ABSTRACT
Extant rhinoceroses are represented only by five species and are characterized by the presence of a nasal horn. In the past, they were much more diverse, with one of the best-known groups being the aceratheriines, i.e., hornless rhinoceroses. Chilotheres are a group of hornless rhinos that inhabited Eurasia during the Late Miocene. Their westernmost geographic range reached Eastern Europe, where overall eight species have been erected. Four of these were described based on material from the Upper Miocene of Samos Island (Greece), two of which are not considered valid anymore. Unfortunately, the type skulls of all four species are lost and there are several issues concerning their taxonomy. Therefore, we herein designate two skulls housed in historical collections from Samos as neotypes for the first two species, Chilotherium schlosseri (Weber, 1905) and Eochilotherium samium (Weber, 1905), and provide detailed comparisons for the separation of the species from each other and from any other chilotheres. Our results prove that the two species are valid and justify their separation on a generic level. Chilotherium schlosseri seems to be more closely affiliated with the other European Chilotherium species, whereas E. samium is more similar to the Chinese ‘Chilotherium’ wimani and ‘Chilotherium’ primigenium, based on their more plesiomorphic characters.
INTRODUCTION
Rhinoceroses are among the last remnants of megafauna, comprising only five species today. In the past they were much more common, representing some of the most typical faunal elements in vertebrate assemblages during the Neogene and Quaternary in the Old World (Heissig, Citation1996, Citation1999). During this time diverse rhinoceros assemblages existed, with taxa that belonged to different groups, including the group of todays horned rhinos, the extinct elasmotheriines and the hornless rhinos (e.g., Kampouridis et al., Citation2022a; Pandolfi, Citation2016). The Late Miocene of Eurasia is an especially interesting time period for fossil rhinos, with several species being able to coexist in the same locality (Antoine, Citationin press; Antoine & Saraç, Citation2005; Deng, Citation2006b; Fortelius et al., Citation2003; Heissig, Citation1999; Pandolfi, Citation2016). The Balkan–Iranian province (Bonis et al., Citation1992; Kampouridis et al., Citation2023; Spassov et al., Citation2019) is one of the best-studied regions with world-renowned Upper Miocene fossil localities such as Pikermi (Gaudry, Citation1862; Roussiakis et al., Citation2019; Wagner, Citation1848) and Samos (Kostopoulos et al., Citation2003; Koufos et al., Citation2011; Osborn, Citation1899; Solounias et al., Citation2010) in Greece, Hadjidimovo (Hristova et al., Citation2003; Spassov, Citation2002; Spassov & Geraads, Citation2004) in Bulgaria, Karaslari (Garevski, Citation1974; Schlosser, Citation1921; Spassov et al., Citation2018) in North Macedonia, and Maragheh (Ataabadi et al., Citation2013; Bernor, Citation1986; Hullot et al., Citation2022; Mecquenem, Citation1908; Pohlig, Citation1886) in Iran. Despite the abundance of material that has been excavated for over a century, many issues continue to exist regarding the rhinos. Samos has been known to yield vertebrate fossils for over a century (Forsyth-Major, Citation1888; Koufos, Citation2009; Solounias & Ring, Citation2007) and is of particular interest when fossil rhinos are concerned. Over the years, the existence of six species has been proposed (Andree, Citation1921; Weber, Citation1904, Citation1905). Most prominently, four chilothere species (originally referred to the genus Aceratherium Kaup, Citation1832) have been erected based on material from Samos (Andree, Citation1921; Weber, Citation1905). Weber (Citation1905) erected Aceratherium schlosseri Weber, Citation1905, and Aceratherium samium Weber, Citation1905, based on a rich fossil collection, which, however, show a very worn-down dentition and Andree (Citation1921) erected Aceratherium wegneri Andree, Citation1921, and Aceratherium angustifrons Andree, Citation1921, based on further complete cranial material. All of these were later (Heissig, Citation1975; Ringström, Citation1924) considered to belong to Chilotherium Ringström, Citation1924. Unfortunately, the type material of all four species has been lost and only the associated type mandible of Chilotherium wegneri is preserved in the collection of GPMM (Kampouridis et al., Citation2022b). This adds to the confusion that concerns this group with different synonymies having been proposed (e.g., Deng, Citation2006a; Giaourtsakis, Citation2003; Heissig, Citation1975; Kampouridis et al., Citation2022b).
The taxonomic and phylogenetic issues regarding the chilotheres became much more complicated, due to the description of four additional species assignable to the genus Chilotherium in Eastern Europe alone (; Geraads & Koufos, Citation1990; Korotkevich, Citation1958; Niezabitowski, Citation1912; Pavlow, Citation1913) and many more in East Asia (e.g., Deng, Citation2006a; Qiu et al., Citation1987; Ringström, Citation1924; Sun et al., Citation2018). The first European chilotheres are the herein revised species C. schlosseri and E. samium from Upper Miocene deposits of Samos in Greece (Weber, Citation1905), ranging from late early Turolian (MN 11) to the earliest late Turolian (MN 13), based on recent biochronological and magnetostratigraphic data (Kostopoulos et al., Citation2003; Koufos et al., Citation2009, Citation2011). Later, Niezabitowski (Citation1912, Citation1913) described a fragmentary skull from Odessa in Ukraine, for which he established the species Teleoceras ponticus Niezabitowski, Citation1912. This species was later extensively reviewed and synonymized with C. schlosseri by Kiernik (Citation1913), who suggested a Maeotian age, based on the sediment matrix and associated invertebrate remains. Pavlow (Citation1913) described a large amount of rhino material from the Upper Miocene deposits of Grebeniki in Ukraine, which is considered to be of early Maeotian age (Vangengeim & Tesakov, Citation2013). This material was attributed to the new species Aceratherium kowalevskii Pavlow, Citation1913, which was subsequently placed in the genus Chilotherium (Ringström, Citation1924). Almost half a century later, another species, Chilotherium sarmaticum Korotkevich, Citation1958, was erected based on ample material from Berislav, a late Sarmatian locality in Ukraine (Vangengeim & Tesakov, Citation2013). More recently, the species Aceratherium kiliasi Geraads & Koufos, Citation1990, was described based on material from the Vallesian locality Pentalophos-1 in Greece (Geraads & Koufos, Citation1990). However, the material was a mixed sample of fossils that belong to Acerorhinus neleus Athanassiou et al., Citation2014, and a chilothere (Fortelius et al., Citation2003) that is herein synonymized with E. samium.
TABLE 1. Updated list of the European chilotheres species (modified after Kampouridis et al., Citation2022b).
Overall, the European representatives of chilotheres include a variety of hornless rhino species that cover a stratigraphic range from the Vallesian to the late Turolian. Their inclusion in the genus Chilotherium was mainly based on the separated parietal crests, the wide mandibular symphysis with the large, flattened tusk-like lower incisors, the complicated upper tooth morphology, and their short and robust appendicular skeleton. Some of these features also occur in E. samium, which, however, also exhibits more plesiomorphic features that would sustain a generic separation.
The aim of the present study is to investigate the preserved aceratheriine fossils from the Upper Miocene of Samos housed in different collections throughout Europe and address the taxonomic issues revolving around this material and the other chilotheres from Eastern Europe, by assigning neotypes to the two valid aceratheriines from Samos. This revision allows us to re-establish the classical two species Chilotherium schlosseri and Eochilotherium samium and place them in separate genera, thus shedding light on some of the issues concerning the chilotheres.
Institutional Abbreviations—AMPG, Athens Museum of Palaeontology and Geology of the National and Kapodistrian University of Athens, Athens, Greece; BSPG, Bayerische Staatssammlung für Paläontologie und Geologie, Munich, Germany; GMM, Geomuseum of the University of Münster, Münster, Germany; GPIH, Geologisch-Paläontologisches Institut der Universität Hamburg, Hamburg, Germany; GPIT, Geologisch-Paläontologisches Institut der Universität Tübingen, Tübingen, Germany; HLMD, Hessisches Landesmuseum Darmstadt, Darmstadt, Germany; MNHN, Muséum national d’Histoire naturelle, Paris, France; NHMW, Naturhistorisches Museum, Vienna, Austria; NMB, Naturhistorisches Museum Basel, Basel, Switzerland.
GEOLOGICAL SETTING
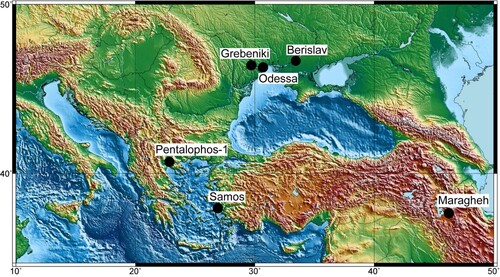
The island of Samos is situated in the easternmost part of the Aegean Sea in Greece, close to Asia Minor () and is one of the richest Upper Miocene localities in the Eastern Mediterranean. All material studied herein comes from these fossiliferous Upper Miocene deposits (Drevermann, Citation1930; Gürich, Citation1911; Lehmann, Citation1984). Recent studies elucidated the stratigraphic context of these deposits (Kostopoulos et al., Citation2003, Citation2009; Koufos et al., Citation2009; Solounias & Mayor, Citation2004). The Neogene sediments of Samos Island are deposited on metamorphic and non-metamorphic rocks of Middle Triassic to Late Jurassic age, associated with the alpine orogenesis. The fossil vertebrate sites are all located in the Mytilinii Basin, in the eastern part of the island. The Neogene stratigraphy of Mytilinii Basin has been studied by many geologists in the past, mostly for the sake of the vertebrate fossils found there (see Kostopoulos et al., Citation2009). We follow the most recent study of Kostopoulos et al. (Citation2009), who divided the Neogene sediments into five formations, the Basal Formation, the Mayradzei Formation, the Hora Formation, the Mytilinii Formation, and the Kokkarion Formation. The Mytilinii Formation, which comprises all fossiliferous layers, mainly constitutes debris-flows in complex with ephemeral lake and overflow deposits.
FIGURE 1. Geographic map indicating the type localities of the Chilotheriina species found in the Eastern Mediterranean: Samos (Aceratherium schlosseri Weber, Citation1905, Aceratherium samium Weber, Citation1905, Aceratherium wegneri Andree, Citation1921, and Aceratherium angustifrons Andree, Citation1921) and Pentalophos-1 (Aceratherium kiliasi Geraads and Koufos Citation1990) in Greece; Odessa (Teleoceras ponticus Niezabitowski, Citation1912), Grebeniki (Aceratherium kowalevskii Pavlow, Citation1913), and Berislav (Chilotherium sarmaticum Korotkevitch, Citation1958) in Ukraine; and Maragheh (Rhinoceros persiae Pohlig, Citation1886) in Iran. The map was made with GMT6 (Wessel et al. Citation2019).
The Mytilinii Formation has been divided into four members. At the bottom are the Old Mill Beds, overlain by the Gravel Beds, both of which consist of green to brown silty sands in alternation with sandy tuffs and conglomerates. These are overlain by the White Beds member, which consists mainly of white marls and marly-sandy limestones. These lower members include only very few fossiliferous localities. The Main Bone Beds member consists primarily of brownish to reddish silty sands, but includes also white tuffites, yellow to brownish conglomerates and tuffaceous sandstones. At the top of the Mytilinii Formation characteristic marker tuffs are found that comprise red tuffaceous sandy silts with intercalations of massive tuffs.
The Main Bone Beds member includes the vast majority of all vertebrate fossil sites discovered on the island of Samos and the material studied herein probably comes from these deposits. Most collectors did not specify the exact location or stratigraphic context of the excavated material. Thus, stratigraphic correlations between the different collections of fossil material from Samos, including the material studied herein, is not possible (see also Kostopoulos et al., Citation2009). The only knowledge on the stratigraphic context of the two chilothere species is that provided by Weber (Citation1905), who was not in the field himself and only stated that they come from different horizons, based on the different sediment adhering to the type material. During the most recent excavations on Samos (Koufos, Citation2009), no chilothere material was found (Giaourtsakis, Citation2009). Koufos et al. (Citation2009) tried to assess the potential position of the two species in the stratigraphic column of Samos, based on the associated fauna for C. schlosseri, that is well-known from the historical collections of Samos and according to the suggested stratigraphic distribution by previous authors that was based on its possible existence in other localities for E. samium (Heissig, Citation1975, Citation1996). It is likely that the two species do not co-occur in the same strata and that E. samium is found in the stratigraphically older layers, whereas C. schlosseri is present in the younger fossiliferous horizons of Samos. However, the lack of stratigraphic information for the specimens prevents their association with any of the fossiliferous horizons; and thus, inhibits certain stratigraphic correlations.
MATERIAL AND METHODS
For the purpose of the present work, material from several collections was studied including the NHMW (Austria), MNHN (France), GMM, GPIH, GPIT, HLMD, SMF, and SMNS (Germany), AMPG (Greece), and NMB (Switzerland). The goal was to find the most appropriate specimens to be designated as neotypes to compensate for the loss of the holotypes of Aceratherium schlosseri Weber, Citation1905, and Aceratherium samium Weber, Citation1905. The names Aceratherium schlosseri and Aceratherium samium will be used only to refer to the type material of the two species that was described by Weber (Citation1905). The names Chilotherium schlosseri and Eochilotherium samium will be used to refer to the species in general and when only the neotypes are concerned the specimen numbers (GPIH 3015 and SMF M 3601) will be stated, to avoid confusion.
Qualifying Conditions for the Designation of the Neotypes
The International Code on Zoological Nomenclature (ICZN) lists seven qualifying conditions (ICZN Art. 75.3) that ought to be met for a neotype designation to be valid (Ride et al., Citation1999). Based on these, the situation of both C. schlosseri and E. samium justifies the designation of neotypes.
The two Samian chilothere species C. schlosseri and E. samium have a problematic taxonomic history. At the moment, it is extremely difficult to assign any new material to either of these species, because the comparison can only be based on old illustrations. This is especially the case for E. samium, for which virtually no additional material from its type locality has ever been described after its erection. Also, no certain attribution has been made from other localities (see also Fortelius et al., Citation2003). Thus, the first qualifying condition (ICZN Art. 75.3.1) is met. The skull GPIH 3015 and its associate mandible are definitely assignable to C. schlosseri based on the morphological similarities, which include the very wide parietal crests and the complex tooth morphology, with a very strong crochet, antecrochet, protocone constriction, and the existence of a crista in some teeth that closes off the medifossette. The taxonomic attribution of GPIH 3015 to C. schlosseri was also encouraged by previous authors (Giaourtsakis, Citation2022; Lehmann, Citation1984). Specimen SMF M 3601, on the other hand, lacks the depression in the frontals, its parietal crests are much closer together, and the secondary folds in the maxillary teeth are not as prominent. These features allow the identification of SMF M 3601 as E. samium. This attribution was also supported by Geraads and Spassov (Citation2009), thus covering the second qualifying condition (ICZN Art. 75.3.2). The specimens herein designated as the neotypes of Aceratherium schlosseri Weber, Citation1905 (GPIH 3015) and Aceratherium samium Weber, Citation1905 (SMF M 3601) will be described and illustrated (, S1–2) in detail and 3D surface models of both specimens are provided (ICZN Art. 75.3.3). The type material of both Aceratherium schlosseri and Aceratherium samium, including all material described by Weber (Citation1905), was formerly housed in the BSPG, but cannot be relocated and it is believed that it was destroyed in the Second World War (Giaourtsakis, Citation2003, Citation2022; Kampouridis et al., Citation2022b), during the bombing of Munich by the Allied Royal Air Force on April 24, 1944 (Nothdurft & Smith, Citation2002), thus meeting the condition of ICZN Art. 75.3.4. As mentioned above, the skulls GPIH 3015 and SMF M 3601, which are selected as neotypes exhibit the same key features as the lectotypes of Aceratherium schlosseri and Aceratherium samium, respectively. These include the notably depressed frontal bones, widely separated (over 70 mm) parietal crests, and the complex dental morphology for C. schlosseri, and the convex frontal bones, the more closely situated parietal crests (approx. 40 mm), and the somewhat less developed secondary folds in the upper teeth in E. samium (ICZN Art. 75.3.5). The material described by Weber (Citation1905), as well as the material studied herein and selected as the neotypes, all come from the Upper Miocene deposits of the Mytilinii Basin on Samos Island (Greece). During the excavations on Samos in the course of the 19th and beginning of the 20th century only little attention was paid to documenting the exact location and stratigraphic context of the findings (Koufos, Citation2009). Therefore, almost nothing is known about it for these specimens, except for the fact that they come from the same basin, where the Turolian fossiliferous deposits on Samos Island are found (Kostopoulos et al., Citation2003), thus being the best option to meeting the conditions of ICZN Art. 75.3.6. Specimen GPIH 3015 has been part of the geological-paleontological collections of the University of Hamburg, an official university in Germany, since at least 1911 (Gürich, Citation1911; Lehmann, Citation1984). These collections are currently administered by the Leibniz Institute for the Analysis of Biodiversity. Similarly, specimen SMF M 3601 has been part of the collection of the Senckenberg Research Institute in Frankfurt, a scientific institution in Germany, since 1910 (R. Brocke, pers. comm.). In both cases, the material is housed in proper collections and will be accessible for further study in perpetuity (ICZN Art. 75.3.7); thereby, meeting all qualifying conditions set forth by ICZN Art. 75.3.
SYSTEMATIC PALEONTOLOGY
Class MAMMALIA Linnaeus, Citation1758
Order PERISSODACTYLA Owen, Citation1848
Family RHINOCEROTIDAE Gray, Citation1821
Subfamily ACERATHERIINAE Dollo, Citation1885 (sensu Lu, Deng, & Pandolfi, Citation2023)
Tribe ACERATHERIINI Dollo, Citation1885 (sensu Lu, Deng, & Pandolfi, Citation2023)
Subtribe CHILOTHERIINA Qiu, Xie, and Yan, Citation1987
Included Genera—Chilotherium Ringström, Citation1924, Shansirhinus Kretzoi, Citation1942, and Eochilotherium Geraads and Spassov, Citation2009.
Amended Diagnosis—Aceratheriine rhinocerotids that feature the following autapomorphic traits: separated parietal crests; upper molars featuring a marked protocone constriction and a moderate to strong antecrochet with a trend to turn lingually; a mandible that is characterized by a very wide mandibular symphysis that features a concave ventral side; large, sexually dimorphic i2s with a trend to become flattened and tusk-like that are separated from each other by a wide diastema and from the p2 by a long diastema with a marked crest. The group also exhibits an upper first deciduous premolar retained into adulthood, whereas the first lower deciduous premolar, when present, is shed and not replaced by a permanent one. Additionally, the upper cheek teeth have generally pronounced secondary enamel folds, and the appendicular skeleton is notably shortened and relatively robust, especially the metapodials.
Remarks—The clade Chilotheriini (sensu Qiu et al., Citation1987) was erected as a tribe to encompass the genera Chilotherium and Shansirhinus. However, the rank of this group is not clear. Different phylogenetic analyses have placed the clade of the hornless rhinos variably as a subfamily, Aceratheriinae (e.g., Cerdeño, Citation1995; Fortelius & Heissig, Citation1989; Heissig, Citation1999; Lu et al., Citation2023), or as a tribe, Aceratheriini (e.g., Antoine, Citation2002; Pandolfi, Citation2016). This leads to an ambiguous rank for the clade that includes the chilotheres, which in the latest phylogenetic analysis concerning hornless rhinos was placed as a subclade (Chilotherium + Shansirhinus) within the tribe Aceratheriini that includes both Chilotherium and Aceratherium (Lu et al., Citation2023). Therefore, we tentatively place Chilotheriina as a subtribe that includes the three genera Chilotherium, Shansirhinus, and Eochilotherium.
Genus CHILOTHERIUM Ringström, Citation1924
Type Species—Chilotherium anderssoni Ringström, Citation1924.
Type Locality—Lok. 30 Shanxi, China (Ringström, Citation1924).
Included Species— Chilotherium persiae (Pohlig, Citation1886), Chilotherium habereri (Schlosser, Citation1903), Chilotherium schlosseri (Weber, Citation1905), Chilotherium kowalevskii (Pavlow, Citation1913), ‘Chilotherium’ wimani Ringström, Citation1924, Chilotherium sarmaticum Korotkevich, Citation1958, Chilotherium orlovi Bayshashov, Citation1982, ‘Chilotherium’ primigenium Deng, Citation2006a, Chilotherium licenti Sun et al., Citation2018.
Amended Diagnosis—Aceratheriine rhinocerotids that feature the following autapomorphic characters: flattened and depressed frontal region; well-developed postorbital processes; moderately to widely separated parietal crests; highly placed orbits; very wide mandibular symphysis; very large, flattened, tusk-like second lower incisors, with a scalene triangle cross section and upturned, dorsomedially oriented wear facets; reduced premaxillary bones that lack upper incisors; and very strong secondary enamel folds, including a lingually flattened and strongly constricted protocone in the molars. It is also characterized by a relatively short length of the premolars compared with the molars, mainly due to the reduced size of the P2 and p2; and notably shortened metapodials and relative robust appendicular skeleton (Geraads & Spassov, Citation2009; Giaourtsakis, Citation2022; modified after Ringström, Citation1924).
Differential Diagnosis—Differs from Eochilotherium in having a less dolichocephalic skull; concave frontals; flat dorsal profile of the skull; more complicated enamel folds in the upper teeth, including constricted protocones in P2 and P3, more strongly constricted molars, and more prominent antecrochets; and much larger lower incisors that are more flattened. Differs from Shansirhinus in having concave frontals; a wider mandibular symphysis; more reduced second upper premolar; less pronounced lingual cingula; much weaker paracone folds; and less enamel plications.
CHILOTHERIUM SCHLOSSERI (Weber, Citation1905)
()
Neotype—A well-preserved skull (GPIH 3015) with an associated mandible (GPIH 3015a), by present designation under the provisions of ICZN Art. 75.
Type Locality—Upper Miocene deposits (Turolian age, Mytilinii Formation) of Samos Island; exact locality unknown.
Junior Synonyms—Teleoceras ponticus Niezabitowski, Citation1912, Aceratherium wegneri Andree, Citation1921, and Aceratherium angustifrons Andree, Citation1921.
Amended Diagnosis—A large Chilotherium species characterized by widely separated parietal crests (minimal width always over 70 mm in adult individuals), notably depressed frontal and nasal bones and a unique combination of dental characters: very long crochet; very strong mesial and distal constriction of the protocone, which is lingually flattened, resulting in a very long antecrochet that usually closes off the median valley at an early wear stage in all teeth; a prominent mesial constriction of the hypocone; crista frequently present that closes the medifossette; and a discontinuous lingual cingulum that is occasionally moderately developed in the premolars; often a closed prefossette is present in the P2; in addition to sporadically present enamel plications in the upper teeth; and discontinuous lingual and buccal cingulids in the lower teeth.
Remarks—The lectotype (designated by Giaourtsakis, Citation2022) is a relatively well-preserved adult cranium with its associated mandible described and figured as Aceratherium schlosseri by Weber (Citation1905:table 8, figs. 1–4; table 9, fig. 1). It was housed in the BSPG, but it is believed to have been destroyed during the Second World War (Giaourtsakis, Citation2009, Citation2022). Due to the loss of the type material, a neotype must be designated to solve the taxonomic issues and to secure the stability of this species. We herein choose the specimen GPIH 3015 as the neotype of C. schlosseri. It is housed in the collections of the GPIH in Germany and comes from the same locality as the lectotype of Aceratherium schlosseri (Weber, Citation1905:table 8, figs. 1–4; table 9, fig. 1), the Upper Miocene deposits of Samos Island (Greece). It was described as Chilotherium schlosseri and illustrated by Lehmann (Citation1984:pl. 3, figs. 2–3). In fact, it shows all morphological features characteristic for this species, as also supported by Giaourtsakis (Citation2022). Therefore, all qualifying conditions for the designation of a neotype (see ICZN Art. 75.3) are met.
Chilotherium schlosseri is much more common in the material collected from Samos than E. samium. Several almost complete skulls of C. schlosseri are available in different collections (e.g., NHMW, HLMD). Herein, we select GPIH 3015 as the neotype as it is virtually complete, preserves its associated mandible and its teeth are at a wear stage that allows the detailed study of the dental morphology.
The lower incisors of the neotypic mandible (GPIH 3015a) are very damaged and only their bases are preserved. Nonetheless, it can be seen that they were very large (i2 width = ∼45 mm). Based on the very wide i2s, it can be assumed that the skull (GPIH 3015) with its mandible (GPIH 3015a) belonged to a male individual.
Description
Skull—The skull is almost complete, lacking only the premaxillary bones, part of the left zygomatic arch, which has been modeled with plaster, parts of the postglenoid and posttympanic processes, and the left D1 and P2 (). Additionally, some areas on the dorsal side of the skull seem to have been modeled, covered with plaster but do not affect any important morphological traits. The specimen has not been affected by any other kind of distortion or deformation.
FIGURE 2. Neotype skull of Chilotherium schlosseri (Weber, Citation1905) (GPIH 3015) from the Upper Miocene of Samos Island (Greece) in dorsal (A) and ventral view (B). Scale bar equals 10 cm.
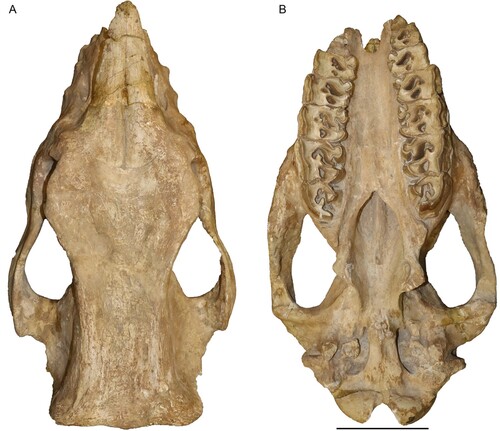
FIGURE 3. Neotype skull of Chilotherium schlosseri (Weber, Citation1905) (GPIH 3015) from the Upper Miocene of Samos Island in left lateral (A) and right lateral view (B). Scale bar equals 10 cm.
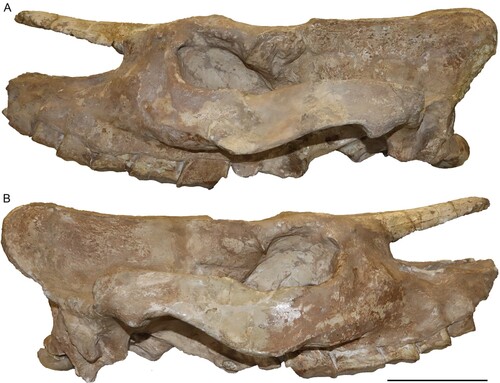
FIGURE 4. Neotype upper dentition of Chilotherium schlosseri (Weber, Citation1905) (GPIH 3015) from the Upper Miocene of Samos Island. Left (A) and right (B) upper toothrow. Scale bar equals 5 cm.
In lateral view (), the dorsal profile of the skull seems flat, with a very slight elevation of the nuchal crest in the posterior portion of the skull. The nasal bones extend slightly past the D1, anteriorly. The nasal notch is almost U-shaped and terminates at the level above the middle of the M1. On the maxillary bone, three infraorbital foramina are present within the nasal notch. There is no apparent facial crest. The orbit is located very high in the skull, with its anterior margin being above the mesial portion of the M2. A small lacrimal tubercle is visible, but the number of lacrimal foramina cannot be assessed, due to the presence of sediment in that area. The postorbital process on the frontal bones is very strong and marks the widest portion of the skull roof, as well as the beginning of the parietal crests. Another, weaker postorbital process is also present on the zygomatic arch, slightly more anteriorly than the one on the frontal bones. The zygomatic arches exhibit a very slight depression behind the postorbital process. The postglenoid process is broken on both sides. But it is visible that they are very close to the posttympanic processes without contacting them. The paroccipital process is also broken.
In dorsal view (), the nasal bones are rather narrow. Their tip is slightly rugose, and they are separated from each other by the internasal suture that forms a noticeable longitudinal groove. The suture between the nasals and the frontal remains visible despite the adult age of the individual. The suture is a curved line approximately at the level of the orbital margin. The frontal bones are strongly depressed. The whole skull roof is transversely concave from the nasal bones until the parietal crests. The parietal crests are widely separated, with a minimal distance of 89 mm between them and end in a well-developed nuchal crest.
In ventral view (), the anterior end of the choana is slightly pointed and reaches the medial part of the M3. The tips of the pterygoid processes are damaged but seem simple and laterally extending. The intercondylar notch is U-shaped and the condyles are widely separated.
In posterior view, the upper portion of the skull forming the nuchal crest is broken, but the general shape seems to be somewhat trapezoidal. The nuchal tubercle is only weakly developed, from which moderately developed lateral occipital crests start and might have reached the nuchal crest. Laterally to them, weak outer lateral crests exist.
Upper Dentition—The upper dentition is almost completely preserved, only lacking the right D1 and P2 (, ). Most teeth are at a moderate wear stage. All of them preserve a layer of cement buccally and lingually. The D1 is retained into adulthood but is heavily worn. The hypocone is larger than the protocone. A large fossette, probably represents the closed median valley, whereas a much smaller fossette should correspond to the postfossette. The P2 is well-worn revealing its morphology in much detail. The protocone is slightly smaller than the hypocone. The protocone is constricted mesially and distally, forming an antecrochet that is connected to the hypocone closing off the median valley. The hypocone is only mesially constricted. A crochet is present, along with a very small crista, with which it may fuse during a more advanced wear stage to form a closed medifossette. The prefossette remains open at this wear stage, but would close when slightly more worn, while the postfossette is already closed. Lingually, an almost continuous cingulum is present, whereas buccally the ectoloph is covered by cement. Nonetheless, a discontinuous buccal cingulum seems to be present. In the P3, the protocone and the hypocone are similarly well-developed. The protocone is strongly constricted mesially and distally, forming a long antecrochet that connects with the hypocone, closing off the median valley. The hypocone does not exhibit a constriction; this is very likely due to the fusion with the antecrochet and the advanced wear stage. A crochet is present, and the crista is very small, almost closing off the medifossette; in the right P3 the small crista is followed by an enamel plication. No prefossette is visible, but the postfossette is closed. A discontinuous lingual and buccal cingulum is present. The P4 features a strongly constricted protocone, with a prominent antecrochet, which fuses with the hypocone, resulting in a closed median valley. A crochet is present; in the right P4 it is much longer and closes off the medifossette. In the left P4, a weak enamel bulge is present that corresponds to the crista. A large postfossette is present. A discontinuous lingual cingulum is present on the right P4, whereas on the left this cannot be observed. On the ectoloph, both P4s exhibit a discontinuous cingulum.
The M1 is well-worn and exposes a very strongly constricted protocone, both mesially and distally, that forms a long, lingually projecting antecrochet. The protocone is lingually flattened and has an almost sub-triangular shape. The hypocone is mesially strongly constricted and connects to the antecrochet, closing off the median valley. A closed medifossette and postfossette are present. Both lingually and buccally, discontinuous cingula are present, which form enamel pillars in the entrance of the median valley. Enamel plications are visible within the median valley and the postfossette in both M1s. Lastly, the M1 exhibits a hypoplasia visible only at the base of the lingual side, because the ectoloph is covered by cement. The M2 is less worn than the previous teeth. It exhibits a lingually flattened and strongly constricted protocone that results in a strong antecrochet, which turns lingually. The hypocone is only mesially constricted; at a more advanced wear stage the hypocone and the antecrochet would fuse and cut off the median valley. The crochet is strong but does not close the medifossette at this wear stage. The postfossette is just about to close. Both M2s feature discontinuous cingula lingually. Moreover, in lingual view, close to the base of the tooth crown a hypoplasia is visible. The M3 is the least worn tooth in this specimen. The protocone is lingually flattened and bears strong mesial and distal constrictions, which form a long lingually projecting antecrochet. A strong crochet is present, along with a crista and an additional enamel plication. Distally the cingulum is represented by a high enamel bar. Finally, a hypoplasia is present in the M3, only visible on the lingual side, due to the presence of cement coverage buccally on the ectoloph.
Mandible—The mandible is very well preserved, including almost the complete dentition and only lacking the left articular process and the coronoid process on both sides (). The symphysis is very wide with a diastema of 82.3 mm between the incisors. On the ventral side the symphysis is markedly concave and bears two large foramina that are also visible in anterior view. A small foramen is visible posterior to the large one on the left hemimandible. Other foramina are not visible due to the presence of plaster that covers part of the ventral surface. The anterior end of the symphysis forms a thin edge between the two incisors. In dorsal view, the symphysis ends at the posterior end of the p3s and is 127 mm long. Between the i2 and the p2 there is a long diastema of 60 mm, which is marked by a well-developed dorsal ridge between these two teeth. The dorsal surface of the symphysis, between these ridges is concave. In lateral view, on the left side one large and one small mental foramen are visible below the p2 and p3. On the left side the foramina are covered by plaster. The height of the mandibular body remains similar throughout its length, with a slight increase posteriorly. The vertical rami are only partially preserved, with the coronoid processes missing in both hemimandibles. The condylar process for the articulation to the skull is 78.5 mm wide. The condylar process becomes medially thinner. Lastly, the mandibular angle bears a strong masseteric tuberosity.
FIGURE 5. Neotype mandible of Chilotherium schlosseri (Weber, Citation1905) (GPIH 3015) from the Upper Miocene of Samos Island in dorsal (A), anterior (B), right lateral (C), and left lateral view (D). Scale bar equals 20 cm in A, 15 cm in B, and 30 cm in C and D.
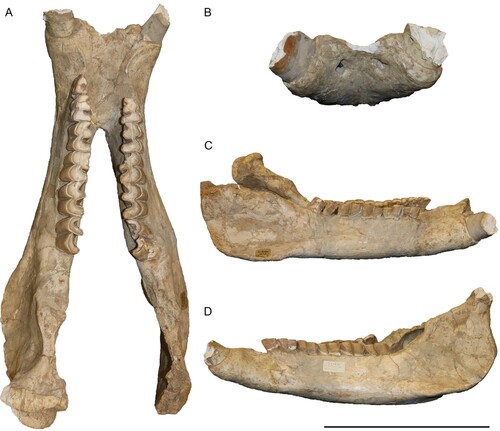
Lower Dentition—The lower dentition only lacks the right p2 and the incisors are broken off (Fig. S1). Despite the damage to the incisors, it is visible that they would have been very large, and their wear facet is placed dorsally and turned abaxially, with enamel only on the ventral side. The cross section of the roots of the incisors is almond shaped with a width of 45 mm. Some of the cheek teeth preserve remnants of their cement. The premolar/molar length ratio (sensu Athanassiou et al., Citation2014) is 72%. All of the cheek teeth bear discontinuous lingual cingulids, the premolars also feature discontinuous buccal cingulids. The p2 has a thin mesially projecting paralophid and features a shallow anterior valley and a weak anterior groove on the ectolophid. Rather strong mesial and distal cingulids are also present, which slightly extend towards the buccal side of the side. Otherwise, the lower cheek teeth have a rather simple morphology that is relatively uniform. They have a paralophid that is slightly shorter than the metaconid. The metaconid and entoconid are relatively thick. The anterior valley is not as deep as the posterior one and the ectolophid groove is deep until a very advanced wear stage.
Genus EOCHILOTHERIUM Geraads & Spassov, Citation2009
Type and Only Species—Aceratherium samium Weber, Citation1905.
Diagnosis— Same as for the type and only species.
Remarks—Eochilotherium was coined by Geraads and Spassov (Citation2009) as a subgenus, Chilotherium (Eochilotherium), with Aceratherium kiliasi as its type species and the authors also included Aceratherium samium in it. Herein, we elevate Eochilotherium to the genus level, this does not affect Aceratherium kiliasi as its type species according to the provisions of the ICZN Art. 61.2.2. However, Aceratherium kiliasi is a junior synonym of E. samium, as stated also in previous studies (Athanassiou et al., Citation2014:table 3; Giaourtsakis, Citation2022). The type species of Eochilotherium is now fixed (under ICZN Article 70.3) as Aceratherium samium Weber, Citation1905, due to the misidentification of Aceratherium kiliasi Geraads and Koufos, Citation1990.
EOCHILOTHERIUM SAMIUM (Weber, Citation1905)
()
Neotype—A well-preserved skull with associated mandible of an old individual (SMF M 3601), by present designation under the provisions of ICZN Art. 75.
Type Locality—Upper Miocene deposits (Turolian, Mytilinii Formation) on Samos Island; exact locality unknown.
Junior Synonym—Aceratherium kiliasi Geraads and Koufos, Citation1990.
Amended Diagnosis—Aceratheriine rhinocerotid of medium size with a unique combination of craniodental features separating it from all other chilotheres: dolichocephalic skull; transversally concave dorsal surface of the parietal bones, while their frontal bones are convex in the anterior part, becoming flattened posteriorly; moderately separated parietal crests (∼40 mm); weak to absent groove that would separate the nasal bones from each other; raised posterior part of the skull; wide mandibular symphysis with moderately enlarged and slightly flattened second lower incisors; the relative length of the premolars with respect to the molars is reduced, primarily due to the reduction of the size of the second upper and lower premolars; and complex enamel folds: moderate crochet and constriction of the protocone of the molars, with a strong antecrochet that bends lingually; neither the protocone nor the hypocone of the P2 are constricted; no crista in the molars; and discontinuous lingual cingula.
Differential Diagnosis—Differs from Chilotherium spp. in having flat to convex frontal bones and lacking the frontal depression characterizing the genus Chilotherium; the parietal crests are usually not as widely separated from each other as in Chilotherium spp.; the posterior portion of the skull is notably elevated, compared with the only weakly elevated or flat posterior part in Chilotherium spp.; in the P2 and P3 the protocone is not constricted; the hypocone is either not constricted or only weakly constricted; the lower incisor is narrower and less flattened in E. samium compared with Chilotherium spp. Differs from Shansirhinus spp. in lacking the complicated enamel folds that include the strongly constricted protocone and hypocone in all teeth and the presence of enamel plications in Shansirhinus spp. Furthermore, Shansirhinus spp. exhibits a prominent paracone fold, which is much weaker in E. samium.
Remarks—The lectotype (designated by Geraads & Koufos, Citation1990:p. 163) is a partially preserved adult cranium with associated mandible described and figured as Aceratherium samium by Weber (Citation1905:table 9, fig. 5; table 10, fig. 1, 2). It was housed in the BSPG, but it is believed to have been destroyed during the Second World War (Giaourtsakis, Citation2009, Citation2022). We herein embrace the notion of Geraads and Spassov (Citation2009), who considered specimen SMF M 3601 as the only specimen that can be assigned to E. samium, and designate this skull (SMF M 3601) as the neotype of Aceratherium samium. Specimen SMF M 3601 is housed in the collections of SMF in Germany and comes from the same locality as the lectotype of Aceratherium samium (Weber, Citation1905:table 9, fig. 5; table 10, fig. 1, 2), Upper Miocene deposits of Samos Island (Greece). This specimen was initially illustrated by Drevermann (Citation1930:fig. 6) as C. schlosseri, without any detailed description or comparison. Geraads and Spassov (Citation2009) mentioned that this is the only specimen that can be assigned to E. samium. It has to be noted that Giaourtsakis (Citation2022) seemingly disagreed with the identification of SMF M 3601 as C. schlosseri by Drevermann (Citation1930:fig. 6), but preferred to keep SMF M 3601 as Chilotherium sp., without mentioning a potential assignment to E. samium that was already suggested by Geraads and Spassov (Citation2009). Herein, we follow Geraads and Spassov (Citation2009), because specimen SMF M 3601 shows the key features in the morphology of the skull and teeth of E. samium, as discussed below. Therefore, all qualifying conditions for the designation of a neotype (see ICZN Art. 75.3) are met.
Eochilotherium samium is a rare species in the Upper Miocene deposits of Samos (Greece). In total, only three skulls of this species have been mentioned: the lectotype and a second skull described by Weber (Citation1905:table 9, fig. 5; table 10, figs. 1, 2), and specimen SMF M 3601. The first two were lost during the Second World War. Therefore, SMF M 3601 is the only reasonable choice for the neotype of this species.
In the neotype mandible (SMF M 3601) only the right lower incisor is preserved. It is almost triangular in shape and is relatively small (i2 width = 30 mm). Based on the small triangular i2, it can be assumed that the skull (SMF M 3601) with its mandible belonged to a female individual.
Description
Skull—The skull SMF M 3601 is almost complete, missing only the right premolars, except the P4, the premaxillary bones, parts of the nasal bones and of the left zygomatic arch, the tips of the paroccipital processes, and the posterior part of its skull roof is damaged (). Beside this damage to the skull, the specimen has not undergone any kind of deformation and is symmetrically preserved. Due to missing premaxillary bones, we cannot directly assess the potential existence of upper incisors; however, the i2 in the mandible is only moderately worn, despite the advanced age of the individual, which might suggest that there was no upper incisor that would have worn it down. Also, the wear facet in the preserved i2 covers the whole tooth and is most similar to Chilotherium, which lacks upper incisors. Therefore, it is very likely that E. samium also lacked upper incisors.
FIGURE 6. Neotype skull of Eochilotherium samium (Weber, Citation1905) (SMF M 3601) from the Upper Miocene of Samos Island in dorsal (A) and ventral view (B). Scale bar equals 10 cm.
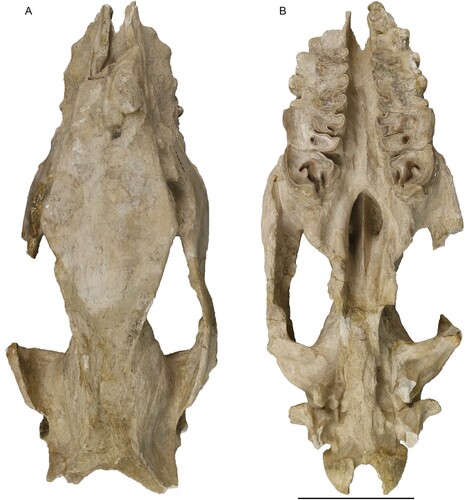
FIGURE 7. Neotype skull of Eochilotherium samium (Weber, Citation1905) (SMF M 3601) from the Upper Miocene of Samos Island in left lateral (A) and right lateral view (B). Scale bar equals 10 cm.

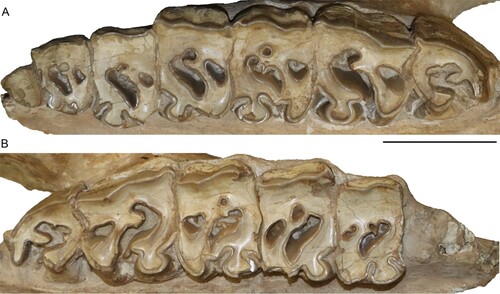
FIGURE 8. Neotype upper dentition of Eochilotherium samium (Weber, Citation1905) (SMF M 3601) from the Upper Miocene of Samos Island. Left (A) and right upper toothrows (B). Scale bar equals 5 cm.

In lateral view (), the dorsal profile of the skull seems flat; however, this is in part due to the damaged posterior part of the skull roof. Otherwise, the dorsal profile of the skull is slightly concave. The anterior part of the nasal bones is missing; therefore, its exact shape and orientation are not known. The nasal notch has an almost trapezoidal shape and terminates at the level above the anterior part of the M1. On the maxillary, laterally to the nasal notch two rather prominent infraorbital foramina are present. A facial crest is partially present on both sides. The orbit is located high in the skull, with its anterior margin being above the distal portion of the M2. A lacrimal tubercle is visible, but the number of lacrimal foramina cannot be assessed. The postorbital process on the frontal is present, but damaged. Another process is also present on the zygomatic arch, roughly at the same level as the one on the frontal. The preserved right zygomatic arch exhibits a strong depression behind the postorbital process. The postglenoid and posttympanic processes are close to each other but remain at least 5 mm apart and do not contact each other. They are both similarly long and extent slightly below the lower limit of the occipital condyle. The paroccipital process is much more ventrally extending than the posttympanic one.
In dorsal view (), the preserved portion of the nasal bones is rather narrow. The internal nasal suture is not visible and there does not seem to have been a very prominent longitudinal groove present, though on their ventral side a weak longitudinal bulge is present. The frontals do not show the typical depression seen in most Chilotherium species but are in fact slightly convex. The posterior part of the frontals becomes flat but not concave at any point. The parietals are concave due to the elevation of the parietal crests. These end in the nuchal crest, which is not preserved in SMF M 3601, and despite being damaged the minimal distance between the parietal crests can be estimated to about 40 mm.
In ventral view (), the anterior end of the choana is slightly pointed and reaches the mesial part of the M3. On the palatine bone two foramina are present at the level of the M2 on the right side. The other side is slightly compressed, and the foramina cannot be seen. The tips of the pterygoid processes are broken but seem simple. The intercondylar notch is U-shaped, and the condyles are widely separated.
In posterior view, the upper portion of the skull forming the nuchal crest is broken, but the general shape seems to be somewhat trapezoidal. The nuchal tubercle is only weakly developed, from which marked lateral occipital crests start and might have reached the nuchal crest. Laterally to them, prominent outer lateral crests exist.
Upper Dentition—The upper dentition is almost completely preserved, only lacking the right D1, P2, and P3. However, the teeth are very worn due to the old age of the individual. Most teeth preserve a thin cement layer on their enamel. The D1 is retained into adulthood and despite the old age of the individual is only moderately worn. It exhibits a lingually projecting parastyle, a very small protocone in contrast to the more prominent hypocone, a closed postfossette and a lingual cingulum. The P2 is almost completely worn and not much of its enamel is preserved, but a closed prefossette is visible and a weak cingulum seems to be present. It can be observed that neither the protocone nor the hypocone exhibit a constriction and that the median valley remains open until the tooth is completely worn off. The P3 does not retain any enamel and its morphology cannot be evaluated. The P4 is similarly well preserved on both sides, the only difference being that the right one preserves the enamel that represents the closed prefossette, whereas on the left side this is already worn off. On both sides, the constriction of the protocone is visible and a portion of the antecrochet which connected with the hypocone and resulted in a closed median valley. Moreover, a prominent enamel pillar is situated at the entrance of the median valley.
The M1 is also extremely worn and only the right one preserves some remains of enamel that confirm the presence of a closed postfossette. Also, both the protocone and the hypocone are constricted and a lingually projecting antecrochet is present, closing off the median valley. The M2 is less worn than the previous teeth, but is nonetheless very worn, with less than 1 cm high enamel remaining on its ectoloph. The closed postfossette is well-visible, the protocone is constricted both mesially and distally, with the presence of a lingually projecting antecrochet, while the hypocone is only mesially constricted. Despite the advanced wear stage and the prominent antecrochet, the median valley remains open in both M2s. The M3 is the least worn tooth in this specimen. It bears mesial and distal constrictions only in the protocone, which form a lingually projecting antecrochet. Additionally, a small crochet is present and distally a cingulum is present.
Mandible—The mandible is very well preserved, including almost the complete dentition (). The symphysis is very wide with a diastema of 58 mm between the incisors. On the ventral side the symphysis is slightly concave and bears two foramina that are also visible in anterior view. Other foramina are not visible due to the presence of sediment and plaster that cover part of the ventral surface. The anterior end of the symphysis forms a thin edge between the two incisors. In dorsal view, the symphysis ends posteriorly at the middle of the p3s and is 119 mm long. Between the i2 and the p2 there is a long diastema of 65 mm, which is marked by a well-developed dorsal ridge between these two teeth. The dorsal surface of the symphysis, between these ridges is concave. In lateral view, only one mental foramen is visible on right horizontal ramus below the p3. On the left side this foramen is covered by some plaster. The height of the mandibular body remains almost the same throughout its length. The vertical rami are almost completely preserved, only the right one lacking the tip of the coronoid process. They almost form a 90° angle to the horizontal rami. The preserved left coronoid process is 63 mm high and the condylar process for the articulation to the skull is 68 mm wide. The condylar process becomes medially much thinner. Lastly, the mandibular angle bears a strong masseteric tuberosity.
FIGURE 9. Neotype mandible of Eochilotherium samium (Weber, Citation1905) (SMF M 3601) from the Upper Miocene of Samos Island in dorsal A, anterior, B, right lateral, C, and left lateral view, D. Scale bar equals 20 cm in A, 15 cm in B, and 30 cm in C and D.
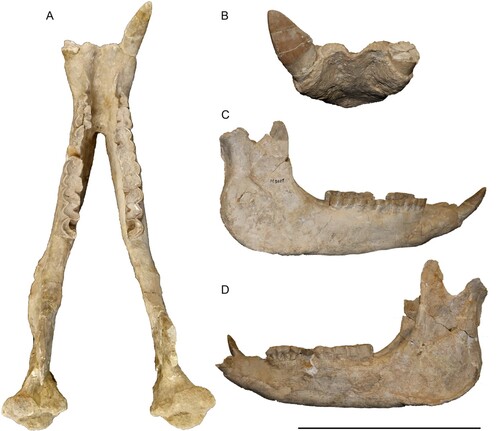
Lower Dentition—The lower dentition only lacks the left i2, p4, and parts of the p2. It is better preserved than the upper dentition and is slightly less worn. The right i2 is only moderately worn despite the high age of the individual. Its crown has a triangular shape with a length of about 52 mm and a width of 30 mm. The labial side of the tooth preserves the complete enamel, whereas on the lingual side only a small surface of enamel is preserved, which nonetheless shows that the tooth was completely covered by enamel, both labially and lingually. The cross section of the root is oval.
Some of the cheek teeth preserve remnants of their cement. The premolar/molar length ratio (sensu Athanassiou et al., Citation2014) is 66.9%. Most cheek teeth are heavily worn, and their morphology cannot be accurately assessed, only the m3 is moderately worn, allowing a more detailed description of its morphology. The p2 has a mesially projecting paralophid and features a shallow anterior valley and a weak anterior groove on the ectolophid. None of the cheek teeth bear lingual or buccal cingulids. In most teeth neither mesial nor distal cingulids can be observed, due to the advanced wear stage, but in the m3 a distal cingulid is well visible and a slight extension of the mesial cingulid towards the lingual side can be traced.
COMPARISON
Both species described above, C. schlosseri and E. samium, come from the Upper Miocene of Samos, though the stratigraphic context is unknown, and they probably were not found in the same fossiliferous horizons (Weber, Citation1905). The taxonomy of the latter one has been very controversial, to the extent where it has been suggested that usage of the species name E. samium should be restricted only to its type material (Fortelius et al., Citation2003). Therefore, the first part of the comparison is dedicated to the comparison of the herein designated neotypes of the two Samian species to the lectotypes. This proves the attribution of GPIH 3015 to C. schlosseri and SMF M 3601 to E. samium. Next, the two Samian species, C. schlosseri and E. samium, will be compared with each other, pointing out the morphological features distinguishing the two taxa. This confirms the existence of two distinct chilothere species in the Upper Miocene deposits on Samos Island, as shown by the prominent morphological differences, though no evidence exists that the two species occurred in the same stratigraphic layer. Afterwards, each of them will be carefully compared with other relevant chilothere species across Eurasia, to verify them as valid species and investigate their potential relationships.
Comparison to the Lectotypes of Aceratherium schlosseri Weber, Citation1905 and Aceratherium samium Weber, Citation1905
The comparison of the two specimens selected as neotypes for C. schlosseri (GPIH 3015) and E. samium (SMF M 3601) to the descriptions and illustrations provided by Weber (Citation1905) for the original type material of Aceratherium schlosseri and Aceratherium samium is crucial for their proper establishment as neotypes. The lectotype of Aceratherium schlosseri (Weber, Citation1905:pl. 8, figs. 1–3) is an almost complete skull with an associated mandible, the teeth of which were in an advanced wear stage (Giaourtsakis, Citation2022). Weber (Citation1905) mentioned another rather complete skull with its mandible and several maxillary and mandibular elements with teeth that he assigned to this species. The lectotype of Aceratherium samium (Weber, Citation1905:pl. 10, figs. 1, 2) is a skull with its mandible, which is posteriorly damaged (Athanassiou et al., Citation2014; Geraads & Koufos, Citation1990). Weber (Citation1905) assigned another partially preserved skull that lacked almost all teeth with its mandible and a maxillary element of a juvenile individual to this species. All this material is now considered lost.
There are several characters that allow the association of the neotypes to their respective species. In general, the skulls of Aceratherium samium and SMF M 3601 are more dolichocephalic and smaller than the ones of Aceratherium schlosseri and GPIH 3015 (, ). Weber (Citation1905) mentioned that the most prominent feature in the two skulls of Aceratherium schlosseri is the depression in the frontal bones. For Aceratherium samium, Giaourtsakis (Citation2022) mistakenly translated the description of Weber (Citation1905) of the frontal region as “slightly depressed,” whereas Weber (Citation1905:p. 354) wrote “etwas gewölbte Stirnregion,” which should be translated as “slightly convex frontal region,” thus meaning the opposite. A frontal depression is clearly present in GPIH 3015 as in Aceratherium schlosseri, whereas in SMF M 3601 the frontal region is convex as in Aceratherium samium (). Weber (Citation1905) also mentioned that in Aceratherium schlosseri the nasal bones are separated by an anteroposterior longitudinal groove, which is well visible on its lectotype (Weber, Citation1905:pl. 8, fig. 2). He also mentioned that a longitudinal bulge is present on the ventral side of the nasal bones. Both the groove on the dorsal side and the bulge on the ventral side of the nasal bones are present in GPIH 3015, with the groove stretching posteriorly until the nasofrontal suture (). In Aceratherium samium, this groove is only weakly visible, more similar to SMF M 3601, where the groove is not visible due to some damage in that region. If it was present, when SMF M 3601 was complete, it must have been rather weak and definitely did not extent as posteriorly as in C. schlosseri (). Moreover, Weber (Citation1905) mentioned that the nasal bones of Aceratherium schlosseri slightly slope upwards, as is the case in GPIH 3015, in contrast to Aceratherium samium where they are completely straight, which also seems to be the case in SMF M 3601 (). He also mentioned that the orbit is placed very highly in the skull of Aceratherium schlosseri, as in GPIH 3015, whereas in the figure of the skull of Aceratherium samium it is visible that is not the case (Weber, Citation1905:table 10, fig. 1). In SMF M 3601 the orbit also seems to be placed somewhat lower. Another feature that Weber (Citation1905) pointed out in Aceratherium schlosseri is the big minimal distance between the parietal crests, which is 90 mm in the lectotype. This was also discussed by Kampouridis et al. (Citation2022b) who considered a minimal distance between the parietal crests of over 70 mm as a diagnostic feature of C. schlosseri that is not seen in other chilotheres (). In GPIH 3015 this distance is 89 mm, thus very close to the lectotype of Aceratherium schlosseri. In the lectotype of Aceratherium samium this portion is heavily damaged, but the right parietal crest is adequately preserved to assess that the minimal distance would be close to 40 mm, as also pointed out by Andree (Citation1921). In SMF M 3601, this portion is also damaged, but the minimal distance between the parietal crests can be estimated to about 40 mm (), thereby being the same as in Aceratherium samium. In general, the parietal region in the lectotype of Aceratherium schlosseri and in GPIH 3015 is much wider than in SMF M 3601 (). Additionally, in Aceratherium schlosseri the skull roof is very flat and posteriorly slopes up only very slightly as in GPIH 3015 (). In Aceratherium samium and SMF M 3601 the posterior part of the skull roof is damaged but based on the preserved parts it can be assumed that in both skulls it would slope up more abruptly. The zygomatic arches in Aceratherium schlosseri are posteriorly widening, as in GPIH 3015, whereas in Aceratherium samium and SMF M 3601 they are positioned closer to the skull and do not become wider posteriorly ().
TABLE 2. Comparison of the minimal distance between the parietal crests (in mm) of Chilotheriina (modified after Kampouridis et al., Citation2022b).
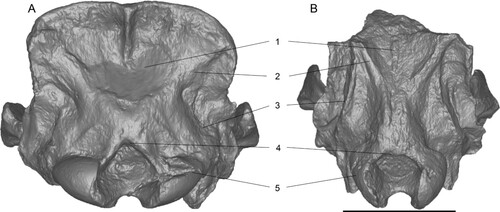
Moreover, in posterior view there are several features distinguishing GPIH 3015 and SMF M 3601 (compare in ). The illustration of the lectotype of Aceratherium schlosseri in posterior view (Weber, Citation1905:pl. 8, fig. 3), however, is very simplistic and many of these features are not visible and the posterior portion of the lectotype of Aceratherium samium was severely damaged and was never illustrated by Weber (Citation1905). The only features comparable between Aceratherium schlosseri and GPIH 3015 are the generally wide skull in posterior view with very wide occipital condyles, which are much higher and longer in SMF M 3601 () and the fact that the lateral occipital crests are relatively weak and widely spaced in comparison to the more prominent and closer situated occipital crests in SMF M 3601.
Weber (Citation1905) described the mandibles of both species in the same manner without pointing out any differentiating features that might be diagnostic. Giaourtsakis (Citation2022:438) mentioned that the mandible of the lectotype of Aceratherium samium does not feature a masseteric tuberosity, which is mentioned for the mandible of the male individual described by Weber (Citation1905:356). This could be explained through some damage to that region that is not clearly depicted in the figure or by a potential simplification of the morphology in the drawing of the lectotype of Aceratherium samium; a strong masseteric tuberosity is definitely present in the mandible of SMF M 3601, which belongs to a female individual just like the lectotype of Aceratherium samium. In the mandibles of Aceratherium schlosseri and GPIH 3015 this tuberosity is clearly visible. In SMF M 3601 the symphysis is rather narrow when compared with Aceratherium schlosseri and GPIH 3015. The preserved lower incisor of SMF M 3601 has also a more triangular shape than GPIH 3015. The shape and size of this tooth is heavily affected by sexual dimorphism in chilotheres (Chen et al., Citation2010), but the narrow triangular shape and rather small size in SMF M 3601 is a more plesiomorphic feature, not seen in material that is assignable to C. schlosseri.
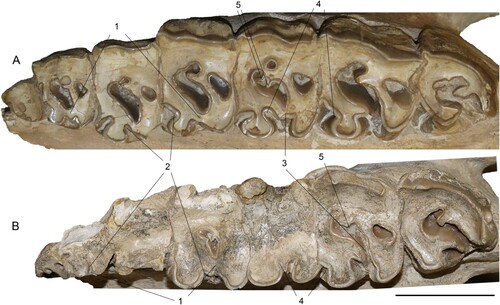
Considering differentiating features in the upper cheek teeth of these two species is more difficult, especially for E. samium, because both the lectotype of Aceratherium samium and SMF M 3601 belonged to ontogenetically very old individuals and their dentition is very worn, more so in SMF M 3601. Nonetheless, there are some features that can be used to attribute specimens GPIH 3015 and SMF M 3601 to C. schlosseri and E. samium (see ). As also pointed out by previous studies (e.g., Geraads & Koufos, Citation1990; Giaourtsakis, Citation2022) the enamel folds in E. samium are not as complex as in C. schlosseri. This is easily visible when the toothrows of the two lectotypes are compared (Weber, Citation1905:table 9, fig. 1, 5). The lectotype of Aceratherium schlosseri features an extremely strong protocone constriction, with a very strong antecrochet that closes off the median valley, which is not the case in the lectotype of Aceratherium samium. In GPIH 3015 the protocone is also much more strongly constricted than in SMF M 3601 and the resulting antecrochet would close off the median valley in all teeth, whereas in SMF M 3601 it remains open in the P2 and M2 despite the advanced wear stage. Another important feature seen in both the lectotype of Aceratherium schlosseri and GPIH 3015 is the strong crochet that results in a closed medifossette in most teeth, whereas in the lectotype of Aceratherium samium and SMF M 3601 the crochet is not as prominent, and most teeth do not feature a closed medifossette. Thereby, GPIH 3015 can safely be attributed to C. schlosseri and SMF M 3601 respectively to E. samium.
Comparison Between Chilotherium schlosseri and Eochilotherium samium
The two Samian species exhibit many important features that distinguish them from each other that not only allow their specific separation but would also justify their placement in different genera. The general shape of the two skulls is very different, with the skull of C. schlosseri being generally larger, but relatively shorter and wider (). The skull of E. samium is much more dolichocephalic and narrower, as seen in the narrow palate and the narrow parietal region. The most striking evidence that they represent distinct taxa is the marked depression in the frontal bones that also lowers the position of the nasals in C. schlosseri, whereas in E. samium the frontal bones are slightly convex anteriorly and become flat posteriorly. On the nasals, in C. schlosseri a deep longitudinal groove is found that reaches the nasofrontal suture, whereas in E. samium this groove is either absent or does not extend as far posteriorly. The parietal crests are much wider apart in C. schlosseri (always >70 mm in adult individuals, n=7) than in E. samium (∼40 mm) and the zygomatic arches become wider in the posterior part in C. schlosseri, whereas in E. samium they are generally closer to the skull and do not show an important degree of widening. In lateral view, despite the damage to the posterior portion of the lectotype and the neotype skulls of E. samium, the posterior skull portion would have been much more raised than in C. schlosseri, where the skull is almost flat (). It is also visible that in C. schlosseri the dorsal border of the orbit is positioned higher than the nasals, whereas in E. samium it is situated at a lower position. In posterior view, several striking differences are visible, despite some damage to the neotypes of both species (). The skull of C. schlosseri is generally much wider than E. samium. This is also the case for the occipital condyles, which are very wide and rounded in C. schlosseri, whereas in E. samium they are in relation much higher, transversally narrower, but anteroposteriorly longer and more angular. The lateral occipital crests are also broader and more rounded in C. schlosseri, compared with E. samium, where they are closer to each other and are more pronounced. The occipital fossa is also deeper in C. schlosseri. The outer lateral crests are much more prominent and longer in E. samium, connecting to the lateral crests, in contrast to C. schlosseri, where they are more subtle and rounded. In C. schlosseri, the foramen magnum features an incision at its dorsal border, which does not exist in E. samium.
FIGURE 10. Comparison of 3D surface models of the neotype skulls of Chilotherium schlosseri (Weber, Citation1905) (GPIH 3015) (A) and Eochilotherium samium (Weber, Citation1905) (SMF M 3601) (B) from the Upper Miocene of Samos Island (Greece) in dorsal view. Abbreviations—1, longitudinal groove on nasal bones: present in C. schlosseri (A) and weak or absent in E. samium (B); 2, shape of frontal bones: strongly depressed in C. schlosseri (A) and slightly convex in E. samium (B); 3, distance between parietal crests: very widely separated (>70mm) in C. schlosseri (A) and moderately separated in E. samium (B); and 4, shape of zygomatic archs: posteriorly widening in C. schlosseri (A) and constant width in E. samium (B). Scale bar equals 10 cm.
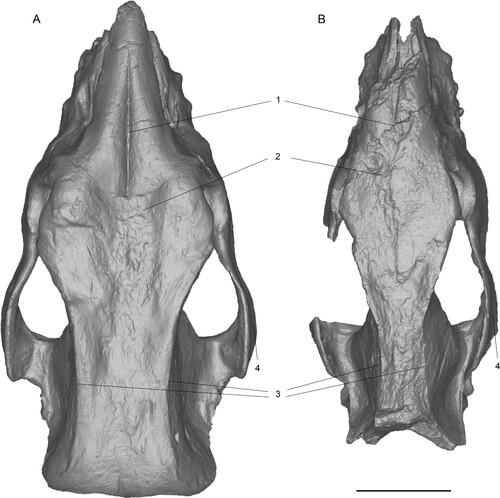
FIGURE 11. Comparison of 3D surface models of neotype skulls of Chilotherium schlosseri (Weber, Citation1905) (GPIH 3015) (A) and Eochilotherium samium (Weber, Citation1905) (SMF M 3601) (B) from the Upper Miocene of Samos Island (Greece) in right lateral view. Abbreviations—1, relative position of nasal bones to dorsal wall of the orbit: at the same level in C. schlosseri (A) and lower position of orbit in E. samium (B). Scale bar equals 10 cm.
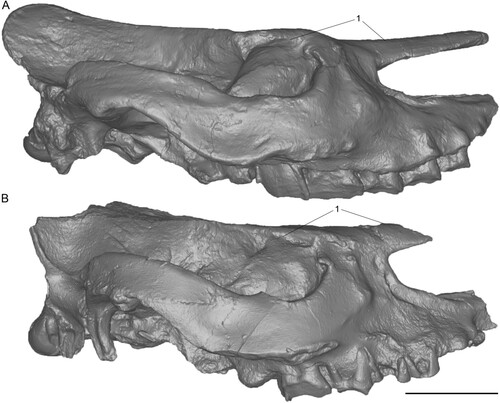
FIGURE 12. Comparison of of 3D surface models of neotype skulls of Chilotherium schlosseri (Weber, Citation1905) (GPIH 3015) (A) and Eochilotherium samium (Weber, Citation1905) (SMF M 3601) (B) from the Upper Miocene of Samos Island (Greece) in posterior view. Abbreviations—1, occipital fossa: deep in C. schlosseri (A) and shallow in E. samium (B); 2, lateral occipital crests: broad and not marked in C. schlosseri (A) and narrow but more pronounced in E. samium (B); 3, outer lateral occipital crests: subtle and rounded in C. schlosseri (A) and more prominent and longer in E. samium (B); 4, dorsal shape of the foramen magnum: dorsal incision in C. schlosseri (A) and rounded dorsal border in E. samium (B); and 5, shape of occipital condyles: wide in C. schlosseri (A) and narrow and high in E. samium (B). Scale bar equals 10 cm.
The upper teeth of both the neotype and the lectotype of E. samium are very worn, due to the high ages of the individuals; nonetheless, some differentiating characters for the two chilothere species are still visible (). The P2 in E. samium has an unconstricted protocone and in the P4 the protocone is only moderately constricted, in contrast to the strongly constricted protocone in all three permanent premolars in C. schlosseri. Additionally, in the P2 the protocone and the hypocone remain separated and in the P4 they barely touch in E. samium, despite their advanced wear stage. In C. schlosseri, the protocone and the hypocone are connected in all premolars. Similarly, in the M2 of E. samium the median valley remains open and would only close when the tooth is almost completely worn down, whereas in the M1 of C. schlosseri they are already connected, and in the M2 they would connect at an earlier wear stage than in E. samium. Both the M2 and the M3 of E. samium feature small crochets that do not close off the medifossette, whereas in the M1 of C. schlosseri the medifossette is closed off, in the M2 the crochet is relatively small due to the early wear stage and in M3 the crochet is already large and would close off the medifossette at a slightly more advanced wear stage. In general, the molars of C. schlosseri feature much stronger constricted protocones than in E. samium. Interestingly, in both species the protocone constriction leads to strong antecrochets that are lingually oriented, a feature common among chilotheres, but somewhat more pronounced in C. schlosseri. Lastly, C. schlosseri differs from E. samium in sporadically exhibiting enamel plications, which seems to be absent in E. samium.
FIGURE 13. Comparison of the left upper tooth row of the neotypes of Chilotherium schlosseri (Weber, Citation1905) (GPIH 3015) (A) and Eochilotherium samium (Weber, Citation1905) (SMF M 3601) (B) from the Upper Miocene of Samos Island (Greece) in occlusal view. Abbreviations—1, closure of the median valley in the premolars: at an early wear stage in C. schlosseri (A) and at a late wear stage or not at all in E. samium (B); 2, protocone constriction in the premolars: very strong in C. schlosseri (A) and weak or absent in E. samium (B); 3, closure of the median valley in the molars: at a moderate wear stage in C. schlosseri (A) and at a late wear stage in E. samium (B); 4, proto- and hypocone constriction in molars: very strong in C. schlosseri (A) and moderate in E. samium (B); and 5, enamel plications: present C. schlosseri (A) and absent in E. samium (B). Scale bar equals 5 cm.
The mandibles of the two species also seem to offer some clues for their separation. However, they must be taken with much caution, as the lectotype and the neotype of E. samium seem to have belonged to female individuals, whereas the neotype of C. schlosseri belonged to a male individual. This is mainly based on the wider symphysis in C. schlosseri with larger incisors. The width of the incisors at their base is 45 mm in C. schlosseri and 30 mm in E. samium. The most prominent differences are found in the anterior part of the mandible. The symphysis is very wide in both species, reaching posteriorly the p3 in both species and is similarly long, with a diastema between the i2 and the p2 of 60 mm in C. schlosseri and 65 mm in E. samium. Despite the similar length, the symphysis is much wider in C. schlosseri, with the incisors being 82.3 mm apart, whereas in E. samium they are only 58 mm apart. This makes the symphyseal area of E. samium look much more elongated than C. schlosseri.
All these characters prove that C. schlosseri and E. samium show significant differences to each other. This not only confirms that they represent distinct species but also justifies a separation at the generic level with C. schlosseri being a member of the genus of Chilotherium that was erected by Ringström (Citation1924) and E. samium belonging to Eochilotherium that was initially erected by Geraads and Spassov (Citation2009) as a subgenus of Chilotherium and is herein raised to the genus level. Moreover, a clear separation of the two taxa is possible based on consistent morphological features that do not seem to vary importantly in the more ample material of C. schlosseri crania from Samos (Greece). The diagnostic traits of the two species do not overlap at all, making a subjective synonymy practically impossible.
Comparison of Chilotherium schlosseri to Other Species of Chilotherium
The species C. schlosseri belongs in the Chilotheriina based on the wide mandibular symphysis, the hypsodont teeth and their complex enamel folds, including the strong constrictions of the protocone and hypocone and the very long antecrochet that bends lingually. However, it differs from the genera Eochilotherium, as mentioned above, and Shansirhinus (Kretzoi, Citation1942). Deng (Citation2005) described an almost complete adult skull of the species Shansirhinus ringstroemi (Kretzoi, Citation1942). It does not exhibit the prominent depression in the frontal bones seen in C. schlosseri, and has thicker nasal bones, closer situated parietal crests (∼40 mm), a narrower mandibular symphysis, with smaller and more triangular lower incisors (Deng, Citation2005). However, both species exhibit similarly developed secondary enamel folds, such as a crochet that often closes the medifossette, strong protocone constrictions, the connection between protocone and hypocone in the premolars, and the variable presence of enamel plications in the upper teeth. Though, S. ringstroemi exhibits a much more prominent paracone fold and the hypocone is additionally distally constricted, as seen in the M1 (Deng, Citation2005:fig. 6), in contrast to C. schlosseri. Based on features in its cranial morphology like the very widely separated parietal crests, the prominent frontal depression, the flat dorsal profile of the skull, along with its rather complex tooth morphology C. schlosseri can be attributed to the genus Chilotherium. The comparison of C. schlosseri will involve the following members of Chilotherium sensu stricto Chilotherium persiae from Maragheh in Iran (Pandolfi, Citation2016; Pohlig, Citation1886), Chilotherium habereri from various localities in China (Killgus, Citation1922, Citation1923; Ringström, Citation1924; Schlosser, Citation1903), Chilotherium kowalevskii from Grebeniki in Ukraine (Korotkevich, Citation1970; Pavlow, Citation1913), Chilotherium anderssoni from Loc. 30 in China (Ringström, Citation1924), Chilotherium sarmaticum from Berislav in Ukraine (Korotkevich, Citation1958, Citation1970), and Chilotherium orlovi from Palvodar in Kazakhstan (Bayshashov, Citation1982, Citation1993). These species have a very similar cranial and dental morphology. They share all typical features known for the genus Chilotherium, such as the depression in the frontal bones, the separated parietal crests, the highly placed orbit, the hypsodont teeth with very complicated enamel folds and the wide mandibular symphysis with large tusk-like incisors that are dorsolaterally upturned, with large wear facets and have an obtuse triangular shape in cross section (Geraads & Spassov, Citation2009; Ringström, Citation1924). Additionally, another feature that seems to be similar in these species is the shape of the zygomatic arches, which are relatively robust and tend to become wider posteriorly. In C. schlosseri, some of these features are, however, more developed than in the other species of Chilotherium. For instance, the frontal depression in C. schlosseri is very strong and also involves the nasal bones, which as a result are placed more ventrally in comparison to the orbit (). Thereby, even in lateral view the ‘nasofrontal’ depression is easily distinguishable, due to the placement of the nasal bones (; Weber, Citation1905:pl. 8, fig. 1), a feature that is more rarely observed in other Chilotherium species. Another important trait is that C. schlosseri exhibits parietal crests that are always separated from each other by at least 70 mm (n = 7). This represents an autapomorphic feature of this species, not seen in any other Chilotherium. The only other Chilotherium species where the minimal distance between the parietal crests may reach over 70 mm, but not consistently, seem to be C. sarmaticum and C. orlovi, varying from 51–76 mm (n = 4; Korotkevich, Citation1970) and from 45–75 mm (n = 3; Bayshashov, Citation1982), respectively (). These two species have been given only little attention since their first description and are not considered in most recent studies about Chilotherium. In almost all other species the parietal crests are always less than 70 mm apart (; Kampouridis et al., Citation2022b:table 2).
The comparison of the upper teeth of Chilotherium spp. is somewhat more complicated, because an important degree of intraspecific variability exists and has to be taken into account. The dentition of C. schlosseri () exhibits relatively hypsodont teeth, with very complicated secondary enamel folds. Most prominently, the crochet is usually very strong and the protocone is always very strongly constricted, leading to a prominent antecrochet that usually closes off the median valley. Specifically in the molars, the protocone constriction is very strong and it has a flat lingual side. This leads the protocone to have a subtriangular shape. These features are usually also observed in C. persiae, C. habereri, C. kowalevskii, C. anderssoni, C. sarmaticum, and C. orlovi. A lingual cingulum, which seems to be almost continuous, is always present in the premolars of C. schlosseri but may also be present in these species. In C. schlosseri, a crista can also be present both in the premolars and the molars. This enamel fold is usually lacking in most Chilotherium species, like in C. habereri and C. anderssoni, where it is only occasionally present, and C. kowalevskii, where it seems to be lacking completely. In C. schlosseri, the crochet very frequently leads to the closure of the medifossette, in which the crista is often involved, whereas in most other species, this is not as common. Although, in the holotype of C. orlovi figured by Bayshashov (Citation1993), a closed medifossette exists in most premolars and in the M1. Another dental feature that seems to separate C. schlosseri from most other Chilotherium species is the fact that it frequently exhibits small enamel plications in the upper cheek teeth, as seen in the right M1 in GPIH 3015 (). These plications are not constantly present, and their appearance may vary intra-specifically and depending on the wear stage of the teeth. Nonetheless, the upper teeth of the well-sampled species C. persiae almost never exhibit such plications. Similarly, in the figured toothrows assigned to C. kowalevskii by Pavlow (Citation1913), to C. anderssoni by Ringström (Citation1924), and to C. sarmaticum by Korotkevitch (Citation1970), also no plications are visible in the permanent dentition and the authors also do not mention any such features.
The mandible bears maybe the most prominent feature of the group: the wide mandibular symphysis with widely separated large incisors (Ringström, Citation1924). The width of the symphysis and the size and shape of the incisors is characterized by notable sexual dimorphism, with males having wider symphyses and large tusks, whereas females have somewhat narrower symphyses and notably smaller incisors (e.g., Chen et al., Citation2010; Weber, Citation1905). The neotype of C. schlosseri does not preserve the complete crown of the lower incisors, only the bases of the teeth, where they are very wide (about 45 mm). The mandibular symphysis is, however, almost perfectly preserved and is very massive, reaches posterior the p3s and the incisors are widely separated from each other. These features clearly separate C. schlosseri from any non-chilothere rhino; however, within the genus Chilotherium these features are rather common and relatively uniform, not allowing a specific identification of a mandible (e.g., Kampouridis et al., Citation2022b). Nonetheless, the features of the skull and upper dentition allow the distinction of C. schlosseri from all other chilothere species.
Comparison of Eochilotherium samium to Aceratherium kiliasi
The problematic taxonomic status of Aceratherium kiliasi has been discussed by several authors (Athanassiou et al., Citation2014; Fortelius et al., Citation2003; Geraads & Spassov, Citation2009; Giaourtsakis, Citation2003; Heissig, Citation1996, Citation1999; Kampouridis et al., Citation2022b). It was originally erected by Geraads and Koufos (Citation1990) for aceratheriine material from the Vallesian locality Pentalophos-1 in Northern Greece. Later, it was pointed out that the material belongs to two distinct aceratheriine taxa (Fortelius et al., Citation2003). The holotype and some other specimens belong to a chilothere (herein E. samium), whereas the rest of the material belongs to Acerorhinus neleus (Athanassiou et al., Citation2014). A potential close relationship between Aceratherium kiliasi and E. samium was already hinted by Heissig (Citation1996) and Giaourtsakis (Citation2022) suggested that Aceratherium kiliasi actually represents a junior synonym of E. samium.
We herein agree with the synonymy of these two species, because the holotype of Aceratherium kiliasi (PNT 135) exhibits the key features that characterize E. samium. More specifically Aceratherium kiliasi has flattened frontal bones, lacking the depression seen in Chilotherium. It also lacks the longitudinal groove in the middle of the nasal bones, just like E. samium. Regarding the upper dentition, the holotypic skull of Aceratherium kiliasi has a quite worn dentition, yet less worn than the dentitions of the lectotype and neotype of E. samium. The premolars feature either weakly constricted or unconstructed proto- and hypocones, similar to the P2 of E. samium. In the P2, despite the advanced wear stage, the protocone and the hypocone remain separated, as in E. samium. Similarly, the M1 is almost completely worn, and yet the protocone and the hypocone are barely connected, without being fully fused. This is very similar to the M2 in the neotype of E. samium. The protocone constriction in the molars in Aceratherium kiliasi also seems to be weaker than in Chilotherium and similar to E. samium. Both in the M2 and M3 the crochet is similarly developed to the lectotype and neotype of E. samium. Thus, the two species represent synonyms, with the earlier described E. samium (Weber, Citation1905), having priority over Aceratherium kiliasi that was described much later (Geraads & Koufos, Citation1990) according to the Principle of Priority (ICZN Art. 23.3).
Comparison of Eochilotherium samium to ‘Primitive’ Chilotherium Species
The species E. samium belongs in the Chilotheriina based on the wide mandibular symphysis, the hypsodont teeth, and their relative complex enamel folds. However, it differs from the genera Chilotherium, as mentioned above, and Shansirhinus. Despite the fact that Eochilotherium and Shansirhinus exhibit several similarities such as the lack of the prominent depression in the frontal bones seen in Chilotherium, the moderately separated parietal crests (∼40 mm in both species), a comparably narrower mandibular symphysis, with smaller and more triangular lower incisors (Deng, Citation2005), the two taxa exhibit several characters that distinguish them. Shansirhinus ringstroemi exhibits more prominent secondary enamel folds, such as a crochet that often closes the medifossette, stronger protocone and hypocone constrictions, a stronger paracone fold, a connection between protocone and hypocone in the premolar, and the existence of enamel plications in the upper teeth. Therefore, E. samium is placed in a different genus. The comparison of E. samium will only include the more ‘primitive’ chilotheres ‘C.’ wimani and ‘C.’ primigenium. These species lack the autapomorphic traits seen in the typical Chilotherium species. All three species lack the depression in the frontal bones that is visible in Chilotherium, as seen in C. schlosseri () and C. anderssoni (Ringström, Citation1924:table 2, figs. 1, 2). On the contrary, in E. samium, ‘C.’ wimani and ‘C.’ primigenium, the frontal bones are convex and become flat posteriorly. In E. samium and ‘C.’ wimani the parietal bones are transversally concave as in typical Chilotherium species, whereas in ‘C.’ primigenium they are only very slightly separated, forming a sagittal crest, a feature not seen in any other chilothere species. All three species exhibit convex frontal bones that posteriorly become more flattened and in E. samium and ‘C.’ wimani the surface between the parietal crests is transversally concave, in contrast to the convex sagittal crest seen in ‘C.’ primigenium, though Deng (Citation2006a) mentioned that ‘C.’ wimani may also form a sagittal crest. Also, the parietal region, in which the brain would be situated is transversally generally narrower in these three species than in typical Chilotherium. The skulls of E. samium and ‘C.’ wimani are also rather dolichocephalic, whereas ‘C.’ primigenium seems to have a comparably more brachycephalic skull. Although, in E. samium, the occipital region seems to not have been as highly elevated as in ‘C.’ wimani and ‘C.’ primigenium.
Regarding the mandible, both ‘C.’ primigenium and ‘C.’ wimani exhibit a very wide mandibular symphysis with very large tusk-like incisors. In E. samium the mandibular symphysis is somewhat narrower, and the incisors are much smaller, most likely due to the mandible of SMF M 3601 belonging to a female individual. The existence of prominent sexual dimorphism is known in chilotheres and especially well-documented in ‘C.’ wimani (Chen et al., Citation2010). Otherwise, both the mandible and the lower dentition do not seem to show any differentiating features. Though, in ‘C.’ primigenium both the upper and lower dentition is extremely worn and only badly preserved. In the upper teeth, however, it is visible that all three species exhibit weakly constricted or unconstricted premolars, as seen in the completely unconstructed protocone and hypocone in the P2 that is preserved in all three species (; Deng, Citation2001, Citation2006a), thereby showing that E. samium must be closer affiliated with “C.” wimani and possibly “C.” primigenium than with the species of the genera Chilotherium and Shansirhinus.
DISCUSSION
Taxonomy
The Eastern European hornless rhinos have remained rather elusive, despite their existence in several localities during the Late Miocene (e.g., Athanassiou et al., Citation2014; Geraads & Spassov, Citation2009; Korotkevich, Citation1970). This is mainly due to the high number of described species that have been primarily associated with the genus Chilotherium and the fact that most of the type material of these new species has been unavailable for further study (Kampouridis et al., Citation2022b). This is also the case for the type skulls of the four species of hornless rhinos that have been erected based on material from the Upper Miocene deposits of Samos Island in Greece (Giaourtsakis, Citation2022; Kampouridis et al., Citation2022b). The four species C. schlosseri, E. samium, C. wegneri, and C. angustifrons were initially placed in the genus Aceratherium (Andree, Citation1921; Weber, Citation1905) and later incorporated into the genus Chilotherium (Heissig, Citation1975; Ringström, Citation1924). The type material of all four species is considered lost, with the exception of the type mandible of C. wegneri, which was relocated in the collections of the GMM (Kampouridis et al., Citation2022b). After careful comparisons, based on the illustrations of the type material, the two species C. wegneri and C. angustifrons can undoubtedly be considered junior synonyms of C. schlosseri, supporting the notion of previous studies (Giaourtsakis, Citation2022; Kampouridis et al., Citation2022b). Thereby only C. schlosseri and E. samium remain, which in fact are the first chilothere species that were described from Europe. This makes the affirmation of their validity even more important. For this purpose, we decided to establish neotypes to replace the destroyed lectotypes of both species that Weber (Citation1905) described. The skulls that were selected as neotypes (GPIH 3015 for C. schlosseri and SMF M 3601 for E. samium) show all essential characters that are known from the lectotypes of Aceratherium schlosseri and Aceratherium samium, as described and illustrated by Weber (Citation1905).
The two taxa also exhibit important differences from each other, primarily in their skull morphology (), confirming that they represent distinct species. These differences include: a generally much wider skull in C. schlosseri compared with the dolichocephalic skull of E. samium (); the presence of a prominent depression in the frontal bones in C. schlosseri () that leads to a lower position of the nasal bones (), whereas in E. samium the frontal bones are convex in the anterior part and posteriorly become flat (, ); slightly upwards turning nasal bones with a deep longitudinal groove in the middle in C. schlosseri, in contrast to the straight nasal bones without a prominent groove in E. samium (); widely separated parietal crests (>70 mm) in C. schlosseri, whereas in E. samium they are more closely situated (∼40 mm) (; ); and the posteriorly widening zygomatic arches in C. schlosseri compared with the straight zygomatic arches with a constant width seen in E. samium ().
The upper dentition of the neotype of C. schlosseri (GPIH 3015) is very well preserved and only moderately worn (), thereby revealing the dental morphology of all teeth. This specimen shows that C. schlosseri has a very complex tooth morphology that involves many secondary enamel folds like a very strong crochet, which along with the potential crista close off the medifossette, an early closure of the postfossete, strongly constricted protocones in all teeth that create a prominent antecrochet that closes off the median valley. These are all features that are also visible in the lectotype of Aceratherium schlosseri (Weber, Citation1905:table 9, fig. 1), including also the presence of some enamel plications, which seem to be variably present in the teeth of this species, in contrast to most other Chilotherium species, where usually no plications are present. This makes C. schlosseri a highly derived member of the chilotheres. Unfortunately, the teeth in the neotype of E. samium (SMF M 3601) are almost completely worn down and badly preserved, so only little can be said about its dental morphology. It seems that the secondary folds are well developed in comparison to other genera of rhinoceroses, but not as prominent as in C. schlosseri () and other members of the genus Chilotherium, as seen by the crochet in the left M2, which despite its advanced wear stage is only moderately long and does not close off the medifossette and the antecrochet in the same tooth does not cut off the median valley. In both P4s of SMF M 3601 the antecrochet barely connects to the hypocone and closes the median valley. The dentition of the lectotype of Aceratherium samium seems to offer similar insights, as in the P2, P4, M1, and M2 no medifossette exists and the median valley in the P4 remains open. These are generally more plesiomorphic features among chilotheres and separate E. samium from all species of Chilotherium, justifying an attribution to a distinct genus, which is herein applied to bring clarity into the confusion concerning the chilotheres.
Relationships with Other Chilothere Species
The complex secondary enamel folds, the features of the skull and the wide mandibular symphysis with large incisors of C. schlosseri show that it is a typical representative of the genus Chilotherium. Moreover, it seems to be closely aligned to the Vallesian species C. sarmaticum from Berislav (Ukraine) and the Turolian C. orlovi from Pavlodar (Kazakhstan), based on the distance between the parietal crests. Additionally, the dentition of C. schlosseri seems to be very similar to C. kowalevskii from the Turolian of Grebeniki (Ukraine), with complicated enamel folds such as the strong protocone constriction that results in a very strong antecrochet and the infrequently present cingulum in the upper premolars. A close relationship between C. schlosseri and C. kowalevskii is also suggested by the similar dimensions of their metapodials that differ from those of C. sarmaticum (Korotkevich, Citation1970; Krokos, Citation1917; Weber, Citation1905). Thus, it seems likely that these species share a close relationship and are more distantly related to the other chilotheres known from Europe and Asia.
Eochilotherium samium seems to share a more distant relationship to the other European species and is probably closer aligned to the “primitive” chilotheres found in China: ‘C.’ wimani and ‘C.’ primigenium. They share some of the defining features that are also present in the typical members of Chilotherium, such as the wide mandibular symphysis with large tusk-like incisors and a small P2/p2 (Deng, Citation2001, Citation2006a). Additionally, E. samium and ‘C.’ wimani also exhibit a transversally concave parietal region. However, they also exhibit plesiomorphic features such as slightly convex frontal bones that flatten posteriorly, straight nasal bones, without a prominent longitudinal groove, and a raised occipital region of the skull. In addition, E. samium features also a somewhat simpler tooth morphology than ‘C.’ wimani (Deng, Citation2006a). Thus, E. samium lacks many of the apomorphic characters seen in Chilotherium, justifying its placement in a different genus. A potential inclusion of ‘C.’ wimani in Eochilotherium is more ambiguous, and the species is herein provisionally kept in its original genus, pending further studies clarifying its generic attribution. ‘Chilotherium’ primigenium, on the other hand, exhibits an unusual combination of characters. It features a very wide mandibular symphysis with large tusk-like incisors and a small p2, which suggests a close relationship to the genus Chilotherium. However, ‘C.’ primigenium includes also more plesiomorphic traits than Eochilotherium such as the closely situated parietal crests that form a sagittal crest instead of more widely separated crests with a concave interior, while also exhibiting convex frontal bones that lack the characteristic frontal depression of the genus Chilotherium. ‘Chilotherium’ primigenium additionally exhibits rather thick nasal bones that are long, and completely straight, without any longitudinal groove separating the nasal bones from each other. Moreover, in lateral view the posterior part of the skull is extremely elevated and in caudal view the skull is bell-shaped. These features separate ‘C.’ primigenium from the typical representatives of Chilotherium, but also from the ‘primitive’ E. samium and ‘C.’ wimani and suggest that it should probably be placed in a different genus. However, further study is needed to clarify its exact taxonomic status and phylogenetic affinities. For the time being, we therefore decided to temporarily keep it in its original genus, while pointing out the issues and suggesting that a generic separation might be preferable.
The comparison of the three genera included in the Chilotheriina, Chilotherium, Eochilotherium, and Shansirhinus, demonstrates their shared similarities, such as the wide mandibular symphysis, the tusk-like lower incisors, the moderately to widely separated parietal crests, the hypsodont teeth, and the very complex enamel folds in the upper teeth. However, it also illustrates the important differences, such as the morphology of the frontal bones, which are notably depressed in Chilotherium, but are flat to convex in Eochilotherium and Shansirhinus, the posterior portion of the skull that is transversally rather wide in Chilotherium, whereas in the other two genera it is narrower, the size and form of the lower incisors, which are large, flat, asymmetrical and tusk-like in Chilotherium and somewhat smaller, more symmetrical and less flattened in Eochilotherium and Shansirhinus. Additionally, the tooth morphology is variably developed in these genera, with Shansirhinus having the most complicated secondary folds in the Chilotheriina. This supports that they represent distinct genera and shows that ‘C.’ wimani and ‘C.’ primigenium do not exactly fit the description of either genus and might actually represent different genera.
CONCLUSIONS
The European chilotheres have a very complicated taxonomic history, with the erection of over eight species. Herein, we attempt to solve some of the issues involving this group by designating neotypes for the first two described chilothere species from Europe, Chilotherium schlosseri and Eochilotherium samium, both from the Upper Miocene deposits of Samos Island (Greece). The revision showed that they represent two distinct taxa, with E. samium being placed in a distinct genus, based on some cranial features such as the missing depression in the frontal bones and its less complex dental morphology, such as the less pronounced protocone constriction. Chilotherium schlosseri represents one of the most derived species of the genus, based on its very widely separated parietal crests and its very complex tooth morphology, which frequently includes enamel plications. This species is closely allied with other Eastern European Chilotherium species, Chilotherium kowalevskii, Chilotherium sarmaticum from Ukraine and the Asian species Chilotherium orlovi from Kazakhstan. Eochilotherium samium on the other hand, seems to be more similar to the Chinese ‘Chilotherium’ wimani and ‘Chilotherium’ primigenium.
AUTHOR CONTRIBUTIONS
Conceptualization: P.K., G.S., M.B.; Investigation: P.K., G.S., N.K., N.S., M.B.; Project Administration: N.S., M.B.; Validation: P.K., N.S., M.B.; Writing original draft: P.K.; Review and approval: P.K., G.S., N.K., N.S., M.B.
SUPPLEMENTARY FILES
Supplementary Data 1: figures of lower teeth and table with the most important features of the Samian chilotheres.
Supplemental Material
Download MS Word (645.1 KB)ACKNOWLEDGMENTS
We would like to thank S. Roussiakis, G. Theodorou (AMPG), O. Sandrock, M. Blume, M. Kollbacher (HLMD), M. Bertling, D. Theda (GMM), U. Kotthoff (GPIH), I. Werneburg (GPIT), C. Argot (MNHN), U. Göhlich, F. Zachos, A. Bibl (NHMW), L. Costeur, F. Dammeyer (NMB), R. Brocke, G. Riedel, L. Kraus (SMF), and E. Amson (SMNS), for allowing us to study material under their care. We thank P.-O. Antoine (University of Montpellier, France), A. Athanassiou (Ephorate of Palaeoanthropology-Speleology at the Hellenic Ministry for Culture, Greece), B. U. Bayshashov (Institute of Zoology in Akademgorodok, Kazakhstan), I. Giaourtsakis (Ludwig-Maximilians-University of Munich, Germany) and K. Heissig (Staatliche Naturwissenschaftlichen Sammlungen Bayerns, Germany) for fruitful discussions and for providing literature about fossil rhinoceroses. We thank the Editor A. Friscia (University of California, U.S.A.), an anonymous reviewer and P.-O. Antoine (University of Montpellier, France) for their helpful comments that improved this study. This research received support from the SYNTHESYS + project (http://www.synthesys.info/) which is financed by European Community Research Infrastructure Action under the H2020 Integrating Activities Programme, Project number 823827 (AT-TAF-TA3-9 and AT-TAF-TA4-29 and FR-TAF-TA4-71).
LITERATURE CITED
- Andree, J. (1921). Rhinocerotiden aus dem Unterpliocän von Samos. Paläontologische Zeitschrift, 3, 189–212.
- Antoine, P.-O. (2002). Phylogénie et évolution des Elasmotheriina (Mammalia, Rhinocerotidae). Mémoires du Muséum National d’Histoire Naturelle, 188, 1–359.
- Antoine, P.-O. (In Press). Rhinocerotids from the Siwalik faunal sequence. In C. Badgley, D. Pilbeam, & M. Morgan (Hrsg.), At the Foot of the Himalayas: Paleontology and Ecosystem Dynamics of the Siwalik Record of Pakistan. Johns Hopkins University Press.
- Antoine, P.-O., & Saraç, G. (2005). Rhinocerotidae (Mammalia, Perissodactyla) from the late Miocene of Akkașdaği, Turkey. Geodiversitas, 27(4), 601–632.
- Ataabadi, M. M., Bernor, R. L., Kostopoulos, D. S., Wolf, D., Orak, Z., Zare, G., Nakaya, H., Watabe, M., & Fortelius, M. (2013). Recent Advances in Paleobiological Research of the Late Miocene Maragheh Fauna, Northwest Iran. In M. Fortelius, X. Wang, & L. Flynn (Hrsg.), Fossil Mammals of Asia (S. 546–565). Columbia University Press. https://doi.org/10.7312/columbia/9780231150125.003.0025
- Athanassiou, A., Roussiakis, S. J., Giaourtsakis, I. X., Theodorou, G. E., & Iliopoulos, G. (2014). A new hornless rhinoceros of the genus Acerorhinus (Perissodactyla: Rhinocerotidae) from the Upper Miocene of Kerassiá (Euboea, Greece), with a revision of related forms. Palaeontographica Abteilung A, 303(1–3), 23–59. https://doi.org/10.1127/pala/303/2014/23
- Bayshashov, B. U. (1982). A new rhinoceros species of the genus Chilotherium from Pavlodar. In Mesozoic and Cenozoic vertebrate fauna and flora of North-Eastern and southern Kazakhstan (Bd. 8, S. 72–83). Academy of Sciences of Kazakhstan.
- Bayshashov, B. U. (1993). Neogene rhinoceroses of Kazakhstan. Academy of Sciences of Kazakhstan.
- Bernor, R. L. (1986). Mammalian biostratigraphy, geochronology, and zoogeographic relationships of the Late Miocene Maragheh fauna, Iran. Journal of Vertebrate Paleontology, 6(1), 76–95. https://doi.org/10.1080/02724634.1986.10011600
- Bonis, L. de, de Brunet, M., Heintz, E., & Sen, S. (1992). La province Greco-irano-afghane et la répartition des faunes mammaliennes au Miocène supérieur. Paleontologia i Evolucio, 24–25, 103–112.
- Cerdeño, E. (1995). Cladistic Analysis of the Family Rhinocerotidae (Perissodactyla). American Museum Novitates, 3143, 28.
- Chen, S., Deng, T., Hou, S., Shi, Q., & Pang, L. (2010). Sexual Dimorphism in Perissodactyl Rhinocerotid Chilotherium wimani from the Late Miocene of the Linxia Basin (Gansu, China). Acta Palaeontologica Polonica, 55(4), 587–597. https://doi.org/10.4202/app.2009.0001
- Deng, T. (2001). New material of Chilotherium wimani (Perissodactyla, Rhinoceritdae) from the Late Miocene of Fugu, Shaanxi. Vertebrata Palasiatica, 39(2), 129–138.
- Deng, T. (2005). New cranial material of Shansirhinus (Rhinocerotidae, Perissodactyla) from the Lower Pliocene of the Linxia Basin in Gansu, China. Geobios, 38(3), 301–313. https://doi.org/10.1016/j.geobios.2003.12.003
- Deng, T. (2006a). A primitive species of Chilotherium (Perissodactyla, Rhinocerotidae) from the Late Miocene of Linxia Basin (Gansu, China). Cainozoic Research, 5(1–2), 93–102.
- Deng, T. (2006b). Neogene Rhinoceroses of the Linxia Basin (Gansu, China). Courier Des Forschungsinstituts Senckenberg, 256, 43–56.
- Dollo, L. (1885). Rhinocéros vivants et fossiles. Revue de Questions Scientifiques, 17, 293–299.
- Drevermann, F. (1930). Aus der Zeit des dreizehigen Pferdes. Natur und Museum, 60(1), 2–13.
- Forsyth-Major, C. I. (1888). Sur un gisement d’ossements fossiles dans l’île de Samos contemporains de l’âge de Pikermi. Comptes Rendus Hebdomadaires des Séances de l’Académie des Sciences, 107, 1178–1181.
- Fortelius, M., & Heissig, K. (1989). The phylogenetic relationships of the Elasmotherini. Mitteilungen der Bayerischen Staatssammlung für Paläontologie und historische Geologie, 29, 227–233.
- Fortelius, M., Heissig, K., Saraç, G., & Sen, S. (2003). Rhinocerotidae (Perissodactyla). In Geology and Paleontology of the Miocene Sinap Formation, Turkey (S. 282–307). Columbia University Press.
- Garevski, R. (1974). Beitrag zur Kenntnis der Pikermifauna Mazedoniens. Fossilreste der Chalicotheriiden. Fragmenta Balcanica, 9, 201–209.
- Gaudry, A. (1862). Animaux fossiles et géologie de l’Attique. F. Savy.
- Geraads, D., & Koufos, G. (1990). Upper Miocene Rhinocerotidae (Mammalia) from Pentalophos-1, Macedonia, Greece. Palaeontographica Abteilung A, 210, 151–168.
- Geraads, D., & Spassov, N. (2009). Rhinocerotidae (Mammalia) from the Late Miocene of Bulgaria. Palaeontographica Abteilung A, 287(4–6), 99–122. https://doi.org/10.1127/pala/287/2009/99
- Giaourtsakis, I. X. (2003). Late Neogene Rhinocerotidae of Greece: Distribution, diversity and stratigraphical range. Deinsea, 10, 235–253.
- Giaourtsakis, I. X. (2009). The Late Miocene Mammal Faunas of the Mytilinii Basin, Samos Island, Greece: New Collection 9. Rhinocerotidae. Beiträge Zur Paläontologie, 31, 157–187.
- Giaourtsakis, I. X. (2022). The Fossil Record of Rhinocerotids (Mammalia: Perissodactyla: Rhinocerotidae) in Greece. In E. Vlachos (Hrsg.), Fossil Vertebrates of Greece Vol. 2 (S. 409–500). Springer International Publishing. https://doi.org/10.1007/978-3-030-68442-6_14
- Gray, J. E. (1821). On the natural arrangemenet of vertebrose animals. London Medical Repository, 15, 297–310.
- Gürich, G. (1911). Fossile Säugetierreste aus Samos. Verhandlungen des Naturwissenschaftlichen Vereins in Hamburg, 3(19), 79.
- Heissig, K. (1975). Rhinocerotidae aus dem Jungtertiär Anatoliens. Geologisches Jahrbuch (B), 15, 145–151.
- Heissig, K. (1996). The stratigraphical range of fossil rhinoceroses in the Late Neogene of Europe and the Eastern Mediterranean. In The evolution of westermn Eurasian Neogene Mammal Faunas (S. 339–347). Columbia University Press.
- Heissig, K. (1999). Family Rhinocerotidae. In The Miocene land mammals (S. 175–188). Pfeil.
- Hristova, L., Kovachev, D., & Spassov, N. (2003). Hipparion brachypus Hensel, 1862 from Hadjidimovo, souwestern Bulgaria (late Miocene). Comptes rendus de l’Académie bulgare des Sciences, 56(2), 77–84.
- Hullot, M., Antoine, P.-O., Spassov, N., Koufos, G. D., & Merceron, G. (2022). Late Miocene rhinocerotids from the Balkan-Iranian province: Ecological insights from dental microwear textures and enamel hypoplasia. Historical Biology, 1–18. https://doi.org/10.1080/08912963.2022.2095910
- Kampouridis, P., Hartung, J., Ferreira, G. S., & Böhme, M. (2022a). Reappraisal of the late Miocene elasmotheriine Parelasmotherium schansiense from Kutschwan (Shanxi Province, China) and its phylogenetic relationships. Journal of Vertebrate Paleontology, 41(6), e2080556. https://doi.org/10.1080/02724634.2021.2080556
- Kampouridis, P., Ratoi, B., & Ursachi, L. (2023). New evidence for the unique coexistence of two subfamilies of clawed perissodactyls (Mammalia, Chalicotheriidae) in the Upper Miocene of Romania and the Eastern Mediterranean. Journal of Mammalian Evolution. https://doi.org/10.1007/s10914-023-09657-5
- Kampouridis, P., Svorligkou, G., Kargopoulos, N., & Augustin, F. J. (2022b). Reassessment of ‘Chilotherium wegneri’ (Mammalia, Rhinocerotidae) from the late Miocene of Samos (Greece) and the European record of Chilotherium. Historical Biology, 34(3), 412–420. https://doi.org/10.1080/08912963.2021.1920939
- Kaup, J. (1832). Über Rhinoceros incisivus Cuv., und eine neue Art, Rhinoceros schleiermacheri. Isis von Oken, (8), 898–904.
- Kiernik, E. (1913). Über einen Aceratheriumschädel aus der Umgebung von Odessa. Bulletin international de l’Académie des sciences de Cracovie, 1913, 808–864.
- Killgus, H. (1922). Die Unterpliocaenen Chinesischen Säugetierreste der Tafelschen Sammlung zu Tübingen [Ph.D. Dissertation]. Eberhard-Karls University of Tübingen.
- Killgus, H. (1923). Unterpliozäne Säuger aus China. Paläontologische Zeitschrift, 5(3), 251–257. https://doi.org/10.1007/BF03160368
- Korotkevich, O. L. (1958). A new Chilotherium species from the Sarmatian deposits of the Ukraine. Dopovidi Akad. Nauk Ukrainskoi RSR, 12, 1372–1376.
- Korotkevich, O. L. (1970). The mammals of the Berislav late Sarmatian Hipparion-fauna. In The Natural Environment and the fauna of the past (5. Aufl., S. 24–121). Naukova Dumka.
- Kostopoulos, D. S., Koufos, G. D., Sylvestrou, I. A., Syrides, G. E., & Tsombachidou, E. (2009). The Late Miocene Mammal Faunas of the Mytilinii Basin, Samos Island, Greece: New Collection: 2. Lithostratigraphy and Fossiliferous Sites. Beiträge Zur Paläontologie, 31, 13–26.
- Kostopoulos, D. S., Sen, S., & Koufos, G. D. (2003). Magnetostratigraphy and revised chronology of the late Miocene mammal localities of Samos, Greece. International Journal of Earth Sciences, 92(5), 779–794. https://doi.org/10.1007/s00531-003-0353-8
- Koufos, G. D. (2009). The Late Miocene Mammal Faunas of the Mytilinii Basin, Samos Island, Greece: New Collection: 1. History of the Samos Fossil Mammals. Beiträge Zur Paläontologie, 31, 1–12.
- Koufos, G. D., Kostopoulos, D. S., & Vlachou, T. D. (2009). The Late Miocene Mammal Faunas of the Mytilinii Basin, Samos Island, Greece: New Collection 16. Biochronology. Beiträge Zur Paläontologie, 31, 397–408.
- Koufos, G. D., Kostopoulos, D. S., Vlachou, T. D., & Konidaris, G. E. (2011). A synopsis of the late Miocene Mammal Fauna of Samos Island, Aegean Sea, Greece. Geobios, 44(2–3), 237–251. https://doi.org/10.1016/j.geobios.2010.08.004
- Kretzoi, M. (1942). Bemerkungen zum System der Nachmiozänen Nashorn-Gattungen. Földtani Közlöny, 72(1), 4–12.
- Krokos, W. I. (1917). Aceratherium schlosseri Web. Du village de Grebeniki du gouvernement de Kherson. Memoires of the Agricultural Society of Southern Russia, 82(2), 1–96.
- Lehmann, U. (1984). Notiz für Säugerreste von der Insel Samos in der Sammlung des Geologisch-Paläontologischen Instituts und Museums Hamburg. Mitteilungen des Geologisch-Paläontologischen Instituts der Universität Hamburg, 57, 147–156.
- Linnaeus, C. (1758). Systema Naturae. Laurentii Salvii.
- Lu, X.-K., Deng, T., & Pandolfi, L. (2023). Reconstructing the phylogeny of the hornless rhinoceros Aceratheriinae. Frontiers in Ecology and Evolution, 11, 1005126. https://doi.org/10.3389/fevo.2023.1005126
- Mecquenem, R. de. (1908). Contribution a l'étude du gisement des vertébrés de Maragha et de ses environs. Annales d'histoire Naturelle, 1, 27–98.
- Niezabitowski, E. von L. (1912). Teleoceras ponticus n. Sp. Vorläufige Notiz (S. 2). Nowy Targ.
- Niezabitowski, E. von L. (1913). Über das Schädelfragment eines Rhinocerotiden (Teleoceras pοnticus Niez.) von Odessa. Bulletin International de I’Academic des Sciences de Cracovie, Series B, 223–235.
- Nothdurft, W., & Smith, J. B. (2002). The lost dinosaurs of Egypt. Random House.
- Osborn, H. F. (1899). On Pliohyrax kruppi Osborn, a fossil hyracoid from Samos, Lower Pliocene in the Stuttgart collection. A new type and first known Tertiary hyracoid. Proceedings of the 4th International Congress of Zoology, 172–173.
- Owen, R. (1848). Description of teeth and portions of jaws of two extinct anthracotherioid quadrupeds (Hyopotamus vectianus and Hyop. Bovinus) discovered by the Marchioness of Hastings in the Eocene deposits on the N.W. coast of the Isle of Wight; with an attempt to develop Cuvier’s idea of the classification of pachyderms by the number of their toes. Quarterly Journal of the Geological Society of London, 4, 103–141.
- Pandolfi, L. (2016). Persiatherium rodleri, gen. Et sp. Nov. (Mammalia, Rhinocerotidae) from the upper Miocene of Maragheh (northwestern iran). Journal of Vertebrate Paleontology, 36(1), e1040118. https://doi.org/10.1080/02724634.2015.1040118
- Pavlow, M. (1913). Mammifères tertiaires de la Nouvelle Russie, 1. Partie: Artiodactyla, Perissodactyla (Aceratherium kowalevskii n.s.). Nouveaux Mémoires de la Société Impériale des Naturalistes de Moscou, 17, 1–68.
- Pohlig, H. (1886). On the Pliocene of Maragha, Persia, and its resemblance to that of Pikermi in Greece. Quaterly Journal of the Geological Society of London, 42, 177–179.
- Qiu, Z. X., Xie, J. Y., & Yan, D. F. (1987). A new chilothere skull from Hezheng, Gansu, China, with special reference to the Chinese “Diceratherium”. Scientia Sinica B, 5, 545–552.
- Ride, W. D. L., Cogger, H. G., Dupris, C., Kraus, B., Minelli, A., Thompson, F. C., & Tubbs, P. K. (1999). International Code of Zoological Nomenclature. International Trust for the Zoological Nomenclature and the British Museum.
- Ringström, T. (1924). Nashörner der Hipparion-Fauna Nord-Chinas. Palaeontologia Sinica, 1(4), 1–156.
- Roussiakis, S., Filis, P., Sklavounou, S., Giaourtsakis, I. X., Kargopoulos, N., & Theodorou, G. (2019). Pikermi: A classical European fossil mammal geotope in the spotlight. European Geologist, 48, 28–32.
- Schlosser, M. (1903). Die fossilen Säugethiere Chinas nebst einer Odontographie der recenten Antilopen. Abhandlungen der Koniglichen Bayerischen Akademie der Wissenschaften, 22, 1–221.
- Schlosser, M. (1921). Die Hipparionenfauna von Veles in Mazedonien. Abhandlungen der Bayerischen Akademie der Wissenschaften, 24(4), 1–55.
- Solounias, N., & Mayor, A. (2004). Ancient references to the fossils from the land of Pythagoras. Earth Sciences History, 23(2), 283–296.
- Solounias, N., & Ring, U. (2007). Samos Island, Part II: Ancient history of the Samos fossils and the record of earthquakes. Journal of the Virtual Explorer, 28(3), 1–18. https://doi.org/10.3809/jvirtex.2007.00179
- Solounias, N., Rivals, F., & Semprebon, G. M. (2010). Dietary interpretation and paleoecology of herbivores from Pikermi and Samos (late Miocene of Greece). Paleobiology, 36(1), 113–136. https://doi.org/10.1666/0094-8373-36.1.113
- Spassov, N. (2002). The Turolian Megafauna of West Bulgaria and the character of the Late Miocene ‘'Pikermian biome”. Bollettino della Società Paleontologica Italiana, 41(1), 69–81.
- Spassov, N., & Geraads, D. (2004). Tragoportax Pilgrim, 1937 and Miotragocerus Stromer, 1928 (Mammalia, Bovidae) from the Turolian of Hadjidimovo, Bulgaria, and a revision of the late Miocene Mediterranean Boselaphini. Geodiversitas, 26(2), 339–370.
- Spassov, N., Geraads, D., Hristova, L., Markov, G. N., Garevska, B., & Garevski, R. (2018). The late Miocene mammal faunas of the Republic of Macedonia (FYROM). Palaeontographica Abteilung A, 311(1–6), 1–85. https://doi.org/10.1127/pala/2018/0073
- Spassov, N., Geraads, D., & Markov, G. (2019). The late Miocene mammal fauna from Gorna Sushitsa, southwestern Bulgaria, and the early/middle Turolian transition. Neues Jahrbuch Für Geologie Und Paläontologie - Abhandlungen, 291(3), 317–350. https://doi.org/10.1127/njgpa/2019/0804
- Sun, D.-H., Li, Y., & Deng, T. (2018). A new species of Chilotherium (Perissodactyla, Rhinocerotidae) from the Late Miocene of Qingyang, Gansu, China. National Science Review, 56(3), 216–228. https://doi.org/10.19615/j.cnki.1000-3118.180109
- Vangengeim, E., & Tesakov, A. (2013). Late Miocene mammal localities of Eastern Europe and Western Asua: Toward bistratigraphic synthesis. In Fossil Mammals of Asia. Neogene Bistratigraphy and Chronology (S. 521–537). Columbia University Press.
- Wagner, A. (1848). Urweltliche Säugetier-Überreste aus Griechenland. Abhandlungen der Bayerischen Akademie der Wissenschaften, 5, 335–378.
- Weber, M. (1904). Ueber Tertiäre Rhinocerotiden von der Insel Samos. Bulletin de la Société Impériale des Naturalistes de Moscou, 17(4), 477–501.
- Weber, M. (1905). Über Tertiäre Rhinocerotiden von der Insel Samos. II. Bulletin de la Société Imperériale des Naturalistes de Moscou, 18(4), 344–363.
- Wessel, P., Luis, J. F., Uieda, L., Scharroo, R., Wobbe, F., Smith, W. H. F., & Tian, D. (2019). The Generic Mapping Tools version 6. Geochemistry, Geophysics, Geosystems, 20, 5556–5564. https://doi.org/10.1029/2019GC008515
