Abstract
Objective: Severe asthma exacerbations are often treated with short courses of oral corticosteroids (OCS). This study assessed the incidence of OCS being prescribed in asthmatic children of various age groups and calculated their chances of receiving subsequent OCS prescriptions. Methods: Longitudinal Dutch community pharmacy data of 2272 children who were regular users of asthma medication was analyzed retrospectively. Incidence rates for first, second and third prescriptions of OCS were calculated, stratified by age and sex. Probabilities of receiving first, second or third OCS prescriptions were assessed with Kaplan–Meier analysis. Results: Incidence rates for first OCS prescriptions were 4.5 for the 1st year of life per 100 person-years (100PY); 3.9 for the 2nd; 4.6 for the 3rd; 4.2 for the 4th, and 4.7 for the 5th year of life per 100PY. This was relatively high compared to incidence rates for children between the ages of 6 and 11 (ranging between 2.2 per 100PY (age 9) and 3.7(age 11)). Incidence rates for second and third OCS prescriptions were very high: 78.2(95%CI: 45.0–123.7) and 241.2(95%CI: 81.2–583.4) per 100PY for infants, respectively. The chances of receiving a first OCS prescription was higher in males (P value < 0.01). Conclusions: In the Netherlands, the incidence of OCS being prescribed to children being treated with asthma medication in early childhood is relatively high for first OCS prescriptions and extremely high for second and third OCS prescriptions compared to other ages. Furthermore, there is a high probability of receiving a further OCS prescription shortly after an OCS prescription.
Introduction
Childhood asthma creates a substantial burden on the affected children and their families by restricting the child's activities and increasing the risk of school absences Citation[1]. The diagnosis of asthma, however, is complicated in preschool children for various reasons; lung function tests, for example, are difficult to perform in this age group Citation[2, 3]. Nevertheless, it is well known that asthma-like symptoms frequently occur in this period of life. Cloutier et al. reported asthma-like symptoms in 34% of children aged <5 years in an urban community (Hartford, CT, USA) Citation[4]. In general, there is a lot of controversy on how to diagnose asthma in young children (under the age of 5), and there is a paucity of data regarding the efficacy of available treatments in this age group, as well Citation[5].
Longitudinal cohort studies have shown that asthma-like symptoms at a young age persist in only a minority of cases. The lack of strict criteria for the diagnosis of asthma results in an empirical therapeutic approach in children below the age of 4. In both children and adults with overt asthma, short-acting β2;-adrenergic agonists (SABA) can be used to relieve symptoms. If patients remain symptomatic despite SABA use, long-term control therapy with inhaled corticosteroids (ICS) can be prescribed, sometimes in combination with add-on, long-acting β2-adrenergic agonists. The treatment for acute asthma exacerbations can include short acting β2-adrenergic agonists (SABA) and oxygen. Furthermore, short courses of oral corticosteroids (OCS) are commonly prescribed for acute asthma exacerbations Citation[3, 6–10]. Although these treatment guidelines are supported by vast amounts of literature for patients with documented asthma, the efficacy of these drugs are less well documented in young children with asthma-like symptoms.
Furthermore, the use of OCS is limited by significant side effects. Most of these side effects only occur during long-term use; however hyperglycemia, gastro-intestinal side-effects and mood changes may occur even with short-term use Citation[5]. Thus, we were interested in examining the extent to which OCS were being prescribed to children in various age groups, as the optimization of asthma therapy in groups with a high burden of exacerbations might help to reduce the amount of OCS prescriptions.
Many studies have investigated the prevalence of OCS use for exacerbations in children with asthma, as well as the proportion of children with asthma that use OCS; however, these studies have not investigated age-categorized incidence of use Citation[4, 11–14]. For that reason, this study examined the incidence of OCS prescriptions in Dutch children with asthma from different age groups to determine if there were differences in OCS prescription incidence rates in different age categories. We also assessed a child's chances of receiving a second or third OCS prescription in different age groups. In brief, we tried to identify high risk groups of children who were prone to developing asthma exacerbations (and therefore receiving OCS prescriptions), who should then be more carefully monitored.
Methods
Study population and data collection
Longitudinal deidentified data on medication was available for 3575 children (age: 4–12 years) who were eligible for inclusion in the PACMAN (Pharmacogenetics of Asthma medication in Children: Medication with Anti-inflammatory effects) cohort study (). Children were selected from Dutch community pharmacy databases based on their regular use of asthma medication (ATC code R03) (≥3 prescriptions in the last two years and ≥1 prescription in the last 6 months) with the help of the Utrecht University's UPPER network. The design and rationale of the PACMAN study has been described elsewhere Citation[15]. The PACMAN study has been approved by the Medical Ethics Committee of the University Medical Centre Utrecht Citation[15].
Figure 1. Flowchart of the prescribed medications and OCS for our study population. *If the difference between two OCS prescriptions were less than 10 days, it counted as continuing of the previous course of treatment.
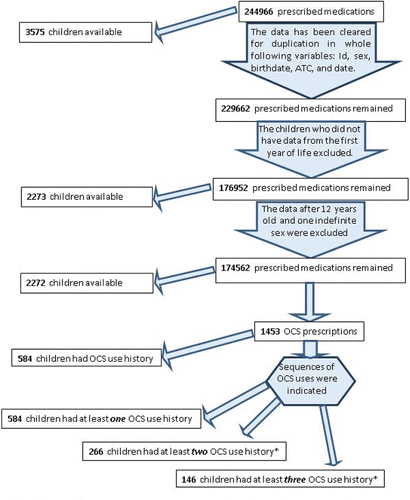
For this study, we excluded children who had no medication data available starting with their first year of life, and one child whose gender was unknown. All OCS prescriptions for the rest of the population (2272 children) were identified until the end of follow-up (time of extraction medication data), or until the child's 13th birthday (). If there were two prescriptions for the same OCS on the same day we calculated this as one prescription. If the difference between two OCS prescriptions was more than 10 days, they were considered as one exacerbation episode.
Incidence measurements
We assessed the incidence rates per person year (the product of the number of years times the number of members of a population who were at risk for an event) for the first prescriptions of OCS at every year of age. The frequencies of the first OCS prescriptions for every child in each age category (all prescriptions within 1-year-olds, 2-year-olds, etc.) were used as a numerator to calculate incidence.
For the denominator, the sum of the years or part of the years (Days/365.25) the patients were at risk for the prescription of OCS was calculated for each age category. For this calculation we excluded the time after the patient started taking OCS. After the first prescription of OCS, children were at risk for a second prescription. We calculated the years (days/365.25) after the first prescription as “time at risk for second prescription of OCS.” We did this in a similar way for the risk of a third prescription. We also calculated the “days at risk” separately for both sexes. We compared the incidence rates of the various age groups using chi-square or Fisher tests when the sample sizes were too small (less than 5 observations per cell) using R statistics software.
Probability measurement
The probability of receiving a first, second or third OCS prescription during follow-up visits and the differences between sexes were assessed with Kaplan–Meier analysis. Statistical analyses were performed using R software/environment and SPSS v.20.0 (IBM Corp., Armonk, NY, USA).
Results
Characteristics of the study population
Our population consisted of 2272 children; 65% of our study population were males, and the median age was 8.3 years (IQR = 6.0–10.7) at the time of data extraction (). In our study population, the age distribution at the time of data extraction showed a relatively uniform pattern, meaning that the number of children in each year of age was almost equal. The total time at risk for 1st, 2nd and 3rd prescription was 15377, 1594 and 549 person-years, respectively. The mean time of medication follow-up was 8.3 years (see Figure 3).
Table 1. Characteristics of the population.
Amount of prescribed medicines
Our population received 174562 drug prescriptions (all drug classes) in total. Most of the children in our study (98.0%) received prescriptions for inhaled beta agonists at least once, and ICS were prescribed for 93.9% of the children. Moreover, OCS was prescribed for 584 children (25.7%) at least once during their follow-up. Half of these children (n = 266, 11.7%) also received a second prescription for OCS, and 146 (6.4%) children received 3 or more prescriptions for OCS.
Incidence of OCS prescriptions
First OCS prescription
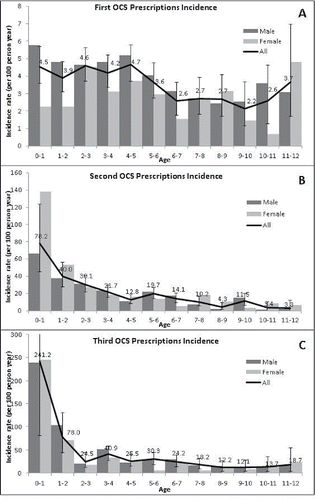
The incidence rates of OCS prescriptions are shown in . The overall incidence rates for first OCS prescriptions for age groups were statistically significantly different (P value < 0.01). The incidence rates for a first OCS prescription in the first 5 years of life were as follows: first year of life = 4.5 (95% confidence interval [CI]: 3.6 to 5.7); second year of life = 3.9 (95% CI: 3.1 to 4.8); third year of life = 4.6 (95% CI: 3.7 to 5.6); fourth year of life = 4.2 (95% CI: 3.3 to 5.2); fifth year of life = 4.7 (95% CI: 3.7 to 5.7) per 100 person-years. These rates are relatively high in comparison with the incidence rates for children between the ages of 6 and 11, with the lowest incidence rate being 2.2 (95% CI: 1.1 to 3.7) at age 9 and the highest incidence rate 3.7 (95% CI: 1.8 to 7.0) OCS prescriptions per 100 person-years at age 11 Citation[16].
Second and third OCS prescriptions
The incidence rates for second and third OCS prescriptions were very high for children under the age of one: 78.2 (95% CI: 45.0 to 123.7) and 241.2 (95% CI: 81.2 to 583.4) per 100 person-years, respectively. The incidence rate for a second OCS prescription was lowest at the age of 11: 3.3 (95% CI: 0.4 to 12.0) per 100 person-years. Children at the age of 9 had the lowest incidence rate of a third OCS prescription: 12.1 (95% CI: 4.0 to 28.5) per 100 person-years. The overall incidence rates for age groups were different for second OCS prescriptions (P value < 0.01) and third OCS prescriptions (P value < 0.01).
Probability of OCS prescriptions related to the time of previous OCS prescription
In Figure 3, a Kaplan–Meier plot illustrates the probability of a child receiving no OCS prescriptions at each age.
Figure 3. Probability of receiving OCS prescriptions (a) per age category in the total population and stratified per sex; (b) after first prescription. Follow-up time in years after first OCS prescription; (c) after 2nd prescription. Follow-up time in years after 2nd OCS prescription. “Time = 0” is the time of previous OCS prescription.
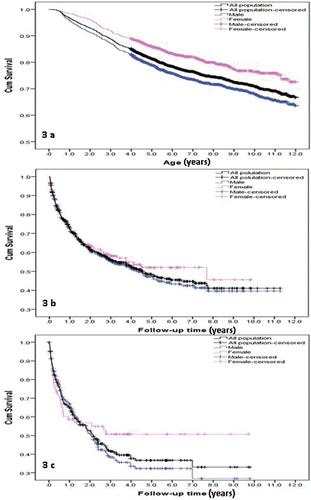
The slope of the Kaplan-Meier curve is relatively steep in early childhood, indicating that the probability of receiving an OCS prescription in early childhood is high for our study population. The chance of ever receiving a prescription for OCS was approximately 14.8% for a 4-year-old child with asthma; however, this chance increased to approximately 25.5% for an 8-year-old child with asthma. There was a statistically significant difference between males and females with regards to their chances of receiving a first OCS prescription. Males had a 1.5 (95% CI: 1.2–1.8) times higher chance of receiving OCS at all ages compared to females (P value < 0.01).
The probability of receiving a second OCS prescription during the 2 years following the first OCS prescription was 38.5%; similarly, the probability of receiving a third OCS prescription in the 2 years after the second OCS prescription was 47.5%. There seemed to be a difference in the risk of receiving a second or third OCS prescription between males and females; however these differences did not reach statistical significance.
Discussion
This study assessed the incidence of OCS prescriptions in a population of children who had used asthma medication. Higher OCS prescriptions' incidence rates were found in early childhood (age: 0 to 4 years) compared to school age children (age: 6 to 10 years).
Our data showed that OCS were prescribed in high numbers in children with asthma. In our study population, 26% of the children had been prescribed OCS. Other studies that had previously investigated the incidence or prevalence of OCS prescriptions were conducted within shorter timeframes. For example, in France over the course of three months, Mahut et al. reported even higher numbers Citation[17]. They reported 31% severe exacerbations in 359 children treated for asthma Citation[17]. Mudd et al. assessed OCS use over a period of 6 months in asthmatic children (age: 2–9 years) who had used ≥3 asthma medication nebulizers in the past 30 days and who had had an Emergency Department visit in the last 12 months. They showed that 45% of the children had received one OCS prescription Citation[11]. Although the inclusion criteria of these different studies are not fully comparable, these data stress the common use of OCS as a treatment modality, as well as the high impact of asthma in the young children.
Furthermore, in our study the probability of a child receiving another OCS prescription shortly after their first or second OCS prescription was high. When he or she received an OCS course for respiratory symptoms, there was an almost 50% chance that the child would receive another course of OCS within 2 years. Half of the children that had received a first OCS prescription in our study also received a second OCS prescription. These findings are in line with Miller et al., who included 2780 severe or difficult-to-treat, physician-diagnosed asthma patients (≥12 years of age) and assessed their records after 1.5 years. They compared the severity of asthma between a group of patients with non-recent, severe exacerbations and a group of patients with recent, severe exacerbations, and they concluded that recent exacerbations are a strong independent predictor of future exacerbations Citation[18].
This high prevalence of repeated OCS prescriptions is also in line with the earlier mentioned study of Mudd et al., who showed that 14.8% of the children had received more than one OCS prescriptions in the 6-month study period Citation[11]. These combined data strongly demonstrate the necessity of extra attention being paid to children who have recently experienced asthma exacerbations, as they are at the highest risk for repeated OCS prescriptions.
Our data also showed striking differences in OCS prescriptions between males and females; males had a higher chance of receiving their first OCS prescription during childhood (0 to 12 years).
The age-dependent approach in this study also highlights the high risk for OCS prescriptions in young children (<2 years of age). Second and third OCS prescriptions incidences were especially high in the first and second year of age. There are two possible explanations for this: firstly, since viral respiratory infections that frequently trigger wheezing and asthma-like symptoms are common in this young age group, OCS could have been prescribed as a result. Nevertheless, most studies that have evaluated the efficacy of OCS among preschool children with episodic wheezing have not demonstrated beneficial effects Citation[19]. Therefore, even short-term OCS use may lead to side-effects, and need to be prescribed cautiously. A second reason may be that children in this age group are ineffectively treated with ICS, or cannot yet inhale the asthma medication adequately. In a drug utilization study like ours, it is impossible to disentangle the different reasons for the frequent use of OCS. However, optimization of drug treatment, especially in children who have already received a first prescription for OCS is important, and might help to prevent subsequent second and/or third prescriptions.
An important strength of this study was the use of a large dataset, in which the prescription data from 100 Dutch community pharmacies was combined. However, the sole use of prescription data obtained from community pharmacies might have led to an underestimation of the incidence rates, as OCS prescriptions might have also been provided by hospital pharmacies. Furthermore, the number of children who received OCS and had follow-up data for second and third OCS prescription available was limited, therefore there was not enough power in our study to show a statistically significant difference between males and females.
Conclusions
In conclusion, in the Netherlands the incidence of OCS prescriptions in children treated with asthma medication is relatively high for first OCS prescriptions and extremely high for second and third OCS prescriptions in early childhood as compared to other age groups (>6 years). Further research is needed to assess the risks and benefits of OCS use in this young age group. Furthermore, the study also shows that there is a high probability of receiving another OCS prescription shortly after an OCS prescription. Therefore, children who have recently received an OCS prescription are prone to receiving another one, and should be carefully monitored.
Declaration of interest
Jan A. M. Raaijmakers is a part-time professor at the Utrecht University and he was Vice-president External Scientific Collaborations for GSK in Europe, and holds stock in GSK. Cornelis K. van der Ent received unrestricted grants from GSK and Grunenthal. Furthermore, the Division of Pharmacoepidemiology and Clinical Pharmacology, Utrecht Institute for Pharmaceutical Sciences, employing authors Ali Arabkhazaeli, Susanne J.H. Vijverberg, Jan A.M. Raaijmakers, and Anke-Hilse Maitland-van der Zee, has received unrestricted research funding from the Netherlands Organisation for Health Research and Development (ZonMW), the Dutch Health Care Insurance Board (CVZ), the Royal Dutch Pharmacists Association (KNMP), the private-public funded Top Institute Pharma (http://www.tipharma.nl website, includes co-funding from universities, government, and industry), the EU Innovative Medicines Initiative (IMI), EU 7th Framework Program (FP7), the Dutch Medicines Evaluation Board, the Dutch Ministry of Health and industry (including GSK, Pfizer, and others). The authors alone are responsible for the content and writing of the article.
Funding
The PACMAN cohort study has been funded by a strategic alliance between the Utrecht Institute for Pharmaceutical Sciences (UIPS) and GlaxoSmithKline (GSK).
References
- World Health Organization (WHO). Asthma 2014. Available from: http://www.who.int/respiratory/asthma/en/index.html [last accessed 20 January 2014].
- Stein RT, Martinez FD. Asthma phenotypes in childhood: lessons from an epidemiological approach. Paediatr Respir Rev 2004;5:155–161.
- The Global Strategy for the Diagnosis and Management of Asthma in Children 5 Years and Younger. 2009; 2014. Available from: http://www.ginasthma.org [last accessed 20 January 2014].
- Cloutier MM, Wakefield DB, Hall CB, Bailit HL. Childhood asthma in an urban community: prevalence, care system, and treatment. Chest 2002;122:1571–1579.
- Pocket Guide for Asthma Management and Prevention in Children 5 Years and Younger | Documents / Resources | GINA. 2016. Available from: http://www.ginasthma.org/documents/3 [last accessed 10 January 2016].
- Fuhlbrigge AL, Lemanske RF,Jr, Rasouliyan L, Sorkness CA, Fish JE. Practice patterns for oral corticosteroid burst therapy in the outpatient management of acute asthma exacerbations. Allergy Asthma Proc 2012;33:82–89.
- Barnes PJ. Chapter 254. Asthma. Harrison's Principles of Internal Medicine; 18e 2012.
- Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, Gibson P, Ohta K, O'Byrne P, Pedersen SE, Pizzichini E, Sullivan SD, Wenzel SE, Zar HJ. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J 2008;31:143–178.
- Camargo CA,Jr, Rachelefsky G, Schatz M. Managing asthma exacerbations in the emergency department: summary of the National Asthma Education and Prevention Program Expert Panel Report 3 guidelines for the management of asthma exacerbations. J Emerg Med 2009;37:S6–S17.
- NHG-Standaard Astma bij kinderen | NHG. 2014. Available from: https://www.nhg.org/standaarden/volledig/nhgstandaard-astma-bij-kinderen#Richtlijnendiagnostiek [last accessed 6 August 2014].
- Mudd K, Bollinger ME, Hsu VD, Donithan M, Butz A. Pharmacy fill patterns in young urban children with persistent asthma. J Asthma 2006;43:597–600.
- McQuaid EL, Vasquez J, Canino G, Fritz GK, Ortega AN, Colon A, Klein RB, Kopel SJ, Koinis-Mitchell D, Esteban CA, Seifer R. Beliefs and barriers to medication use in parents of Latino children with asthma. Pediatr Pulmonol 2009;44:892–898.
- Chauliac ES, Silverman M, Zwahlen M, Strippoli MP, Brooke AM, Kuehni AC. The therapy of pre-school wheeze: appropriate and fair? Pediatr Pulmonol 2006;41:829–838.
- Bianchi M, Clavenna A, Bonati M. Inter-country variations in anti-asthmatic drug prescriptions for children. Systematic review of studies published during the 2000-2009 period. Eur J Clin Pharmacol 2010;66:929–936.
- Koster ES, Raaijmakers JA, Koppelman GH, Postma DS, van der Ent CK, Koenderman L, Bracke M, Maitland-van der Zee AH. Pharmacogenetics of anti-inflammatory treatment in children with asthma: rationale and design of the PACMAN cohort. Pharmacogenomics 2009;10:1351–1361.
- Armitage P BG. Statistical Methods in Medical Research. Oxford, UK: Blackwell, 1994.
- Mahut B, Trinquart L, Delclaux C. Influence of age on the risk of severe exacerbation and asthma control in childhood. J Asthma 2011;48:65–68.
- Miller MK, Lee JH, Miller DP, Wenzel SE, TENOR Study Group. Recent asthma exacerbations: a key predictor of future exacerbations. Respir Med 2007;101:481–489.
- Collins AD, Beigelman A. An update on the efficacy of oral corticosteroids in the treatment of wheezing episodes in preschool children. Ther Adv Respir Dis 2014;8:182–190.