ABSTRACT
Objective: Characterize fluticasone propionate (Fp) and combination fluticasone propionate and salmeterol (FS) pharmacokinetic and safety profiles, delivered via a novel, inhalation-driven, multidose dry powder inhaler (MDPI). Methods: This multicenter, open-label, four-period crossover, single-dose study randomized patients aged ≥12 years with persistent asthma to Fp MDPI 200 mcg, FS MDPI 200/12.5 mcg, Fp dry powder inhaler (DPI) 500 mcg (250 mcg × 2 inhalations), or FS DPI 500/50 mcg. Blood samples for determination of Fp and salmeterol pharmacokinetic parameters including Cmax, AUC0–t, AUC0–inf, tmax, and t½ were collected predose through 36 h postdose (14 time points). Safety assessments comprised adverse events, vital signs, and physical examinations. The institutional review board approved the study protocol. Results: The pharmacokinetic analysis set and safety population each included 40 patients. Fp systemic exposure (Cmax, AUC0–t, and AUC0–inf) was highest for Fp DPI 500 mcg and similar for Fp MDPI 200 mcg, FS MDPI 200/12.5 mcg, and FS DPI 500/50 mcg. Fp geometric mean t½ values were similar across treatments. Salmeterol Cmax was 20% lower and AUC0–t and AUC0–inf were approximately 50% lower with FS MDPI versus FS DPI. Median tmax and geometric mean t½ were similar between FS MDPI and FS DPI. Adverse events were similar across treatments with no relevant changes in vital signs, physical examinations, or hematology test results. Conclusions: Fp MDPI and FS MDPI produced similar or lower systemic exposure to Fp and salmeterol, despite lower doses, versus conventional DPI devices, suggesting improved efficiency due to formulation and device differences.
Introduction
Persistent asthma in adolescents and adults is characterized by the presence of asthma symptoms occurring more often than 2 days per week, or, in some cases, daily or throughout the day Citation(1). The treatment goal for all patients with persistent asthma, regardless of severity, is total symptom control Citation(2). Current practice guidelines advocate the initiation of controller treatment as soon as possible after an asthma diagnosis Citation(2). Patients with persistent asthma require daily, long-term use of controller medications as preventive or maintenance treatment, and rescue medication for prompt relief from bronchoconstriction and acute airway obstruction as well as during asthma exacerbation Citation(1).
Ideally, asthma therapy should provide effective, long-term control using the least amount of medication in order to minimize the risk of side effects Citation(3). When initiating therapy, a step-wise approach is currently recommended Citation(1,3), starting with inhaled corticosteroids (ICS) Citation(2), which are considered safe, well tolerated, and consistently effective in the long-term management of asthma Citation(2,3). When low- to medium-dose ICS monotherapy provides inadequate control of asthma symptoms, addition of a long-acting beta agonist (LABA) to ICS is the preferred treatment for sustained control of asthma symptoms, regardless of the previous ICS regimen Citation(2–4).
Fluticasone propionate (Fp) is an ICS approved in 1994 for use in patients 4 years of age and older with asthma Citation(5). Studies have demonstrated that adding the LABA salmeterol to moderate doses of ICS provides greater clinical benefit than increased doses of ICS monotherapy Citation(4,6,7). The combination of Fp/salmeterol (FS) was approved for asthma maintenance therapy in 2000 Citation(8).
The ability of patients and caregivers to properly coordinate the actuation and inhalation required with some inhalers (e.g., metered-dose inhalers) may limit the effectiveness of the medication Citation(9–12). A novel, inhalation-driven, multidose dry powder inhaler (MDPI; Teva Pharmaceutical Industries, Frazer, PA, USA) was developed that is intuitive and simple for patients to use Citation(13). The MDPI does not require the patient to coordinate actuation and inhalation and has increased the efficiency of dose delivery compared with a conventional dry powder inhaler (DPI) Citation(9,14). Greater efficiency of the MDPI device is suggested from other studies in which the efficacy of Fp MDPI 25, 50, or 100 mcg was demonstrated to be similar to Fp DPI 100 mcg and the efficacy of FS MDPI 100/12.5 mcg was similar to FS DPI 100/50 mcg with lower systemic exposure to salmeterol Citation(15,16).
The objective of the present study was to assess the pharmacokinetic and safety profiles of single doses of Fp MDPI and FS MDPI versus Fp DPI and FS DPI in patients 12 years of age and older with persistent asthma.
Methods
Study description
This was a phase 1, multicenter, open-label, randomized, four-period crossover, single-dose study (ClinicalTrials.gov registration identifier: NCT02437604). Prior to study initiation, the institutional review board (Chesapeake IRB, Columbia, MD, USA) granted approval for the research protocol. The study was conducted in accordance with International Council for Harmonisation Good Clinical Practice Consolidated Guideline (E6) and all applicable national and local laws and regulations.
Patients
Patients were eligible to participate if they were aged 12 years or older and had persistent asthma that was diagnosed at least 3 months prior to screening but was stable (i.e., no exacerbations or changes in asthma medication) for ≥30 days before providing informed consent. For adult patients (aged 18 years and older, or as applicable per local regulations), the written informed consent form was signed and dated by the patient before any study-related procedures were conducted. For adolescent patients (aged 12 to 17 years, or as applicable per local regulations), the written informed consent form was signed and dated by the parent/legal guardian and the written assent form was signed and dated by the patient (if applicable) before commencement of any study-related procedure. Eligible patients had forced expiratory volume in 1 s (FEV1) ≥60% of the predicted value for age, height, sex, and race, as per National Health and Nutrition Examination Survey III reference values Citation(17) at the screening visit; used an ICS at a stable dose (i.e., no change in dose) for ≥30 days before screening; and were able to achieve a peak inspiratory flow (PIF) rate of ≥60 L/min on an inhaler training device.
Patients were excluded from participation in the study if they could not demonstrate acceptable, reproducible inhalation technique with the MDPI device and the comparator device or were unable to withhold rescue medication for ≥6 h before the screening and all other visits where spirometry was performed. Additional exclusion criteria were: a history of a life-threatening asthma exacerbation (defined as an asthma episode that required intubation and/or was associated with hypercapnia, respiratory arrest, or hypoxic seizures); pregnancy, lactation, or planned pregnancy during the study period or ≤30 days after the last study visit; smoking at the time of the screening visit or smoking history ≥10 pack-years; and history of alcohol or drug abuse ≤2 years preceding the screening visit. At the screening visit, patients were excluded if they had a culture-documented or suspected bacterial or viral infection of the upper respiratory tract, lower respiratory tract, sinus, or middle ear within the previous 2 weeks, had asthma exacerbation requiring systemic corticosteroids within the previous 30 days, were hospitalized for asthma within the previous 2 months, or used immunosuppressive medications within the previous 4 weeks. Patients with untreated oral candidiasis at screening were ineligible to participate in the study. Patients who had clinically significant abnormalities in laboratory hematology values, or had a disease or condition that would, in the medical judgment of the investigator, compromise the safety of the patient through participation, were excluded from the study.
Patients whose FEV1 was <60% of the predicted value, whose PIF rate was <60 L/min, or who could not continue to demonstrate correct use of each device at the training visit or during the in-patient treatment period visits were not eligible to continue in the study.
Study design
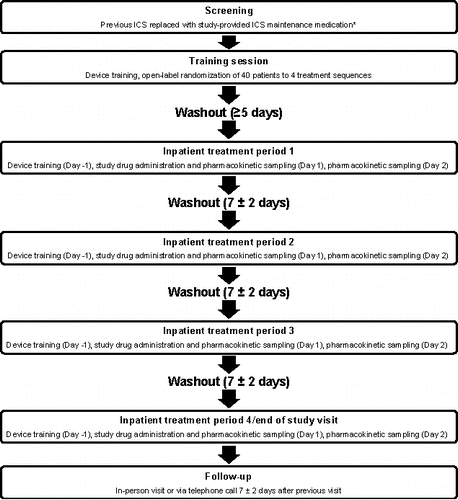
The study design is summarized in . Eligible patients were randomized to one of four treatment sequences (ABDC, BCAD, CDBA, or DACB): A) Fp MDPI 200 mcg; B) FS MDPI 200/12.5 mcg; C) Fp DPI 500 mcg (250 mcg × 2 inhalations); or D) FS DPI 500/50 mcg. At the screening visit, patients' previous ICS was replaced with mometasone furoate 220 mcg (1 inhalation twice daily), which was to be used until the final treatment period. Mometasone furoate was selected as the replacement ICS as it was previously determined to not interfere with the bioanalytical quantitation of either fluticasone or salmeterol. Patients observed a washout period of ≥5 days before the start of the treatment period. Each of the four treatment periods consisted of an inpatient stay, during which the patient was trained on the device (day −1 of the treatment period), administration of study drug between 0700 and 0900 (±30 min; day 1), and collection of blood samples for pharmacokinetic assessments of plasma Fp and salmeterol (days 1 and 2). A washout period of 7 ± 2 days was observed between treatment periods. A follow-up visit (7 ± 2 days after the last study visit) consisted of a telephone call or visit to the investigational center at the investigator's discretion to assess treatment-emergent adverse events (TEAEs).
Pharmacokinetics
Plasma concentrations of Fp and salmeterol were determined from blood samples (6 mL) collected approximately 30 min before dosing and at 5, 15, 30, and 45 min and 1, 2, 4, 8, 12, 16, 24, 30, and 36 h postdose. Plasma Fp and salmeterol concentrations were analyzed using a validated liquid chromatography–tandem mass spectrometry method. The primary pharmacokinetic parameters were area under the plasma concentration-vs-time curve from time 0 to the last measurable concentration up to 36 h following dosing (AUC0–t) and maximum observed plasma concentration (Cmax). Other pharmacokinetic parameters of interest included AUC from time 0 extrapolated to infinity (AUC0–inf), percentage of AUC0–inf due to extrapolation (%AUCextrap), time to Cmax (tmax), and terminal half-life of elimination (t½).
Safety
Safety was assessed by monitoring and recording adverse events (AEs), vital signs, physical examination findings, clinical laboratory hematology testing, spirometry measurements, and concomitant medication usage. AEs were coded according to their preferred terms in version 18.0 of the Medical Dictionary for Regulatory Activities (MedDRA). A serious AE was defined as an AE occurring at any dose that resulted in death, a life-threatening AE, inpatient hospital admission, prolongation of existing hospitalization, persistent or significant disability or incapacity, congenital anomaly, or birth defect.
Statistics
The intent-to-treat population (ITT) included all randomized patients. The full analysis set (FAS) included all patients in the ITT population who received at least one dose of study drug and had at least one evaluable pharmacokinetic parameter. The FAS was used for supportive analyses of the pharmacokinetic parameters. The pharmacokinetic analysis set consisted of all randomized patients who received ≥1 dose of study drug and had sufficient data to calculate the pharmacokinetic parameters AUC0–t and Cmax from any treatment period without a major protocol violation. The pharmacokinetic analysis set was the primary analysis set for analyses of the pharmacokinetic parameters. The safety population consisted of all randomized patients who received ≥1 dose of study drug. The safety population was used for all analyses of safety data.
Plasma concentrations of Fp and salmeterol were summarized descriptively (mean, standard deviation, standard error of mean, median, minimum, maximum, geometric mean, and coefficient of variation) by analyte, by treatment, and at nominal time points. The lower limit of quantitation for both Fp and salmeterol was 0.50 pg/mL; concentrations below that threshold were treated as 0 in calculations of summary statistics for plasma drug concentrations. Pharmacokinetic parameters were calculated through noncompartmental methods using Phoenix® WinNonlin® version 6.3 (Certara LP, Princeton, NJ). Summary statistics were provided for the pharmacokinetic parameters, including the mean, geometric mean, and coefficient of variation. If inferential statistics were computed, they were to include least square (LS) mean and standard error of the LS mean.
For Fp and salmeterol, pairwise comparisons of AUC0–t and Cmax between treatments were performed using a parametric analysis of variance (ANOVA) model with terms for sequence, period, and treatment and a random effect of patient within sequence. Treatment differences for Fp and salmeterol and the associated 90% confidence intervals (CIs) estimated from the ANOVA on the log scale were back-transformed to obtain the estimated ratio of geometric means between treatments and the 90% CI. Because this study did not evaluate any formal hypotheses, these standard bioequivalence statistical analyses were not used for a formal bioequivalence assessment, but rather for the purpose of comparison between Fp MDPI versus Fp DPI and FS MDPI versus FS DPI.
For secondary pharmacokinetic parameters (tmax, AUC0–inf, %AUCextrap, and t½), pairwise comparisons of tmax and t½ between treatments were performed using the Wilcoxon signed-rank test. AUC0–inf was analyzed using the same statistical methods used for AUC0–t and Cmax. %AUCextrap was summarized using descriptive statistics based on data from both the pharmacokinetic analysis set and the FAS. These standard bioequivalence statistical analyses were not used for a formal bioequivalence assessment, but rather for the purpose of comparison.
Results
Patients
In total, 45 patients were screened for enrollment. Of the 43 patients who were enrolled in the study, 3 patients withdrew before taking any study drug and 40 patients received ≥1 dose of study drug (FAS, pharmacokinetic analysis set, and safety population) (). Demographic and baseline characteristics of the ITT population are presented in . Prior to the start of the study, 34 (79%) patients were being treated with salbutamol, 20 (47%) patients with Fp, and 10 (23%) patients with FS.
Figure 2. Patient disposition. *Of the 43 enrolled patients, 3 patients withdrew consent before receiving any study drug. FAS, full analysis set; Fp DPI, fluticasone propionate dry powder inhaler; Fp MDPI, fluticasone propionate multidose dry powder inhaler; FS DPI, fluticasone propionate/salmeterol dry powder inhaler; FS MDPI, fluticasone propionate/salmeterol multidose dry powder inhaler; PK, pharmacokinetic analysis set.
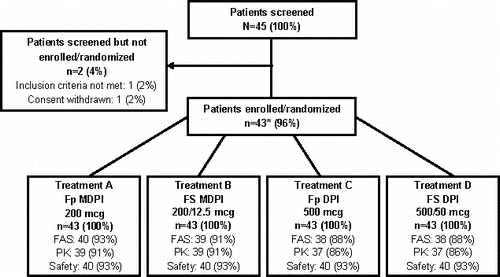
Table 1. Patient demographics and baseline characteristics, intent-to-treat population.
Pharmacokinetics
Fluticasone propionate
The mean plasma concentration-vs-time profiles for Fp for each treatment are shown in . For all treatments, all patients had quantifiable Fp concentrations at the first postdose time point of 5 min. Systemic exposure to Fp, as represented by geometric mean estimates of Cmax, AUC0–t, and AUC0–inf, was the highest in patients treated with Fp DPI and similar among patients receiving the other three treatments (). Overall, systemic exposures for Fp following Fp MDPI were approximately 20% lower than those observed with Fp DPI. The ratios of geometric LS means for Cmax, AUC0–t, and AUC0–inf are presented in and demonstrate less systemic exposure between Fp MDPI and Fp DPI and similar systemic exposure between FS MDPI and FS DPI. The ratio of geometric LS means for the comparison between Fp MDPI and FS MDPI was close to unity for Fp Cmax (). Peak Fp concentrations for all four treatment groups were reached by 4 h postdose, with the median tmax for Fp highest (2.0 h) for patients treated with FS MDPI and similar (1.0 h) for the other three treatment groups (). There was little or no difference in tmax between Fp MDPI and Fp DPI as well as between FS MDPI and FS DPI (). Fp geometric mean t½ values ranged from 10.7 to 11.2 h with a slight difference in comparisons between Fp MDPI and Fp DPI and FS MDPI and FS DPI ( and ).
Figure 3. Plasma concentration-vs-time profiles for fluticasone propionate (a) and salmeterol (b), pharmacokinetic analysis set. Fp DPI, fluticasone propionate dry powder inhaler 500 mcg; Fp MDPI, fluticasone propionate multidose dry powder inhaler 200 mcg; FS DPI, fluticasone propionate/salmeterol dry powder inhaler 500/50 mcg; FS MDPI, fluticasone propionate/salmeterol multidose dry powder inhaler 200/12.5 mcg.
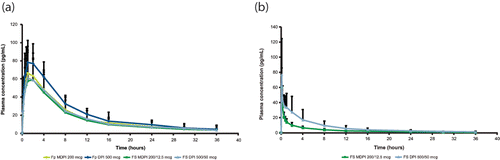
Table 2. Fluticasone propionate plasma pharmacokinetic parameters, pharmacokinetic analysis set.
Table 3. Fluticasone propionate treatment comparisons, pharmacokinetic analysis set.
Salmeterol
Salmeterol concentrations were quantifiable in all patients for all treatments at the first measured postdose time point of 5 min (). Systemic exposure to salmeterol, as represented by geometric mean estimates of Cmax, AUC0–t, and AUC0–inf, was lower for FS MDPI compared with FS DPI (). In patients treated with FS MDPI, Cmax was approximately 20% lower and AUC0–t and AUC0–inf were approximately 50% lower compared with patients treated with FS DPI ( and ). Peak plasma concentrations of salmeterol occurred between 5 and 45 min in patients treated with FS MDPI and between approximately 5 min and 2 h in patients treated with FS DPI. There was little to no difference in median tmax for salmeterol between patients treated with FS MDPI compared with those treated with FS DPI ( and ). Salmeterol geometric mean t½ estimates were similar between treatments ().
Table 4. Salmeterol plasma pharmacokinetic parameters, pharmacokinetic analysis set.
Table 5. Salmeterol treatment comparisons, pharmacokinetic analysis set.
Safety
Of the 40 patients who received ≥1 dose of study drug, 13 (33%) experienced a TEAE. None of the TEAEs were serious or considered by the investigators to be related to study drug, and none resulted in study discontinuation. No AE-related deaths or asthma exacerbations were reported during the study. The most frequently reported TEAE was nausea, which occurred in two patients during treatment with Fp MDPI, one patient during treatment with FS MDPI, and one patient during treatment with FS DPI. No clinically meaningful trends in mean changes from baseline for clinical laboratory hematology values, vital signs, or percent-predicted FEV1 were observed during the study.
Discussion
In this prospective, randomized, crossover study, single doses of Fp MDPI 200 mcg and FS MDPI 200/12.5 mcg were safe and well tolerated by adolescent and adult patients with persistent asthma. Following oral inhalation of Fp MDPI 200 mcg and FS MDPI 200/12.5 mcg, Fp and salmeterol concentrations were quantifiable in all patients 5 min after dosing. Plasma concentrations of Fp peaked up to 4 h after dosing with Fp MDPI 200 mcg and Fp DPI 500 mcg, and concentrations of salmeterol peaked between 5 and 45 min after dosing with FS MDPI 200/12.5 mcg and between approximately 5 min and 2 h for FS DPI 500/50 mcg. Systemic exposure to the ICS Fp was approximately 20% lower in patients treated with Fp MDPI 200 mcg compared with patients treated with Fp DPI 500 mcg, while systemic exposure to Fp was similar between patients treated with FS MDPI 200/12.5 mcg and FS DPI 500/50 mcg. Systemic exposure to salmeterol was between 20% and 50% lower in patients treated with FS MDPI 200/12.5 mcg compared with those treated with FS DPI 500/50 mcg. The tmax and t½ for Fp were similar in patients treated with Fp MDPI 200 mcg and Fp DPI 500 mcg, as were the tmax and t½ for salmeterol in patients treated with FS MDPI 200/12.5 mcg and FS DPI 500/50 mcg. Patients treated with Fp MDPI 200 mcg or FS MDPI 200/12.5 mcg had similar values for Fp plasma Cmax, AUC0–t, and AUC0–inf, indicating that there is no interaction for the systemic exposure between Fp and salmeterol when administered together as FS MDPI. Interestingly, the exposure to Fp in FS DPI was approximately 20% less than that in Fp DPI, indicating that the two comparator products do not have similar exposure to Fp despite delivering the same dose.
For this study, the doses of Fp MDPI 200 mcg and FS MDPI 200/12.5 mcg were selected based on three dose-ranging studies conducted in patients with persistent asthma Citation(15,16,18). In studies by Kerwin et al. Citation(16) and Bernstein et al. Citation(18), Fp AUC0–t and Cmax increased approximately dose proportionally across the dose levels tested (Fp MDPI 12.5, 25, 50, 100, 200, and 400 mcg). Both Fp MDPI 25 and 50 mcg in the Kerwin et al. study had similar clinical efficacy outcomes compared with Fp DPI 100 mcg but with lower systemic exposures. In a crossover study by Miller et al. Citation(15) to assess FS MDPI doses of 100/6.25, 100/12.5, 100/25, and 100/50 mcg compared with FS DPI 100/50 mcg and Fp MDPI 100 mcg, improvements in pulmonary function in patients treated with FS MDPI 100/12.5 and 100/25 mcg were comparable to improvements in patients treated with FS DPI 100/50 mcg with lower systemic exposure to salmeterol. The doses of Fp DPI and FS DPI (500 mcg and 500/50 mcg, respectively) used in this study were chosen as comparators based on current prescribing information and their clinical efficacy profiles, which are similar to Fp MDPI 200 mcg and FS MDPI 200/12.5 mcg, respectively. Similar to the studies by Kerwin et al. and Miller et al., patients treated with Fp MDPI 200 mcg and FS MDPI 200/12.5 mcg in the present study had either similar or lower exposure to Fp and salmeterol compared with Fp DPI 500 mcg or FS DPI 500/50 mcg when used at a lower dose. Since Fp has negligible oral bioavailability, the systemic exposure following inhaled administration is attributed to lung delivery and deposition Citation(19,20). The results of these previously published studies and of the present study suggest more efficient delivery of Fp and salmeterol via the MDPI device compared with the DPI device.
Safety profiles were similar across the four treatments. The absence of serious TEAEs, discontinuations due to a TEAE, and asthma exacerbations demonstrated that single doses of Fp 200 mcg and salmeterol 12.5 mcg used in Fp MDPI 200 mcg and FS MDPI 200/12.5 mcg in the present study were safe and well tolerated.
Device selection is an important consideration when deciding the appropriate medication for a patient. Poor inhaler technique, sometimes stemming from an inability to coordinate inhalation with actuation, has the potential to lead to poor patient outcomes Citation(21). A recent article evaluated patient satisfaction and ease of use of albuterol delivered using an MDPI delivery device similar to that used in the present study Citation(13). Greater than 90% of patients ≥4 years of age with asthma or chronic obstructive disease responded that they were “very satisfied,” “satisfied,” or “somewhat satisfied” when asked about the ease of use in handling and holding the inhaler, in using the inhaler, and in inhaling a dose from the MDPI Citation(13).
The generalizability of the results from this phase 1 study may be limited by the small sample size (n = 40) and open-label design, which could have introduced patient bias toward the approved medications. However, the PK analyses were blinded, so this is unlikely to represent bias in the interpretation of the results. The patient population deemed eligible for participation in this study may not reflect the wider patient population suffering from persistent asthma because of inclusion criteria regarding prior medical history (e.g., prior and concomitant therapies, comorbidities, body mass index, and body weight). Greater monitoring of treatment adherence and correct administration of the study drug in the controlled setting of this clinical trial, in comparison to the real-world setting where the wide spectrum in patient health, adherence to therapy, and ability to correctly administer medication is readily apparent, also may be considered a limitation of this study. Similarly, the crossover design of the present study may be considered a limitation by the possibility of introducing a crossover effect. However, a washout period of 5 to 7 days was implemented in between treatment visits and study drug administration was randomized in order to prevent a crossover effect.
Conclusions
The results of this phase 1 study demonstrated that Fp MDPI 200 mcg and FS MDPI 200/12.5 mcg are safe and well tolerated in adolescent and adult patients with persistent asthma. Fp MDPI 200 mcg and FS MDPI 200/12.5 mcg produced similar or lower systemic exposure to Fp and salmeterol despite lower doses than the conventional DPI device, suggesting improved efficiency due to formulation and device differences.
Declaration of interest
This study was sponsored by Teva Branded Pharmaceutical Products R&D, Inc. Medical writing assistance was provided by Lisa Feder, PhD, of Peloton Advantage and was funded by Teva Branded Pharmaceutical Products R&D, Inc. Teva provided a full review of the article. Gloria Yiu, Courtney Nugent, and Sharon Song are employees of Teva Pharmaceutical Industries, Frazer, PA, USA. Cynthia Caracta was an employee of Teva Pharmaceutical Industries, Frazer, PA, USA, at the time of study conduct and manuscript preparation.
Acknowledgments
The authors wish to thank the clinical personnel and the patients who participated in this study, and Dimitry Golubovsky, clinical programmer, Ave Beatty, data management, and Steve Gorman, bioanalytical of DMPK, all of whom are employees of Teva Pharmaceutical Industries, Frazer, PA, USA. The authors gratefully acknowledge Paul H. Ratner, MD, MBA, for his contribution.
References
- Expert Panel Report 3 (EPR3). Guidelines for the diagnosis and management of asthma. Bethesda, MD: National Heart, Lung, and Blood Institute, US Department of Health and Human Services; 2007 7/2007.
- Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention. Bethesda, MD: National Heart, Lung, and Blood Institute; 2016.
- Urbano FL. Review of the NAEPP 2007 Expert Panel Report (EPR-3) on asthma diagnosis and treatment guidelines. J Manag Care Pharm. 2008;14(1):41–9.
- Bateman ED, Boushey HA, Bousquet J, Busse WW, Clark TJ, Pauwels RA, et al. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL study. Am J Respir Crit Care Med. 2004;170(8):836–44.
- Flovent Diskus [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2014 5/2014.
- Masoli M, Weatherall M, Holt S, Beasley R. Moderate dose inhaled corticosteroids plus salmeterol versus higher doses of inhaled corticosteroids in symptomatic asthma. Thorax. 2005;60(9):730–4.
- Ducharme FM, Ni Chróinín M, Greenstone I, Lasserson TJ. Addition of long-acting beta2-agonists to inhaled corticosteroids versus same dose inhaled corticosteroids for chronic asthma in adults and children. Cochrane Database Syst Rev. 2010(5):CD005535.
- Advair Diskus [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2014 July 2014.
- Price DB, Pearce L, Powell SR, Shirley J, Sayers MK. Handling and acceptability of the Easi-Breathe device compared with a conventional metered dose inhaler by patients and practice nurses. Int J Clin Pract. 1999;53(1):31–6.
- Virchow JC, Crompton GK, Dal Negro R, Pedersen S, Magnan A, Seidenberg J, et al. Importance of inhaler devices in the management of airway disease. Respir Med. 2008;102(1):10–9.
- O'Conor R, Wolf MS, Smith SG, Martynenko M, Vicencio DP, Sano M, et al. Health literacy, cognitive function, proper use and adherence to inhaled asthma controller medications among older adults with asthma. Chest. 2015;147(5):1307–15.
- Giraud V, Roche N. Misuse of corticosteroid metered-dose inhaler is associated with decreased asthma stability. Eur Respir J. 2002;19(2):246–51.
- Given J, Taveras H, Iverson H. Prospective, open-label evaluation of a new albuterol multidose dry powder inhaler with integrated dose counter. Allergy Asthma Proc. 2016;37(3):199–206.
- Vutikullird AB, Gillespie M, Song S, Steinfeld J. Pharmacokinetics, safety, and tolerability of a new fluticasone propionate multidose dry powder inhaler compared with fluticasone propionate diskus® in healthy adults. J Aerosol Med Pulm Drug Deliv. 2016;29(2):207–14.
- Miller DS, Yiu G, Hellriegel ET, Steinfeld J. Dose-ranging study of salmeterol using a novel fluticasone propionate/salmeterol multidose dry powder inhaler in patients with persistent asthma. Allergy Asthma Proc. 2016;37(4):291–301.
- Kerwin EM, Gillespie M, Song S, Steinfeld J. Randomized, dose-ranging study of a fluticasone propionate multidose dry powder inhaler in adolescents and adults with uncontrolled asthma not previously treated with inhaled cortico-steroids. J Asthma. 2017;54(1):89–98.
- The Third National Health and Nutrition Examination Survey (NHANES III). 1988–1994 Series 11, no. 9A Data Release. Atlanta, GA: Centers for Disease Control and Prevention; 2001. Available from: https://wwwn.cdc.gov/nchs/data/nhanes3/9a/Readme.txt.
- Bernstein DI, Gillespie M, Song S, Steinfeld J. Safety, efficacy, and dose response of fluticasone propionate delivered via the novel MDPI in patients with severe asthma: a randomized, controlled, dose-ranging study. J Asthma. 2017;54(6):559–69.
- Falcoz C, Oliver R, McDowall JE, Ventresca P, Bye A, Daley-Yates PT. Bioavailability of orally administered micronised fluticasone propionate. Clin Pharmacokinet. 2000;39 Suppl 1:9–15.
- Bye A. The oral systemic availability of fluticasone propionate in man–preliminary data. Br J Clin Pharmacol. 1993;36(2):136–7.
- Levy ML, Hardwell A, McKnight E, Holmes J. Asthma patients' inability to use a pressurised metered-dose inhaler (pMDI) correctly correlates with poor asthma control as defined by the Global Initiative for Asthma (GINA) strategy: a retrospective analysis. Prim Care Respir J. 2013;22(4):406–11.