicates a key physiological shift within feathered dinosaur. Nature 507:350–353.
- Lindgren, J., P. Uvdal, P. Sjövall, D. E. Nilsson, A. Engdahl, B. Schultz, and V. Thiel. 2012. Molecular preservation of the pigment melanin in fossil melanosomes. Nature Communications 3:824.
- Lindgren, J., P. Sjövall, R. M. Carney, P. Uvdal, J. A. Gren, G. Dyke, B. Pagh Schultz, M. D. Schwkey, K. R. Barnes., and M. J. Polcyn. 2014. Skin pigmentation provides evidence of convergent melanism in extinct marine reptiles. Nature 506:484–488.
- Lindgren, J., A. Moyer, M. H. Schweitzer, P. Sjövall, P. Uvdal, D. E. Nilsson, J. Heimdal, A. Engdahl, J. A. Gren, B.P. Schultz, and B. P. Kear. 2015. Interpreting melanin-based coloration through deep time: a critical review. Proceedings to the Royal Society B. 282:20150614.
- Lindgren, J., T. Kuriyama, H. Madsen, P. Sjövall, W. Zheng, P. Uvdal, A. Engdahl, A. E. Moyer, J. A. Gren, N. Kamezaki, S. Ueno, and M. H. Schweitzer. 2017. Biochemistry and adaptive coloration of an exceptionally preserved juvenile fossil sea turtle. Scientific Reports 7:13324.
- Lindgren, J., D. Nilsson, P. Sjövall, M. Jarenmark, S. Ito, K. Wakamatsu, B. P. Kear, B. P. Schultz, R. L. Sylvestersen, H. Madsen, J. R. LaFountain Jr., C. Alwmark, M. E. Eriksson, S. A. Hall, P. Lindgren, I. Rodríguez-Meizoso, and P. Ahlberg. 2019. Fossil insect eyes shine light on trilobite optics and the arthropod pigment screen. Nature 573:122–125.
- Linnaeus, C. 1758. Systema Naturae. Tenth edition, vol. 1. Stockholm, 824 pp.
- Lindow, B. E. K., and G. J. Dyke. 2006. Bird evolution in the Eocene: Climate change in Europe and a Danish fossil fauna. Biological Reviews 81:483–499.
- Lindow, B. E. K., and G. J. Dyke. 2007. A small galliform bird from the lower Eocene Fur Formation, north-western Denmark. Bulletin of the Geological Survey of Denmark 55:59–63.
- McNamara, M. E., D. E. G. Briggs, P. J. Orr, D. J. Field and Z. Wang. 2013. Experimental maturation of feathers: implications for reconstructions of fossil feather colour. Biology Letters 9:20130184.
- Milàn, J., J. Kofoed, and R. G. Bromley. 2010. Crocodylian-chelonian carnivory: bite traces of dwarf caiman, Paleosuchus palpebrosus, in red-eared slider, Trachemys scripta, carapaces. New Mexico Museum of Natural History and Science Bulletin 51:195–200.
- Milàn, J., B. E. K. Lindow, and B. W. Lauridsen. 2011. Bite traces in a turtle carapace fragment from the middle Danian (Lower Paleocene) bryozoan limestone, Faxe, Denmark. Bulletin of the Geological Society of Denmark 59:61–67.
- Mitlehner, A. G. 1996. Palaeoenvironments in the North Sea Basin around the Paleocene-Eocene boundary: evidence from diatoms and other siliceous microfossils; pp. 255–273 in R.W. O’B. Knox, R.M. Corfield, and R.E. Dunay eds., Correlation of the early Paleogene in Northwest Europe. Geological Society of London, Special Publication 101.
- Moody, R. T. J. 1968. A turtle, Eochelys crassicostata (Owen), from the London Clay of the Isle of Sheppey. Proceedings of the Geologists’ Association 79:129–140.
- Moody, R. T. J. 1997. The Paleogeography of Marine and Coastal Turtles of the North Atlantic and Trans-Saharan Regions; pp. 259–278 in J.M. Callaway and E.L. Nicholls, (ed.), Ancient Marine Reptiles. Academic Press, San Diego, California, USA.
- Myrvold, K. S., J. Milàn, and J. A. Rasmussen. 2018. Two new find remains from the Danian and Selandian (Paleocene) deposits of Denmark with evidence of predation by crocodilians and sharks. Bulletin of the Geological Society of Denmark 66:211–218.
- Nessov, L. A. 1987. The Paleogene sea turtles of southern Kazakhstan and the phylogenetic relationships between the Toxochelyidae and the Cheloniidae. Journal of Paleontology 4:76–87.
- Nielsen, E. 1959. Eocene turtles from Denmark. Meddelelser fra Dansk Geologisk Forening 14:96–115.
- Parham, J. F., and N. D. Pyenson. 2010. New sea turtle from the Miocene of Peru and the iterative evolution of feeding ecomorphologies since the Cretaceous. Journal of Paleontology 84:231–247.
- Pedersen, G. K., and B. Buchardt. 1996. The calcareous concretions (cementastan) in the Fur Formation (Paleogene, Denmark): isotopic evidence of early diagenetic growth. Bulletin of the Geological Society of Denmark 43:78–86.
- Pedersen, G. K., and F. Surlyk. 1983. The Fur Formation, a late Paleocene ash-bearing diatomite from northern Denmark. Bulletin of the Geological Society of Denmark 32:43–65.
- Pedersen, G. K., S. A. S. Pedersen, J. Stefensen, and C. S. Pedersen. 2004. Clay content of a clayey diatomite, the early Eocene Fur Formation, Denmark. Bulletin of the Geological Society of Denmark 51:153–174.
- Rasmussen, J. A., H. Madsen, B. P. Schultz, R. L. Sylvesternsen, and N. Bonde. 2016. The lowermost Eocene deposits and biota of the western Limjord region, Denmark- Field Trip Guidebook. 2nd International Mo-clay Meeting, 2–4 November 2016, at Museum, Skive and Fossil and Mo-clay Museum, Museum Mors, Nykøbing Mors.
- Rodríguez, C. E., A. M. H. Duque, J. Steinberg, D. B. Woodburn. 2018. Chelonia; pp.825–854 in K.A. Terio, D. McAloose, and J. St. Leger (eds.), Pathology of Wildlife and Zoo Animals. Elsevier, London, United Kingdom.
- Rothschild, B. M., H. P. Schultze, and R. Pellegrini. 2013. Osseous and other hard tissue pathologies in turtles and abnormalities of mineral deposition; pp.501–534 in D.B. Brinkman (ed.), Morphology and Evolution of turtles. Springer, Dordrecht, United Kingdom.
- Sánchez-Villagra, M. R., C. Mitgutsch, H. Nagashima, and S. Kuratani. 2007. Autopodial development in the sea turtles Chelonia mydas and Caretta caretta. Zoological Science 24:257–263.
- Schwimmer, D. R. 2010. Bite marks of the giant crocodilian Deinosuchus on Late Cretaceous (Campanian) bones. New Mexico Museum of Natural History and Science Bulletin 51:183–190.
- Schwimmer, D. R., J. D. Stewart, and G. D. William. 1997. Scavenging in sharks of the genus Squalicorax in the late Cretaceous of North America. PALAIOS 12(1):71–83.
- Shimada, K., and Hooks, G. E. 2004. Shark-bitten protostegid turtles from the upper Cretaceous Mooreville Chalk, Alabama. Journal of Paleontology 78:205–210.
- Stokke, E. W., E. J. Liu, and M. T. Jones. 2020. Evidence of explosive hydromagmatic eruptions during emplacement of the North Atlantic Igneous Province. Volcanica 3(2):227–250
- Sutherland, R. W., and E. G. Sutherland. 2003. Status of the flatback sea turtle (Natator depressus) rookery on Crab Island, Australia, with notes on predation by crocodiles. Chelonian Conservation and Biology 4:612–619.
- Tong, H., and R. Hirayama. 2008. A new species of Argillochelys (Testudines: Cryptodira: Cheloniidae) from the Ouled Abdoun phosphate basin, Morocco. Bulletin de la Société Géologique de France 179:623–630.
- Tong, H., R. Hirayama, and J. Tabouelle. 2012. Puppigerus camperi (Testudines: Cryptodira: Cheloniidae) from the Ypresian (early Eocene) of Ouled Abdoun basin, Morocco. Bulletin de la Société Géologique de France 183:635–640.
- Tong H., R. Hirayama, E. Makhoul, and F. Escuillié. 2006. Rhinochelys (Chelonioidea: Protostegidae) from the late Cretaceous (Cenomanian) of Nammoura, Lebanon. Atti della Socitá italiana di Scienze naturali e del Museo civico Storia naturale in Milano 147:113–138
- Waterhouse, D. M., B. E. Lindow, N. V. Zelenkov, and G. J. Dyke. 2008. Two new parrots (Psittaciformes) from the lower Eocene Fur Formation of Denmark. Paleontology 51:573–582.
- Weems, R. E. 2014. Paleogene chelonians from Maryland and Virginia. PaleoBios 31:1–32.
- Weems, R. E., and K. M. Brown. 2017. More-complete remains of Procolpochelys charlestonensis (Oligocene, South Carolina), an occurrence of Euclastes (upper Eocene, South Carolina), and their bearing on Cenozoic pancheloniid sea turtle distribution and phylogeny. Journal of Paleontology 91:1228–1243.
- Whiting, S. D., and A. U. Whiting. 2011. Predation by the saltwater crocodile (Crocodylus porosus) on sea turtle adults, eggs, and hatchlings. Chelonian Conservation and Biology 10:198–205.
- Wilson, L. A., and N. J. Butterfield. 2014. Sediment effects on the preservation of Burgess Shale-type compression fossils. PALAIOS 29:145–153.
- Wood, R. C., J. Gove, E. S. Gaffney, and K. E Maley. 1996. Evolution and phylogeny of leatherback turtles (Dermochelyidae), with descriptions of new fossil taxa. Chelonian Conservation and Biology 2:266–286.
- Wyneken, J. 2001. The anatomy of sea turtles. U.S. Department of Commerce NOAA Technical Memorandum NMFS-SEFSC 470:1–172.
- Zangerl, R. 1969. The turtle shell; pp.311–339 in C. Gans, A. d’A.Bellairs, and T. S. Parsons, (eds.) Biology of the Reptilia. Volume 1, Morphology A. Academic Press, New York, New York.
- Zangerl, R. 1980. Patterns of phylogenetic differentiation in the toxochyelyid and cheloniid sea turtles. American Zoologist 20:585–596.
- Zvonok, E. A., E. V. Syromyatnikova, I.G. Danilov, and A.F. Bannikov. 2019. A sea turtle (Cheloniidae) from the middle Eocene of North Caucasus. Paleontological Journal 53:530–539.
