Abstract
The supplementation of natural populations of Pacific salmon Oncorhynchus spp. with hatchery fish poses unique management challenges. Two such challenges addressed in this study are limiting the number of hatchery fish spawning with natural-origin fish and maximizing the number of natural-origin fish in the supplementation broodstock. In this study, we focus on stock enhancement of Sockeye Salmon O. nerka in Hidden Lake, Alaska, where the Trail Lakes Hatchery supplements the natural population with hatchery-raised fry. Production in Hidden Lake is limited by the availability of spawning habitat and not by juvenile rearing capacity. The hatchery collects broodstock from the lake and releases fry with thermally marked otoliths at one of two primary natural spawning sites in Hidden Lake each year. During this study, an average of 58% of the fish returning to the lake through a weir on the outlet stream were of hatchery origin. However, an average of 88% of the fish at the release site were hatchery-origin fish, indicating a nonrandom distribution of hatchery-origin spawners. This pattern is consistent with homing to specific sites within the lake of either or both hatchery- and wild-origin fish. However, this distribution results in a larger-than-desirable proportion of hatchery-origin fish spawning with natural-origin fish at the release site. The proportion of hatchery-origin fish used for brood is also larger than desirable because the site is also the broodstock collection site. We propose that releasing hatchery fish at a new location removed from the primary spawning areas and the hatchery broodstock collection site will reduce the proportion of hatchery-origin fish spawning with wild-origin fish and increase the proportion of wild-origin fish in the broodstock, if our results are due, at least in part, to homing of hatchery fish.
Received May 12, 2012; accepted May 20, 2013
Pacific salmon Oncorhynchus spp. typically spawn in streams or lakes, spend a variable amount of time as fry in freshwater, and move into marine waters for 2 to 7 years before returning as adults to spawn in freshwater (Quinn Citation2005). Sockeye Salmon O. nerka are finely adapted to local conditions that influence reproductive success and survival (Taylor Citation1991; Fraser et al. Citation2011) and hence home to their natal sites on a small spatial scale to spawn (Quinn et al. Citation2006). The tendency to home to natal sites to spawn produces reproductive isolation between populations and demographic independence among populations that must be taken into account in the management of wild and enhanced populations supporting commercial fisheries.
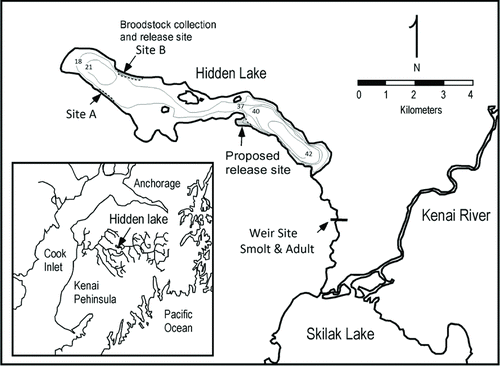
FIGURE 1 Bathymetric map of Hidden Lake, Alaska, showing Sockeye Salmon spawning areas, broodstock collection sites, and fry release sites. Depth is shown in meters.
In the face of declining harvests and habitat changes, large salmon hatchery programs were developed in Alaska, British Columbia, and the Pacific Northwest beginning in the late 19th century (Roppel Citation1982; Mahnken et al. Citation1998). These hatchery programs currently produce large numbers of fish that may pose ecological and genetic risks to wild populations (Reisenbichler and McIntyre Citation1977; Campton Citation1995; Naish et al. Citation2007; Grant Citation2012). Recent studies show that hatchery rearing can reduce fitness in the wild (Kostow Citation2004; Araki et al. Citation2007, 2008) and that hybridization between hatchery and wild fish can lower the overall fitness of wild populations (Ford Citation2002).
In recognition of these risks, the Alaska Department of Fish and Game (ADFG) has implemented regulations to protect wild salmon stocks from the undesirable effects of hatcheries. These guidelines are incorporated into hatchery management protocols to address specific areas of concern. One particular unwanted effect is interbreeding between hatchery-reared and wild fish. The ADFG has attempted to reduce the incidence of hatchery–wild fish interbreeding in the enhancement of small populations of salmon at several sites. For example in an enhancement program for Chinook Salmon O. tshawytscha on the Ninilchik River, Alaska, returning hatchery fish are identified by adipose fin clips and not allowed to pass a weir to the spawning grounds (Booz and Kerkvliet Citation2011). In other cases, the otoliths of hatchery-reared fish are thermally marked (Volk et al. Citation1990, 1999) so that the origins of the broodstock can be identified. The progeny of these fish can be allocated to specific programs to meet release goals. The English Bay Lakes, Alaska, Sockeye Salmon enhancement program provides an example of this type of program, in which progeny of wild-origin parents are released back into the lake of origin and progeny from hatchery-origin parents are released into terminal areas with no wild stocks. These procedures reduce interactions between hatchery- and natural-origin fish. The use of otolith marking, however, is somewhat limited, because it permits only retrospective analysis and cannot be used in real time to manage spawning populations.
In this study, we address similar enhancement management challenges by describing the distributions of hatchery-reared and natural Sockeye Salmon in Hidden Lake, Alaska (), and by using this information to guide program management to reduce hatchery–wild interactions. The goal of this evaluation was to determine whether returning hatchery- or wild-origin Sockeye Salmon home within Hidden Lake. To test this we compared the proportions of hatchery- and natural-origin fish at the hatchery release site to the proportions of fish entering the lake. The null hypothesis is that the distribution of hatchery- and wild-origin fish would be the same at the entry site as the release site. Based on the results of our study, the program can be modified to take advantage of the homing behavior to reduce risks associated with hatchery- and wild-origin fish spawning and hatchery broodstock collection.
The homing of salmon to natal spawning areas, especially Sockeye Salmon, has been the focus of much research (Dittman and Quinn Citation1996; Quinn et al. Citation1999). Mature Sockeye Salmon generally spawn in streams associated with a lake, but some adults spawn in the lake itself. Fry spend 0–2 years in freshwater before migrating to sea as smolts where they spend 1–4 years, before returning as adults to natal streams or lakes to spawn (Quinn et al. Citation1999). Sockeye Salmon tend to show higher levels of homing fidelity than other Pacific salmon (Hendry et al. Citation2004). This homing behavior isolates spawning groups so that random genetic drift and local selection produce differences among groups, which can often be detected with molecular markers (Grant et al. Citation1980; Hendry et al. Citation1996, 2000; Seeb et al. Citation2000). Although lake and stream spawners often differ genetically, less variation among spawning groups occurs within lakes (Varnavskaya et al. Citation1994; Burger et al. Citation1995; Seeb et al. Citation2000; Habicht et al. Citation2007; Gomez-Uchida et al. Citation2011).
The Hidden Lake Sockeye Salmon enhancement program was started by the State of Alaska in 1976 and has been operated by the Cook Inlet Aquaculture Association since 1988 to take advantage of unused rearing capacity (Kyle et al. Citation1990). Population size in Hidden Lake appears to be limited by available spawning habitat and not by juvenile rearing capacity (Simpson and Edmundson Citation1999). Hidden Lake does not have significant perennial streams flowing into it so that fish spawn almost exclusively on beaches. The lake has some of the largest zooplankton biomass in lakes on the Kenai Peninsula and produces some of the largest Sockeye Salmon smolts of any lake system in Alaska (Simpson and Edmundson Citation1999). The current goal of the enhancement program is to produce an annual return of at least 30,000 spawners to the lake, and this is achieved by stocking an average 830,000 unfed fry (2006–2011).
Eggs are stripped from mature beach-spawning fish, fertilized, and incubated at nearby Trail Lakes Hatchery. The otoliths of developing embryos are thermally marked (Volk et al. Citation1990, 1999). Hatchery-origin spawners can be identified with 100% certainty, because a sample of fry is certified by ADFG before fish are released into the lake. Developing larvae are maintained at the hatchery for a few months until fry have absorbed their yolk sac. Unfed fry are trucked from the hatchery, transferred to a boat, which takes them to a single release site (Site B, ), where they are released from the transport tank. Fry are typically released in the late morning or early afternoon and have been observed to swim down into the substrate in the immediate area (G. Fandrei, personal observation). When the project was initiated in 1976, naturally spawning fish were used as broodstock, but after hatchery fish returned to the lake to spawn, broodstock were taken without regard to the origins of the spawners, because it was not possible to determine the origins of fish in real time. Otolith temperature marking was first applied in 1996, so that returning hatchery-origin fish could be identified in 1999.
TABLE 1 Number of hatchery-origin (otolith-marked) and natural-origin Sockeye Salmon at the outlet weir and at Site A and Site B (Figure 1), where hatchery fish are not stocked and are stocked, respectively, in Hidden Lake, Alaska, by year. Percent of hatchery fish and total sample sizes (N) are provided.
METHODS
Study Site.—Hidden Lake (60°29’ N 150°16’ W) is an oligomesotrophic system 86 m above sea level and drains a watershed of 37.4 km2 (Kyle et al. Citation1990) with average annual precipitation of 44 cm and estimated water residence time of 11.7 years (http://www.ciaanet.org/Projects/HIDDEN%20RPT%2007.pdf). The lake is 683 hectares in size, with a mean depth of 20 m and a maximum depth of 45 m. It has 22.5 km of shoreline and one seasonally inflowing stream at the southwest end of the lake. The lake is drained by an outlet stream flowing into Skilak Lake, which is part of the Kenai River system and is 96 km upstream from Cook Inlet. The fish assemblage is dominated by beach-spawning Sockeye Salmon with an average return to the lake of 23,300 fish (2006–2011), counted at a weir across the outlet stream. The lake also supports kokanee or residual Sockeye Salmon, Coho Salmon O. kisutch, Dolly Varden Salvelinus malma, and Lake Trout S. namaycush. Two primary Sockeye Salmon spawning areas are located on beaches at the northwest end of the lake about 1 kilometer apart (, Sites A and B). There are other spawning locations in the lake but the numbers of fish are generally low (<50) on these small scattered areas. Specific spawner counts are unavailable, but similar numbers of fish generally spawn at Sites A and B (G. Fandrei, personal communication). Broodstock are collected at only Site B, where unfed fry are released.
Sample collection.—Total escapement to Hidden Lake, as counted at the weir, ranged from 11,002 (2009) to 40,503 (2010) fish during this study. Returning fish were sampled at the weir three times (1–2 week apart) during the run and were representative of the run. Fish in spawning condition were collected using a beach seine at Sites A and B. Otoliths were collected from these fish or from freshly spawned-out carcasses at least three times at Site B throughout the egg-take period from 2008 to 2010. Otoliths were also collected from spawners at Site A at the same times. No Sockeye Salmon spawn in the lake's tributaries. Otoliths were mounted on a glass slide and ground before examining them with a microscope for thermal banding (Volk et al. Citation1990).
Statistical analysis.—In order to determine if year should be controlled across these tests, we first tested the hypothesis that the proportion of hatchery-origin fish at the weir was homogenous across years. The goal of this project was to test the hypothesis that after entering the lake hatchery- and wild-origin fish are randomly distributed among spawning areas. We tested the hypothesis that the proportion of hatchery-origin fish found at Site B (hatchery release site) was equal to the proportion of hatchery-origin fish sampled at the weir. Secondarily, we tested the hypothesis that the proportion of hatchery-origin fish found at Site A was equal to the proportion of hatchery-origin fish sampled at the weir. These tests were performed through chi-square tests of contingency tables followed by Fisher's method to combine the results across annual tests. Critical value was set at 0.05 and adjusted for multiple tests using the Bonferroni correction when applicable. Sample sizes provided statistical power to detect an expected difference as low as 10%, 95% of the time.
RESULTS
Thermal banding patterns on otoliths in sampled fish from three sites (2008–2010) indicated that, on average, 58% of returning adults sampled at the weir were of hatchery origin, and that 53% sampled at Site A and 88% sampled at Site B were of hatchery origin (). The chi-square test of the hypothesis that the proportions of hatchery-origin fish at the weir across years was highly significant (P < 0.001) indicating that these proportions were not consistent across years. This result made it necessary to control for year in the remaining tests. Chi-square statistics testing the null hypothesis that the proportion of hatchery-origin fish found at Site B (hatchery release site) was equal to the proportion of hatchery-origin fish sampled at the weir was highly significant for every year (P < 0.001), with higher proportions of hatchery-origin fish at Site B than at the weir in all three years. Fisher's Method result across all years was highly significant for this hypothesis test (P < 0.001). The chi-square test of the proportion of hatchery-origin fish found at Site A was equal to the proportion of hatchery-origin fish sampled at the weir was highly significant only in 2009 (P < 0.001), with a lower proportion of hatchery-origin fish at Site A than at the weir. In 2008 and 2010, no deviation was observed between the proportions of hatchery-origin fish at Site A and at the weir, after adjustment for multiple tests (P = 0.02 and 0.89, respectively). Fisher's method result across all years was also was highly significant (P < 0.001) for this hypothesis test.
DISCUSSION
The results of this study show a complex pattern of migration to spawning areas in Hidden Lake. While 58% of fish entering the lake were of hatchery origin, 88% of the fish collected at release Site B were of hatchery origin. A larger proportion of hatchery-origin fish returning to Site B was detected over all 3 years, indicating that this behavior is a persistent feature of Sockeye Salmon spawning in Hidden Lake. However, the origins of these differences are uncertain. Consistent with these results, a significant chi-square test suggested a lower proportion of hatchery-origin fish at Site A in 2009, relative to the proportion of fish entering the lake but not in 2008 or 2010.
The nonrandom distribution of spawning fish may be due to homing within the lake by either wild-origin or hatchery-origin fish. The higher proportion of hatchery-origin fish at Site B may be due to the homing of hatchery-origin fish to Site B where they were released or to the homing of wild-origin fish. Because abundance estimates at the two sites are unavailable, it is not possible to determine which explanation is correct. Importantly, both explanations indicate homing behavior within a small lake system. Natural populations of stream and lake spawning Sockeye Salmon are thought to home to natal sites with some precision (Groot and Margolis Citation1991; Hendry et al. Citation1996; Quinn et al. Citation1999; Gomez-Uchida et al. Citation2011), so that genetic differences between spawning populations might be expected. However, genetic differences among spawning groups of Sockeye Salmon within a lake were not observed in three Bristol Bay lakes (Varnavskaya et al. Citation1994; Habicht et al. Citation2007), in three lakes in Cook Inlet (Burger et al. Citation1995; Seeb et al. Citation2000), and in one of the two lakes in Russia (Varnavskaya et al. Citation1994). Only a single instance of genetic differentiation between lake-spawning aggregations has been reported. In this case, Kuril Lake is geographically much larger than Hidden Lake and supports the largest lake-spawning Sockeye Salmon population in Russia (Varnavskaya et al. Citation1994). While the amount of genetic segregation between spawning groups in Hidden Lake is unknown, the identification of hatchery-origin and wild-origin fish using otolith marks indicates the nonrandom mixing among spawning areas.
The results of our study can help to guide the enhancement program in Hidden Lake, regardless of the behavioral processes producing differences between spawning areas. A routine practice in this program is the release of early-stage unfed fry into the lake. This reduces the amount of time fry are cultured in the hatchery, reducing opportunities for domestication, and the early release facilitates exposure to the same environmental cues at a time when these fish are imprinted. Coho Salmon, released as unfed fry, also exhibit life history traits that are more similar to wild salmon than to fish released as smolts (Thériault et al. 2010). After release, unfed hatchery fry seek cover in the beach gravel in the immediate vicinity (G. Fandrei, personal observation), increasing the amount of time spent at that location. Fed fry have already made the ontogenic shift from benthic to pelagic habitats selection. When older fry leave the bottom and move around the lake to forage, they abandon their place of birth (Groot and Margolis Citation1991), reducing opportunities for imprinting on the physical cues of the release site.
Most hatcheries in Alaska attempt to segregate the hatchery population from wild populations by using only hatchery salmon returning to the hatchery for broodstock. Even though population sizes of segregated hatcheries are large so that change through random drift is small, genetic changes can still occur through the selection of traits that enhance survival during hatchery culture (domestication). In fact, selection is more efficient in large than in small populations because the countering effects of random drift are less important in large populations. In many hatcheries, the intensity of domestication selection has been reduced by more closely integrating broodstock for hatchery production with wild populations (HSRG 2004; Naish et al. Citation2007).
Based on the results of this study, several steps can be taken to reduce the impact of hatchery-origin fish on wild populations in Hidden Lake. One step would be to collect broodstock at spawning sites with a high proportion of natural fish. In particular, broodstock collection is being moved from Site B to Site A, where the proportion of hatchery-origin fish is significantly less than at Site B, where 88% of the fish were of hatchery origin. Our results show that 33–60% of spawners at site B would likely be natural-origin adults.
Another step would be to release unfed hatchery fry at a new release site farther from the broodstock collection site (Site B) and closer to the outlet stream (). If our results are due to homing of hatchery-origin fish to their release site, this step would direct returning hatchery-origin fish away from beaches with naturally spawning fish (Sites A and B). This procedure could have three effects. First, it would decrease the number of hatchery-origin fish breeding with natural-origin fish in the wild. Second, it would decrease the proportion of hatchery-origin fish used as hatchery broodstock. Third, it would decrease the survival of hatchery-origin progeny because of suboptimal environmental conditions (coarse substrate) at the new release site. These desired effects hinge on homing fidelity to the new release site for spawning.
Even though these practices would not entirely eliminate the inadvertent inclusion of hatchery-origin fish into the broodstock, they would nevertheless reduce the proportion of hatchery-origin fish and help to reduce genetic risks to natural populations. The Cook Inlet Aquaculture Association, the ADFG, and the U.S. Fish and Wildlife Service have developed a work plan that details program monitoring. This includes ongoing baseline data collection and sampling to examine how these program modifications alter the proportions of hatchery-origin fish in wild and hatchery-broodstock aggregates when the first fish from the modified program return in 2017. The inclusion of detailed fish counts at all sites within Hidden Lake will facilitate better understanding of hatchery- and wild-origin Sockeye Salmon homing behavior as this project continues in the future.
ACKNOWLEDGMENTS
We thank J. Jasper for providing statistical advice and C. Cline at the Cook Inlet Aquaculture Association for preparing and reading the otoliths used in the study. Sara Turner, Bill Templin, and Eric Volk provided insightful comments on the manuscript and the comments of four anonymous reviews helped to shape the manuscript considerably. Funding for manuscript preparation and publication were provided by State of Alaska general funds. This is Professional Publication Number PP-273 of the Commercial Fisheries Division of the Alaska Department of Fish and Game.
© Christopher Habicht, Terri M. Tobias, Gary Fandrei, Nathan Webber, Bert Lewis, and W. Stewart Grant
REFERENCES
- Araki , H. , Berejikian , B. A. , Ford , M. J. and Blouin , M. S. 2008 . Fitness of hatchery-reared salmonids in the wild . Evolutionary Applications , 1 : 342 – 355 .
- Araki , H. , Cooper , B. and Blouin , M. S. 2007 . Genetic effects of captive breeding cause a rapid, cumulative fitness decline in the wild . Science , 318 : 100 – 103 .
- Booz , M. D. and Kerkvliet , C. M. 2011 . Ninilchik River Chinook Salmon stock assessment and supplementation, 2008 . Alaska Department of Fish and Game, Fishery Data Series 11-54, Anchorage. Available: www.adfg.alaska.gov/FedAidpdfs/FDS11-54.pdf. (May 2012)
- Burger , C. V. , Finn , J. E. and Holland-Bartels , L. 1995 . Pattern of shoreline spawning by Sockeye Salmon in a glacially turbid lake: evidence for subpopulation differentiation . Transactions of the American Fisheries Society , 124 : 1 – 15 .
- Campton , D. E. 1995 . “ Genetic effects of hatchery fish on wild populations of Pacific salmon and steelhead: what do we really know? ” . In Uses and effects of cultured fishes in aquatic ecosystems , Edited by: Schramm , H. L. Jr. and Piper , R. G. 337 – 353 . Bethesda , Maryland : American Fisheries Society, Symposium 15 .
- Dittman , A. H. and Quinn , T. P. 1996 . Homing in Pacific salmon: mechanisms and ecological basis . Journal of Experimental Biology , 199 : 83 – 91 .
- Ford , M. J. 2002 . Selection in captivity during supportive breeding may reduce fitness in the wild . Conservation Biology , 16 : 815 – 825 .
- Fraser , D. J. , Weir , L. K. , Bernatchez , L. , Hansen , M. M. and Taylor , E. B. 2011 . Extent and scale of local adaptation in salmonid fishes: review and meta-analysis . Heredity , 106 : 404 – 420 .
- Gomez-Uchida , D. , Seeb , J. E. , Smith , M. J. , Habicht , C. , Quinn , T. P. and Seeb , L. W. 2011 . Single nucleotide polymorphisms unravel hierarchical divergence and signatures of selection among Alaskan Sockeye Salmon (Oncorhynchus nerka) populations . BMC Evolutionary Biology [online serial] , 11 article 48
- Grant , W. S. 2012 . Understanding the adaptive consequences of hatchery–wild interactions in Alaska salmon . Environmental Biology of Fishes , 94 : 325 – 342 .
- Grant , W. S. , Milner , G. B. , Krasnowski , P. and Utter , F. M. 1980 . Use of biochemical genetic variants for identification of Sockeye Salmon (Oncorhynchus nerka) stocks in Cook Inlet, Alaska . Canadian Journal of Fisheries and Aquatic Sciences , 37 : 1236 – 1247 .
- Groot , C. and Margolis , L. 1991 . Pacific salmon: life histories , Vancouver : University of British Columbia Press .
- Habicht , C. , Seeb , L. W. and Seeb , J. E. 2007 . Genetic and ecological divergence defines population structure of Sockeye Salmon populations returning to Bristol Bay, Alaska, and provides a tool for admixture analysis . Transactions of the American Fisheries Society , 136 : 82 – 94 .
- Hendry , A. P. , Castric , V. , Kinnison , M. T. and Quinn , T. P. 2004 . “ The evolution of philopatry and dispersal: homing versus straying in salmonids ” . In Evolution illuminated: salmon and their relatives , Edited by: Hendry , A. P. and Stearns , S. C. 52 – 91 . New York : Oxford University Press .
- Hendry , A. P. , Quinn , T. P. and Utter , F. M. 1996 . Genetic evidence for the persistence and divergence of native and introduced Sockeye Salmon (Oncorhynchus nerka) within Lake Washington, Washington . Canadian Journal of Fisheries and Aquatic Sciences , 53 : 823 – 832 .
- Hendry , A. P. , Wenburg , J. K. , Bentzen , P. , Volk , E. C. and Quinn , T. P. 2000 . Rapid evolution of reproductive isolation in the wild: evidence from introduced salmon . Science , 290 : 516 – 518 .
- HSRG (Hatchery Scientific Review Group) . 2004 . Hatchery reform: principles and recommendations of the HSRG , Seattle : Long Live the Kings . Available: www.hatcheryreform.org. (January 2012)
- Kostow , K. E. 2004 . Differences in juvenile phenotypes and survival between hatchery stocks and a natural population provide evidence for modified selection due to captive breeding . Canadian Journal of Fisheries and Aquatic Sciences , 61 : 577 – 589 .
- Kyle , G. B. , Litchfield , D. S. and Todd , G. L. 1990 . Enhancement of Hidden Lake Sockeye Salmon (Oncorhynchus nerka): summary of fisheries production (1976–1989) , Juneau : Alaska Department of Fish and Game, Division of Fisheries Rehabilitation, Enhancement, and Development, Report 102 .
- Mahnken , C. , Ruggerone , G. , Waknitz , W. and Flagg , T. 1998 . A historical perspective on salmonid production from Pacific Rim hatcheries . North Pacific Anadromous Fish Commission Bulletin , 1 : 38 – 53 .
- Naish , K. A. , Taylor , J. E. III , Levin , P. S. , Quinn , T. P. , Winton , J. R. , Huppert , D. and Hilborn , R. 2007 . An evaluation of the effects of conservation and fishery enhancement hatcheries on wild populations of salmon . Advances in Marine Biology , 53 : 61 – 194 .
- Quinn , T. P. 2005 . The behavior and ecology of Pacific salmon and trout , Bethesda , Maryland : American Fisheries Society .
- Quinn , T. P. , Stewart , I. J. and Boatright , C. P. 2006 . Experimental evidence of homing to site of incubation by mature Sockeye Salmon, Oncorhynchus nerka . Animal Behaviour , 72 : 941 – 949 .
- Quinn , T. P. , Volk , E. C. and Hendry , A. P. 1999 . Natural otolith microstructure patterns reveal precise homing to natal incubation sites by Sockeye Salmon (Oncorhynchus nerka) . Canadian Journal of Zoology , 77 : 766 – 775 .
- Reisenbichler , R. R. and McIntyre , J. D. 1977 . Genetic differences in growth and survival of juvenile hatchery and wild steelhead trout, Salmo gairdneri . Journal of the Fisheries Research Board of Canada , 34 : 123 – 128 .
- Roppel , P. 1982 . Alaska's salmon hatcheries, 1891–1959 . Alaska Department of Fish and Game, Division of Fisheries Rehabilitation, Enhancement, and Development, Juneau
- Seeb , L. W. , Habicht , C. , Templin , W. D. , Tarbox , K. E. , Davis , R. Z. , Brannian , L. K. and Seeb , J. E. 2000 . Genetic diversity of Sockeye Salmon of Cook Inlet, Alaska, and its application to management of populations affected by the Exxon Valdez oil spill . Transactions of the American Fisheries Society , 129 : 1223 – 1249 .
- Simpson , E. M. and Edmundson , J. A. 1999 . Hidden Lake Sockeye enhancement project: technical review . Alaska Department of Fish and Game, Division of Commercial Fisheries, Regional Information Report 2A99-16, Anchorage
- Taylor , E. B. 1991 . A review of local adaptation in Salmonidae, with particular reference to Pacific and Atlantic salmon . Aquaculture , 98 : 185 – 207 .
- Théiault , V. , Moyer , G. R. and Banks , M. A. 2010 . Survival and life history characteristics among wild and hatchery Coho Salmon (Oncorhynchus kisutch) returns: how do unfed fry differ from smolt releases? . Canadian Journal of Fisheries and Aquatic Sciences , 67 : 486 – 497 .
- Varnavskaya , N. V. , Wood , C. C. , Everett , R. J. , Wilmot , R. L. , Varnavsky , V. S. , Midanaya , V. V. and Quinn , T. P. 1994 . Genetic differentiation of subpopulations of Sockeye Salmon (Oncorhynchus nerka) within lakes of Alaska, British Columbia, and Kamchatka, Russia . Canadian Journal of Fisheries and Aquatic Sciences , 51 ( Supplement 1 ) : 147 – 157 .
- Volk , E. C. , Schroder , S. L. and Fresh , K. L. 1990 . “ Inducement of unique otolith banding patterns as a practical means to mass-mark juvenile Pacific salmon ” . In Fish-marking techniques , Edited by: Parker , N. C. , Giorgi , A. E. , Heidinger , R. C. , Jester , D. B. Jr. , Prince , E. D. and Winans , G. A. 203 – 215 . Bethesda , Maryland : American Fisheries Society, Symposium 7 .
- Volk , E. C. , Schroder , S. L. and Grimm , J. J. 1999 . Otolith thermal marking . Fisheries Research , 43 : 205 – 219 .