Abstract
Objective: To describe health care resource utilization (HCRU) and associated costs in adult patients referred for specialist asthma care in Southwest Finland, by disease severity and blood eosinophil count (BEC).
Methods: This non-interventional, retrospective registry study (GSK ID: HO-17-17558) utilized data from patients >18 years of age on the hospital register of the Hospital District of Southwest Finland. Data extraction was from January 1, 2004 to December 31, 2015; the index date was the first hospital visit within this period with an International Classification of Diseases-10 diagnosis code for asthma or acute severe asthma. Patients were categorized by asthma severity (based on medication use) and BEC (<300 or ≥300 cells/μL). Total and asthma-related HCRU and estimated costs were recorded the year following index and for calendar years 2004–2015.
Results: Overall, 14,398 patients were included; 388 had severe asthma at index. BEC was available for 3781 patients; 1434 had a BEC ≥300 cells/μL and 2347 had a BEC <300 cells/μL. A total of 1241 patients had severe asthma; 270 patients had severe eosinophilic asthma (severe asthma and a BEC ≥300 cells/μL). Patients with severe versus non-severe asthma had higher total- and asthma-related outpatient visits, inpatient days, emergency room visits and costs per patient year; those with BEC ≥300 cells/μL versus <300 cells/μL had more outpatient visits. All recorded HCRU and associated costs were highest in patients with severe eosinophilic asthma.
Conclusion: This study demonstrated a substantial burden associated with severe and/or eosinophilic asthma for adults in Finland.
Introduction
Asthma is a heterogeneous disease characterized by chronic airway inflammation and respiratory symptoms (Citation1,Citation2) and is thought to affect approximately 235 million people worldwide (Citation3,Citation4). The prevalence of asthma has increased in many countries in recent decades, and in Finland the prevalence of asthma in adults is estimated to be around 10% (Citation5).
Some patients suffer from severe asthma, which is defined in European Respiratory Society/American Thoracic Society guidelines as asthma that requires treatment with high-dose inhaled corticosteroids (ICS) plus a second controller and/or systemic corticosteroids for ≥50% of the year to maintain asthma control or asthma that remains uncontrolled despite these treatments (Citation1). There is substantial heterogeneity in the presentation of severe asthma and several distinct severe asthma phenotypes have been identified (Citation1,Citation2). One of these is severe eosinophilic asthma, characterized by poor asthma control, eosinophilic inflammation, frequent exacerbations and reduced lung function (Citation1,Citation2,Citation6–8).
The prevalence of severe asthma has been estimated to be approximately 5–10% among patients with asthma (Citation1,Citation9), although the use of different disease definitions means that there is a high degree of variation among estimates (Citation10–13). Despite the relatively low prevalence of severe asthma, it places a high burden on health care systems. In particular, severe asthma is estimated to account for more than 50% of total asthma-related health care costs (Citation13,Citation14). In Sweden, it is reported that patients with severe asthma on regular oral corticosteroid (OCS) treatment have total health care costs that are double those of patients using OCS periodically, and three times higher than patients who do not use OCS (Citation15). Comorbidities are a substantial cost driver in these patients (Citation15). In addition, health care costs have been shown to be higher in patients with severe eosinophilic asthma compared with those without an eosinophilic phenotype (Citation13).
There remains a need for further real-world data on the level of health care resource utilization (HCRU) and related costs for patients with asthma, according to disease severity and phenotype, as this would help to identify those patients who are not achieving adequate disease control and who may benefit from additional therapy. The aims of the current study were to describe the HCRU and associated costs in patients with asthma in a specialty care hospital setting in Finland, according to disease severity and eosinophil level, and to determine the burden of comorbidities and mortality in these patient groups.
Methods
Study design
This was a non-interventional, retrospective registry study (GSK ID: HO-17–17558) utilizing data from the hospital register of the Hospital District of Southwest Finland (HDSWF). Adult asthma is typically managed within primary care services in Finland; however, patients with asthma are likely to be referred to specialist care centers due to poor asthma control, diagnostic problems, or comorbidities requiring specialist management. To fully assess the burden of adult asthma in Finland, this study therefore analyzed data from patients referred to specialist care hospitals. Patient-level data were linked via national identity codes to Statistics Finland, a nationwide database, for causes of death and to the drug reimbursement register of the Social Insurance Institution (SII) of Finland for reimbursed medication purchase data. The study data extraction period ran from January 1, 2004 to December 31, 2015. For each patient, data extraction began from the first visit in specialty care with an asthma diagnosis (index date) and continued until December 31, 2015 or death; this was defined as the follow-up period. Notably, the index date visit was the first hospital visit during which an asthma diagnosis was recorded, but asthma did not have to be the main cause for the visit. This registry-based study was approved by the Auria Biobank Scientific Steering Committee (decision number AB17-7796), Finland.
Patients
Adult patients with asthma (>18 years of age) who were receiving specialist care at either Turku University Hospital, or Salo, Loimaa, or Vakka-Suomi district hospitals between 2004 and 2015 and had an International Classification of Diseases (ICD)-10 diagnosis code of J45 (asthma) or J46 (acute severe asthma) were included. Patients with both asthma and chronic obstructive pulmonary disease (J44) were excluded. The majority of adults receive specialist care at public hospitals, in contrast to children who typically receive care at private centers. Private care data were not available, therefore only adult patients were included in this study.
Patients were categorized according to asthma severity and blood eosinophil count. At index date, patients were classified as having either non-severe or severe asthma. Patients with non-severe asthma either remained in the non-severe category throughout the follow-up period or presented with severe asthma during follow-up and were therefore classified as having transitioning asthma. The transition date was recorded where applicable.
The classification of non-severe or severe asthma was based on medication purchases from the SII register. Severe asthma was defined as use of high-dose ICS (>1000 μg/day fluticasone propionate or equivalent [Supplementary Table S1]) plus a second controller (e.g. a long-acting β2-agonist [LABA]) and/or OCS (≥5 mg prednisolone daily or equivalent) (Citation1). ICS and OCS use were calculated based on a sliding window of three consecutive purchases, with average usage calculated as total purchased items (defined daily dose) divided by the window length. Medication data from ≥2 years prior to the index date were excluded.
At index date, patients were also divided into groups based on blood eosinophil count throughout the whole follow-up period. Patients with no blood eosinophil count data available were categorized as blood eosinophil count unknown. Those with data available were divided into two groups: <300 cells/μL throughout the follow-up period and ≥300 cells/μL at any time during the follow-up period. This cutoff was selected as it is supported by current treatment guidelines used in clinical practice in Finland and by a database study in secondary health care in Finland (Citation16). Severe eosinophilic asthma was defined as severe asthma with a blood eosinophil count of ≥300 cells/μL.
Endpoints and assessments
Endpoints included total HCRU (hospital inpatient days and visits, outpatient visits, emergency room [ER] visits, hospital laboratory tests, and procedures), and asthma-related HCRU (hospital inpatient days and visits, outpatient visits, ER visits) in the year following the index date and for calendar years between 2004 and 2015 (total events, events per patient, and events per patient year). Costs associated with total and asthma-related HCRU, estimated based on standard item direct costs, were also assessed. Comorbidities were assessed at the end of follow-up based on ICD-10 codes and reported if their prevalence was >10% of the total asthma population. In addition, the Charlson comorbidity index was calculated from the ICD-10 codes (Citation17,Citation18); this index is designed to predict mortality, taking into account the number and seriousness of chronic comorbid diseases. All-cause and asthma-related mortality figures were obtained through Statistics Finland. Asthma-related mortality was defined as mortality where asthma was the direct, indirect or contributing cause of death.
Complete details of hospital medications for asthma and comorbidities were not available in the hospital register. Outpatient medications were only used to categorize patients according to asthma severity. Therefore, the direct costs associated with specialist care, but not medication costs, were assessed in this study. Standard HCRU costs used were €110 for each outpatient visit, €195 for each ER visit, and €330 for each hospital inpatient day (all 2017 price listings from HDSWF). Procedures, operations, and laboratory measures were priced using procedure codes and prices initially evaluated from the HDSWF 2017 price list; any items not available from this list were priced using other appropriate Finnish sources.
Sample size and statistical analysis
Descriptive statistics were produced for continuous and categorical variables for the population stratified by asthma severity and blood eosinophil count. Asthma severity stratification was performed for each analysis as follows: for comorbidity analyses of overall diagnoses, patients were divided into non-severe or severe categories based on the most severe category they had reached during follow-up; for Kaplan–Meier analyses of mortality patients were divided by baseline severity (non-severe vs severe); in Cox-proportional hazard models, severity was handled as a time-varying covariate; for HCRU and cost analyses, patients were stratified according to time spent in the non-severe and severe categories during follow-up.
Between-group differences in mortality were evaluated using a log-rank test. The effect of premature mortality was assessed using univariable and multivariable Cox-proportional hazards models, with asthma severity as a time-varying covariate. Model 1 adjusted for severity only (non-severe, severe) and Model 2 adjusted for severity, age, gender, body mass index, Charlson comorbidity index (Citation17), blood eosinophil count, forced expiratory volume in 1 s (FEV1), and peak expiratory flow. Cause-specific mortality was assessed using competing risk models as implemented in the R-package “cmprsk.”
Results
Patient population
A total of 14,398 adult patients with asthma were included in the analysis. At the index date, 388 patients had severe asthma and 14,010 patients had non-severe asthma. Of the patients with non-severe asthma, 853 presented with severe asthma during follow-up and were therefore classified as having transitioning asthma. In all, 1241 (8.6%) patients had severe asthma at any time within the study period and of these, 578 (46.6%) were regular users of OCS. The mean follow-up time was 5.2 years in patients with non-severe asthma (n = 13,157), 7.2 years in patients with transitioning asthma (n = 853), and 4.7 years in patients with severe asthma (n = 388).
Blood eosinophil counts were available for 3781 (26.3%) patients; 1434 (37.9%) had blood eosinophil counts ≥300 cells/μL and 2347 (62.1%) had blood eosinophil counts <300 cells/μL. Of the 3177 patients with non-severe asthma and available eosinophil data, 1164 (36.6%) had a blood eosinophil count ≥300 cells/μL. Of the 152 patients with severe asthma at index and available eosinophil data, 71 (46.7%) had a blood eosinophil count ≥300 cells/μL. Transitioning asthma was identified in 452 patients with available eosinophil data, 199 (44.0%) of whom had a blood eosinophil count ≥300 cells/μL. A total of 270 patients had severe eosinophilic asthma, defined as severe asthma and a blood eosinophil count ≥300 cells/μL at any time within the study period. Baseline characteristics by asthma severity and eosinophil status are shown in .
Table 1. Baseline characteristics by asthma severity and eosinophil status.
HCRU and costs
For the analysis of HCRU and costs, 14,010 patients were grouped as having non-severe asthma and 1241 patients were grouped as having severe asthma. Patients with severe asthma had a greater number of all-cause and asthma-related outpatient visits, inpatient days, and ER visits per patient year compared with patients with non-severe asthma. The mean annual rate of all-cause ER visits for patients with severe asthma was 0.76 visits per patient year and for non-severe asthma 0.51 visits per patient year. Compared with all patients who had severe asthma, those with severe eosinophilic asthma showed a 1.4 to 1.7-fold increase in all-cause and asthma-related outpatient visits and inpatient days (). Greater numbers of total outpatient visits were also observed in patients with blood eosinophil counts ≥300 cells/μL versus <300 cells/μL (). The number of asthma-related ER visits and inpatient days was low for all patients. Total and asthma-related HCRU was highest during the first year after index date regardless of asthma severity and decreased over time in patients with non-severe and severe asthma (). After the first year, HCRU was higher among patients with severe asthma than those with non-severe asthma. This difference was greater for asthma-related HCRU than for total HRCU ().
Figure 1. HCRU per patient year by (A) asthma severity* and (B) blood eosinophil count†. *Patients were divided into two groups according to time spent in the non-severe and severe categories. †Blood eosinophil count categories: <300 cells/μL (throughout the follow-up period); ≥300 cells/μL (at any time during the follow-up period); unknown (no blood eosinophil count available). Severe eosinophilic asthma was defined as severe asthma with a blood eosinophil count of ≥300 cells/μL. ‡Outpatient visits include scheduled and ER outpatient visits and scheduled telephone calls from which data were recorded in the patient files. BEC: blood eosinophil count; HCRU: health care resource utilization; ER: emergency room.
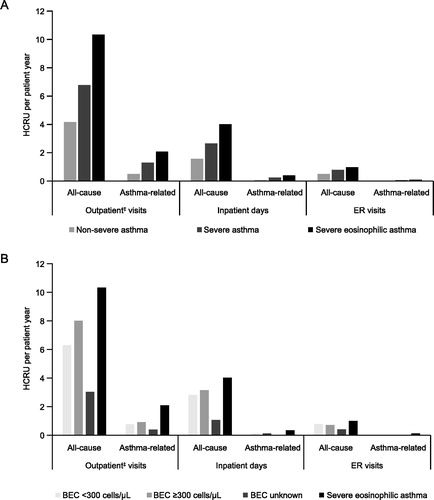
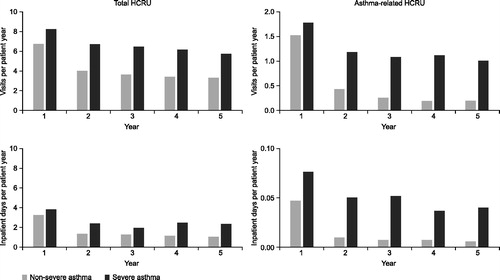
Figure 2. Annual HCRU from index date to Year 5 of follow-up in patients according to asthma severity. *Patients were divided into two groups according to time spent in the non-severe and severe categories. HCRU: health care resource utilization.
Total- and asthma-related HCRU costs (per patient and per patient year) were higher in patients with severe asthma compared with those with non-severe asthma, and in patients with a blood eosinophil count ≥300 cells/μL versus <300 cells/μL (). For example, total HCRU had a cost of €1878 per patient year in patients with non-severe asthma, compared with €3147 per patient year in those with severe asthma. Severe eosinophilic asthma was associated with a cost of €5143 per patient year; patients with severe eosinophilic asthma had higher total and asthma-related HCRU costs (per patient and per patient year) than those who either had severe asthma or a blood eosinophil count ≥300 cells/μL (). Total costs were greater than asthma-related costs regardless of asthma severity or blood eosinophil count.
Table 2. HCRU-Associated total and asthma-related costs by asthma severity and blood eosinophil count.
Comorbidities
Comorbidities were assessed by the most severe asthma category reached during follow-up, so that all patients with transitioning asthma were included in the severe asthma category. Comorbidities reported by patients with severe asthma (n = 1241) or non-severe asthma (n = 13,157) were compared; those that occurred in >10% of patients and were more common in patients with severe than non-severe asthma included essential hypertension, pneumonia, sleep disorders, type 2 diabetes, atrial fibrillation and flutter, and age-related cataract (all p < 0.05) (Supplementary Table S2).
Mortality
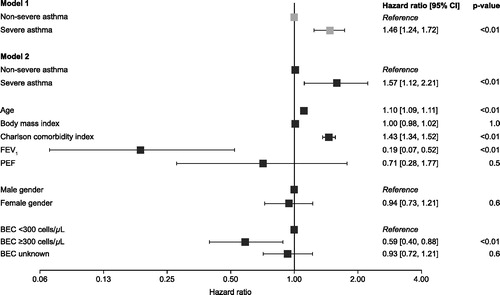
Of the deaths reported, most were not asthma-related. Asthma-related deaths occurred in <5% of patients across all asthma severity groups during the follow-up period. Overall survival was reduced in patients with severe asthma compared with non-severe asthma (p = 0.009) (). However, patients with a blood eosinophil count ≥300 cells/μL had improved overall survival compared with those with a blood eosinophil count <300 cells/μL (p < 0.0001) (). Changes in asthma-related mortality (as measured by competing risk models) were not statistically significant in patients grouped by asthma severity or blood eosinophil count (). In an adjusted Cox regression model, the hazard ratio for all-cause mortality was found to be increased in patients with severe asthma compared with non-severe asthma, and increased with age and Charlson comorbidity index (). The hazard ratio for all-cause mortality was lower in patients with a blood eosinophil count of ≥300 cells/μL versus <300 cells/μL and in patients with higher FEV1 ().
Figure 3. Overall survival by (A) asthma severity* and (B) blood eosinophil count† and competing risk models for non-asthma and asthma-related mortality by (C) asthma severity* and (D) blood eosinophil count†. *Patients were divided into two groups by baseline disease severity status: non-severe and severe. †Blood eosinophil count categories: <300 cells/μL (throughout the follow-up period); ≥300 cells/μL (at any time during the follow-up period); unknown (no blood eosinophil count available). BEC: blood eosinophil count.
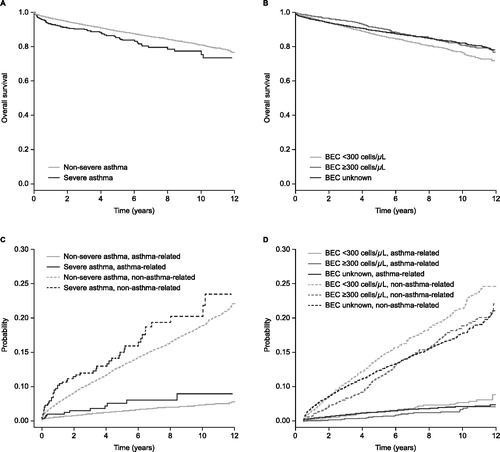
Figure 4. Cox regression models of all-cause mortality by disease severity* and patient characteristics. *To account for patients with transitioning asthma (non-severe asthma at index but presented with severe asthma during follow‐up), severity was modeled as a time-varying covariate. CI: confidence interval; BEC: blood eosinophil count; FEV1: forced expiratory volume in 1 s; PEF: peak expiratory flow.
Discussion
The results of this retrospective, non-interventional registry study in Finland showed high HCRU and costs in adult patients with asthma. Patients with severe asthma had higher total- and asthma-related HCRU compared with those with non-severe asthma. In addition, we found that total costs were 1.7 times higher and asthma-related costs were three times higher in patients with severe asthma compared with those with non-severe asthma. Total costs in patients with severe eosinophilic asthma were also 1.6 times higher than in the overall severe asthma group. These findings demonstrate the high burden associated with asthma in Finland.
In the current study, 47% of patients with severe asthma and 37% of patients with non-severe asthma had eosinophilic asthma (based on a blood eosinophil count of ≥300 cells/μL). Eosinophil measurements were only available in 26% of patients with asthma, representing 49% and 24% of patients with severe and non-severe asthma, respectively. If the proportion of patients with a blood eosinophil count ≥300 cells/μL is the same among all patients with severe asthma, our results suggest that the proportion of patients with severe eosinophilic asthma in the total cohort may be approximately 4%. This is like estimates from other recent studies. For example, a hospital-based study in Finland found that severe uncontrolled eosinophilic asthma occurred in approximately 2% of patients (Citation9). In addition, results from a population-based study in the UK found that 10% of patients had severe asthma, but only 2% and 1% had uncontrolled severe asthma and uncontrolled severe eosinophilic asthma, respectively (Citation19).
Several of our findings indicate that the health care burden was highest in those with severe eosinophilic asthma compared with the severe and non-severe groups. This likely reflects the high comorbidity burden in patients with severe eosinophilic asthma. These findings are in line with a growing body of literature highlighting the high disease burden and poor prognosis of patients with persistent eosinophilic disease (Citation1,Citation2,Citation16,Citation19–21). For example, blood eosinophil counts ≥300 cells/μL have previously been associated with higher rates of hospital admissions and outpatient visits, compared with blood eosinophil counts <300 cells/μL (Citation16). We chose the threshold for eosinophil count of ≥300 cells/μL, in line with previous publications (Citation16,Citation22–25) and current treatment guidelines used in clinical practice in Finland. As reported previously and substantiated in this study, this threshold clearly differentiates patients with higher disease burden.
We observed reduced overall survival in patients with severe asthma versus non-severe asthma. However, a blood eosinophil count ≥300 cells/μL was associated with better overall survival than a blood eosinophil count <300 cells/μL. The Charlson comorbidity index scores were similar regardless of blood eosinophil group. Therefore, it is plausible that the differing asthma-related mortality risk between these two groups may be linked to increased HCRU or an improved response to treatment in patients with a blood eosinophil count ≥300 cells/μL.
Annual levels of HCRU and associated costs have been reported in other Finnish studies and in other countries around the world. For example, a US retrospective analysis of data from 1762 patients with severe asthma in an administrative claims database reported that the mean annual ER visit count was 1.52–2.02 per patient year (Citation26). A multicenter, cross-sectional study of 500 patients with asthma in Italy also found that the mean annual rate of ER visits was 1.41 per patient year in individuals with severe, persistent asthma (n = 39) (Citation27). In our study, the mean annual rate for patients with severe asthma was higher than the rate for patients with non-severe asthma (0.76 vs 0.51 ER visits per patient year, respectively), but was lower than that reported in other studies. This may reflect the standard of asthma care and guided self-management that is routine clinical practice in Finland. For example, the implementation of Finnish national asthma and allergy programs has been associated with a marked reduction in hospitalizations, plus a reduction in lost productivity (and associated costs) (Citation28).
Our findings indicate that less than 10% of health care costs were directly related to asthma in the patient populations studied (7% in patients with severe and 4% in patients with non-severe asthma), although it is likely that asthma will influence costs related to comorbidities such as pneumonia. In a Swedish study, mean total- and asthma-related costs per year for patients with severe uncontrolled asthma (on the basis of regular OCS use) were €5615 and €834, respectively (Citation15), compared with €5143 and €354, respectively, in patients with severe eosinophilic asthma and €3147 and €228 in patients with severe asthma in our study. However, it should be noted that comparison of HCRU and associated costs between countries is difficult due to considerable variations in healthcare systems. Moreover, there are variations in calculation of HCRU use and associated costs across studies, with each considering distinct health care categories and taking different cost sources and standard costs into consideration. Nonetheless, the common finding across these studies is a higher use of HCRU and associated costs with severe versus non-severe asthma and/or eosinophilic versus non-eosinophilic asthma (Citation16,Citation19,Citation26,Citation29), highlighting a need for more effective treatments to improve asthma control in this patient subset.
Several limitations should be considered when interpreting the results of the present study. First, there are several limitations typically associated with retrospective database analyses, as well as those associated with cohort selection. These include the risk that some information may not have been consistently recorded for all patients, potentially impacting on the population size and other outcomes. However, as long as patients purchased their medications in Finland, this should have been recorded systematically. In addition, we used a medication-based definition for severe asthma, but our analysis did not evaluate whether poor asthma control and the use of high-dose medication was caused by factors other than severe asthma (e.g. comorbidities with asthma-like symptoms or poor inhalation technique). We also did not evaluate the effect of noncompliance on HCRU. Because asthma severity assessment was based on purchased medicines, non-compliant patients would have been classified as non-severe; this would likely have reduced the difference in HCRU between patients with severe and with non-severe asthma.
Eosinophil data were not available for a large proportion of patients, which may have led to an over- or underestimation of the prevalence of severe eosinophilic asthma. Furthermore, patients with eosinophilic disease may have been treated with corticosteroids prior to the index date, lowering their eosinophil level and potentially leading to their inclusion in the <300 cells/μL rather than the ≥300 cells/μL group. In addition, health care associated costs are likely to have been underestimated as imaging and other specialist examinations were not included, nor were those for medication or primary and/or private care. Hospital medications for asthma and for comorbidities would likely have been a significant cost driver, but these were not available for analysis. Finally, indirect costs, such as loss of productivity, were not included. Patients who experience poor asthma control despite the use of optimized controller therapies may receive biologic therapies that increase medication costs but also may also decrease indirect costs by improving asthma control. However, during the data extraction period only one biologic therapy (omalizumab) was available and was given only to a minority of patients with severe asthma at the hospitals included in this study. Despite these limitations, the present study clearly demonstrates differences in health care burden among patients with varying disease severity and eosinophil status, and our results provide a basis for further research in this area.
Conclusions
This real-world study demonstrates that adult asthma represents a significant disease burden in Finland, with an increased burden in patients with severe and/or eosinophilic disease.
Declaration of interest
AV has served as a scientific advisory board member for AstraZeneca, GSK and Novartis, has received lecture fees from AstraZeneca, Chiesi, Boehringer-Ingelheim, Mundipharma, and Novartis, and has participated in congresses and educational lectures with support from AstraZeneca, Boehringer-Ingelheim, Chiesi, Novartis, and Roche. MIL and IT are employees of Medaffcon Oy. AK has received lecture fees from Bayer. LV and JJI-H are employees of GSK and JJI-H holds shares in GSK. TL has served as a scientific advisory board member for GSK, has performed research sponsored by GSK, and has received funding from GSK to participate in a scientific conference.
Authors contributions
MIL, IT, LV, JJI-H and TL made substantial contributions to the conception or design of the work, and IT and AK were involved with the acquisition of data (e.g., study investigator). All authors were involved in the data analysis or interpretation, drafting the work or revising it critically for important intellectual content, gave final approval of the version to be published, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Supplemental Material
Download MS Word (19.7 KB)Acknowledgements
This study was funded by GlaxoSmithKline (GSK ID: HO-17-17558). The authors would like to thank the staff at the Center for Clinical Informatics at Turku University Hospital for their assistance with data–extract-transfer-load processes and source data harmonization. Editorial support (in the form of writing assistance, including development of the initial draft based on author direction, assembling tables and figures, collating authors’ comments, grammatical editing, and referencing) was provided by Laura Pearce PhD, at Fishawack Indicia Ltd, UK, and was funded by GSK.
Data availability statement
GSK makes available anonymized individual participant data and associated documents from interventional clinical studies which evaluate medicines, upon approval of proposals submitted to www.clinicalstudydatarequest.com. To access data for other types of GSK sponsored research, for study documents without patient-level data and for clinical studies not listed, please submit an enquiry via the website. The sharing of a de-identified dataset of this study is restricted by Finnish law (Data Protection Act (1050/2018)). The dataset can only be requested through permit authorization process from Turku Clinical Research Center for justifiable research projects (http://www.turkucrc.fi/en).
Additional information
Funding
References
- Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, Adcock IM, Bateman ED, Bel EH, Bleecker ER, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–373. doi:10.1183/09031936.00202013.
- Wenzel S. Severe asthma: from characteristics to phenotypes to endotypes. Clin Exp Allergy 2012;42:650–658. doi:10.1111/j.1365-2222.2011.03929.x.
- G. B. D. Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. 2017;5:691–706. doi:10.1016/S2213-2600(17)30293-X.
- World Health Organization. Asthma. Key facts 2017. http://www.who.int/news-room/fact-sheets/detail/asthma. Accessed Sep, 2018.
- Jousilahti P, Haahtela T, Laatikainen T, Makela M, Vartiainen E. Asthma and respiratory allergy prevalence is still increasing among Finnish young adults. Eur Respir J. 2016;47:985–987. doi:10.1183/13993003.01702-2015.
- Pavord ID. Eosinophilic phenotypes of airway disease. Ann Am Thorac Soc. 2013;10: S143–S149. doi:10.1513/AnnalsATS.201306-168AW.
- Walsh CJ, Zaihra T, Benedetti A, Fugere C, Olivenstein R, Lemiere C, Hamid Q, Martin JG. Exacerbation risk in severe asthma is stratified by inflammatory phenotype using longitudinal measures of sputum eosinophils. Clin Exp Allergy 2016;46:1291–1302. doi:10.1111/cea.12762.
- Demarche SF, Schleich FN, Paulus VA, Henket MA, Van Hees TJ, Louis RE. Asthma control and sputum eosinophils: a longitudinal study in daily practice. J Allergy Clin Immunol Pract. 2017;5:1335–1343.e5. doi:10.1016/j.jaip.2017.01.026.
- Ilmarinen P, Tuomisto LE, Niemela O, Kankaanranta H. Prevalence of patients eligible for anti-IL-5 treatment in a cohort of adult-onset asthma. J Allergy Clin Immunol Pract. 2019;7:165–174.e4. doi:10.1016/j.jaip.2018.05.032.
- Hekking PP, Wener RR, Amelink M, Zwinderman AH, Bouvy ML, Bel EH. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135:896–902. doi:10.1016/j.jaci.2014.08.042.
- Larsson K, Stallberg B, Lisspers K, Telg G, Johansson G, Thuresson M, Janson C. Prevalence and management of severe asthma in primary care: an observational cohort study in Sweden (PACEHR). Respir Res. 2018;19:12. doi:10.1186/s12931-018-0719-x.
- Varsano S, Segev D, Shitrit D. Severe and non-severe asthma in the community: A large electronic database analysis. Respir Med. 2017;123:131–139. doi:10.1016/j.rmed.2016.12.017.
- Chen S, Golam S, Myers J, Bly C, Smolen H, Xu X. Systematic literature review of the clinical, humanistic, and economic burden associated with asthma uncontrolled by GINA Steps 4 or 5 treatment. Curr Med Res Opin. 2018;34:1–14. doi:10.1080/03007995.2018.1505352.
- Nunes C, Pereira AM, Morais-Almeida M. Asthma costs and social impact. Asthma Res Pract. 2017;3:1.doi:10.1186/s40733-016-0029-3.
- Janson C, Lisspers K, Stallberg B, Johansson G, Telg G, Thuresson M, Nordahl Christensen H, Larsson K. Health care resource utilization and cost for asthma patients regularly treated with oral corticosteroids - a Swedish observational cohort study (PACEHR). Respir Res. 2018;19:168. doi:10.1186/s12931-018-0855-3.
- Mäkelä MJ, Christensen HN, Karlsson A, Rastogi S, Kettunen K. Health care resource utilization and characteristics of patients with eosinophilic asthma in secondary health care in Finland. Eu Clin Respir J 2018;5:1458560. doi:10.1080/20018525.2018.1458560.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi:10.1016/0021-9681(87)90171-8.
- Thygesen SK, Christiansen CF, Christensen S, Lash TL, Sørensen HT. The predictive value of ICD-10 diagnostic coding used to assess Charlson comorbidity index conditions in the population-based Danish National Registry of Patients. BMC Med Res Methodol. 2011;11:83. doi:10.1186/1471-2288-11-83.
- Kerkhof M, Tran TN, Soriano JB, Golam S, Gibson D, Hillyer EV, Price DB. Healthcare resource use and costs of severe, uncontrolled eosinophilic asthma in the UK general population. Thorax 2018;73:116–124. doi:10.1136/thoraxjnl-2017-210531.
- Price D, Wilson AM, Chisholm A, Rigazio A, Burden A, Thomas M, King C. Predicting frequent asthma exacerbations using blood eosinophil count and other patient data routinely available in clinical practice. J Asthma Allergy 2016;9:1–12. doi:10.2147/JAA.S97973.
- Zeiger RS, Schatz M, Li Q, Chen W, Khatry DB, Gossage D, Tran TN. High blood eosinophil count is a risk factor for future asthma exacerbations in adult persistent asthma. J Allergy Clin Immunol Pract. 2014;2:741–750. doi:10.1016/j.jaip.2014.06.005.
- Chupp GL, Bradford ES, Albers FC, Bratton DJ, Wang-Jairaj J, Nelsen LM, Trevor JL, Magnan A, ten Brinke A. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med. 2017;5:390–400. doi:10.1016/S2213-2600(17)30125-X.
- Bel EH, Wenzel SE, Thompson PJ, Prazma CM, Keene ON, Yancey SW, Ortega HG, Pavord ID. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med 2014;371:1189–1197. doi:10.1056/NEJMoa1403291.
- Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, Ortega H, Chanez P. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet 2012;380:651–659. doi:10.1016/S0140-6736(12)60988-X.
- Buhl R, Humbert M, Bjermer L, Chanez P, Heaney LG, Pavord I, Quirce S, Virchow JC, Holgate S. Severe eosinophilic asthma: a roadmap to consensus. Eur Respir J. 2017;49:1700634. doi:10.1183/13993003.00634-2017.
- Chastek B, Korrer S, Nagar SP, Albers F, Yancey S, Ortega H, Forshag M, Dalal AA. Economic burden of illness among patients with severe asthma in a managed care setting. J Manage Care Spec Pharm. 2016;22:848–861. doi:10.18553/jmcp.2016.22.7.848.
- Antonicelli L, Bucca C, Neri M, De Benedetto F, Sabbatani P, Bonifazi F, Eichler HG, Zhang Q, Yin DD. Asthma severity and medical resource utilisation. Eur Respir J. 2004;23:723–729. doi:10.1183/09031936.04.00004904.
- Haahtela T, Herse F, Karjalainen J, Klaukka T, Linna M, Leskelä R-L, Selroos O, Reissell E. The Finnish experience to save asthma costs by improving care in 1987-2013. J Allergy Clin Immunol. 2017;139:408–414. e402. doi:10.1016/j.jaci.2016.12.001.
- Bahadori K, Doyle-Waters MM, Marra C, Lynd L, Alasaly K, Swiston J, FitzGerald JM. Economic burden of asthma: a systematic review. BMC Pulm Med. 2009;9:24.doi:10.1186/1471-2466-9-24.
