ABSTRACT
Objective: Bronchial thermoplasty (BT) as an add-on therapy for uncontrolled severe asthma is an alternative to biologic therapies like omalizumab (OM). We conducted an indirect treatment comparison (ITC) to appraise comparative effectiveness of BT and OM. Methods: A systematic literature review identified relevant randomized controlled trials. The ITC followed accepted methodology. Results: The ITC comprised a sham-controlled trial of BT (AIR2) and two placebo-controlled trials of OM (INNOVATE; EXTRA). Comparing the BT post-treatment period to ongoing treatment with OM, showed no significant differences in the rate ratios (RRs) for severe exacerbations (RR of BT versus OM = 0.91 [95% CI: 0.64, 1.30]; p = 0.62) or hospitalizations (RR = 0.57 [95% CI: 0.17, 1.86]; p = 0.53); emergency department visits were significantly reduced by 75% with BT (RR = 0.25 [95% CI: 0.07, 0.91]; p = 0.04); the proportions of patients with clinically meaningful response on the asthma quality-of-life questionnaire were comparable (RR = 1.06 [95% CI: 0.86, 1.34]; p = 0.59). The RR for exacerbations statistically favours OM over the total study period in AIR2 (RR = 1.50 [95% CI: 1.11, 2.02]; p = 0.009) likely reflecting a transient increase in events during the BT peri-treatment period. Conclusions: The ITC should be interpreted cautiously considering the differences between patient populations in the included trials. However, based on the analysis, BT compares well with a potentially more costly pharmacotherapy for asthma. Clinicians evaluating the relative merits of using these treatments should consider the totality of evidence and patient preferences to make an informed decision.
Abbreviations
| AQLQ | = | asthma quality-of-life questionnaire |
| BT | = | bronchial thermoplasty |
| ITC | = | indirect treatment comparison |
| OAT | = | optimized asthma therapy |
| OCS | = | oral cortical steroids |
| OM | = | omalizumab |
| RCT | = | randomized controlled trial |
| RR | = | rate ratio |
Introduction
Treatment options for asthma include long-acting beta-agonists, short-acting beta-agonists, and corticosteroids, delivered in order according to the Global Initiative for Asthma (GINA) guidelines [Citation1]. However, for approximately 5% of the patients, these options are insufficient to provide control of asthma symptoms [Citation2]. For these patients, current GINA guidelines [Citation1] specify other
treatment options, including bronchial thermoplasty (BT) and monoclonal antibody therapies such as omalizumab (OM) and mepolizumab, which are effective only in specific asthma phenotypes [Citation3–5].
BT is a minimally invasive, once per lifetime intervention for the treatment of uncontrolled severe asthma. The BT procedure involves delivery of controlled thermal energy to the airway wall as a means of reducing excess airway smooth muscle mass and constriction of the airways. This has been shown to reduce the number and severity of asthma exacerbations and their associated treatment requirements. BT is currently indicated for both allergic and non-allergic asthma patients [Citation6]. OM is a once- or twice-monthly subcutaneously injected monoclonal antibody therapy that targets serum-free immunoglobulin E (IgE) to interrupt the molecular signals that trigger the allergic inflammatory cascade in asthma. OM is indicated only for treating subtypes of allergic asthma [Citation7]. The ideal patient population for BT remains unknown.
Both BT and OM therapies were evaluated and proven to be safe and effective in their own clinical trials and gained regulatory approval. In the pivotal trial for BT (Asthma Intervention Research 2 Trial – AIR2), BT was compared to sham treatment [Citation6, Citation8], while in the OM registration trials, OM was compared to placebo injection [Citation9–11]. Clinicians and patients who wanted to make informed decisions regarding treatment choices and payers who were required to take decisions regarding reimbursement may benefit from the comparison of BT and OM. Recently published allergist guidelines suggest consideration of BT and OM as part of a sustained ‘step-up’ of therapeutic options for asthma [Citation12]. However, to date, no trial has directly compared BT and OM and, while a randomized controlled trial (RCT) comparing these two treatments would be ideal, there would be significant, perhaps intractable, challenges in maintaining subject blinding when comparing an interventional procedure with an injected drug. For this reason, the use of an indirect treatment comparison (ITC) should be considered as this would, at a minimum, afford the asthma community, currently grappling with a directional understanding of the comparative efficacy of these two treatments, some comparison of these two add-on treatment options. Thus, the objective of this study was to explore the comparative effectiveness of BT and OM utilizing an ITC.
Methods
The methodological approach was consistent with the health technology assessment guidelines for submissions to the Australian Medical Services Advisory Committee and Pharmaceutical Benefits Advisory Committee [Citation13, Citation14]. Briefly, RCTs were identified through a rigorous systematic review of the published literature. Double-blinded randomized trials represent the study-type least susceptible to bias and are generally regarded as the best quality evidence available; non-blinded randomized trials were therefore identified but not included in the ITC. The treatments were then compared by an indirect meta-analysis of the study results, via a common non-therapeutic reference, following the method of Bucher, using fixed-effect models [Citation15]. To examine the comparability of baseline characteristics of the trial populations, average means and pooled standard deviations were aggregated to BT-pooled and OM-pooled values and a T-test was used for the total study populations to generate the p-value.
Systematic literature review of trials and inclusion/exclusion criteria
The systematic literature review searched EMBASE, MEDLINE, Cochrane Library, and Clinical Trial Registries. Search terms included main concepts for the population, disease area and intervention. The strategies used for the EMBASE + MEDLINE searches are provided in full in the Supplementary Material Online (e-Appendix 1). Included trials were RCTs which accepted patients with uncontrolled severe asthma despite treatment with asthma therapy consistent with Step 4 of the GINA guidelines. Identified trials were limited to those in which BT or OM was used as an add-on therapy option to a patient's existing optimized asthma therapy (OAT) and the comparator was given OAT alone or OAT with the addition of a non-therapeutic treatment (i.e., a sham procedure or placebo) for the purposes of blinding. No limitations were placed upon the inclusion of trials based on language of publication, or outcome(s) studied.
Outcomes
The outcomes of interest were as follows: the rate ratios (RRs) of severe asthma exacerbations and asthma exacerbation-related events (hospitalizations, emergency department visits, unscheduled doctor's office visits, etc.), and change in total score on the asthma-related quality-of-life questionnaire (AQLQ) [Citation16]. A standardized form was used for the extraction of the data and all data were agreed upon consensus of two reviewers. No adjustment was made for potential differences in the definition of a severe asthma exacerbation in each trial (definitions of severe asthma exacerbation used in each trial included in the ITC are provided in e-).
Table 1. Main characteristics of randomized controlled trials identified in literature search.
ITC
The Bucher method examines the magnitude of treatment effect in studies of different treatments (against a common reference, usually placebo) and compares those treatment effects while preserving the randomization of the originally assigned patient groups. Adopting this approach, it is possible, even if control and treatment groups across trials differ in their baseline characteristics, to obtain an unbiased estimate of treatment effect. The only requirement is that the magnitude of treatment effect remains consistent across trials’ differences in the populations’ baseline characteristics [Citation15].
Results
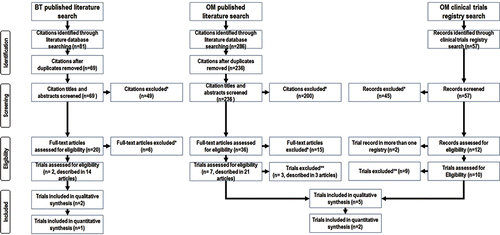
Results of the systematic literature review are shown in a PRISMA diagram (). A total of 424 publications (citations and clinical trials’ registry records) were identified from the initial searches. After a review of the publications and application of the inclusion and exclusion criteria, seven RCTs [Citation6,Citation17–22] were considered for further review (), of which the sham-controlled trial of BT (AIR2 [NCT00231114]) [Citation6] and the two placebo-controlled trials of OM (INNOVATE [NCT00046748]) [Citation20] and EXTRA [NCT00314574]) [Citation19] were included in the ITC, using the sham and placebo arms as a common reference (). These three trials were selected for the ITC on the basis that they each were double-blinded trials and thus represented the least bias best quality data available for each treatment.
Figure 1. PRISMA flow diagram showing the results of the systematic literature search. BT, bronchial thermoplasty; OM, omalizumab. Searches of the clinical trials’ registries identified no further trials meeting search inclusion criteria for which data were available. *Not RCT; wrong intervention and/or comparator; wrong population. **Small trial; outcome measures not relevant; population broader than specified by search criteria and no subgroup analysis reported; Limited information available—trial reported only as a conference abstract. Searches were conducted in June 2014.
Comparability of trial populations and feasibility of ITC
Overall, the populations included in the BT and OM trials reflect patients with severe asthma which was uncontrolled despite treatment with GINA Step 4 asthma therapy including high dose inhaled corticosteroids and a long-acting beta-agonist. However, scrutiny of the trial entry criteria and the baseline characteristics of subjects indicates differences across the trials (): the BT trials exclusively involved adult patients, whereas the OM trials included both adults and adolescents; the BT trials did not exclude subjects on the basis of atopy, whereas the OM trials included only allergic asthma; the populations included in the OM trials represent patients that could be interpreted to be more severely affected by their asthma than those included in the BT trials based on average baseline characteristics including slightly younger and greater proportion male subjects, lower FEV1, worse morning peak expiratory flow, a lower asthma symptom score comparable to minimal patient-perceptible difference, a lower AQLQ though the absolute difference not meeting minimal clinical relevance, higher maintenance dose of inhaled corticosteroids, more likely to be on maintenance corticosteroid therapy, more frequent asthma exacerbations requiring treatment with systemic steroids, and a higher rate of hospitalizations and emergency department visits.
Table 2. Key inclusion criteria in the sham/placebo-controlled trials.
Table 3. Baseline characteristics of the subjects included in the sham/placebo-controlled trials.
Table 4. Asthma medications at baseline in the sham/placebo-controlled trials.
Results of the ITC
Severe asthma exacerbations and related event rates
As shown in , and the Supplementary Material Online (e-), when comparing the post-treatment period of the AIR2 BT trial (i.e., the follow-up period after completion of all three BT procedures) to the ongoing treatment period with OM in the INNOVATE and EXTRA trials, there is a statistically significant difference (∼75% reduction: p = 0.035) in favour of BT for the RR for asthma-related emergency department visits. There is no statistical difference between the treatments for the RRs of severe asthma exacerbations, asthma-related hospitalizations and unscheduled doctor's office visits. Notably, the comparative effect of OM and BT in preventing exacerbations is measured on the relative reduction in exacerbations.
Table 5. Results of the indirect treatment comparison.
Figure 2. Asthma-related asthma exacerbations. BT, bronchial thermoplasty; OAT, optimized asthma therapy; OM, omalizumab.
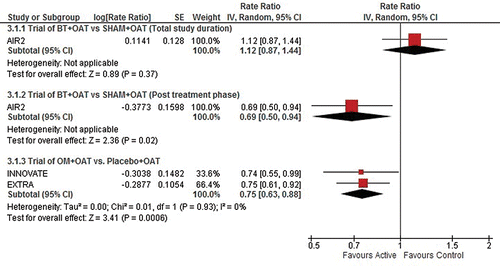
Figure 3. Asthma-related hospitalizations. BT, bronchial thermoplasty; OAT, optimized asthma therapy; OM, omalizumab.
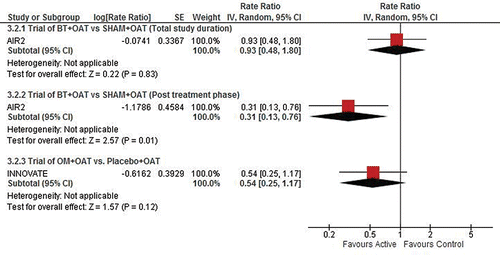
Figure 4. Asthma-related emergency department visits. BT, bronchial thermoplasty; OAT, optimized asthma therapy; OM, omalizumab.
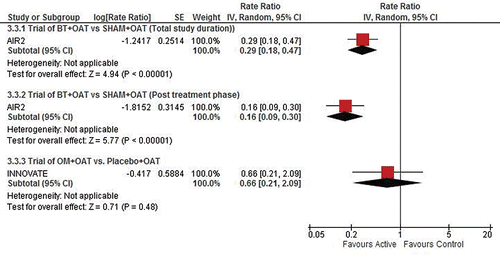
also shows results for the indirect comparison of events occurring during the total BT study duration in the AIR2 trial (peri-treatment period as well as the 46-week post-treatment follow-up period) to those during the ongoing treatment period in the OM trials. There is no statistical difference between the treatments in terms of the RRs of asthma-related hospitalizations and emergency department visits. However, there is a statistically significant difference which favours OM in terms of the RR for severe asthma-related exacerbations (∼33% rate reduction compared to BT; p = 0.009).
Asthma-related quality of life
The indirect comparison of AQLQ outcomes with BT and OM shows similar, clinically significant improvements in the AQLQ total scores and comparability in terms of the proportions of patients achieving the minimal clinical important difference ( and e-Figure 1B and C).
Discussion
Clinicians, patients and payers frequently must choose between available treatment options, based on the relative clinical and cost effectiveness. The authors acknowledge that the best supporting evidence would be an RCT, but this is not feasible with a SHAM trial of BT, now being considered unethical by most authorities. In addition, there would be insurmountable challenges arising when attempting to compare a surgical intervention with a chronic drug treatment. Accordingly, there are situations when evidence derived from ITC methodologies must be considered to draw conclusions on comparative efficacy. In the absence of RCT data, ITCs are perhaps necessary in order to make informed decisions, despite inherent weaknesses in the methodology.
The analysis presented here suggests some favourable outcomes for BT over OM with regard to emergency room visits during the post-treatment period, with a statistically and clinically significant 75% reduction with BT compared to OM (RR = 0.25; p = 0.04). There were no significant differences in the RR for severe exacerbations or hospitalizations, and the proportions of patients achieving a clinically meaningful response on the AQLQ were comparable.
These analyses did not specifically examine events in the ‘peri-procedure’ period. However, statistically significant results observed in favour of OM in terms of the RR for asthma-related exacerbations when considering the total study period of the AIR2 BT trial likely reflect a transient increase in asthma-related exacerbations observed during the peri-procedure period. While the occurrence of these adverse events in the short term may detract from considering BT as the first treatment intervention, due consideration should be given also to the sustained reduction of these events over a protracted period in the longer term, for example, out to five years in published studies [Citation8,Citation23]. Extended follow-up post-OM is not available within the published data with the longest follow-up being two years and this was an open label study.
A key assumption of indirect comparisons is the similarity of relative treatment effect across the respective sets of clinical trials. However, when faced with differences in patient characteristics or study methods, the indirect comparison will remain valid when there is no treatment effect modification within each side of the indirect comparison or trials’ populations [Citation20].
In regard to the study designs of the BT and OM trials, both sides of the comparison included only randomized controlled double-blinded trials. In regard to the control arms, a non-therapeutic treatment (i.e., sham procedure or a placebo injection) was used in addition to patients’ existing OAT for blinding purposes. For the purpose of this study, it has been necessary to consider these non-active elements sufficiently similar to allow the control arms of the trials to serve as an appropriate common reference for the ITC, although clearly this may be an imperfect assumption. The outcomes of interest were similarly defined across the trials, notwithstanding some minor differences between AIR2 and INNOVATE trials as to what constituted a severe asthma exacerbation: a worsening of asthma requiring treatment with oral or intravenous corticosteroids or a doubling of the baseline inhaled corticosteroid dose, or any temporary increase in the dosage of oral corticosteroids (AIR2) [Citation6]; a worsening of asthma symptoms requiring treatment with systemic corticosteroids (INNOVATE) [Citation20]; a worsening of asthma symptoms requiring treatment with systemic corticosteroids or a 20 mg or more increase in the average daily dose of oral prednisone or a comparable dose of another systemic corticosteroid (EXTRA) [Citation19]. Differences across the trials in terms of the duration of follow-up are acknowledged. In regard to the baseline characteristics of the patients, acknowledged differences, inferring that the patients in the BT trials were less severely affected by their asthma than the patients in the OM trial, may place potential limitations on the interpretation of the results of the analysis. However, it is important to note that asthma exacerbation-related events are compared as RRs, which means that the comparative effect of OM and BT in preventing events is on a relative scale. As outlined earlier, provided there is no modification of treatment effect due to greater asthma severity, the two treatments would be expected to be comparable on a relative scale.
The absence of a BT treatment modification effect either way cannot be confirmed based on the AIR2 trial data. Logistic regression analysis data from the AIR2 trial did not confirm any treatment effect modification based on baseline variables, including those indicative of baseline asthma severity. Moreover, evidence from the early RISA clinical trial [Citation22] and several case series [Citation24–29] shows BT to be similarly effective in patients more severely affected by their asthma than those included in the AIR2 trial. On the OM side of the comparison, a pooled analysis of data from seven RCTs involving 4308 patients, 93% of which had severe asthma, has shown that baseline characteristics do not reliably predict benefit with OM, although there was a suggestion of a greater treatment effect in patients with lower percentage-predicted FEV1 values and higher IgE [Citation30–33], and more recently, in those with a higher serum eosinophil count, serum periostin or fractionated exhaled nitric oxide [Citation34].
Given the assumption that the BT treatment effect is not reduced and the OM treatment effect is unchanged or increased with increased asthma severity, a conclusion of comparable efficacy between BT post-treatment and OM, based on the ITC, is reasonable, potentially biased against BT due to the OM trials’ populations potentially skewing more to populations that may be associated with an enhanced treatment effect. Specifically, OM is used in patients with specific patient characteristics, which at least initially were believed to be indicative of a likely treatment response, whereas no such selection process occurs for patients receiving BT.
Treatment choice based on relative clinical merit is frequently hampered by the absence of direct comparative clinical trial evidence. ITCs may help clinicians and treatment providers to make more informed decisions regarding treatment choices and funding [Citation35]. While it must be acknowledged that the findings of this ITC are best considered indicative rather than definitive, in the absence of RCT evidence which directly compares BT and OM or which allows an indirect or mix treatment comparison in more comparable severe asthma populations, it provides the best available quantitative assessment of the relative efficacy of these two therapies. Final treatment choice should therefore consider factors in addition to apparent relative efficacy, for example, toxicity, convenience and cost.
Conclusion
Based on the ITC of healthcare-utilization events which occurred during the post-treatment period alone, BT compares favourably to OM in terms of relative impact on exacerbation-related events including severe exacerbations, emergency department visits, and hospital admissions, as well as impact on asthma-related quality of life. Using the indirect comparison of severe exacerbations occurring during the total study duration in the AIR2 trial, the less favourable result for BT likely reflects the transient increase in events observed only during the peri-treatment period.
These data need to be interpreted with caution due to the indirect nature of the methodology. However, in the absence of an impractical, potentially unethical and time-consuming RCT, which would be the ‘gold standard’ for directly comparing these two treatments, and despite the inherent weakness of the indirect comparison as described here, our observations nevertheless provide one analysis of the potential relative benefits of OM and BT. Clinicians and treatment providers evaluating these two add-on treatments should consider the totality of evidence as well as patient preferences to make an informed decision.
Declaration of interests
Authors contributions
DPT and SC contributed to the performance of the systematic review, data extraction, meta-analysis, and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed to the manuscript preparation, review, and response to peer-review comments.
Financial/non-financial disclosures
This study was funded by Boston Scientific, Marlborough, MA, USA. MJC and MRS are currently employed by Boston Scientific, which manufactures and markets Alair™, a device system for BT. DPT and SC are the employees of THEMA Consulting Pty Ltd, which was paid to conduct research described in this manuscript. NSS is a Consultant to Boston Scientific Corp. and was an employee of Boston Scientific when this study was being performed. RMN has received personal fees and non-financial support from Novartis, Chiesi and Boehringer Ingelheim, and personal fees from GSK, Boston Scientific, Astra Zeneca, Teva and Vectura.
Notation of prior abstract publication/presentation
This work was presented at the European Respiratory Society 2015 International Congress, Amsterdam, Netherlands.
The study is based on a systematic literature review and analysis of previously published data. As such, approval from an institutional review board was not required.
Supplementary_Material_Online_content_only_Revised_10032017.docx
Download MS Word (135.9 KB)References
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2016 [cited 2016 Sept 19]. Available from: wwwginaasthma.org
- Peters SP, Ferguson G, Deniz Y, Reisner C. Uncontrolled asthma: a review of the prevalence, disease burden and options for treatment. Respir Med 2006;100(7):1139–1151.
- Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med 2009;360(10):973–984.
- Normansell R, Walker S, Milan SJ, Walters EH, Nair P. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev 2014;(1):CD003559.
- Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet 2012;380(9842):651–659.
- Castro M, Rubin AS, Laviolette M, Fiterman J, De Andrade LM, Shah PL, et al. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med 2010;181(2):116–124.
- Eisner MD, Zazzali JL, Miller MK, Bradley MS, Schatz M. Longitudinal changes in asthma control with omalizumab: 2-year interim data from the EXCELS Study. J Asthma 2012;49(6):642–648.
- Wechsler ME, Laviolette M, Rubin AS, Fiterman J, Lapa e Silva JR, Shah PL, et al. Bronchial thermoplasty: Long-term safety and effectiveness in patients with severe persistent asthma. J Allergy Clin Immunol 2013;132(6):1295–1302.
- Busse W, Corren J, Lanier BQ, McAlary M, Fowler-Taylor A, Cioppa GD, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol 2001;108(2):184–190.
- Holgate ST, Chuchalin AG, Hebert J, Lotvall J, Persson GB, Chung KF, et al. Efficacy and safety of a recombinant anti-immunoglobulin E antibody (omalizumab) in severe allergic asthma. Clin Exp Allergy 2004;34(4):632–638.
- Soler M, Matz J, Townley R, Buhl R, O'Brien J, Fox H, et al. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J 2001;18(2):254–261.
- Chipps BE, Corren J, Israel E, Katial R, Lang DM, Panettieri RA et al. Asthma Yardstick -Practical recommendations for a sustained step-up in asthma therapy for poorly controlled asthma. Ann Allergy Asthma Immunol 2017;118(2):113–142.
- Australian Government Department of Health. The Pharmaceutical Benefits Advisory Committee Guidelines (Version 4.5). 2015 [cited 2016 Sept 19]. Available from: https://pbac.pbs.gov.au/information/printable-version-of-guidelines.html.
- Australian Governemnt Department of health. Technical Guidelines for preparing assessment reports for the Medical Services Advisory Committee - Medical Service Type: Therapeutic (Version 2.0). 2016 [cited 2016 Sept 19]. Available from: http://www.msac.gov.au/internet/msac/publishing.nsf/Content/0BD63667C984FEEACA25801000123AD8/$File/TherapeuticTechnicalGuidelines-Final-March2016-Version2.0-accessible.pdf
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol 1997;50(6):683–691.
- Juniper EF, Guyatt GH, Ferrie PJ, Griffith LE. Measuring quality of life in asthma. Am Rev Respir Dis 1993;147(4):832–838.
- Omalizumab Use and Asthma-Related Quality of Life in Patients With Severe Persistent Allergic Asthma. 2011 [cited 2016 Sept 19]. Available from: https://clinicaltrials.gov/ct2/show/NCT00567476
- Bousquet J, Siergiejko Z, Swiebocka E, Humbert M, Rabe KF, Smith N, et al. Persistency of response to omalizumab therapy in severe allergic (IgE-mediated) asthma. Allergy 2011;66(5):671–678.
- Hanania NA, Alpan O, Hamilos DL, Condemi JJ, Reyes-Rivera I, Zhu J, et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Ann Intern Med 2011;154(9):573–582.
- Humbert M, Beasley R, Ayres J, Slavin R, Hebert J, Bousquet J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy 2005;60(3):309–316.
- Niven R, Chung KF, Panahloo Z, Blogg M, Ayre G. Effectiveness of omalizumab in patients with inadequately controlled severe persistent allergic asthma: an open-label study. Respir Med 2008;102(10):1371–1378.
- Pavord ID, Cox G, Thomson NC, Rubin AS, Corris PA, Niven RM, et al. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med 2007;176(12):1185–1191.
- Pavord ID, Thomson NC, Niven RM, Corris PA, Chung KF, Cox G, et al. Safety of bronchial thermoplasty in patients with severe refractory asthma. Ann Allergy Asthma Immunol 2013;111(5):402–407.
- Bicknell S, Chaudhuri R, Lee N, Shepherd M, Spears M, Pitman N, et al. Effectiveness of bronchial thermoplasty in severe asthma in ‘real life’ patients compared with those recruited to clinical trials in the same centre. Ther Adv Respir Dis 2015;9(6):267–271.
- Burn J, Sims A, Bousfield D, Patrick H, Welham S, Heaney L. P171 Efficacy and safety of bronchial thermoplasty in clinical practice: early results from a national registry. Thorax 2013;68(Suppl 3):A152–A153.
- Burn J, Sims AJ, Keltie K, Patrick H, Welham S, Niven RM, et al. S12 Efficacy of bronchial thermoplasty in clinical practice using the British Thoracic Society UK Difficult Asthma Registry and Hospital Episode Statistics. Thorax 2015;70(Suppl 3):A11.
- Chakir J, Haj-Salem I, Gras D, Joubert P, Beaudoin E-L, Biardel S, et al. Effects of bronchial thermoplasty on airway smooth muscle and collagen deposition in asthma. Ann ATS 2015;12(11):1612–1618.
- Fernandez-Bussy S, Labarca G, Caviedes I, Folch E, Majid A. Bronchial thermoplasty for severe asthma: Initial experience in Chile. Arch Bronconeumol 2016;52(1):52.
- Riddell P, Lawrie I, Lane S, Egan J. Bronchial Thermoplasty For Severe Persistent Asthma, An Irish Experience. The Spy Who Loved Bronchoscopy: Advances in International Interventional Pulmonary Procedures. American Thoracic Society; 2014. p. A4409.
- Bousquet J, Cabrera P, Berkman N, Buhl R, Holgate S, Wenzel S, et al. The effect of treatment with omalizumab, an anti-IgE antibody, on asthma exacerbations and emergency medical visits in patients with severe persistent asthma. Allergy 2005;60(3):302–308.
- Bousquet J, Rabe K, Humbert M, Chung KF, Berger W, Fox H, et al. Predicting and evaluating response to omalizumab in patients with severe allergic asthma. Respir Med 2007;101(7):1483–1492.
- Holgate S, Buhl R, Bousquet J, Smith N, Panahloo Z, Jimenez P. The use of omalizumab in the treatment of severe allergic asthma: A clinical experience update. Respir Med 2009;103(8):1098–1113.
- Sandstrom T. Omalizumab in the management of patients with allergic (IgE-mediated) asthma. J Asthma Allergy 2009;2:49–62.
- Hanania NA, Wenzel S, Rosen K, Hsieh HJ, Mosesova S, Choy DF, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med 2013;187(8):804–811.
- Jansen JP, Fleurence R, Devine B, Itzler R, Barrett A, Hawkins N, et al. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value Health 2011;14(4):417–428.
