ABSTRACT
The Mediterranean Basin has been subjected to important geological and paleogeographic events that have shaped the evolution of the faunal assemblages that inhabit it. Two main events can be highlighted, the closure of its eastern margin by the Gomphotherium Land Bridge, and the temporal closure of the connection to the Atlantic Basin during the Messinian Salinity Crisis. These two events shaped the diversity of the chondrichthyan assemblages of the Mio–Pliocene Mediterranean, and their consequences can be observed even in the modern faunal composition. To better understand the taxonomic and ecological composition of the Mediterranean chondrichthyan faunas between the aforementioned events, and how they affected their evolutionary dynamics, we have performed a complete systematic and paleoenvironmental study of the chondrichthyan assemblage recovered from various Late Miocene localities at the municipality of Alcoy Basin (Alicante, eastern Spain). Our analysis allowed us to recognize up to 20 taxa of sharks and rays recovered from Miocene deposits. In addition, we reconstruct the paleobathymetry of the El Muro fossil site, based on the estimation of the Oceanization Index for foraminifera, as well as by the elasmobranch fossil assemblage via weighted bootstrap analysis. We expect that this first description and study of the Miocene chondrichthyan taxa from this area of eastern Spain could help us create a comparative framework to compare these communities with other areas, as well as show the changes in the elasmobranch species that have inhabited the Mediterranean Basin during and after the Messinian Salinity Crisis.
INTRODUCTION
The Mediterranean Sea is one of the biodiversity hot spots on the planet, hosting up to 18% of the global macroscopic sea life (Bianchi & Morri, Citation2000). This biodiversity has been partially shaped by its paleogeographic evolution, which is closely linked to its unique geological and paleoclimatic history, primarily associated with the tectonic plate interactions that occurred during the last 20 Ma in the region (Popov et al., Citation2006; Rögl, Citation1999). Among them, two events can be highlighted, the development of the Gomphotherium Land Bridge during the Early Miocene (∼19 Ma), which closed the connection with the Indian Ocean via the Eastern Mediterranean (Crespo, Citation2017; Hamon et al., Citation2013); and the Messinian Salinity Crisis (MSC) (∼5.9 Ma), derived from the collision of the European and African plates during the Late Miocene, which caused the closing of the Atlantic Ocean connection and the subsequent drastic reduction in water mass in the Mediterranean Basin (Gibert et al., Citation2013, Krijgsman et al., Citation1999; Rögl, Citation1999). These Neogene paleogeographic changes, mainly the MSC, had drastic environmental consequences (Marramà et al., Citation2019; Marsili, Citation2008; Pérès, Citation1985) and had a substantial impact on the Mediterranean marine biota, resulting in a drop in Mediterranean marine biodiversity (Agiadi et al., Citation2020; Coll et al., Citation2010; Monegatti & Raffi, Citation2010) including invertebrates e.g., corals (Vertino et al., Citation2014), echinoids (Borghi & Garilli, Citation2022), and vertebrates (Marsili, Citation2008), with chondrichthyans (sharks, rays, and chimaeras) being particularly affected (Villafaña et al., Citation2023).
At present, the Mediterranean Sea is recognized as one of the most diverse regions on the planet (Coll et al., Citation2010), which has its origin in the repopulation from the Atlantic Ocean through the Strait of Gibraltar once it reopened about 5.33 million years ago during the Zanclean flood (Coll et al., Citation2010; Gibert et al., Citation2013). Interestingly, elasmobranchs constitute one of the most evident examples of this repopulation, being represented currently in the Mediterranean by 49 species of sharks, 35 species of batoids, and two species of chimaera (Ebert & Dando, Citation2020). At the same time, the group shows a relatively high extinction-risk—30% of their known species are in some category of threat by the IUCN, 36% if the Data Deficient species are included (Davidson et al., Citation2022)—mainly related to the important environmental changes that are affecting the region (Dulvy et al., Citation2016). Considering that a better understanding of the history of the group in the region can help us understand how the different past environmental changes affected their diversity dynamics, any new data could be of great significance to predict how extant species may react to similar changes.
Within this framework, here we present the first complete systematic and paleoenvironmental analysis of the chondrichthyan assemblage recovered from various Late Miocene localities at the Alcoy Basin, western Mediterranean (Alicante, eastern Spain). The aim of this work is to expand the very limited knowledge of the poorly studied, but abundant Neogene elasmobranch faunas from Spain. We expect that our study could help us in the future to propose accurate conservation policies for the group, not just in the Mediterranean, but on a worldwide scale.
Previous Fossil Chondrichthyan Studies at the Western Mediterranean Area
Neogene outcrops from the Eastern Iberian Peninsula have yielded abundant Miocene and Pliocene-age fossil shark teeth (Adnet et al., Citation2010; Ferrón et al., Citation2019; Herraiz et al., Citation2020, Citation2023; Marín, Citation1992; Martínez-Pérez et al., Citation2018; Mendiola, Citation1996), allowing us to establish the presence of an important chondrichthyan faunal assemblage in the Western Mediterranean Sea. From north to south, several outcrops have yielded a diverse chondrichthyan community, highlighting the remains of †Otodus megalodon, †Galeocerdo aduncus, †Hemipristis serra, or †Carcharodon hastalis, from Langhian deposits of Tarragona (Herraiz et al., Citation2020). Of similar age, outcrops located in Villafranca del Penedès complete this faunal list with other taxa such as †Carcharias acutissima and †Carcharias cuspidatus (Bauzá Rullán & Plans, Citation1973).
Additional Miocene outcrops from eastern Spain include the elasmobranch faunal assemblage described from the El Chorrillo fossil site (Alicante, southeastern Spain) (Martínez-Pérez et al., Citation2018). This locality has yielded a shark assemblage characterized by microremains of at least seven taxa (Deania calcea, †Isistius triangulus, Squaliolus cf. †S. schaubi, †Paraetmopterus sp., Pristiophorus sp., Scyliorhinus sp., and a cf. Squaliformes indet.) of three different orders (Squaliformes, Pristiophoriformes, and Carcharhiniformes). In addition, associated macroremains have also been found, including teeth of †Carcharodon hastalis, Isurus sp., †Hemipristis serra, Odontaspis sp., Carcharhinus spp., and †Otodus megalodon (Ferrón et al., Citation2019; Martínez-Pérez et al., Citation2018). Also in the Alicante province, macrofossils—including an associated Carcharodon carcharias dentition—have been recovered from the Pliocene Guardamar del Segura fossil locality (Adnet et al., Citation2010), as well as the recent first description in the Mediterranean region of Trigonognathus sp. teeth from the Middle Miocene Ferriol fossil site (Herraiz et al., Citation2022). The Ferriol fossil site, along with other localities such as Vallongas and Castelar, has also been historically a source of isolated teeth belonging to multiple elasmobranch taxa including among them “†Hexanchus primigenius,” †Odontaspis cuspidata, Isurus cf. oxyrhinchus, †Isurus hastalis, Galeocerdo cuvieri, †Hemipristis serra, †Carcharhinus priscus, Sphyrna sp., “†Carcharocles megalodon” and a new Rhincodon species (Marín, Citation1992; Mendiola, Citation1996).
In addition to the Miocene elasmobranch faunas known from the Eastern Iberian Peninsula, there have been many documented findings of similar faunas from the Balearic Archipelago, mainly from the Mallorca and Menorca islands, where a variety of elasmobranch taxa were described, such as †Myliobatis meridionalis, Carcharias cf. taurus, and †Hemipristis serra (Bauzá Rullán, Citation1969; Bauzá Rullán & Mercadal, Citation1961; Mas, Citation2003, Citation2010; Mas & Fiol, Citation2002).
MATERIAL AND METHODS
All the material described in the present study comes from four localities situated at the municipality of Alcoy (Alicante, southeastern Spain) (). Three of the localities (El Muro, Castillo Barchell, and Bancales) were sampled for chondrichthyan microremains, and material from a fourth locality (Moli Palla) was studied from museum collections. All the outcrops are located inside the natural reserve of Sierra de Mariola, between the provinces of Valencia and Alicante, geologically belonging to the Betic Ranges. As commented above, the fossil localities sampled for the present study were: (1) El Muro (38.704167°N, 0.525117°W), located near the housing estate of Muro Baradello-Sargento, 3.5 km west of Alcoy; (2) Castillo Barchell (38.690667°N, 0.530806°W), near a secondary path towards the eponymous castle, close to the CV-795 road; and (3) Bancales (38.690278°N, 0.534333°W), 300 m Southwest of the castle (). The three outcrops contain the same lithological series with alternating levels of marlstones, calcarenites, and homogeneous sandstones without discernible dips or lateral continuity. These sites seemingly correspond to patches of Miocene outcrops in the geological maps of the area, which had been historically dated as “Serravallian” based on their foraminiferal composition—where †Preorbulina glomerosa circularis, †P. g. curva, †Globorotalia premaenardii, and †G. mayeri were identified (Martínez Díaz, Citation1970; Martínez & Benzaquen, Citation1975). However, previous works suggested a Late Miocene age (Tortonian) due to the presence of the foraminifera †Globorotalia plesiotumida (Banner & Blow, Citation1965) and †Globorotalia lupeae (Martínez Díaz, Citation1970; Martínez & Benzaquen, Citation1975). Further studies are needed to clarify this aspect.
FIGURE 1. Area of study in the Alcoy Basin, highlighting the main geological subdivisions of the Betic Range (above), and location of the El Muro, Bancales, and Castillo Barchell fossil sites referenced in the present study, as well as the Moli Palla locality for some of the fossils from the “Museo Paleontológico y de las Ciencias Isurus (Alcoy).”
Specific samples from the calcarenitic levels found in each of the outcrops were collected for acid digestion, sampling 4.32 kg at Castillo Barchell, 5.3 kg in Bancales, and up to 52.54 kg at El Muro locality. Each sample was dissolved and processed using acetic acid (∼5–8%), and subsequently screened with sieve meshes of 2, 1.25, 0.5, and 0.2 mm. The picking process yielded more than 4,000 pieces including elasmobranch teeth, placoid scales, and osteichthyan remains. In addition to the chondrichthyan fossil sampling, 250 g of sediment from the El Muro outcrop was separated for foraminiferal analyses, for dating and complementary paleoenvironmental studies, following the methodology described in García-Sanz et al. (Citation2018). All the new elasmobranch microremains found, as well as the foraminiferal remains, are housed at the “Museo de Historia Natural de la Universitat de València.” The specimens were photographed using the Hitachi S4800 scanning electron microscope of the University of Valencia (Spain), whereas the macroremains were photographed using the Leica MZ12 stereoscopic binocular microscope and the Leica Application Suite software, located in the Department of Botany and Geology of the University of Valencia (Spain).
Moreover, previously known elasmobranch fossils from the same localities, historically surface-collected and housed at the “Museo Paleontológico y de las Ciencias Isurus (Alcoy),” were studied and included in the present work. Additionally, fossils from a new locality, Moli Palla (38.690694°N, −0.514694°W), were also included as they were historically collected and deposited as part of the collections.
We conducted two paleobathymetric estimations. The first involved assessing foraminiferal assemblages and applying the Oceanization Index for the Western Mediterranean, as outlined in De Rijk et al. (Citation2000). The second estimation utilized a depth estimator employing weighted bootstrap analysis. This method estimated the depth distribution of the chondrichthyan population abundance based on the bathymetric ranges of their closest living relatives, following the approach described by Pérez et al. (Citation2017) and Oyanadel-Urbina et al. (Citation2021).
In the application of this technique, we calculated the mean for both the total depth distribution range and the mean for the common distribution range for each taxon. A bootstrap analysis was then conducted, incorporating the mean differences and using the relative abundance of each taxon as a weight estimator of the mean. The data underwent 10,000 resamplings, and the results were plotted as a histogram, providing the mean depth. The 95% confidence interval was determined using a percentile method.
To ensure ecological accuracy and avoid unnecessary errors, we repeated the analysis while excluding taxa not identified at the genus level or lower. In those cases where conflicting depth reports were presented in the two main references for bathymetric ranges (Ebert et al., Citation2021; Froese & Pauly, Citation2023), we adopted the widest bathymetric range possible by combining these references to include the entire known range. Analyses were performed considering () and excluding Batoidea (), resulting in datasets with 298 and 305 specimens, respectively. Both the raw data and the script are included as supplementary material to this study (S1 and S2).
SYSTEMATIC PALEONTOLOGY
Class CHONDRICHTHYES Huxley, Citation1880
Superorder SQUALOMORPHII Compagno, Citation1973
Order SQUALIFORMES Goodrich, Citation1909
Family ETMOPTERIDAE Fowler, Citation1941
Genus TRIGONOGNATHUS Mochizuki & Ohe, Citation1990
TRIGONOGNATHUS sp.
()
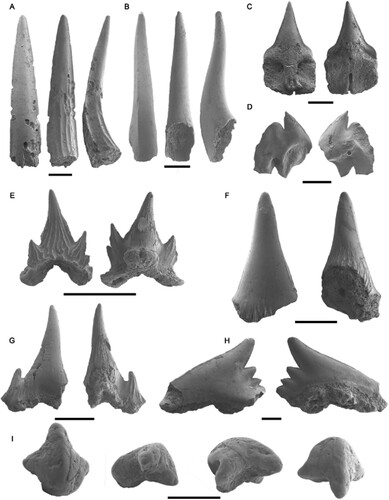
FIGURE 2. Labial and lingual views of the elasmobranch tooth microremains recovered from the Alcoy Basin fossil sites. A, Trigonognathus sp. MGUV-39898; B, Chlamydoselachus sp. MGUV-39949; C, Deania calcea MGUV-39943; D, cf. †Squaliodalatias weltoni MGUV-39944; E, Scyliorhinus canicula MGUV-39950; F, †Megascyliorhinus sp. MGUV-39952; G, Scyliorhinus sp. MGUV-39951; H, Galeorhinus sp. MGUV-39953; I, cf. Rajiformes MGUV-39888. All scale bars represent 500 µm.
Referred Material—Two fragmented anterolateral teeth from Bancales, one specimen figured (MGUV-39898), second non-figured tooth (MGUV-39899).
Description—The best-preserved specimen, MGUV-39898, represents an incomplete antero-lateral tooth crown fragment. Monocuspidate symmetrical crown with transversally convex labial and lingual faces, reaching approximately 5–6 mm in height, and bent toward the lingual face in a slight sigmoid curvature (). Five parallel ridges are present in the lingual face, reaching up to approximately half to two-thirds of its length, starting at the base of the cusp where they are more clearly defined. The lower portion of the specimen is also heavily damaged on its labial face and lacks its basal-most section and the root.
Comparative Remarks—The species of the Trigonognathus genus do not show dignathic heterodonty, with the only differences present on its teeth being the relative positions on the same jaw due to monognathic heterodonty (Cappetta & Adnet, Citation2001). The more distal teeth positions show a wider, shorter, and stockier morphology, and a lesser marked sigmoid curvature when observed laterally; whereas the anterior positions usually show a strong curvature and are bent lingually, with a very slender body (Herraiz et al., Citation2022). The lingual markings and general anatomy of the cusp are very similar to the teeth of Mitsukurina (Cappetta, Citation2012; Vialle et al., Citation2011). However, teeth of Mitsukurina can reach much larger sizes—despite size not being a good diagnostic characteristic—and show a higher concentration of lingual folds, which are also more developed and reach higher in the crown. In addition, Mitsukurina teeth seem to be more robust even in anterior positions (Cappetta, Citation2012:fig. 175).
Chlamydoselachus, another deep-sea shark whose teeth have been recovered in the Neogene of the Mediterranean Basin, possesses tricuspid teeth which are commonly found as a broken and fragmented solitary cusp, which can also be misidentified as Trigonognathus teeth. Chlamydoselachus in the Pliocene of Italy (Cigala Fulgosi et al., Citation2009) shows two different morphologies, one with ridges similar to those of Trigonognathus in both their lingual and labial faces, and a second one with smooth enameloid in both the labial and lingual faces, and absence of ridges. Further differences between these two genera are the more convex labial and lingual faces of Chlamydoselachus, which give it a more rounded section (see Chlamydoselachus sp.; ).
Trigonognathus teeth have been previously found in the Mio-Pliocene of the Caribbean (Aguilera & Rodriguez de Aguilera, Citation2001; Carrillo-Briceño et al., Citation2015), as well as its earliest known representative, Trigonognathus virginiae, from the Lutetian (Eocene) of France (Cahuzac et al., Citation2007; Cappetta & Adnet, Citation2001). In addition to these, the Miocene outcrop of El Ferriol (Alicante, Spain), has also yielded the most abundant Trigonognathus teeth collection, as well as its first description in the Mediterranean Basin (Herraiz et al., Citation2022), with the Alcoy specimen being its second known appearance in the Iberian Peninsula.
The only living species of the genus is Trigonognathus kabeyai (Mochizuki & Ohe, Citation1990). Its known distribution consists of deep waters from the Eastern and Central Pacific Ocean (Ebert et al., Citation2021), being reported from Japan, Taiwan, and Hawaii (Ebert et al., Citation2013; Mochizuki & Ohe, Citation1990; Shirai & Okamura, Citation1992; Wetherbee & Kajiura, Citation2000). The living species, T. kabeyai, has been reported near the bottom of upper continental slopes, at depths between 250–1,000 m, as well as the uppermost slope of seamounts (Compagno et al., Citation2005; Ebert et al., Citation2021; Mochizuki & Ohe, Citation1990). However, some specimens have been captured at 150 m in deep open waters (Ebert et al., Citation2021), showing possible oceanic habitat usage. This species undergoes vertical migrations following prey items to feed on, such as teleosts from the Lophius genus (Yano et al., Citation2003).
Order HEXANCHIFORMES de Buen, Citation1926
Family CHLAMYDOSELACHIDAE Garman, Citation1884
Genus CHLAMYDOSELACHUS Garman, Citation1884
CHLAMYDOSELACHUS sp.
()
Material—Three fragments from El Muro (MGUV-39821 and MGUV-39823), 12 from Castillo Barchell (MGUV-39822, MGUV-39824, MGUV-39825 and MGUV-39826), 16 from Bancales (MGUV-39949, figured; MGUV-39827 and MGUV-39828 non-figured specimens).
Description—Isolated narrow cusps, with markedly convex labial and lingual faces, giving to the cusps a relatively rounded section, becoming flatter toward the apical portion of the tooth. The recovered fragments do not present ornamentation nor folds. The cusps show a sigmoidal curvature in lateral view (). One of the recovered fragments seems to be a central cusp with a small fragment of its root still attached, being rectangular in shape and projected toward the lingual face of the tooth, although in a poor preservation state and incomplete.
Comparative Remarks—All fragments show the genus’ characteristic morphology, with each cusp possessing a long, narrow morphology and cutting edges, where the central cusp is slenderer with a narrow lingually elongated root (Cappetta, Citation2012). All specimens recovered are extremely fragmented and lack most of the crown and the root, with the exception of one that still retains a small portion.
The recovered fragments show the same fracture pattern in their bases, caused during the biostratinomic phase by the break between the circular bottom portion of the cusps at the base of the crown. While the slender, narrow cusps of Chlamydoselachus bear similarities to those of the aforementioned Trigonognathus (see ), Chlamydoselachus can reach larger body sizes and as such, their teeth can end up being significantly larger. In addition, whereas Trigonognathus teeth show just folds in their lingual surface, Chlamydoselachus can show similar folds in both labial and lingual faces of the cusps, or have completely smooth cusps—both morphologies can be seen figured in Cigala Fulgosi et al. (Citation2009). Furthermore, as previously stated, the base of Chlamydoselachus cusps is more convex in both the labial and lingual faces, which gives it a characteristic circular section, whereas Trigonognathus teeth are less convex in their lingual face, and the more lateral positions present a more labiolingually flattened and wide morphology when compared with the anterior teeth.
Fossil remains of this genus have been recovered from Miocene deposits in Austria (Pollerspöck et al., Citation2020), Germany (Barthelt, Citation1991), and the U.S.A. (Phillips et al., Citation1976), with Serravallian-dated remains found also in Parma (Cappetta, Citation2012), as well as teeth extracted from the Miocene of Ecuador (Carrillo-Briceño et al., Citation2014). In the Mediterranean Basin, an abundant record of teeth belonging to this genus were extracted from Pliocene material found in Italy (Cigala Fulgosi et al., Citation2009).
There are two extant representatives of the genus, C. africana (Ebert & Compagno, Citation2009) and C. anguineus (Garman, Citation1884). The currently known distribution of C. africana encompasses both the Atlantic and Indian sectors of the southern portion of Africa, possibly up to the Indian Subcontinent (Ebert et al., Citation2021). The second species, C. anguineus, is present in a wider distribution across both the Atlantic and Pacific basins (Ebert et al., Citation2021). Besides the living representatives, the fossil record of the genus consists of at least 10 extinct species (Carrillo-Briceño et al., Citation2014).
The living species usually inhabit soft bottom deep-water environments, between 17 and 1520 m (Ebert et al., Citation2021), although the genus is generally found between 500 and 1,000 m deep (Froese & Pauly, Citation2023). It has been reported to feed on cephalopods, other sharks, and teleost fishes (Ebert et al., Citation2021; Kubota et al., Citation1991).
Family CENTROPHORIDAE Bleeker, Citation1859
Genus DEANIA Jordan & Snyder, Citation1902
Species DEANIA CALCEA Lowe, Citation1839
()
Material—Two upper teeth from Castillo Barchell, one figured specimen (MGUV-39943), second fragmented specimen (MGUV-39942).
Description—Labio-lingually flattened monocuspid triangular teeth, with the absence of serrated edges in the mesial and distal cutting edges. From the two specimens, the figured one (MGUV-33943) is better preserved and retains most of its root () showing a square root of equal width to the base of the crown. The central portion of the root possesses a well-developed uvula on its labial face, while a foramen is present in the center of the lingual face, as well as a clearly defined meso-lingual channel that reaches the basal extreme of the root ().
Comparative Remarks—Deania calcea presents a dentition with close similarities to Centrophorus, in which a dignathic heterodonty is present, with the lower teeth more high than broad, and labio-lingually compressed; the upper jaw presents smaller teeth with monognathic heterodonty, the more mesial positions possess practically symmetrical teeth, while the lateral positions tend to be similar to the lower jaw (Cappetta, Citation2012). The morphological features of our teeth represent upper jaw elements similar to those found in other fossil Miocene deposits of the Alicante province (Martínez-Pérez et al., Citation2018), as well as those described by Ledoux (Citation1970). The genus is well-documented in the fossil record, with fossil teeth being reported from Miocene sites of different countries worldwide, such as the Tortonian of Italy, or the Middle Miocene of France and Germany, as well as older representatives being found at Paleocene and Eocene deposits of France (Cappetta, Citation2012). There is also a complete fossil record from the Miocene of the western India (Kriwet & Klug, Citation2009), and in the Iberian Peninsula, Miocene-aged remains have been recovered both in Portugal (Antunes & Jonet, Citation1970) and in the Alicante province from Spain (Martínez-Pérez et al., Citation2018).
Deania calcea is an extant representative of its genus with a wide distribution in the Atlantic Ocean, being more frequently reported in the eastern shores, near the Cantabrian Sea and in the south of Africa (Iitembu & Richoux, Citation2015; Rodríguez-Cabello et al., Citation2020; Santos et al., Citation2020). Further areas of distribution include some regions of the Indian Ocean, the western coast of Australia (Rochowski et al., Citation2015), and the western coasts of America (Keggin, Citation2017). This species has been captured at depths ranging between 555 and 1200 m (Ebert et al., Citation1992; Santos et al., Citation2020), although it is generally found at depths of 400–900 m (Ebert et al., Citation2021; Froese & Pauly, Citation2023). Despite the diversity in their diet, its dominant prey items seem to be teleosts of the Myctophydae family such as Diaphus ostenfeldi, one of the most represented in its dietary preferences (Ebert et al., Citation1992).
Family DALATIIDAE Gray, Citation1851
Genus †SQUALIODALATIAS Adnet, Cappetta, & Reynders, Citation2006
cf. †SQUALIODALATIAS WELTONI Adnet, Cappetta, & Reynders, Citation2006
()
Material—Nine individuals, four fragmented teeth from Castillo Barchell (specimen MGUV-39944 figured, MGUV-39896 and MGUV-39945 non-figured) and five non-figured specimens from Bancales (MGUV-39946, MGUV-39947, MGUV-39948, and MGUV-39897).
Description—Although all the specimens are partially broken or eroded, they clearly show a labio-lingually flattened crown with a short and compact single cusp. The crown possesses a pronounced curvature toward the distal margin, and its cutting edges are devoid of serrations. In the labial face, the beginning of a skirt can be seen in one of the sides (, left image). The lingual face of the root shows a mesolingual foramen, although said root is incomplete and fragmented.
Comparative Remarks—The teeth of this taxon show similarities in their morphology to those of Squaliolus schaubi; although both taxa present a lateral skirt and a meso-lingual foramen in the root, S. schaubi possesses a lower cusp, a longer foramen, and taller teeth (Underwood & Schlögl, Citation2012). Additional differences include the absence of a mesolingual furrow on its root, whereas the furrow can be observed in the lingual face of the root of S. weltoni individuals.
This species is extinct nowadays, and its first known records are from the middle Eocene of France, with more recent teeth being recovered from Langhian (Middle Miocene) deposits (Underwood & Schlögl, Citation2012). As this taxon is extinct, a living ecological analog is needed to infer its paleoecological characteristics. Dalatias is a closely related genus, living mainly in the western Mediterranean and Atlantic basins, although it also occurs in the Pacific and Indian oceans (Ebert et al., Citation2021). This genus only has an extant representative, generally found in deep waters, despite occasionally being captured at lesser depths. Its bathymetric range has been reported between 37 and 1800 m; however, it is usually situated deeper than 200 m (Ebert et al., Citation2021) with reports of usual range at 300–600 m (Froese & Pauly, Citation2023). Euprotomicrus bispinatus is another member of the Dalatiidae family with a close phylogenetic relation to †S. weltoni. It is a small, poorly known amphitemperate deep-sea inhabitant that undergoes vertical migrations and can be found at depths ranging from 0–1829 meters, although in mid-ocean waters can reach depths of almost 10,000 meters. Its main prey items seem to be deep-sea cephalopods and other small prey such as crustaceans and bony fishes (Ebert et al., Citation2021).
Order HEXANCHIFORMES de Buen, 1925
Family HEXANCHIDAE Gray, Citation1851
Genus NOTORYNCHUS Ayres, Citation1855
†NOTORYNCHUS sp.
()
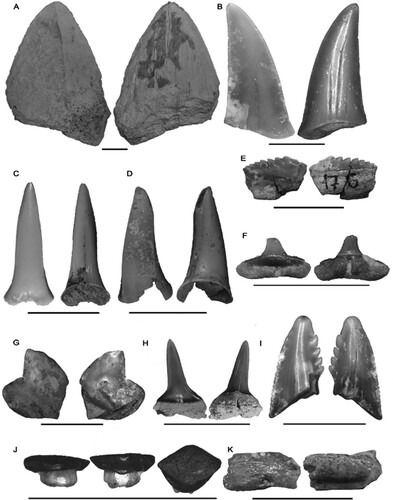
FIGURE 3. Labial and lingual views of the elasmobranch tooth macroremains from the Alcoy collections of the “Museo Paleontológico y de las Ciencias Isurus (Alcoy),” and extracted from the aforementioned fossil sites. A, †Otodus megalodon MGUV-39832; B, †Carcharodon hastalis CVAI00770; C, †Carcharias acutissima CVAI00775; D, Carcharias sp. CVAI00777; E, †Notorynchus primigenius CVAI00779; F, Carcharhinus sp. CVAI00762; G, Galeocerdo sp. CVAI00761; H, cf. Isurus sp. CVAI00773; I, †Hemipristis serra CVAI00764; J, Dasyatidae indet. CVAI00767; K, Myliobatidae indet. CVAI00780. All scale bars represent 1 cm.
Material—One fragmented tooth from El Muro (CVAI00779).
Description—The unique, incomplete, preserved specimen shows a labiolingually compressed tooth with seven defined conules in the crown, situated by size in descending order from the mesial to the distal side of the tooth, with the biggest conule found in the mesial extreme of it. The conules are slanted toward the distal side and are absent of serrations in their cutting edges. The tooth preserves the central portion of the root, more robust than the crown and lacking the mesial and distal extremes as well as its base.
Comparative Remarks—Despite the incomplete nature of the described tooth, with part of its root and crown missing, the distribution of the differently sized conules and their arrangement by size from mesial end to distal end, show similarities to the tooth reported by Ávila et al. (Citation2012) assigned to the genus.
The fossil record of this genus shows a cosmopolitan distribution during the Mio–Pliocene, as evidenced by its rich fossil record in different basins of said age (Ávila et al., Citation2012; De Schutter, Citation2011; Szabó & Kocsis, Citation2020; Visaggi & Godfrey, Citation2010).
The only living species within this genus is Notorynchys cepedianus (Perón, Citation1807). This species is distributed across most temperate inshore continental waters, although it is not present in Northern Atlantic waters (Ebert et al., Citation2021). The living representative has been usually found at depths ranging between less than 1 and at least 570 m, although it is mostly found at less than 100 m deep, inhabiting bays and estuaries, and cruising close to the bottom (Ebert et al., Citation2021).
Order CARCHARHINIFORMES Compagno, Citation1973
Family SCYLIORHINIDAE Gill, Citation1862
Genus SCYLIORHINUS De Blainville, Citation1816
Species SCYLIORHINUS CANICULA Linnaeus, Citation1758
()
Material—Two anterolateral teeth from Castillo Barchell, one figured (MGUV-39950) and a second non-figured individual (MGUV-39809).
Description—Teeth with a main larger slender central cusp and four lateral cusplets in the mesial and distal sides of the crown, arranged in decreasing size from the main cusp toward the edges of the crown. In the labial face, a series of folds run parallel, starting from the base and towards the apical portion of the crown (, left). These folds are not visible in the lingual face, perhaps due to taphonomic processes. The labial face of the crown overhangs the root via a skirt, with the aforementioned folds also being present in the accessory cusplets. The root is robust and preserves a significant lingual protuberance and a flattened base.
Comparative Remarks—Morphological comparison of the recovered teeth with published material and studies regarding different representatives of the genus has allowed the identification of this taxon (Berio et al., Citation2020; Cappetta, Citation2012; Halter, Citation1995; Soldo et al., Citation2000), thanks to its characteristic distribution of cusplets at the sides of the central, slender main cusp, the strongly marked folds in the base of the crown and its robust root, which is covered on its labial face by the base of the crown, and the strong lingual protuberance found in it (Cappetta, Citation2012). Despite the lack of visible foramina due to the preservation state, the rest of the characteristics fall in line with former dental descriptions of this genus and species in the bibliography, such as Cappetta (Citation2012).
This species has been previously reported from the Neogene of the Iberian Peninsula in the Serravallian of the Alicante province (Martínez-Pérez et al., Citation2018), and in the Lower Miocene of Portugal (Antunes et al., Citation1999). The genus is also known from its fossil record from the Cretaceous and during all the Neogene, with representatives still living nowadays, such as the extant S. canicula (Cappetta, Citation2012).
Scyliorhinus canicula can be found in the Mediterranean realm (Abella & Serena, Citation2005; Bertrand et al., Citation2000). In addition to the Mediterranean, it also inhabits sandy and muddy-bottom environments from nearshore up to 800 m—although it is usually found between 80 and 100 m (Froese & Pauly, Citation2023)—on continental shelves and upper slopes of the North-eastern Atlantic, such as the British coasts and Portugal (Bakopoulos et al., Citation2018; Ebert et al., Citation2021; Ellis et al., Citation2005). Its main prey items include marine invertebrates and osteichthyans, with some cases of cannibalism reported (Martinho et al., Citation2012), as well as different habitat use in the different stages of their life history (Bakopoulos et al., Citation2018; Olaso et al., Citation2005).
Family SCYLIORHINIDAE Gill, Citation1862
Genus SCYLIORHINUS De Blainville, Citation1816
SCYLIORHINUS sp.
()
Material— Five fragmented teeth from Bancales (MGUV-39807, MGUV-39810, and MGUV-39812) and two fragmented teeth from Castillo Barchell (MGUV-39951, MGUV-39811, figured specimen MGUV-39802).
Description—Teeth with a main narrow triangular cusp, with a smaller accessory cusplet on its side, less than half the size of the main cusp. In all the specimens the crown is significantly fragmented, lacking some of the accessory cusp (). On the preserved labial face (, left) of the crown and the cusps a series of marked folds and striations runs from the base to the apical end of the cusps, reaching two-thirds of the main cusp’s length. The same folds and striations can be found in the lingual face of the crown, where they are more marked, reaching almost the apex of the cusps. None of the recovered teeth preserves completely the root, although part of the lingual protuberance can be observed.
Comparative Remarks—The recovered teeth are similar to those of other genera of the Scyliorhinidae family that are also found in the same outcrops. They can be distinguished from †Megascyliorhinus by the quasi-monocuspid teeth of the latter, with a main cusp clearly differentiated from the small, sharp accessory cusplets that are occasionally closely attached to its sides (Cappetta, Citation2012), and the weakly marked folds that the Neogene †Megascyliorhinus individuals have (Cappetta, Citation2012). The teeth are remarkably similar to those of Scyliorhinus canicula, but they differ in their labial face morphology of the crown, which presents a more rectangular shape, with a slight concavity and a more subdued union with the root, as well as the absence of the skirt found in S. canicula, and less overhang of the crown over the root. The fragmented specimens also resemble those of the fossil catshark genus †Premontreia, although the presence of ridges on both lingual and labial faces of the crown, sets them apart from the fossil †Premontreia subgenus, which possesses smooth enameloid surface on the lingual face of the crown and shorter parallel ridges on the labial face (Cappetta, Citation2012). Our specimens differs as well from †Oxyscyllium, by the presence in the latter of a salient bulge in the labial face of the teeth (Cappetta, Citation2012), whereas the recovered Scyliorhinus sp. lacks such bulge (, left).
Due to the preservation state, it is difficult to assign our teeth to a specific taxon, but due to the similarities with S. canicula, we believe they could represent a second Scyliorhinus taxon in the same environment. The possible presence of more than one species of the Scyliorhinus genus in the same areas can be seen nowadays in the northern Atlantic, as well as the Mediterranean, where S. canicula, S. duhamelii, and S. stellaris occur naturally (Ebert et al., Citation2021). The presence of the genus extends from the Early Cretaceous to modern times, being found in outcrops from Europe, North America, and Africa (Cappetta, Citation2012).
Other members of the Scyliorhinus genus have been used as ecological analogs based on dental similarities to infer its habitat use and bathymetry, representing small-sized species that feed on small fishes and invertebrates and live near soft bottoms in continental shelf and upper slope areas, being found at depths up to 2,000m (Ebert et al., Citation2021). They usually inhabit shallower waters attached to insular and continental shelves and slopes of the Atlantic, Indian, and Pacific oceans, as well as smaller oceanic basins, such as the Mediterranean and Caribbean—see Scyliorhinus spp. in Ebert et al. (Citation2021). As an example, the extant S. stellaris can be found in continental shelves up to 380 m deep, although it usually occurs at 20–63 m on rocky bottoms or where seaweeds are present and cover the bottom (Compagno, Citation1984; Ebert et al., Citation2021). The other extant species commonly found in the Mediterranean, S. canicula, can be found from nearshore environments up to 800 m deep, although it usually inhabits shallower waters than 450 m, on continental shelves and upper slopes, being found in muddy and sandy bottoms (Ebert et al., Citation2021). Scyliorhinus duhamelii, a recently described species of the Scyliorhinus genus also found in the Mediterranean, is endemic to the Adriatic and can be found in continental shelves at bathyal ranges of 43–75 m (Soares & De Carvalho, Citation2019).
Family SCYLIORHINIDAE Gill, Citation1862
Genus MEGASCYLIORHINUS Cappetta and Ward, Citation1977
†MEGASCYLIORHINUS sp.
()
Material—16 fragmented teeth from Bancales (MGUV-39800 and MGUV-39805), 19 from Castillo Barchell (MGUV-39952, MGUV-39801, MGUV-39803, and MGUV-39804; figured specimen MGUV-39798) and two from El Muro (MGUV-39806).
Description—Monocuspid crown with robust conical morphology, very faint cutting edge and convex labial and lingual faces, which give it a rounded section at the base of the cusp. Said folds are more present in the lingual face of the crown, where they reach higher up the crown. None of the recovered teeth preserve their root portion.
Comparative Remarks—Despite the lack of root and the poor condition of the teeth they retain enough characteristics to assign them to †Megascyliorhinus. Their rounded section at the base could be a cause for misidentification as Chlamydoselachus, but the cusps in the latter are more elongated and slender, as well as having a sigmoid labiolingually curvature, and possessing a different arrangement in their folds, or in some cases lacking said folds and having a smooth enameloid (Cappetta, Citation2012; Cigala Fulgosi et al., Citation2009). The characteristic folds present in Scyliorhinidae members are clearly visible in the described material all across the lower portion of the crown.
Its fossil record shows it inhabited different sea basins in addition to the Mediterranean, where it has been reported from several Spanish fossil sites from the Miocene (Hoffmann et al., Citation2020), having been found also in Eocene British outcrops, as well as in France and Portugal (Cappetta & Ward, Citation1977), and in the Middle Miocene of Panamá (Cione et al., Citation2008).
†Megascyliorhinus (Cappetta & Ward, Citation1977) is an extinct catshark genus with no living representatives, although from different studies regarding the faunal assemblages where it has been found in the fossil record, it has been inferred that this genus would have inhabited continental shelf waters up to 150–200 m (Keyes, Citation1984; Ward & Bonavia, Citation2001). In some formations where it has been recovered, other taxa found alongside its teeth were typical of warm waters, such as †Carcharoides totuserratus (Ameghino, Citation1901), allowing the assumption that this taxa could be a warm water shelf-inhabiting demersal shark genus (Suárez et al., Citation2004).
Family TRIAKIDAE Gray, Citation1851
Genus GALEORHINUS De Blainville, Citation1816
cf. GALEORHINUS sp.
()
Material—One fragmented tooth from El Muro (MGUV-39799), one from Castillo Barchell (MGUV-39953, figured), and one from Moli Palla (CVAI00766).
Description—Incomplete lower tooth with a labiolingually flattened crown, composed by a main cusp slanted toward the distal side of the tooth, with a mesial cutting edge slightly convex at its base and concave on its apical portion, which gives it a slender sigmoidal curvature. The tooth shows a short distal heel that presents three cusplets that decrease in size toward the distal side, which is missing in the recovered piece. The tooth is not well preserved, but it does not seem to have a heavy overhanging of the crown on its labial face, nonetheless this could be caused by the lack of the lowermost portion of the crown, the crown-root boundary being missing. The lingual and labial faces of the crown are completely smooth, without any folds or markings. Although the root is very poorly preserved, a small central portion of the lobes shows that the root is divided, with a central furrow separating them.
Comparative Remarks—Despite lacking most of the root, the triangular shape crown resembles those of representatives of the genus described and figured in Cappetta (Citation2012). Some characteristics of the recovered tooth include the shorter distal heel with up to four cusplets, in addition to the convex outline of the distal cutting edge in the main cusp, and the continuity between the mesial heel and cutting edge (more common in lateral positions). In addition, the central furrow of the root is still present despite the poor preservation state of the piece, as well as a small portion of the two lateral lobes of the root. As noted by Cappetta (Citation2012), this genus is quite similar in its tooth morphology to the upper positions of Chaenogaleus and Hemigaleus, and is probably a close relative to the Hemigaleidae family.
The fossil record of the genus is comprised by remains dating from the Cenomanian (Late Cretaceous) as well as the Paleogene and Neogene, being found in the European (i.e., Spain and Portugal) (Bauzá Rullán & Gómez-Pallerola, Citation1988; Fialho et al., Citation2021) and western African fossil record (Cappetta, Citation2012).
The Galeorhinus genus only has one living representative, G. galeus (Linnaeus, Citation1758), a slender shark that reaches up to 195 cm in total length (Ebert et al., Citation2021). This shark can be found almost worldwide except in the Northwest Pacific and Northwest Atlantic, inhabiting in temperate waters. It occurs mainly on continental shelves, ranging from nearshore shallow waters to offshore depths of up to 800 m, often near the bottom, being a demersal species. It partakes in vertical migrations to feed, mainly on invertebrates and bony fishes (Ebert et al., Citation2021).
Family CARCHARHINIDAE Jordan & Evermann, Citation1896
Genus CARCHARHINUS De Blainville, Citation1816
cf. CARCHARHINUS sp.
()
Material—18 fragmented teeth from El Muro and Castillo Barchell (CVAI00769 and CVAI00772), six from Moli Palla (figured specimen CVAI00762, rest of material CVAI00763 and CVAI00782-784) and one from Bancales (MGUV-39797).
Description—Crown with a single broken narrow cusp, separated from the lateral heels and with a smooth cutting edge. Its cutting edges lack serrations and both the labial and lingual faces of the crown do not show marks or folds, with a smooth surface. In lateral view, the labial face shows a slight convexity without overhanging the root, whereas the lingual face is flat. The root is low and presents elongated mesio-distal extensions with rounded extremes, and its base is almost flat, although a slight concavity can be seen in the central portion. On the lingual face of the root, a mesial protuberance can be appreciated, while the labial face is clearly flat. The lingual face also shows a mesial furrow that extends toward the basal face. Said fold can even be visible from the labial face, where it continues without reaching its upper portion.
Comparative Remarks—The narrow straight morphology of the cusp allows for the identification of the figured tooth as a lower lateral tooth, which would be located in the more distal positions near the margin of the jaw. The poor preservation state of the referred material does not allow for a more precise identification, although it has been assigned to Carcharhinus instead of other Carcharhinidae genera due to the less developed heels and the remarkable similarity to teeth from some of the species of this genus, such as C. limbatus or C. brevipinna—see fig. 185 in Cappetta (Citation2012).
This genus is one of the most diverse groups within Selachimorpha, and its fossil record extends up to the Eocene of North Africa (Cappetta, Citation2012), of which teeth have been recovered from fossil outcrops all around the globe. Living representatives are present in all the major oceanic basins, mainly on temperate and warm/tropical waters, where it can be considered a cosmopolitan genus and its species are some of the main predators of their habitats.
Despite the lack of a positive identification at a species level and the environmental analysis of the outcrop, and taking into account that the ecological parameters of most Carcharhinus species are related to shelf waters, it has been inferred that our taxon would have inhabited warm shallow waters located on the continental shelf, probably less than 50 m deep, although some Carcharhinus species are pelagic in nature and can be readily found in open waters up to at least 200 m deep (Ebert et al., Citation2021).
Family GALEOCERDONIDAE Poey, Citation1875
Genus cf. GALEOCERDO Müller & Henle, Citation1837
cf. GALEOCERDO sp.
()
Material—Six fragmented teeth from El Muro (figured tooth CVAI00761; one non-figured tooth CVAI00760 and four non-figured individuals CVAI00122).
Description—Triangular monocuspidate crown, possessing a convex mesial cutting edge. Despite the poor preservation, the cutting edges show remains of serrations, more clearly seen in the heel of the straight distal cutting edge. Said distal heel is not fully preserved although its diagnostic height and length are still present. A smooth surface without ridges or folds can be seen in both the labial and lingual faces of the crown. In lateral view, the labial face is visibly flat and slightly overhangs the remains of the root, while on the contrary the lingual face shows some convexity. The root is almost completely absent and with little detail, though the remains are thick labiolingually, and the central portion seems to show a concave line of union to the crown.
Comparative Remarks—The lack of a complete crown, in addition to the abrasive damage and the significant missing portions of the root make the identification of the tooth position and a more accurate identification of the taxon difficult. Despite said preservation, the remaining portions of the teeth and the serrations they still retain, in addition to the typical heel of this genus, have allowed us to identify the teeth as belonging to the genus Galeocerdo, showing remarkable similarities to both fossil and extant specimens—e.g., fig. 281 in Cappetta (Citation2012)—figured in the bibliography (Türtscher et al., Citation2021).
The Galeocerdo genus is known from fossil deposits since the Ypresian, forming part of marine faunal assemblages found in Europe, India, South Africa, Japan, and America among other localities (Cappetta, Citation2012). Despite only having a single extant representative, this genus is well represented in the fossil record, with 22 recognized extinct species, although the validity of some of the aforementioned extinct species has been brought to question (Türtscher et al., Citation2021).
The extant representative of the genus, G. cuvier, more commonly known as ‘tiger shark,’ is a macropredatory shark found in the tropical and temperate seas across the globe, normally associated to continental and insular shelves, from the surface and intertidal areas up to depths of 1136 m (Ebert et al., Citation2021). Additionally, it has been found in a wide range of habitats, from estuaries and harbors to coral atolls and lagoons, travelling long distances between localities (Ebert et al., Citation2021). This shark species is an adept swimmer that preys upon a wide array of prey items, from albatross (Ebert et al., Citation2021), to teleosts, sea turtles (Simpfendorfer et al., Citation2001), marine mammals, mollusks, and more (Dicken et al., Citation2017).
Family HEMIGALEIDAE Gill, Citation1862
Genus HEMIPRISTIS Agassiz, Citation1843
cf. †HEMIPRISTIS SERRA Agassiz, Citation1843
()
Material—Three fragmented teeth from El Muro (CVAI00764, figured; CVAI00765).
Description—Incomplete tooth fragments, in all cases the lower portion of the crown missing, along with the root. Triangular tooth shape, with the mesial margin of the crown longer than the distal one, giving the incomplete crown a slant toward the distal side. The distal cutting edge retains four well-preserved serrated denticles, whereas the upper portion of the mesial cutting edge shows eroded denticles, with the small serrations on the more basal portion of the crown still maintained, becoming smaller and in a poorer preservation state as they advance to the apical section. The enameloid of the crown presents parallel markings caused by taphonomic processes that in some cases reach the apical portion of the cusp, both on the lingual and labial faces.
Discussion—†Hemipristis serra teeth show a series of characteristics that have allowed for its identification and distinction from other carcharhiniform taxa despite the poor condition in which the studied tooth was preserved. These characteristics like their distinct serration pattern and crown morphology were compared with other carcharhiniform teeth from bibliographic references (Cappetta, Citation2012), in addition to other published †H. serra material (Chandler et al., Citation2006; Purdy, Citation1998; Reis, Citation2005).
Teeth from this macropredatory taxon have been recovered from Miocene deposits of the Mediterranean basin, such as Menorca, the eastern portion of the Iberian Peninsula, as well as France and Italy (Bauzá Rullán & Mercadal, Citation1961; Gagnaison, Citation2013; Herraiz et al., Citation2020; Marsili et al., Citation2007; Martínez-Pérez et al., Citation2018). In addition, this cosmopolitan taxon has also been recovered from Tertiary deposits from all the major ocean basins (Argyriou et al., Citation2015; Antunes, Citation1977; Cione et al., Citation2011; Frankel, Citation1972; Gillette, Citation1984; Portell et al., Citation2008; Radwanski, Citation1965). Cappetta (Citation2012) remarks on the particular abundance of this taxon in warm water neritic deposits, becoming less common in higher latitude deposits where it can be found even in Pliocene outcrops.
Hemipristis elongata (Klunzinger, Citation1870) is the only living species from this genus currently described (Ebert et al., Citation2021). Its distribution range comprises the coasts from the Indian ocean, being found from northern Australia to Madagascar and other islands (Fricke et al., Citation2018; Last et al., Citation2009; Tyabji et al., Citation2018; Zhou & Griffiths, Citation2008). Hemipristis elongata is a demersal species that usually inhabits warm and temperate waters (Cione et al., Citation2011), up to 130 meters deep (Froese & Pauly, Citation2023; Jaiteh & Momigliano, Citation2015), being found in the continental and insular shelves (Ebert et al., Citation2021).
Order LAMNIFORMES Berg, Citation1958
Family LAMNIDAE Müller & Henle, Citation1838
Genus CARCHARODON Rafinesque, Citation1810
Species †CARCHARODON HASTALIS Agassiz, Citation1843
()
Material—133 fragmented teeth from El Muro (CVAI00770, figured; CVAI00771, CVAI00774, CVAI00781), and two from Castillo Barchell (CVAI00785).
Description—Monocuspid crown, in all cases, the roots are broken. The smooth cutting edges lack serrations, and the teeth show a slant toward the distal margin. The enameloid surface is smooth and flat, without striations or folds on the labial face. However, some specimens show at the lingual face few slight striations probably caused during the biostratinomic stage.
Comparative Remarks—None of the studied fragments preserve their root, and the poor preservation state could lead to a misidentification with other lamniform taxa such as †Isurus subserratus (Agassiz, Citation1843) whose teeth can present a similar morphology. However, †I. subserratus can possess some serrations at their cutting edges (Toscano Grande, Citation2016), whereas the cutting edge of †C. hastalis is void of any serrations. The taxon has been assigned to the genus Carcharodon instead of †Cosmopolitodus following Ehret et al. (Citation2012) and the findings on Neogene outcrops from Peru. Subsequent taxa from the Carcharodon genus, such as †C. hubbelli and C. carcharias developed serrations, †C. hubbelli being considered an intermediate form between †C. hastalis and C. carcharias (Ehret et al., Citation2012). †C. hastalis is a well-represented species in the fossil record, as a cosmopolitan species that has been found in outcrops from all ocean basins (Ávila et al., Citation2012; Bhalla & Prammendra, Citation1988; Canevet, Citation2011; Cappetta, Citation2012).
†Carcharodon hastalis is an extinct species of the Carcharodon genus within Lamnidae family, whose sole living representative nowadays is the great white shark, C. carcharias (Linnaeus, Citation1758). This living taxon is a pelagic macropredatory shark with a bathymetric range and habitat usage of waters from 0–1280 m (Ebert et al., Citation2021), although it is generally found at 150 m or less from the surface (Froese & Pauly, Citation2023). These sharks include within their preferred prey items marine mammals such as pinnipeds and cetaceans (Ebert et al., Citation2021), other shark species, as well as osteichthyans and cephalopods (Hussey et al., Citation2012).
Material—Two fragmented teeth from El Muro, specimen figured (CVAI00773), other specimen (MGUV-39831).
Description—The best-preserved specimen shows a single slender monocuspid crown fully preserved, with smooth cutting edges lacking serrations, and a slight slant toward the distal edge, neither of the cutting edges reach the base of the crown and contact the root. The cusp is quite narrow on its apical portion and most of its length, widening toward its basal third. The flat labial face slightly overhangs the labial portion of the root with a subtle convexity near the base, whereas the lingual face of the crown is more convex. In lateral view, a slight sigmoid curvature can be appraised, with the apical portion bent toward the labial face, and the basal half bent toward the lingual face. The root is partially preserved, with the lateral lobes being absent. The central part of its lingual face presents what seems to be a foramen, although the whole root piece shows considerable surface damage.
Comparative Remarks—Due to the lack of a complete root, a misidentification with other taxa belonging to the Lamniformes order, especially within Lamnidae, could be possible, thus leaving the classification of the taxon as open. Despite the state of the piece, most of its morphology is consistent with teeth belonging to species within the Isurus genus, whereas the lack of lateral cusplets sets it apart from Carcharias and Odontaspis teeth (see Cappetta, Citation2012), and free-swimming Lamna teeth have a triangular crown with straight or slightly curved edges (Purdy & Francis, Citation2007), in addition to having anterior teeth with a broad base in the extant L. nasus (Cappetta, Citation2012).
The only two known extant representatives of the genus, I. oxyrhinchus and I. paucus, are widely distributed pelagic macrophagous sharks that have been found in temperate and warm waters across the globe, although I. paucus is poorly recorded in the vast majority of its possible range (Ebert et al., Citation2021). Both species can reach up to more than four meters in length (Ebert et al., Citation2021), and while the biology and behavior of I. paucus is less known, I. oxyrhinchus is a coastal to epipelagic active swimmer that can be found in depths from 0–888 m (Ebert et al., Citation2021) and preys on fishes and cephalopods, with larger individuals also feeding on small cetaceans and pinnipeds (Ebert & Dando, Citation2020; Ebert et al., Citation2021). The longfin mako (I. paucus) also feeds mainly on fish and squids in open waters, although this species seems to be found at deeper waters than the other extant representatives ranging from 0–1752 meters and possibly being a slower swimmer than the record-bearing I. oxyrhinchus (Ebert et al., Citation2021).
Family †OTODONTIDAE Glikman, Citation1964
Genus †OTODUS Agassiz, Citation1838
Species †OTODUS MEGALODON Agassiz, Citation1835
()
Material—One fragmented tooth from El Muro (MGUV-39832, figured) and one tooth from Castillo de Barchell (CVAI00125).
Description—The recovered tooth from El Muro (MGUV-39832) is poorly preserved, lacking its root and part of the basal portion of the crown, as well as the enameloid cup layer. The labial face of the crown is flat, while the lingual face is significantly convex. The lack of enameloid has also affected the crown, showing parallel folds that run throughout the lingual face towards its apex. No serrations were preserved on the cutting edges.
Comparative Remarks—The referred material can be unquestionably identified as O. megalodon despite its poor preservation condition, thanks to the large size its teeth can attain, as well as diagnostic characteristics that this taxon possesses. Some of these are the robust, triangular morphology, the flat or slightly concave labial face and the strongly convex lingual face of the crown. Other clear indicators for this taxon such as the specific serrations on the cutting edges and the enameloid-lacking V-shaped neck on the basal portion in the lingual face of the crown are not preserved in our specimens.
This cosmopolitan species has been recovered from multiple Mio-Pliocene localities all around the world (Antunes et al., Citation2015; Herraiz et al., Citation2020; Pimiento & Balk, Citation2015; Reolid & Molina, Citation2015).
This extinct macrophagous lamniform is the largest macropredatory shark species discovered, with maximum size estimations ranging from 15–18 meters (Boessenecker et al., Citation2019; Cooper et al., Citation2020; Gottfried, Citation1996; Herraiz et al., Citation2020; Pimiento & Balk, Citation2015; Pimiento et al., Citation2010; Shimada, Citation2021; Shimada et al., Citation2020), and even some questionable larger estimations. Regardless of the maximum size, this cosmopolitan giant shark inhabited temperate and warm waters all around the globe (Herraiz et al., Citation2020; Pimiento et al., Citation2016; Shimada, Citation2021). Due to its size and tooth morphology, as well as its metabolism (Ferrón, Citation2017), the closest extant ecological analog to infer its habitat use and trophic strategies is the living Carcharodon carcharias, like with †C. hastalis. In addition, these extinct taxa are usually found together in many outcrops around the world (Herraiz et al., Citation2020 and references therein).
Family CARCHARIIDAE Müller & Henle, Citation1841
Genus CARCHARIAS Rafinesque, Citation1810
cf. †CARCHARIAS ACUTISSIMA Agassiz, Citation1843
()
Material—Three fragmented teeth from El Muro and Castillo Barchell, figured specimen (CVAI00775) and rest of material (CVAI00776) and four from Castillo Barchell (CVAI00787).
Description—Despite their poor preservation (only a portion of the crown is preserved), the teeth possess a slender monocuspidate crown with a slightly convex lingual face and flatter labial face that shows a slighter convexity at its base, giving the teeth an ellipsoidal or rounded shape in transversal view. Multiple well-developed folds can be observed in the lingual face (reaching more than half the length of the cusp), although appearing very faintly in the lowermost labial face. Absence of serrations in the thin cutting edges, which reach the robust basal-most portion of the crown although weakening as they approach it. The lateral view of the teeth shows a sigmoidal curvature.
Comparative Remarks—This lamniform taxon can be distinguished from the other recovered teeth belonging to a second representative from its family based on the flat labial face and the presence of folds on the crown (Cappetta, Citation2012), and are remarkably similar to those figured and described by Antunes and Balbino (Citation2010) and Marsili et al. (Citation2007). According to Cappeta’s (Citation2012) description of Odontaspis teeth, the latter possess a strongly transversally convex, completely smooth lingual face of the cusp, with additional differences with Carcharias such as a straight profile.
According to the fossil record, †C. acutissima is a cosmopolitan species identified in multiple fossil sites, both in the Mediterranean (Marsili et al., Citation2007) and other ocean basins (Laurito et al., Citation2014).
Nowadays, the only living representative of the genus Carcharias is C. taurus, which has been used as the closest extant analog for this species (see also Carcharias sp.).
Family CARCHARIIDAE Müller & Henle, Citation1841
Genus CARCHARIAS Rafinesque, Citation1810
cf. CARCHARIAS sp.
()
Material—One fragmented tooth from El Muro (CVAI00777, figured) and 28 fragmented teeth from El Muro and Castillo Barchell (CVAI00778).
Description—Slender teeth, the lingual face of the crown is convex whereas the labial face is flat. Both faces are completely smooth and lack folds or striations, and the cutting edge lacks serrations and reaches the base of the crown. All referred individuals lack the root portion due to their poor preservation state.
Discussion—Despite being unable to identify this taxon at a species level due to the lack of root and poor preservation in general, the pointed, slender monocuspid crown, its sigmoid curvature, their large size and smooth cutting edges have allowed for identification at a genus level, due to the lack of sigmoidal profile in Odontaspis teeth according to Cappetta (Citation2012). This genus is known from Oligocene deposits and is quite common in marine fossil localities from the Miocene (Antunes & Balbino, Citation2010), being a cosmopolitan genus that has been identified in outcrops from all continents (Cappetta, Citation2012; Ward & Bonavia, Citation2001). Carcharias taurus (Rafinesque, Citation1810) is the only extant representative of the genus Carcharias currently described. C. taurus can be found in the western Mediterranean and the coasts of all ocean basins, except the central and eastern Pacific (Ebert et al., Citation2021; Pollard & Smith, Citation2009).
Carcharias taurus is a demersal-pelagic shark, being usually found in shelf waters from near the surf-line to at least 232 m deep in offshore reefs, although it can mostly be found close to the coast at 15–25 m deep, and also less frequently in open waters (Ebert et al., Citation2021; Pollard & Smith, Citation2009; Teter et al., Citation2015), feeding on a wide range of prey items that include invertebrates and fishes.
Superorder BATOMORPHII Cappetta, Citation1980
Order MYLIOBATIFORMES Compagno, Citation1973
Family MYLIOBATIDAE Bonaparte, Citation1838
aff. MYLIOBATIDAE indet.
()
Material—One fragmented tooth from El Muro (CVAI00780, figured) and one from Castillo Barchell (CVAI00786).
Description—The specimens represent incomplete teeth with a lateral portion missing, and in poor preservation state. Hexagonal morphology in occlusal view, with uniform width and straight lateral, labial, and lingual faces, which run perpendicularly to the occlusal surface. Both the occlusal and basal faces show multiple round marks, and the occlusal portion of the crown shows a slight convexity. The root is less developed than the crown and more labiolingually flattened, with the crown overhanging significantly. There are no discernible protrusions or bulges in either the labial or lingual faces. Despite the poor preservation state, the remaining root portion does not seem to bend at an angle from the crown.
Comparative Remarks—The hexagonal morphology of the tooth in occlusal view, the straight lateral, labial, and lingual faces, and its comparison with material reported in the bibliography (Cappetta, Citation2012), have allowed the identification of this tooth at a family level, although the poor preservation state of the tooth has made further classification significantly difficult due to the lack of additional morphological characteristics to be described and compared. Many circular markings are found all over the tooth, including the root, as well as the functional occlusal face of the crown, which suggests the aforementioned marks were not caused by use during feeding, and would have probably been caused during the fossilization process.
The Myliobatidae family is well represented in the fossil record, with representatives being found in different regions of South America, (Monsch, Citation1998; dos Reis, Citation2005), as well as European localities among others (Szabó & Kocsis, Citation2020).
Both of the genera that are included within the family, Aetomylaeus and Myliobatis, are benthopelagic species that inhabit warm shallow waters although they can be seen at depths up to 150 meters (Froese & Pauly, Citation2023).
Material—Two individuals from Moli Palla (figured specimen CVAI00767, and CVAI00768), as well as one complete individual from El Muro (MGUV-39886).
Description—The two studied teeth possess a globular crown with irregular surface, showing a rhomboidal morphology in occlusal view, with a more rounded lingual face. The crown lacks cusps and its lingual margin overhangs the mesial portion of the root. None of the crown margins shows striations or folds, although the labial face shows a rugose texture behind the transversal keel, whereas the lingual face is smooth. One of the teeth is partially embedded in the matrix, but the other shows a reduced root, less wide than the crown, with labiolingually developed lobes and a flattened basal face. Said lobes are separated by a well-developed mesial furrow, which is filled with matrix, making the observation of possible foramina impossible.
Comparative Remarks—The globular crown and its irregular surface are typical characteristics from the Dasyatidae family (Cappetta, Citation2012), although due to both teeth being partially embedded in the matrix (one of them almost completely), as well as the preservation state, it was difficult to classify them at a more precise level. In addition, despite the tooth morphology of the specimens described herein showing similarities to the Dasyatidae family, other families lack reliable tooth descriptions, or are completely unknown, even more so in the Mediterranean Realm (Ebert & Dando, Citation2020). As such, the association of the aforementioned teeth to this family is tentative and could be subject to change in the future, with additional findings and descriptions of batoid faunas.
Fossil remains from this family have been recovered from Miocene deposits of the Mediterranean (Balbino & Antunes, Citation2006; Vialle et al., Citation2011), as well as from some regions of Africa and Asia (Marramà et al., Citation2018; Sharma & Patnaik, Citation2013; Stewart, Citation2001).
The living representatives from this family, such as Dasyatis pastinaca (Linnaeus, Citation1758), are demersal species that can be found in warm and temperate marine waters (Cappetta, Citation2012) —and even in freshwater bodies in some genera—where they live near the bottom feeding mainly on hard prey. D. pastinaca can be found in coastal waters and continental shelves at depths up to 140 m, being generally found in waters shallower than 50 m, in the Mediterranean and Atlantic basins (Last et al., Citation2016).
Material—Two teeth from El Muro (figured specimen MGUV-39888, and MGUV-39887).
Description—The root is absent in both teeth due to poor preservation state. The crown possesses a rounded morphology, with a central cusp slanted toward the lingual face, and two smaller lateral lobes located at the base of the crown, also slanted lingually, and more developed in the lower portion of the labial face of the crown. Both, the cusp and the rest of the crown, show a smooth surface without striations or folds besides some taphonomic marks. The root was not preserved, with the only possible remaining element being what appears to be a foramen in the basal face of one of the teeth.
Comparative Remarks—Despite the lack of the root and more details that could help narrow down the classification to a more specific level, the presence of a cusp (Cappetta, Citation2012), as well as the general morphology of the crown, with the more developed basal portion in the labial face, with two lateral lobes that are inclined towards the lingual face like the cusp, have allowed the identification of the specimen as part of the Rajiformes order, resembling members of the Rajidae family, among others—see fig. 349 in Cappetta (Citation2012). Regrettably, the lack of the root and poor general state of preservation make further identification impossible, as the members of this order possess similarities between different families and genera (Herman et al., Citation1994, Citation1996, Citation1998), and as such the authors decided to abstain from attempting deeper classification, leaving the tooth as a tentative member of Rajiformes, with some similarities to the Rajidae family.
Within the Rajiformes, the Rajidae family is well represented in the fossil record, with numerous findings from the Miocene of Denmark, Austria, France in Europe (Bendix-Almgreen, Citation1983; Marramà et al., Citation2018; Vialle et al., Citation2011), as well as Chile and Argentina in South America (Cione et al., Citation2011) and the United States (Cicimurri & Knight, Citation2009) among others.
The current diversity of species within Rajidae in the Mediterranean and Atlantic basins seems to have risen during the Miocene to Pleistocene (Cappetta, Citation2012; Valsecchi et al., Citation2005). Of the 17 living genera within the family, most skates are demersal and inhabit cold waters located on or near the continental slopes and abyssal plains at depths surpassing 4,000 m, although some temperate and cold-water inhabitants occur on the continental shelves, in considerably shallower waters (Last et al., Citation2016). Within the Mediterranean, Ebert and Dando (Citation2020) reported the presence of three species of the Rajidae family as the only representatives of their order.
As a reference species, Raja clavata (Linnaeus, Citation1758), is a demersal skate that occurs in the Mediterranean and eastern Atlantic (Last et al., Citation2016), as well as the southwestern Indian Ocean, being found at depths up to 300 m (Froese & Pauly, Citation2023), but reported at maximum depths of 1020 m (Last et al., Citation2016).
DISCUSSION
The fossil assemblage recovered from Alcoy shows an important abundance of ichthyofauna, with more than 4,000 fragmented specimens, being very well represented by elasmobranch teeth and placoid scales. Among them, a total of 20 selachian taxa were identified, belonging to four Orders of sharks—Lamniformes, Carcharhiniformes, Hexanchiformes, and Squaliformes—with seven taxa being identified at species level: †Carcharodon hastalis (), cf. †Carcharias acutissima (), †Otodus megalodon (), Scyliorhinus canicula (), cf. †Hemipristis serra (), Deania calcea (), and cf. †Squaliodalatias weltoni (). Ten additional taxa were identified at genus level due to the poor preservation of the recovered fossil elements: cf. Carcharias sp. (), †Notorynchus sp. (), cf. Isurus sp. (), Carcharhinus sp. (), cf. Galeocerdo sp. (), Scyliorhinus sp. (), †Megascyliorhinus sp. (), cf. Galeorhinus sp. (), Chlamydoselachus sp. (), and Trigonognathus sp. (). This finding also marks the second record of Trigonognathus in the Neogene of the Mediterranean Realm, after the multiple teeth recovered from the El Ferriol fossil site, also located in Alicante Province, Spain (Herraiz et al., Citation2022). Beside the aforementioned selachian taxa, two different batoid families are also represented: aff. Dasyatidae (), aff. Myliobatidae (), both within the Myliobatiformes Order, as well as an additional member of Rajiformes (cf. Rajiformes indet.; ).
Regarding the relative abundances of the different families recovered, Lamnidae stands out as the most represented, constituting nearly half of the total specimens (44.9%, n = 137) in the sample, with a pronounced predominance of †C. hastalis. Following closely are the catsharks, Scyliorhinidae (15%, n = 46), Carchariidae (13.4%, n = 41), and Carcharhinidae (8%, n = 25), highlighting Lamniformes and Carcharhiniformes as the dominant orders in the environment. With the exception of Chlamydoselachidae (6.8%, n = 21), the remaining families, encompassing both near-shore and deep-water taxa, exhibit lower abundances, hovering around 2%, such as Dalatiidae (2.9%, n = 9) or Galeocerdonidae (1.9%, n = 6), or representing less than 1% of the total abundance, including Triakidae and Hemigaleidae, as well as the teeth associated with aff. Dasyatidae (all at 0.9%, n = 3), Etmopteridae, Centrophoridae, and Otodontidae. Additionally, teeth classified as aff. Myliobatidae and cf. Rajiformes indet. each represent 0.7% (n = 2), and Hexanchidae stands at 0.4% (n = 1).
Paleoecological and Paleoenvironmental Reconstruction
Among the neoselachians identified in the area of study, there seems to be a mixture between shallow and deep-water inhabitants, as well as some pelagic and neritic species, ranging from nearshore to estimated depths of up to approximately 1200 meters (Ebert et al., Citation1992, Citation2021; Santos et al., Citation2020; Froese & Pauly, Citation2023). Despite the heterogeneous nature of the faunal assemblages described, most of the identified taxa do not generally reach more than 200 meters deep, with a significant amount of them being usually found in the upper 100 meters, such as Scyliorhinus spp., Carcharhinus sp., Carcharias sp., and †C. acutissima—with inferred similar habits to the extant C. taurus according to Ebert and Dando (Citation2020), and Ebert et al. (Citation2021)—as well as the unidentified member of Dasyatidae (Ebert & Dando, Citation2020; Last et al., Citation2016). †Otodus megalodon, †Carcharodon hastalis, †Megascyliorhinus sp., †Hemipristis serra and the indeterminate Myliobatidae would probably reach deeper maximum depths but still be mostly confined to the top 200 m based on their closest ecological analog species (Bradford et al., Citation2020; Ebert & Dando, Citation2020; Ebert et al., Citation2021; Last et al., Citation2016).
On the other hand, modern relatives of some taxa from the assemblage usually occur in deeper waters, such as those of Trigonognathus sp., Chlamydoselachus sp. (which can be found at more than 1,000 m) (Ebert et al., Citation2021), †Squalodalatias weltoni and Deania calcea (Ebert et al., Citation2021), as well as most members of the Rajiformes (Ebert & Dando, Citation2020; Last et al., Citation2016).
Regarding habitat use, D. calcea, †S. weltoni, S. canicula, †H. serra, Galeorhinus sp., †Notorynchus sp., Chlamydoselachus sp., Trigonognathus sp., and both representatives of Carcharias are considered demersal taxa that usually occur near the bottom, either in hard or soft substrates or, in the case of some of the aforementioned taxa, in both substrates, based on their closest living ecological relatives (see Systematic Paleontology and references therein). Within this group, Trigonognathus and Chlamydoselachus would not be strictly constricted to near-bottom environments, being considered benthopelagic genera that occur in deep open waters (Ebert et al., Citation2021).
This amalgamation of deep-water, shallow-water, and pelagic inhabitants has been previously documented in other fossil localities, with two primary hypotheses providing explanations. The first hypothesis links this diversity to vertical migrations by deep-water taxa, moving through the water column during night-time feeding. The second hypothesis associates it with taphonomic processes, involving the downslope displacement of shallow-water inhabitants remains (Carrillo-Briceño et al., Citation2015; Cigala Fulgosi et al., Citation2009; Yano et al., Citation2003). In the current study, the vertical migration hypothesis finds stronger support due to the uniform preservation state observed across all fossils considered.
The El Muro foraminiferal assemblage encompasses at least 15 different taxa (refer to ), comprising seven planktonic and eight benthic species, respectively. Analysis of the relative abundances of benthic and planktonic foraminifera estimates a deep-water environment of approximately 873 meters in depth, following the Oceanization Index for the Western Mediterranean proposed by De Rijk et al. (Citation2000). Additionally, the presence of †Globorotalia menardii (d'Orbigny in Parker et al., Citation1865) has facilitated dating the El Muro fossil site to the Tortonian (Late Miocene) age, aligning with findings by Sierro (Citation1985). On the contrary, the weighted bootstrap bathymetric analyses revealed a bimodal distribution when depicting depth probability (). The initial peak is evident around 200 meters, followed by a second, notably higher peak spanning 400–500 meters, with the mean estimated at approximately 420 meters. Notably, this outcome remains consistent whether or not Batoidea taxa are included in the analyses.
TABLE 1. Taxonomic classification, divided in planktonic and benthonic habitat usage and number of specimens of the foraminiferal assemblage extracted from El Muro.
FIGURE 4. Depth estimation employing weighted bootstrap analysis of the elasmobranch faunal composition, considering both A, the members of Batoidea, and B, and excluding them. The mean depth estimation is represented by the line, while the 95% confidence interval for the most probable depth range of the assemblage is illustrated as a shadow.
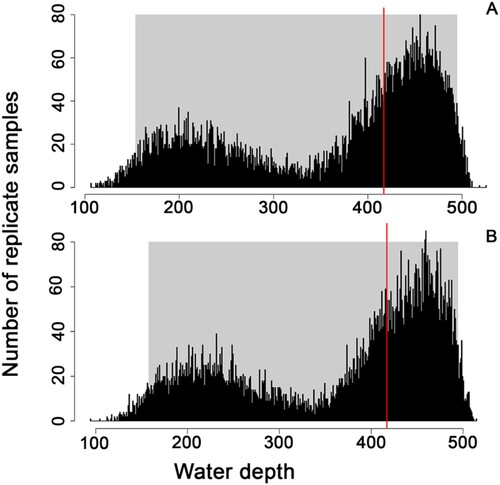
The interpretation of the paleoenvironment and bathymetry at the El Muro locality, based on sediments and foraminifera, holds relevance for all similar locations in Alcoy. However, it appears to diverge from the depth estimates derived from the weighted bootstrap analysis which indicates shallower waters based on the chondrichthyan assemblage. While comparable statistical analyses have been employed by other researchers in prior studies (Carrillo-Briceño et al., Citation2020; Oyanadel-Urbina et al., Citation2021; Pérez et al., Citation2017) the conflicting results, especially when juxtaposed with the foraminiferal assemblage as well as the sedimentology pointing to higher-energy water environments, suggest that these analyses alone may not be sufficient for estimating paleodepths. The presence of components with diverse lifestyles and habitat usages within the faunal composition might be a contributing factor. Vertical migration undertaken by deep-water taxa to feed at night in shallower waters, as highlighted by Martínez-Pérez et al. (Citation2018) or Herraiz et al. (Citation2022), and references therein, along with the broad depth range occupied by some macropredatory taxa, as discussed in Systematic section, could potentially skew depth estimations compared with other methods.
Comparison with Other Localities
The faunal assemblage described here is comparable to other coetaneous Miocene outcrops from the Alicante province in Eastern Spain. The El Chorrillo fossil site, near the municipality of Sax (Alicante, Spain), also yielded common taxa such as Deania, Scyliorhinus, Carcharodon, Hemipristis, and †Otodus, in addition to other taxa absent from our area of study and exclusive to that locality, such as Pristiophorus, Isistius, or †Paraetmopterus. Despite a similar diversity of taxa, the relative abundances of both faunal compositions are starkly different, with Deania calcea being the predominant species found at El Chorrillo, whereas the most abundant at Alcoy seems to be †C. hastalis. Ferrón et al. (Citation2019) also reported on the ecological diversity found in El Chorrillo based on the analysis of the dermal denticles recovered from the site, having identified placoid scales associated with optimized swimming for active pelagic taxa, bioluminescence aiding scales, and denticles associated with demersal inhabitants, both living on hard and soft substrates, as well as deep-water inhabitants. The reported ecological diversity based on the scales is again concurrent with the one described by Martínez-Pérez et al. (Citation2018), and similar to the one found in Alcoy and presented herein. Some of the reported scales from El Chorrillo were identified as bioluminescent, and this ecological group is also represented in the Alcoy faunas with Trigonognathus sp., a representative of the Etmopteridae family that has been stated to possess bioluminescence based on its position within this family and the presence of photophores (Mochizuki & Ohe, Citation1990; Straube et al., Citation2010). In addition, †Squaliodalatias weltoni, belonging to the Dalatiidae family, is also reconstructed as a bioluminescent taxon in Ferrón (Citation2023).
Furthermore, the faunal assemblage from Alcoy could also be compared with another Miocene elasmobranch assemblage described by Marín (Citation1992), from the “Burdigalian” localities of El Ferriol, Castelar, and Vallongas from the municipality of Elche (Alicante, Spain) (see above). The comparison of the described faunal assemblages with the one from Alcoy shows only five taxa in common, the macropredatory †Otodus megalodon, †Carcharodon hastalis, Carcharhinus sp. (reported as †C. priscus), and †Hemipristis serra. Despite not sharing the same species, Galeocerdo sp., reported as G. cuvier, similar families are represented in the Elche localities, such as Carchariidae with the Carcharias genus (†C. cuspidatus), and Hexanchidae with †Notorynchus sp. The differences in faunal assemblages in regard to the apparent lack of small size chondrichthyans and demersal taxa in the Elche outcrops is probably biased by a lack of sampling efforts for microfossils and not by the lack of such ecological groups. These fossil sites have been historically known and exploited by local fossil collectors that focused their surface sampling on the larger and better-preserved fossil pieces, which could explain the faunal differences between those localities and our study. As such, further studies in more depth are probably needed to shed light into the real complexity and elasmobranch diversity of the Elche faunal assemblages.
CONCLUSIONS
The present study represents the first systematic description of the Miocene chondrichthyans of the Alcoy localities (Alicante, Spain), including a total of 20 elasmobranch taxa belonging to six neoselachian orders (Lamniformes, Carcharhiniformes, Squaliformes, Hexanchiformes, Rajiformes, and Myliobatiformes), and 14 families (†Otodontidae, Lamnidae, Carchariidae, Carcharhinidae, Galeocerdonidae, Hemigaleidae, Triakidae, Scyliorhinidae, Etmopteridae, Dalatiidae, Centrophoridae, Hexanchidae, Chlamydoselachidae, Myliobatidae, and Dasyatidae). Additionally, by studying the fossil elasmobranch and foraminiferal assemblages, an estimation of the paleodepth and environment of these localities has been inferred, showing signs of Bathyal environments, no deeper than 1,000 meters according to the foraminiferal assemblage. These results contrast with the weighted bootstrap analysis of the elasmobranch assemblage that suggests shallower environments, with a mean of 420 m approximately. The significant difference between both methods highlights the lack of compatibility of certain faunal assemblages with the bootstrap methodology, which perhaps should be reconsidered for future studies. The presence of the foraminifer †Globorotalia menardii (Kennett & Srinivasan, Citation1983) has allowed to date, at least the El Muro outcrop, as Tortonian (Late Miocene). Comparisons with other Miocene elasmobranch assemblages from Alicante (Spain) show similarities in faunal composition, although many of those fossil sites have been historically known but never studied and published in peer-reviewed literature, or have been subject to exploitation by local collectors. Subsequent sampling and analyses should be undertaken in future studies of those areas to provide a deeper, more comprehensive picture of their faunal composition and the paleoecology of chondrichthyan communities previous to one of the most important biotic crises at the end of the Miocene period.
SUPPLEMENTARY FILES
Supplemental file 1.xls: Raw Data matrix for paleobathymetric estimation, and abundance and depth data for each taxon used.
Supplemental file 2.R: Script for paleobathymetric estimation.
AUTHOR CONTRIBUTIONS
JLH and CMP conceived the study; JLH, AC, and CMP conducted the fieldwork, JLH, AC, IGS, and CMP identified the specimens; HGF conducted the statistical analyses; JLH led the writing with assistance from HGF, HB, and CMP.
Supplemental Material
Download Zip (13.2 KB)ACKNOWLEDGMENTS
The materials studied herein were collected under the project 2018/0820-A of the Conselleria d’Educació, Investigació, Cultura i Esport (Valencia, Spain). H.G.F. is funded by the MCIN and the “European Union NextGenerationEU” [grant number RYC2021-032775-I]. C.M.P has been funded by the Ministry of Science and Innovation of Spain, Research Project PID2020-117373GA-I00.
DISCLOSURE STATEMENT
No potential conflict of interest was reported by the author(s).
LITERATURE CITED
- Abella, A. J., & Serena, F. (2005). Comparison of elasmobranch catches from research trawl surveys and commercial landings at port of Viareggio, Italy, in the last decade. Journal of Northwest Atlantic Fishery Science, 35, 345–356. DOI: 10.2960/J.v35.m526
- Adnet, S., Balbino, A. C., Antunes, M. T., & Marín-Ferrer. J. M. (2010). New fossil teeth of the White Shark (Carcharodon carcharias) from the Early Pliocene of Spain. Implication for its paleoecology in the Mediterranean. Neues Jahrbuch Für Geologie Und Paläontologie-Abhandlungen, 256(1), 7–16. DOI: 10.1127/0077-7749/2009/0029
- Adnet, S., Cappetta, H., & Reynders, J. (2006). New squaliforms genera (Chondrichthyes: Squalomorphii) from the Paleogene of the Landes department (southwestern France). Paläontologische Zeitschrift, 80, 60–67.
- Agassiz, L. (1835). Recherches sur les poissons fossiles, 5th livraison (June 1835). Petitpierre et Prince (text) and H. Nicolet (plates), Neuchâtel, 3, pl. D, 46.
- Agassiz, L. (1838). Recherches sur les poissons fossiles, 11th livraison (November 1838). Petitpierre et Prince (text) and H. Nicolet (plates), Neuchâtel, 3, 73–140.
- Agassiz, L. (1843). Recherches sur les poissons fossiles, v. 3: Neuchâtel. Switzerland, Petitpierre.
- Agiadi, K., Hohmann, N., Carnevale, G., Gliozzi, E., Faranda, C., Lozar, F., Harzhauser, M., Iliopoulos, G., Caruso, A., & Kontakiotis. G. (2020). The Impact of the Messinian Salinity Crisis on Marine Biota. EGU General Assembly 2020, Online, 4–8 May 2020, EGU2020-5799. DOI: 10.5194/egusphere-egu2020-5799
- Aguilera, O., & Rodriguez de Aguilera, D. (2001). An exceptional coastal upwelling fish assemblage in the Caribbean Neogene. Journal of Paleontology, 75(3), 732–742. DOI: 10.1666/0022-3360(2001)0752.0.CO;2
- Ameghino, F. (1901). Notices Préliminaires Sur Des Ongulés Nouveaux Des Terrains Crétacés de Patagonie. Boletín de la Academia Nacional de Ciencias de Córdoba, 16, 349–426.
- Antunes, M. (1977). Late Neogene fish faunas from Angola, their age and significance. Journal of the Paleontological Society of India, 20, 224–229.
- Antunes, M. T., & Jonet, S. (1970). Requins de l’Helvétien Supérieur et Du Tortonien de Lisbonne. Revista Facultad Ciências Lisboa, 16(1), 119–280.
- Antunes, M. T., & Balbino, A. C. (2010). Uppermost Miocene lamniform selachians (Pisces) from the Alvalade basin (Portugal). Ciências da Terra, 15, 141–154.
- Antunes, M. T., Balbino, A. C., & Cappetta, H. (1999). Sélaciens du Miocene terminal du bassin d’Alvalade (Portugal), Essai de synthèse. Ciências Da Terra/Earth Sciences Journal, 13, 115–129.
- Antunes, M. T., Legoinha, P., & Balbino. A. (2015). Megalodon, mako shark and planktonic foraminifera from the continental shelf off Portugal and their age. Geologica Acta, 13(3), 181–190.
- Argyriou, T., Cook, T. D., Muftah, A. M., Pavlakis, P., Boaz, N. T., & Murray, A. M. (2015). A fish assemblage from an early Miocene horizon from Jabal Zaltan, Libya. Journal of African Earth Sciences, 102, 86–101. DOI: 10.1016/j.jafrearsci.2014.11.008
- Ávila, S. P., Ramalho, R., & Vullo, R. (2012). Systematics, palaeoecology and palaeobiogeography of the Neogene fossil sharks from the Azores (Northeast Atlantic). Annales de Paléontologie, 98(3), 167–189. DOI: 10.1016/j.annpal.2012.04.001
- Ayres, W.O. (1855). A shark of a new generic type: Notorynchus maculatus. Proceedings of the California Academy of Sciences, (Series 1), 1, 72–73.
- Bakopoulos, V., Tsepa, E., Diakou, A., Kokkoris, G., Kolygas, M. N., & Athanassopoulou, F. (2018). Parasites of Scyliorhinus canicula (Linnaeus, 1758) in the north-eastern Aegean Sea. Journal of the Marine Biological Association of the United Kingdom, 98(8), 2133–2143. DOI: 10.1017/S0025315417001552
- Balbino, A. C., & Antunes, M. T. (2006). Latest miocene Dasyatidae (Neoselachii, Batomorphii) from the Alvalade Basin, Portugal. Geobios, 39(6), 747–755. DOI: 10.1016/j.geobios.2005.07.002
- Banner, F. T., & Blow, W. H. (1965). Two new taxa of the Globorotaliinae (Globigerinacea, Foraminifera) assisting determination of the late Miocene/middle Miocene boundary. Nature, 207, 1351–1354. DOI:10.1038/2071351a0
- Barthelt, D. (1991). Notizen zu einem Profil der Selachier-Fundstelle Walbertsweiler im Bereich der Miozan Oberen meeresmolasse Suddeutchlands. Munchener Geowiss Abh (A), 19, 195–208.
- Bauzá Rullán, J. (1969). Contribuciones al conocimiento de la ictiología fósil de Mallorca. Bolletí de La Societat d’Història Natural de Les Balears, 15, 93–102.
- Bauzá Rullán, J., & Mercadal, B. (1961). Nuevas contribuciones al conocimiento de la fauna ictiológica fósil de Menorca. Bolletí de La Societat d’Història Natural de Les Balears, 7, 45–48.
- Bauzá Rullán, J., & Plans, J. (1973). Contribución al conocimiento de la fauna ictiológica del Neógeno Catalano Balear. Bolletí de La Societat d’História Natural de Les Balears, 18, 72–131.
- Bauzá Rullán, J., & Gómez-Pallerola, J. E. (1988). Contribución al conocimiento de la ictiología fósil de España. Bolletí de La Societat d’Història Natural de Les Balears, 32, 115–138.
- Bendix-Almgreen, S. E. (1983). Carcharodon megalodon from the Upper Miocene of Denmark, with comments on elasmobranch tooth enameloid: coronoïn. Bulletin of the Geological Society of Denmark, 32, 1–32.
- Berg, L.S. (1940). Classification of fishes, both recent and fossil. Zoology Academy of Sciences of the USSR, 5, 87–517.
- Berg L.S. (1958). System der rezenten und fossilen Fischartigen und Fische. Hochschulbucher fur Biologie, 4, I-XI, Deutschen Verlag der Wissenscahften, Berlin. 310pp.
- Berio, F., Evin, A, Goudemand, N., & Debiais-Thibaud, M. (2020). The intraspecific diversity of tooth morphology in the large-spotted catshark Scyliorhinus stellaris: insights into the ontogenetic cues driving sexual dimorphism. Journal of Anatomy, 237(5), 960–978. DOI: 10.1111/joa.13257
- Bertrand, J., Gil-de-Sola-Simarro, L., Papakonstantinou, C., Relini, G., & Souplet, A. (2000). Contribution on the distribution of elasmobranchs in the Mediterranean (from MEDITS surveys). Biologia Marina Mediterranea, 7,1–15.
- Bhalla, S. N., & Prammendra, D. E. V. (1988). Paleoecology of Baripada bads (Middle Miocene), East Coast of India. Bulletin of Indian Geologist Association, 21,141–153.
- Bianchi, C. N., & Morri, C. (2000). Marine biodiversity of the Mediterranean Sea: situation, problems and prospects for future research. Marine Pollution Bulletin, 40(5), 367–376. DOI: 10.1016/S0025-326X(00)00027-8
- de Blainville, H.M.D. (1816). Prodrome d'une nouvelle distribution systématique du règne animal. Bulletin des Sciences, par la Société Philomatique de Paris, 8, 105–124.
- Bleeker, P. (1859). Enumeratio specierum piscium hucusque in Archipelago indico observatarum. Acta Societatis Scientiarum Indo-Neerlandae, 6, 1–276.
- Boessenecker, R. W., Ehret, D. J., Long, D. J., Churchill, M., Martin, E. & Boessenecker, S. J. (2019). The Early Pliocene extinction of the mega-toothed shark Otodus megalodon: a view from the eastern North Pacific. PeerJ, 7, e6088. DOI: 10.7717/peerj.6088
- Bonaparte CL. (1838). Selachorum tabula analytica. Nuovi Annali della Scienze Naturali, 1(2), 195–214.
- Borghi, E., & Garilli, V. (2022). Climate-driven diversity changes of Mediterranean echinoids over the last 6 Ma. Acta Palaeontologica Polonica, 67(4), 781–805. DOI: 10.4202/app.00993.2022
- Bradford, R., Patterson, T. A., Rogers, P. J., McAuley, R., Mountford, S., Huveneers, C., Robbins, R., Fox, A., & Bruce. B. D. (2020). Evidence of diverse movement strategies and habitat use by white sharks, Carcharodon carcharias, off southern Australia. Marine Biology, 167(96), 1–12. DOI: 10.1007/s00227-020-03712-y
- de Buen, F. (1926). Catálogo ictiológico del Mediterráneo español y de Marruecos. Resultados de las campañas realizadas por acuerdos internacionales, Instituto Español Oceanografía, 2, 153–161.
- Cahuzac, B., Adnet, S., Cappetta, H., & Vullo. R. (2007). Les espèces et genres de poissons sélaciens fossiles (Crétacé, Tertiaire) créés dans le Bassin d’Aquitaine; recensement, taxonomie. Bulletin de La Société Linnéenne de Bordeaux 142, nouvelle série 35(1),3–43.
- Canevet, J.-M. (2011). Les Chondrichthyens du Miocène moyen (Serravallien) de Salles (Gironde, France). Cossmanniana, 13, 59–76.
- Cappetta H. (1980). Les Sélaciens du Crétacé supérieur du Liban. II. Batoïdes. Palaeontographica Abteilung A. 168(56), 149–229.
- Cappetta, H. (2012). Handbook of Paleoichthyology, Vol. 3E: Chondrichthyes Mesozoic and Cenozoic Elasmobranchii: Teeth. Verlag Dr. Friedrich Pfeil, 512 pp.
- Cappetta, H., & Ward, D. J. (1977). A new Eocene shark from the London Clay of Essex. Palaeontology, 20(1),195–202.
- Cappetta, H., & Adnet, S. (2001). Découverte du genre actuel Trigonognathus (Squaliformes: Etmopteridae) dans le Lutétien des Landes (sud-ouest de la France). Remarques sur la denture de l’espèce actuelle Trigonognathus kabeyai. Paläontologische Zeitschrift, 74, 575–581. DOI: 10.1007/BF02988163
- Carrillo-Briceño, J. D., Aguilera, O. A., & Rodríguez, F. (2014). Fossil Chondrichthyes from the central eastern Pacific Ocean and their paleoceanographic significance. Journal of South American Earth Sciences, 51, 76–90. DOI: 10.7717/peerj.9051
- Carrillo-Briceño, J. D., Villafaña, J. A., De Gracia, C., Flores-Alcívar, F. F., Kindlimann, R., & Abella, J. (2020). Diversity and paleoenvironmental implications of an elasmobranch assemblage from the Oligocene–Miocene boundary of Ecuador. PeerJ, 8, e9051. DOI: 10.7717/peerj.9051
- Carrillo-Briceño, J. D., De Gracia, C., Pimiento, C., Aguilera, O. A., Kindlimann, R., Santamarina, P., & Jaramillo, C. (2015). A new Late Miocene chondrichthyan assemblage from the Chagres Formation, Panama. Journal of South American Earth Sciences, 60, 56–70. DOI: 10.1016/j.jsames.2015.02.001.
- Chandler, R. E., Chiswell, K. E. & Faulkner, G. D. (2006). Quantifying a possible Miocene phyletic change in Hemipristis (Chondrichthyes) teeth. Palaeontologia Electronica, 9,1–14.
- Cicimurri, D. J., & Knight, J. L. (2009). Late Oligocene sharks and rays from the Chandler Bridge Formation, Dorchester County, South Carolina, USA. Acta Palaeontologica Polonica, 54(4), 627–647. DOI: 10.4202/app.2008.0077
- Cigala Fulgosi, F., Casati, S., Orlandini, A., & Persico, D. (2009). A small fossil fish fauna, rich in Chlamydoselachus teeth, from the Late Pliocene of Tuscany (Siena, central Italy). Cainozoic Research, 6(1/2), 3–23.
- Cione, A. L., Mennucci, J., Pérez, L., & Barla, M. J. (2008). Megascyliorhinus trelewensis (Neoselachii) in the? Middle-Upper Miocene of Paraná, Central eastern Argentina. Temas de La Biodiversidad Del Litoral Fluvial Argentino III. Miscelánea, 17, 41–48.
- Cione, A. L., Cozzuol, M. A., Dozo, M. T., & Acosta Hospitaleche, C. (2011). Marine vertebrate assemblages in the southwest Atlantic during the Miocene. Biological Journal of the Linnean Society, 103, 423–440. DOI: 10.1111/j.1095-8312.2011.01685.x
- Coll, M., Piroddi, C. Steenbeek, J., Kaschner, K., Lasram, F. B. R., Aguzzi, J., Ballesteros, E., Bianchi, C. N., Corbera, J., & Dailianis, T. (2010). The biodiversity of the Mediterranean Sea: estimates, patterns, and threats. PLoS ONE, 5, e11842. DOI:10.1371/journal.pone.0011842
- Compagno, L., Dando, M., & Fowler, S. (2005). Sharks of the World. Princeton Field Guides. Princeton University Press, Princeton.
- Compagno, L.J.V. (1973). Interrelationships of living elasmobranchs. In: P.H. Greenwood, R.S. Miles & C. Patterson (Eds.) Interrelationships of Fishes, Zoological Journal of the Linnean Society, 1(53), pp.1561.
- Compagno, L. J. (1984). FAO species catalogue. Vol. 4. Sharks of the world. An annotated and illustrated catalogue of shark species known to date. Part2. Carcharhiniformes. FAO Fisheries Synopsis, 125, 251–655.
- Cooper, J. A., Pimiento, C., Ferrón, H. G., & Benton, M. J. (2020). Body dimensions of the extinct giant shark Otodus megalodon: a 2D reconstruction. Scientific Reports, 10, 1–9. DOI: 10.1038/s41598-020-71387-y
- Crespo, V. D. (2017). Los mamíferos del Mioceno Inferior de la Cuenca de Ribesalbes-Alcora (Castelló, España). PhD Thessis Disertation, University of Valencia.
- Davidson, L. N., Jaiteh, V. F., Chin, A., & Jabado, R. W. (2022). Conservation Science for Sharks and Rays; pp. 657–688 in Biology of Sharks and Their Relatives. CRC Press.
- De Rijk, S., Jorissen, F. J., Rohling, E. J, & Troelstra, S. R. (2000). Organic flux control on bathymetric zonation of Mediterranean benthic foraminifera. Marine Micropaleontology, 40(3), 151–166. DOI: 10.1016/S0377-8398(00)00037-2
- De Schutter, P. J. (2011). Carcharias vorax (Le Hon, 1871)(Chondrichthyes, Lamniformes), from the Miocene of Belgium: redescription and designation of a neotype and paraneotype. Geologica Belgica, 14(3–4), 175–192.
- Dicken, M. L., Hussey, N. E., Christiansen, H. M., Smale, M. J., Nkabi, N. Cliff, G., & Wintner, S. P. (2017). Diet and trophic ecology of the tiger shark (Galeocerdo cuvier) from South African waters. PLoS ONE, 12, e0177897. DOI: 10.1371/journal.pone.0177897
- Dulvy, N. K., Allen, D. J., Ralph, G. M., & Walls, R. H. L. (2016). The conservation status of sharks, rays and chimaeras in the Mediterranean Sea. IUCN, Malaga, Spain.
- Ebert, D. A., & Compagno, L. J. (2009). Chlamydoselachus africana, a new species of frilled shark from southern Africa (Chondrichthyes, Hexanchiformes, Chlamydoselachidae). Zootaxa, 2173(1), 1–18.
- Ebert, D. A., & Dando, M. (2020). Field Guide to Sharks, Rays & Chimaeras of Europe and the Mediterranean. Princeton University Press.
- Ebert, D. A., Compagno, L. J. V., & Cowley, P. D. (1992). A preliminary investigation of the feeding ecology of squaloid sharks off the west coast of southern Africa. South African Journal of Marine Science, 12(1), 601–609. DOI:10.2989/02577619209504727
- Ebert, D. A., Dando, M., & Fowler, S. (2021). Sharks of the World a complete guide (Vol. 22). Princeton University Press.
- Ebert, D. A., White, W. T., Ho, H.-C., Last, P. R., Nakaya, K., Seret, B., Straube, N., Naylor, G. J., & De Carvalho, M. R. (2013). An annotated checklist of the chondrichthyans of Taiwan. Zootaxa, 3752, 279–386. DOI: 10.11646/zootaxa.3752.1.17
- Ehret, D. J., Macfadden, B. J., Jones, D. S., Devries, T. J., Foster, D. A., & Salas-Gismondi, R. (2012). Origin of the white shark Carcharodon (Lamniformes: Lamnidae) based on recalibration of the Upper Neogene Pisco Formation of Peru. Palaeontology, 55(6), 1139–1153. DOI: 10.1111/j.1475-4983.2012.01201.x
- Ellis, J. R., Cruz-Martinez, A., Rackham, B. D., & Rogers, S. I. (2005). The distribution of chondrichthyan fishes around the British Isles and implications for conservation. Journal of Northwest Atlantic Fishery Science, 35, 195–213. DOI: 10.2960/J.v35.m485
- Ferrón, H. G. (2017). Regional endothermy as a trigger for gigantism in some extinct macropredatory sharks. PLoS ONE, 12, e0185185. DOI: 10.1371/journal.pone.0185185
- Ferrón, H. G. (2023). Illuminating the evolution of bioluminescence in sharks. Palaeontology, 66(1), e12641. DOI: 10.1111/pala.12641
- Ferrón, H. G., Herraiz, J. L,. Botella, H., & Martínez-Pérez, C. (2019). Pre-Messinian ecological diversity of Mediterranean sharks revealed by the study of their dermal denticles. Spanish Journal of Palaeontology, 34(2), 289–298. DOI: 10.7203/sjp.34.2.16118
- Fialho, P. R., Balbino, A. C., Legoinha, P., & Antunes, M. T. (2021). Shark fossil diversity (Squalomorphii, Squatinomorphii, and Galeomorphii) from the Langhian of Brielas (Lower Tagus Basin, Portugal). Geological Journal, 56(1), 405–421. DOI: 10.1002/gj.3965
- Fowler, H. W. (1941). The fishes of the groups Elasmobranchii, Holocephali, Isospondyli, and Ostariophysi obtained by the United States Bureau of Fisheries Steamer" Albatross" in 1907 to 1910, chiefly in the Philippine Islands and adjacent seas. Bulletin of the US National Museum, 13(100), 1–879.
- Frankel, J. J. (1972). Shark teeth in the tertiary of Zululand. South African Journal of Geology, 75(3), 311–312.
- Fricke, R., Mahafina, J., Behivoke, F., Jaonalison, H., Léopold, M., & Ponton, D. (2018). Annotated checklist of the fishes of Madagascar, southwestern Indian Ocean, with 158 new records. FishTaxa, 3(1), 1–432. DOI: 10.11646/zootaxa.3752.1.17
- Froese, R., & Pauly, D. (2023). FishBase. World Wide Web electronic publication. www.fishbase.org, version (06/2023).
- Gagnaison, C. (2013). Les assemblages de vertébrés dans deux sites paléontologiques du bassin miocène de Savigné-sur-Lathan/Noyant-sous-le-Lude: La Guimardière et Pelmer (Maine-et-Loire, France). Geodiversitas, 35(1), 67–103. DOI: 10.5252/g2013n1a5
- García-Sanz, I., Usera, J., Guillem, J., Giner-Baixauli, A., & Alberola, C. (2018). Geographical and bathymetric distribution of foraminiferal assemblages from the Alboran Sea (western Mediterranean). Quaternary International, 481, 146–156. DOI: 10.1016/j.quaint.2017.11.016
- Garman, S. (1884). An extraordinary shark. Bulletin of the Essex Institute, 16, 47–55.
- Gibert, L., Scott, G. R., Montoya, P., Ruiz-Sánchez, F. J., Morales, J., Luque, L., Abella, J., & Lería, M. (2013). Evidence for an African-Iberian mammal dispersal during the pre-evaporitic Messinian. Geology, 41(6), 691–694. DOI: 10.1130/G34164.1
- Gill, T. (1862). Analytical synopsis of the Order of Squali and revision of the nomenclature of the genera. Annals of the Lyceum of Natural History of New York, 7(32), 367–408. DOI: 10.1111/j.1749-6632.1862.tb00166.x
- Gillette, D. D. (1984). A marine ichthyofauna from the Miocene of Panama, and the Tertiary Caribbean faunal province. Journal of Vertebrate Paleontology, 4(2), 172–186. DOI: 10.1080/02724634.1984.10012001
- Goodrich, E. S. (1909). Vertebrata Craniata (First Fascicle: Cyclostomes and Fishes). In: Lankester, R. (Ed.) A treatise on zoology, Volume 9, (1909), pp.1–518.
- Glikman, L. S. (1964). Sharks of the Paleogene their stratigraphic significance. Nakua Press, Moscow, 229 pp.
- Gottfried, M. D. (1996). Size and skeletal anatomy of the giant” megatooth” shark Carcharodon megalodon. In A. P. Klimley, D. G. Ainley, Great White Sharks: The Biology of Carcharodon carcharias (pp. 55–66). San Diego: Academic Press.
- Gray, J.E. (1851). List of the specimens of fish in the collection of the British Museum. Part I. Chondropterygii. British Museum (Natural History), London, 160 pp.
- Halter, M. C. (1995). Additions to the fish fauna of NW Europe. 3. Three new species of the genus Scyliorhinus from the late Cretaceous (Campanian and Maastrichtian) of the Limburg area (Belgium and the Netherlands) with the reassignment of four additional species to the genus Scyliorhinus sensu stricto. Elasmobranches et Stratigraphie. Professional Paper of the Belgian Geological Survey, 278, 65–110.
- Hamon, N., Sepulchre, P., Lefebvre, V., & Ramstein. G. (2013). The role of eastern Tethys seaway closure in the Middle Miocene Climatic Transition (ca. 14 Ma). Climate of the Past, 9(6), 2687–2702. DOI: 10.5194/cp-9-2687-2013
- Herman, J., Hovestadt-Euler, M., Hovestadt, D. C., & Stehmann, M. (1994). Contributions to the study of the comparative morphology of teeth and other relevant ichthyodorulites in living supra-specific taxa of Chondrichthyan fishes. Part B: Batomorphi, Bulletin de l'Institut royal des sciences naturelles de Belgique, 64, 165–207.
- Herman, J., Hovestadt-Euler, M. Hovestadt, D. C., & Stehmann, M. (1996). Contributions to the study of the comparative morphology of teeth and other relevant ichthyodorulites in living supra-specific taxa of Chondrichthyan fishes. Part B: Batomorphi No. 1c: Order Rajiformes-Suborder Rajoidei-Family: Rajidae-Genera and Subgenera: Arhynchobatis, Bathyraja richardsoni-type. Cruriraja, Irolita, Notoraja, Pavoraja (Insentiraja), Pavoraja (Pavoraja), Pseudoraja, Raja (Atlantoraja), Raja (Okamejei), Bulletin de l'Institut royal des sciences naturelles de Belgique, 66, 179–236.
- Herman, J., Hovestadt-Euler, M., Hovestadt, D., & Stehmann, M. (1998). Contributions to the study of the comparative morphology of teeth and other relevant ichthyodorulites in living supra-specific taxa of Chondrichthyan fishes. Part B: Batomorphi, Bulletin de l'Institut royal des sciences naturelles de Belgique, 68, 145–197.
- Herraiz, J. L., Ribé, J., Botella, H., Martínez-Pérez, C., & Ferrón, H. G. (2020). Use of nursery areas by the extinct megatooth shark Otodus megalodon (Chondrichthyes: Lamniformes). Biology Letters, 16(11), 20200746. DOI: 10.1098/rsbl.2020.0746
- Herraiz, J. L., Carrillo-Briceño, J. D., Ferrón, H. G., Adnet, S., Botella, H., & Martínez-Pérez. C. (2022). First fossil record (Middle Miocene) of the viper shark Trigonognathus Mochizuki and Ohe, 1990, in the Mediterranean realm. Journal of Vertebrate Paleontology, 42(1), e2114360. DOI: 10.1080/02724634.2022.2114360
- Hoffmann, R., Bitner, M. A., Pisera, A., Jäger, M., Auer, G., Giraldo-Gómez, V., Kočí, T., Buckeridge, J., Mueller, M., & Stevens, K. (2020). Late Miocene biota from the Abad Member of the Carboneras-Nijar Basin (Spain, Andalusia): A bathyal fossil assemblage pre-dating the Messinian salinity crisis. Geobios, 59,1–28. DOI: 10.1016/j.geobios.2020.03.002
- Hussey, N. E., McCann, H. M., Cliff, G., Dudley, S. F., Wintner, S. P., & Fisk, A. T. (2012). Size-based analysis of diet and trophic position of the white shark (Carcharodon carcharias) in South African waters. In M. L. Domeier, Global Perspectives on the Biology and Life History of the White Shark (pp. 27–50). CRC Press.
- Huxley, T. H. (1880). A manual of the anatomy of vertebrated animals. D. Appleton.
- Iitembu, J. A., & Richoux, N. B. (2015). Trophic relationships of hake (Merluccius capensis and M. paradoxus) and sharks (Centrophorus squamosus, Deania calcea and D. profundorum) in the Northern (Namibia) Benguela Current region. African Zoology, 50(4), 273–279. DOI: 10.1080/15627020.2015.1079142
- Jaiteh, V. F., & Momigliano, P. (2015). New distribution records of the Vulnerable fossil shark Hemipristis elongata from eastern Indonesia call for improved fisheries management. Marine Biodiversity Records, 8, e79. DOI: 10.1017/S1755267215000548
- Jordan, D.S. & Evermann, B.W. (1896). The fishes of North and Middle America: a descriptive catalogue of the species of fish-like vertebrates found in the waters of North America, north of the Isthmus of Panama. Part I. Bulletin of the United States National Museum, 47, 1–1240.
- Jordan, D.S. & Gilbert, C.H. (1879). Notes on the fishes of Beaufort Harbor, North Carolina. Proceedings of the United States National Museum, 1(55), 365–388.
- Jordan, D. S., & Snyder, J. O. (1902). Descriptions of two new species of squaloid sharks from Japan. Proceedings of the United States National Museum, 25, 79–87.
- Keggin, T. (2017). Population genomics of two deep sea sharks: Centroselachus crepidater and Deania calcea. Master's Thessis. Durham University, 81pp.
- Kennett, J. P., & Srinivasan, M. S. (1983). Neogene Planktonic Foraminifera, A Phylogenetic Atlas: Hutchinson Ross Pub. Co., Stroudsburg, Pennsylvania 265.
- Keyes, I. W. (1984). New records of fossil elasmobranch genera Megascyliorhinus, Centrophorus, and Dalatias (Order Selachii) in New Zealand. New Zealand Journal of Geology and Geophysics, 27, 203–216. DOI: 10.1080/00288306.1984.10422527
- Klunzinger, K. B. (1870). Synopsis Der Fische Des Rothen Meeres. C. Ueberreuter’she Buchdruckerei.
- Krijgsman, W., Hilgen, F. J., Raffi, I., Sierro, F. J., & Wilson, D. S. (1999). Chronology, causes and progression of the Messinian salinity crisis. Nature, 400(6745), 652–655. DOI: 10.1038/23231
- Kriwet, J., & Klug, S. (2009). 2. Fossil Record and Origin of Squaliform Sharks. In: V.F. Gallucci, G.A. McFarlane, G.G. Bargmann (Eds.), Biology and management of dogfish sharks, American Fisheries Society, (2009), pp. 19–38.
- Kubota, T., Shiobara, Y., & Kubodera, T. (1991). Food habits of the Frilled Shark Chlamydoselachus anguineus Collected from Suruga Bay, Central Japan. Nippon Suisan Gakkaishi, 57(1), 15–20. DOI: 10.2331/suisan.57.15
- Last, P., G. Naylor, B. Séret, W. White, M. de Carvalho, and M. Stehmann. 2016. Rays of the World. CSIRO publishing.
- Last, P. R., Stevens, J. D., Swainston, R., & Davis, G. (2009). Sharks and rays of Australia. CSIRO publishing.
- Laurito, C. A., Calvo, C., Valerio, A. L., Calvo, A., & Chacón, R. (2014). Lower Miocene ichthyofauna from the locality of Pacuare de Tres Equis, río Banano formation, Cartago province, Costa Rica and description of a new genus and species of scaridae. Revista Geológica de América Central, 50, 153–192.
- Ledoux, J.-C. (1970). Les dents des squalidés de la Méditerranée occidentale et de l’Atlantique Nord-Ouest africain. Vie et Nature, A30, 309–361.
- Linnaeus, C. (1758). Systema Naturae. Stockholm Laurentii Salvii.
- Lowe, R.T. (1839). A supplement to a synopsis of the fishes of Madeira. Proceedings of the Zoological Society of London, 7, 76–92.
- Marín, J. (1992). Paleoictiología de algunos yacimientos neógenos de la provincia de Alicante (II). Revista Ilicitana Paleontología y Mineralogía, 1, 4–24.
- Marramà, G., Schultz, O., & Kriwet, J. (2019). A new Miocene skate from the central Paratethys (Upper Austria): the first unambiguous skeletal record for the Rajiformes (Chondrichthyes: Batomorphii). Journal of Systematic Palaeontology, 17(11), 937–960. DOI: 10.1080/14772019.2018.1486336
- Marramà, G., Klug, S., De Vos, J., & Kriwet, J. (2018). Anatomy, relationships and palaeobiogeographic implications of the first Neogene holomorphic stingray (Myliobatiformes: Dasyatidae) from the early Miocene of Sulawesi, Indonesia, SE Asia. Zoological Journal of the Linnean Society, 184(4), 1142–1168. DOI: 10.1093/zoolinnean/zly020
- Marsili, S. (2008). Systematic, paleoecologic and paleobiogeographic analysis of the Plio-Pleistocene Mediterranean elasmobranch fauna. Atti Societa Toscana Scienze Naturali, Serie A, 113, 81–88.
- Marsili, S., Carnevale, G., Danese, E., Bianucci, G., & Landini, W. (2007). Early Miocene vertebrates from Montagna della Maiella, Italy. Annales de Paléontologie, 93(1), 27–66. DOI: 10.1016/j.annpal.2007.01.001
- Martínez Díaz, C. (1970). Tres nuevas especies de Foraminíferos en el Andaluciense. Acta Geológica Hispánica, 5(1), 1–3.
- Martínez, W., & Benzaquen, M. (1975). Mapa y Memoria Explicativa de La Hoja 820 (Onteniente) Del Mapa Geológico E. 1: 50.000> Plan Magna, FC. ME.
- Martínez-Pérez, C., Carrillo-Briceño, J. D., Esparza, C., Ferrón, H. G., Manzanares, E., Hammann, C., & Botella, H. (2018). A Serravallian (Middle Miocene) shark fauna from Southeastern Spain and its palaeoenvironment significance. Historical Biology, 30(3), 422–432. DOI:10.1080/08912963.2017.1326111
- Martinho, F., Sá, C., Falcão, J., Cabral, H. N., & Pardal M. Â. (2012). Comparative feeding ecology of two elasmobranch species, Squalus blainville and Scyliorhinus canicula, off the coast of Portugal. Fishery Bulletin, 110(1), 71-84.
- Mas, G. (2003). Presència de Paratodus benedeni (Le Hon, 1871)(Pisces: Chondrichthyes: Otodontidae) al Neogen de Mallorca i Menorca (Illes Balears, Mediterrània occidental). Consideracions taxonòmiques i paleoambientals. Presence of Paratodus benedeni (Le Hon 1871). Bolletí de La Societat d’Història Natural de Les Balears, 46, 85–90.
- Mas, G. (2010). Ictiofauna del Pliocè del barranc de sa Talaia (Mallorca, Illes Balears, Mediterrània Occidental). Implicacions paleoambientals. Bolletí de La Societat d’Història Natural de Les Balears, 53, 43–70.
- Mas, G. & Fiol, G. (2002). Ictiofauna del Messinià de la plataforma sedimentària de Llucmajor (Illes Balears, Mediterrània occidental). Aspectes paleoambientals. Icthyofauna from the Messinian of the Llucmajor sedimentary platform (Balearic Islands, Western Mediterranean). Paleo. Bolletí de La Societat d’Història Natural de Les Balears, 45, 105–116.
- Mendiola, C. (1996). Rhincodon ferriolensis n. sp.(Neoselachii, Orectolobiformes, Rhincodontidae) del Burdigaliense superior de Elche (Sureste de España). Societat Paleontologica d’Elx, 2, 1–6.
- Mochizuki, K., & Ohe, F. (1990). Trigonognathus kabeyai, a new genus and species of the squalid sharks from Japan. Japanese Journal of Ichthyology, 36(4), 385–390. DOI: 10.11369/jji1950.36.385
- Monegatti, P., & Raffi, S. (2010). The Messinian marine molluscs record and the dawn of the eastern Atlantic biogeography. Palaeogeography, Palaeoclimatology, Palaeoecology, 297(1), 1–11. DOI: 10.1016/j.palaeo.2010.06.023
- Monsch, K. A. (1998). Miocene fish faunas from the northwestern Amazonia basin (Colombia, Peru, Brazil) with evidence of marine incursions. Palaeogeography, Palaeoclimatology, Palaeoecology, 143(1–3), 31–50. DOI: 10.1016/S0031-0182(98)00064-9
- Müller, J. & Henle, F.G.J. (1837). Gattungen der Haifische und Rochen nach einer von ihm mit Hrn. Henle unternommenen gemeinschaftlichen Arbeit über die Naturgeschichte der Knorpelfische. Berichte der Königlichen Preussischen Akademie der Wissenschaften zu Berlin, 1837(2), 111–118.
- Müller, J., & Henle, F. G. J. (1838). On the generic characters of cartilaginous fishes, with descriptions of new genera. Magazine of Natural History, 2(3), 34–37.
- Müller, J. & Henle, F.G.J. (1841). Systematische Beschreibung der Plagiostomen. Berlin, Veit, pp. 1–200.
- Olaso, I., Velasco, F., Sánchez, F., Serrano, A., Rodríguez-Cabello, C., & Cendrero, O. (2005). Trophic relations of lesser-spotted catshark (Scyliorhinus canicula) and blackmouth catshark (Galeus melastomus) in the Cantabrian Sea. Journal of Northwest Atlantic Fishery Science, 35, 481–494.
- Oyanadel-Urbina, P., De Gracia, C., Carrillo-Briceño, J. D., Nielsen, S. N., Flores, H., Casteletto, V., Kriwet, J., Rivadeneira, M. M., & Villafaña, J. A. (2021). Neogene Bony Fishes from the Bahía Inglesa Formation, Northern Chile. Ameghiniana, 58(4), 345–368. DOI: 10.5710/AMGH.26.05.2021.3375
- Parker, W. K., Jones T. R, & Brady, H. B. (1865). IV.—On the nomenclature of the Foraminifera. Annals and Magazine of Natural History, 16,15–41.
- Pérès, J. M. (1985). History of the Mediterranean biota and the colonization of the depths. Key Environments: Western Mediterranean, 198–232. Pergamon Press Oxford.
- Pérez, V. J., Pimiento, C., Hendy, A., González-Barba, G., Hubbell G.,, & MacFadden, B. J. (2017). Late Miocene chondrichthyans from Lago Bayano, Panama: functional diversity, environment and biogeography. Journal of Paleontology, 91(3), 512–547. DOI: 10.1017/jpa.2017.5
- Perón, F. A. 1807. Voyage de Découvertes Aux Terres Australes, Exécutés Par Ordre de Sa Majesté l’Empereur et Roi, Sur Les Corvettes Le Géographe, Le Naturaliste, et Le Goëlette Le Casuarina, Pendant Les Années 1800, 1801, 1802, 1803 et 1804; Historique:] Voyage de Découvertes Aux Terres Australes. Historique. De l'Imprimerie impériale, Paris
- Phillips, F. J., Welton B., & Welton, J. (1976). Paleontologic studies of the middle Tertiary Skooner Gulch and Gallaway Formations at Point Arena, California. Proceedings of the Society of Economic Paleontologists and Mineralogists (Pacific Section), San Francisco, United States, 137–154.
- Pimiento, C., & Balk, M. A. (2015). Body-size trends of the extinct giant shark Carcharocles megalodon: a deep-time perspective on marine apex predators. Paleobiology, 41(3),479–490. DOI: 10.1017/pab.2015.16
- Pimiento, C., Ehret, D.J., MacFadden, B. J., & Hubbell, G. (2010). Ancient Nursery Area for the Extinct Giant Shark Megalodon from the Miocene of Panama. PLoS ONE, 5(5), e10552. DOI: 10.1371/journal.pone.0010552
- Pimiento, C., MacFadden, B.J., Clements, C. F., Varela, S, Jaramillo, C., Velez-Juarbe, J., & Silliman, B. R. (2016). Geographical distribution patterns of Carcharocles megalodon over time reveal clues about extinction mechanisms. Journal of Biogeography, 43(8), 1645–1655. DOI: 10.1111/jbi.12754
- Poey, F. (1875). Enumeratio piscium cubensium. Anales de la Sociedad Española de Historia Natural, 4, 75–161.
- Pollard, D., & Smith, A. (2009). The IUCN Red List of Threatened Species, 2009, e.T3854A10132481.
- Pollerspöck, J., Güthner, T., & Straube, N. (2020). Re-evaluation of the Lower Miocene (Burdigalian, Ottnangian) elasmobranch fauna (Elasmobranchii, Neoselachii) from Upper Austria (Allerding, near Schärding, Austria) with comments on the palaeogeographic distribution of the recorded squaliform sharks. Annalen Des Naturhistorischen Museums in Wien. Serie A Für Mineralogie Und Petrographie, Geologie Und Paläontologie, Anthropologie Und Prähistorie, 122, 87–164.
- Popov, S. V., Shcherba, I. G., Ilyina, L. B., Nevesskaya, L. A., Paramonova, N. P., Khondkarian, S. O., & Magyar, I. (2006). Late Miocene to Pliocene palaeogeography of the Paratethys and its relation to the Mediterranean. Palaeogeography, Palaeoclimatology, Palaeoecology, 238(1–4), 91–106. DOI:10.1016/j.palaeo.2006.03.020
- Portell, R. W., Hubbell, G., Donovan, S. K., Green, J. L., Harper D. A.,, & Pickerill, R. (2008). Miocene sharks in the Kendeace and Grand Bay formations of Carriacou, The Grenadines, Lesser Antilles. Caribbean Journal of Science. 44(3). 279–286. DOI: 10.18475/cjos.v44i3.a2
- Purdy, R. W. (1998). The early Miocene fish fauna from the Pollack Farm site, Delaware. In R.N. Benson (Ed.), Geology and Paleontology of the Lower Miocene Pollack Farm Fossil Site, Delaware (pp. 133–139). Delaware Geological Survey Special Publication, 21.
- Purdy, R. W., & Francis, M. P. (2007). Ontogenetic development of teeth in Lamna nasus (Bonnaterre, 1758)(Chondrichthyes: Lamnidae) and its implications for the study of fossil shark teeth. Journal of Vertebrate Paleontology, 27(4), 798–810. DOI: 10.1671/0272-4634(2007)27[798:ODOTIL]2.0.CO;2
- Radwanski, A. (1965). A contribution to the knowledge of Miocene Elasmobranchii from Pinczów (Poland). Acta Palaeontologica Polonica, 10, 267–274.
- Rafinesque, C. S. (1810). Caratteri Di Alcuni Nuovi Generi e Nuove Specie Di Animali e Piante Della Sicilia: Con Varie Osservazioni Sopra i Medesimi. Per le stampe di Sanfilippo, 105 pp.
- Reis, M. A. F. (2005). Chondrichthyan fauna from the Pirabas Formation, Miocene of northern Brazil, with comments on paleobiogeography. Anuário Do Instituto de Geociências, 28, 31–58.
- Reolid, M., & Molina, J. M. (2015). Record of Carcharocles megalodon in the Eastern Guadalquivir Basin (Upper Miocene, South Spain). Estudios Geológicos, 71 (2), e032. DOI: 10.3989/egeol.41828.342.
- Rochowski, B. E. A., Walker, T. I., & Day, R.W. 2015. Geographical variability in life-history traits of a midslope dogfish: the brier shark Deania calcea. Journal of Fish Biology, 87, 728–747. DOI: 10.1111/jfb.12756
- Rodríguez-Cabello, C., Pérez, M., & Grasa, I. (2020). Taxonomic research on Deania calcea and Deania profundorum (Family: Centrophoridae) in the Cantabrian Sea (Northeast Atlantic) with comments on Deania hystricosa. Regional Studies in Marine Science, 37, 101321. DOI: 10.1016/j.rsma.2020.101321
- Rögl, F. 1999. Mediterranean and Paratethys palaeogeography during the Oligocene and Miocene. Hominoid Evolution and Climatic Change in Europe, Vol. 1: The Evolution of the Neogene Terrestrial Ecosystems in Europe. In Agustí, J., Rook, L. & Andrews, P., (Eds.) The Evolution of Neogene Terrestrial Ecosystems. Cambridge University Press, Cambridge, (pp. 8–22).
- Santos, R., Novoa-Pabon, A., Silva, H., & Pinho, M. (2020). Elasmobranch species richness, fisheries, abundance and size composition in the Azores archipelago (NEAtlantic). Marine Biology Research, 16(2), 103–116. DOI: 10.1080/17451000.2020.1718713
- Sharma, K. M., & Patnaik, R. (2013). Additional fossil batoids (skates and rays) from the Miocene deposits of Baripada Beds, Mayurbhanj District, Orissa, India. Earth Science India, 6(4), 160–184.
- Shimada, K. (2021). The size of the megatooth shark, Otodus megalodon (Lamniformes: Otodontidae), revisited. Historical Biology, 33(7), 904–911. DOI: 10.1080/08912963.2019.1666840
- Shimada, K., Bonnan, M. F.,Becker, M. A., & Griffiths, M. L. (2020). Ontogenetic growth pattern of the extinct megatooth shark Otodus megalodon—implications for its reproductive biology, development, and life expectancy. Historical Biology, 33(12), 1–6. DOI: 10.1080/08912963.2020.1861608
- Shirai, S., & Okamura, O. (1992). Anatomy of Trigonognathus kabeyai, with comments on feeding mechanism and phylogenetic relationships (Elasmobranchii, Squalidae). Japanese Journal of Ichthyology, 39(2), 139–150. DOI: 10.11369/jji1950.39.139
- Sierro, F. J. (1985). Estudio de los foraminíferos planctónicos, bioestratigrafía y cronoestratigrafía del Mio-Plioceno del borde occidental de la Cuenca del Guadalquivir (SO de España). Studia Geologica Salmanticensia, 21, 7–85.
- Simpfendorfer, C. A., Goodreid, A. B., & McAuley, R. B. (2001). Size, sex and geographic variation in the diet of the tiger shark, Galeocerdo cuvier, from Western Australian waters. Environmental Biology of Fishes, 61, 37–46. DOI: 10.1023/A:1011021710183
- Soares, K. D., & De Carvalho, M. R. (2019). The catshark genus Scyliorhinus (Chondrichthyes: Carcharhiniformes: Scyliorhinidae): taxonomy, morphology and distribution. Zootaxa, 4601(1), 1–147. DOI: 10.11646/zootaxa.4601.1.1
- Soldo, A., Dulcic, J., & Cetinic, P. (2000). Contribution to the study of the morphology of the teeth of the nursehound Scyliorhinus stellaris (Chondrichthyes: Scyliorhinidae). Scientia Marina, 64(3), 355–356. DOI: 10.3989/scimar.2000.64n3355
- Stewart, K. M. (2001). The freshwater fish of Neogene Africa (Miocene–Pleistocene): systematics and biogeography. Fish and Fisheries, 2, 177–230. DOI: 10.1046/j.1467-2960.2001.00052.x
- Straube, N., Iglésias, S. P., Sellos, D. Y.,Kriwet, J, & Schliewen, U. K. (2010). Molecular phylogeny and node time estimation of bioluminescent lantern sharks (Elasmobranchii: Etmopteridae). Molecular Phylogenetics and Evolution, 56(3), 905–917. DOI: 10.1016/j.ympev.2010.04.042
- Suárez, M., Encinas, A., & Ward, D. (2004). An Early Miocene elasmobranch fauna from the Navidad Formation, Central Chile, South America. Cainozoic Research, 4(1/2), 3–18.
- Szabó, M., & Kocsis, L. (2020). Supplementary data on the Middle Miocene (Badenian) fish assemblage of Nyirád (Hungary): revision and new results on faunal composition and paleoenvironment. Palaeontographica Abteilung A, Palaeozoology – Stratigraphy, 315(5/6), 121–191. DOI: 10.1127/pala/2020/0094
- Teter, S. M., Wetherbee, B. M., Fox, D. A., Lam, C. H., Kiefer, D. A., & Shivji, M. (2015). Migratory patterns and habitat use of the sand tiger shark (Carcharias taurus) in the western North Atlantic. Marine and Freshwater Research, 66(2), 158–169. DOI: 10.1071/MF14129
- Toscano Grande, A. (2016). Vertebrados marinos del neógeno del suroeste de la Península Ibérica. (Doctoral dissertation, Universidad de Huelva), 245 pp.
- Türtscher, J., López-Romero, F. A., Jambura, P. L., Kindlimann, R., Ward, D. J., & Kriwet, J. (2021). Evolution, diversity, and disparity of the tiger shark lineage Galeocerdo in deep time. Paleobiology, 47(4), 574–590. DOI: 10.1017/pab.2021.6
- Tyabji, Z., Jabado, R. W., & Sutaria, D. (2018). New records of sharks (Elasmobranchii) from the Andaman and Nicobar Archipelago in India with notes on current checklists. Biodiversity Data Journal, 6, e28593. DOI: 10.3897/BDJ.6.e28593
- Underwood, C. J., & Schlögl, J. (2012). Deep-water chondrichthyans from the early Miocene of the Vienna Basin (Central Paratethys, Slovakia). Acta Palaeontologica Polonica, 58(3), 487–509. DOI: 10.4202/app.2011.0101
- Valsecchi, E., Pasolini, P., Bertozzi, M., Garoia, F., Ungaro, N., Vacchi, M., Sabelli, B., & Tinti, F. (2005). Rapid Miocene–Pliocene dispersal and evolution of Mediterranean rajid fauna as inferred by mitochondrial gene variation. Journal of Evolutionary Biology, 18(2), 436–446. DOI: 10.1111/j.1420-9101.2004.00829.x
- Vertino, A., Stolarski, J., Bosellini, F. R.,, & Taviani, M. (2014). Mediterranean corals through time: from Miocene to Present. In: Goffredo S, Dubinsky Z (Eds.) The Mediterranean Sea: its history and present challenges. Springer, Dordrecht, pp 257–274. DOI: 10.1007/978-94-007-6704-1_14
- Vialle, N., S. Adnet, & H. Cappetta. 2011. A new shark and ray fauna from the Middle Miocene of Mazan, Vaucluse (southern France) and its importance in interpreting the paleoenvironment of marine deposits in the southern Rhodanian Basin. Swiss Journal of Palaeontology, 130(2), 241–258. DOI: 10.1007/s13358-011-0025-4
- Villafaña, J. A., Rivadeneira, M. M., Pimiento, C., & Kriwet, J. (2023). Diversification trajectories and paleobiogeography of Neogene chondrichthyans from Europe. Paleobiology, 49(2), 329–341. DOI: 10.1017/pab.2022.40
- Visaggi, C. C., & Godfrey, S. J. (2010). Variation in composition and abundance of Miocene shark teeth from Calvert Cliffs, Maryland. Journal of Vertebrate Paleontology, 30(1), 26–35. DOI: 10.1080/02724630903409063
- Ward, D. J., & Bonavia, C. G. (2001). Additions to, and a review of, the Miocene shark and ray fauna of Malta. The Central Mediterranean Naturalist, 3(3), 131–146.
- Wetherbee, B. M., & Kajiura, S. M. (2000). Occurrence of a rare squaloid shark, Trigonognathus kabeyai, from the Hawaiian Islands. Pacific Science, 54(4), 389–394.
- Yano, K., Mochizuki, K., Tsukada, O., & Suzuki, K. (2003). Further description and notes of natural history of the viper dogfish, Trigonognathus kabeyai from the Kumano-nada Sea and the Ogasawara Islands, Japan (Chondrichthyes: Etmopteridae). Ichthyological Research, 50, 251–258. DOI: 10.1007/s10228-003-0165-7
- Zhou, S., & Griffiths, S. P. (2008). Sustainability Assessment for Fishing Effects (SAFE): A new quantitative ecological risk assessment method and its application to elasmobranch bycatch in an Australian trawl fishery. Fisheries Research, 91(1), 56–68. DOI: 10.1016/j.fishres.2007.11.007
