Abstract
Objective
Treatment with benralizumab significantly reduces exacerbations and improves lung function after 1 year and decreases oral corticosteroid (OCS) use after 28 weeks for patients with severe, uncontrolled eosinophilic asthma. We assessed whether these effects on OCS reduction are sustained for up to an additional year of treatment while maintaining an acceptable safety profile.
Methods
Data on OCS maintenance dosage were collected for adult patients with baseline blood eosinophil counts ≥150 cells/μL treated with add-on benralizumab 30 mg (every 4 [Q4W] or 8 weeks [Q8W; first three doses Q4W]) from the 28-week ZONDA study and were integrated with results from the predefined 56-week adult completion phase of the BORA extension study. Efficacy and safety were summarized descriptively.
Results
For patients receiving benralizumab Q8W, the median daily OCS dosage reduction of 75% from baseline to end of treatment achieved in ZONDA was sustained at the end of the BORA extension period (median 67% reduction from baseline). This was estimated to result in a median cumulative OCS dosage of 2.98 g over the 1.5-year period for patients receiving benralizumab Q8W compared with 5.74 g if these patients had remained on their baseline OCS dosages prior to benralizumab initiation. All adverse event rates were similar between the BORA extension and ZONDA periods, with no new or unexpected safety findings.
Conclusion
This benralizumab 1.5-year integrated analysis demonstrates that OCS reductions and safety were maintained with further follow up and supports long-term use of benralizumab for patients with severe, uncontrolled eosinophilic asthma.
Introduction
Patients with severe, uncontrolled asthma experience increased disease burden, including recurrent exacerbations and hospitalizations (Citation1,Citation2). Oral corticosteroids (OCS) are an effective mainstay treatment for asthma exacerbations and symptom control for patients with severe disease. However, cumulative exposure is associated with adverse health-related outcomes (Citation3,Citation4). The detrimental impacts of OCS use on patient health-related quality of life caused by OCS-associated adverse events significantly contribute to OCS noncompliance, which increases risk of future exacerbations (Citation2).
Benralizumab is an interleukin-5 receptor alpha–directed cytolytic monoclonal antibody that induces direct, rapid, and nearly complete depletion of eosinophils via enhanced antibody-dependent cell-mediated cytotoxicity (Citation5,Citation6). The efficacy and safety of benralizumab have been evaluated in three pivotal Phase III studies: SIROCCO (Citation7), CALIMA (Citation8), and ZONDA (Citation9). In the 28-week ZONDA steroid-sparing study, benralizumab reduced OCS dosage from baseline by 75% compared with a 25% reduction with placebo for adult patients with baseline blood eosinophil counts ≥150 cells/μL (Citation9).
Patients who completed SIROCCO, CALIMA, or ZONDA and chose to continue long-term treatment were invited to enroll in the BORA Phase III extension study (Citation10). Adults enrolled in BORA continued treatment for up to 56 weeks, while adolescents could continue treatment for up to 108 weeks. Safety and efficacy data for the first year of BORA for patients in SIROCCO and CALIMA have been evaluated and published (Citation10). Analysis indicated that the safety profile of benralizumab was consistent with published results from the Phase III studies (Citation10).
Here we present analyses focusing on the integration of data from BORA and ZONDA to examine the effect of benralizumab treatment on OCS exposure and safety over a period of up to 1.5 years for adult patients with severe asthma.
Methods
Study design and participants
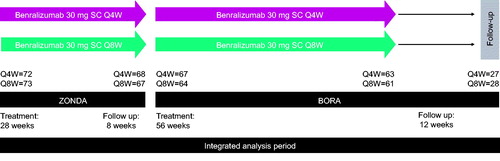
Specific details of the individual study designs have been published (Citation9,Citation10). Patients enrolled in ZONDA were randomized 1:1:1 to receive placebo or benralizumab 30 mg every 4 weeks (Q4W) or 30 mg every 8 weeks (Q8W; first three doses Q4W) for 28 weeks, stratified by baseline blood eosinophil count (≥150–<300/μL vs. ≥300/μL). In this analysis, data were evaluated only for patients who were originally randomized to benralizumab in ZONDA (Citation9) and continued on benralizumab treatment during BORA (up to an additional 56-week on-treatment period and 12 weeks of follow up; ) (Citation10). Patients who received placebo in the pivotal study were randomized to one of the two active treatment groups when they entered BORA. Because these patients did not remain on the same treatment throughout the 52-week follow-up period, they were not included in the integrated analyses. No additional OCS reduction was included in the BORA protocol. Additional information is provided in the online supplement.
Figure 1. Benralizumab 1.5-year integrated analysis study design. Q4W, every 4 weeks; Q8W, every 8 weeks (first three doses Q4W); SC, subcutaneously.
In the integrated analysis, patients receiving benralizumab 30 mg subcutaneously, either Q4W or Q8W, with high-dosage inhaled corticosteroids plus long-acting β2-agonists in the pivotal studies who continued into BORA were assessed, including those who transitioned into the ongoing open-label, 130-week safety extension study MELTEMI (NCT02808819). Full inclusion and exclusion criteria for the pivotal studies and BORA used in the benralizumab integrated analysis have been reported (Citation9,Citation10).
Ethics
All patients provided written informed consent at enrollment. This study was conducted in accordance with the principles of the Declaration of Helsinki and was consistent with the International Council for Harmonisation/Good Clinical Practice. Additional information is provided in the online supplement.
Outcomes
The long-term efficacy endpoint for the integrated analysis of patients who were originally in ZONDA was maintenance of the changes from baseline in OCS dosage achieved at the end of ZONDA over a further year of benralizumab treatment. Change in OCS dosage was defined by either percentage reduction between groups or median reduction while maintaining asthma control.
OCS maintenance dosage data were used to estimate annualized OCS dosage (annualized dosage = 365.25*cumulative dosage observed in follow up [through to final assessment]/duration of follow up) and subsequently median cumulative OCS exposure for benralizumab-treated patients over the 1.5-year treatment period. Median cumulative OCS exposure was calculated for benralizumab-treated patients compared with estimated exposure if those patients had remained on their ZONDA baseline OCS dosages. In ZONDA, only patients receiving baseline OCS ≤12.5 mg/day could discontinue OCS treatment under the protocol-defined OCS titration schedule. Therefore, we also estimated median cumulative OCS exposure for patients with baseline OCS ≤12.5 and >12.5 mg/day. Exposure from rescue OCS use was not included in this analysis.
Additional efficacy assessments included reduction in asthma exacerbations (annual asthma exacerbation rate [AER] and AER associated with hospitalizations and emergency department [ED] visits), lung function (change from baseline in prebronchodilator [BD] forced expiratory volume in 1 s [FEV1]), and change in Asthma Control Questionnaire 6 (ACQ-6) scores. The long-term safety and tolerability endpoints were adverse events (AEs), serious adverse events (SAEs), hypersensitivity, and immunogenicity. Treatment exposure and rate of AEs per 100-patient-years (100-PYs) at risk were determined.
Statistical analysis
The OCS analysis set (OAS) comprised patients who received benralizumab, completed ZONDA, and received at least one dose of benralizumab in BORA, including those who transitioned into MELTEMI (NCT02808819). Additional information is provided in the online supplement.
Analyses of disposition were based on the all-patients analysis set (APS), which included all patients from ZONDA, regardless of enrollment into BORA and later enrollment into MELTEMI. The full analysis set (FAS) included patients who received benralizumab, completed ZONDA, and received at least one dose of benralizumab in BORA.
No placebo comparison is available for the long-term integrated data. Therefore, data were summarized with descriptive statistics (mean, standard deviation [SD], median, range), qualitative summaries, and 95% confidence intervals. All analyses were conducted with SAS® System (SAS Institute Inc., Cary, NC, United States) version 9.4 or later.
Results
Patients
Of the 220 patients in ZONDA, 145 received benralizumab (Q4W, n = 72; Q8W, n = 73), and 75 patients were in the placebo group (Figure S1, Supplementary material). From ZONDA, 131 patients receiving benralizumab entered BORA (Q4W, n = 67; Q8W, n = 64). During the integrated period, the most frequent reasons for discontinuation of benralizumab were patient decision (3.2%) and AE (1.8%). Of the 124 patients who completed treatment for the 56-week extension period, 55 patients remained in BORA and 69 patients entered MELTEMI. Patients who began treatment in BORA and later enrolled into MELTEMI were considered to have completed treatment. Taking into consideration the small sample sizes, demographics and clinical characteristics were, in general, balanced between treatment groups (previously published) (Citation9). The ZONDA OAS consisted of 131 patients. Baseline daily dosages of OCS are provided in .
Table 1. Study entry and baseline optimized OCS dosage for patients enrolled in ZONDA (OAS).
Most patients in the Q8W group (93.5%) had at least 18 months of benralizumab exposure with a mean on-treatment duration of 586.55 days (range: 400.00–604.00, Table S1, Supplementary material). Results were similar across the treatment groups. Because the OAS included an increased proportion of patients in BORA who later enrolled into MELTEMI, the mean and median durations of benralizumab exposure were shorter than those observed in the FAS and more variable between treatment groups. Despite this, most patients in the Q4W (58.2%) and Q8W (68.8%) groups had ≥60 weeks of treatment.
Efficacy outcomes
OCS dosage reduction
OCS dosage reduction observed at the end of the ZONDA study (28 weeks) with benralizumab treatment was maintained during an additional 56 weeks of treatment, defined by either percentage reduction groupings () or median reduction (). In the benralizumab Q8W group at ZONDA end of treatment (EOT) for patients who entered BORA, 70.3% of patients were able to reduce their daily OCS dosages by ≥50%, and 42.2% of patients were able to reduce it by ≥90%. At BORA Week 24, the percentage of patients who were able to reduce their daily OCS dosages by ≥50% and ≥90% were similar to those at ZONDA EOT (75.9% and 39.7%, respectively). This effect was maintained at extension Week 56 (75.0% and 39.3% of patients reduced by ≥50% and ≥90%, respectively). However, fewer patients were enrolled in BORA at this time point. Results were similar in the benralizumab Q4W group ().
Figure 2. Daily OCS dosage reduction from baseline with benralizumab treatment during the 84 weeks integrated analysis (OAS, On-Treatment Period). CI, confidence interval; OAS, OCS analysis set; OCS, oral corticosteroid; Q4W, every 4 weeks; Q8W, every 8 weeks (first three doses Q4W). Error bars represent 95% CI.
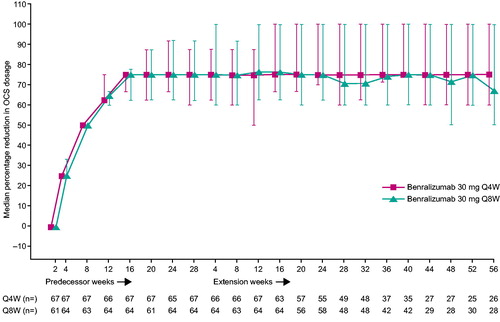
Table 2. Maintenance of OCS dosage reduction during the extension period of the 2-year integrated analysis study (OAS, on-treatment period, blood eosinophil counts ≥150 cells/μL).
In the benralizumab Q8W group, the median optimized daily OCS dosage at randomization was 10.0 mg. At ZONDA EOT, the median daily OCS dosage was 5.0 mg, and the median percentage reduction from baseline was 75.0%. This reduction was maintained throughout the BORA extension period. At Week 56, median OCS daily dosage was 5.0 mg and median percentage reduction in OCS dosage was 67.4%. Results were similar in the Q4W group.
Estimated annualized cumulative OCS dosage and predicted OCS exposure reduction
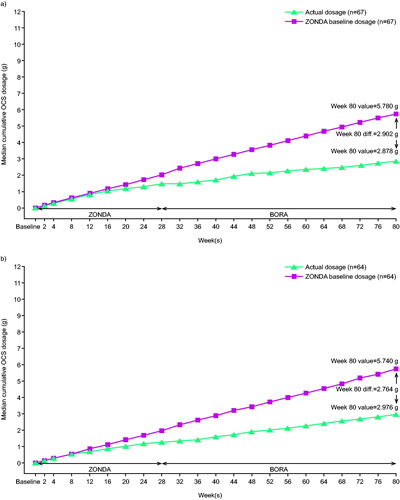
Mean annualized cumulative OCS dosages were estimated at 3.11 g and 2.52 g overall for the benralizumab Q4W and Q8W groups, respectively. Of those with baseline OCS dosages ≤12.5 mg/day, mean annualized cumulative dosages were 1.37 g (Q4W; n = 37) and 1.40 g (Q8W; n = 38). For the group with baseline OCS dosages >12.5 mg/day, mean annualized cumulative dosages were 5.35 g (Q4W; n = 30) and 4.15 g (Q8W; n = 26).
Median cumulative OCS exposure over 1.5 years for patients receiving benralizumab Q8W was estimated at 2.98 g compared with 5.74 g if those same patients had remained on their baseline OCS dosages. This corresponded to an estimated median cumulative OCS exposure reduction of 2.76 g over a 1.5-year period for patients receiving benralizumab Q8W () without accounting for OCS as rescue medication. For patients receiving baseline OCS ≤12.5 and >12.5 mg/day, median cumulative OCS exposures associated with benralizumab Q8W at 1.5 years were 0.87 g and 5.11 g, respectively, with corresponding reductions compared with those remaining on baseline OCS dosages of 4.74 g and 6.12 g, respectively (Figures S2, S3, Supplementary material).
Additional efficacy outcomes
Of the 64 ZONDA patients in the benralizumab Q8W group of the OAS, 37.5% (n = 24) of patients experienced ≥1 exacerbation during the integrated on-treatment period, all of which required the use of systemic corticosteroids (including OCS). Of patients in the Q8W group, 3.1% had ≥1 exacerbation that resulted in hospitalization or an ED visit. Exacerbation rates remained stable over time (0.77 and 0.40 at ZONDA EOT, and 0.56 and 0.57 at BORA EOT for the Q4W and Q8W groups, respectively).
Of ZONDA patients in the FAS, pre-BD FEV1 improved from baseline for both benralizumab dosages at ZONDA EOT. Improvements were, in general, maintained during BORA (FAS, Figure S4, Supplementary material). Baseline ACQ-6 scores were also improved at ZONDA Week 28 for both benralizumab dosages and improvements were in general maintained at BORA Week 56 (FAS, Figure S5, Supplementary material).
Safety outcomes
Unless noted, data reported in this section are for patients in the FAS who received benralizumab, completed ZONDA, received at least one dose of benralizumab in BORA, and did not transition into MELTEMI. The overall incidences of AEs and SAEs were 90.3% (n = 56) and 24.2% (n = 15), respectively (details in Table S2, Supplementary material). The treatment-emergent adverse event (TEAE) rate reported during the extension period was numerically lesser than that reported during ZONDA (73.07 and 141.71 events per 100-PYs, respectively). The overall incidence of TEAEs during the integrated on-treatment period was 90.3% (n = 28) in the benralizumab 30 mg Q8W group, and 25.8% (n = 8) of patients reported SAEs. Overall, patterns in incidence of TEAEs for patients in the benralizumab 30 mg Q4W group were comparable with the Q8W group. The most common AEs reported for ZONDA patients who received benralizumab and entered BORA (n = 62) were viral upper respiratory tract infection (35.5%, n = 22) and bronchitis (22.6%, n = 14). Asthma was the most common SAE (4.8% of all patients receiving benralizumab in the FAS, n = 3), with an overall event rate of 3.05 per 100-PYs ().
Table 3. Adverse events reported during the on-treatment period in any category for predecessor ZONDA patients (FAS, on-treatment period).
One patient (3.2%, event rate 2.06 per 100-PYs) receiving benralizumab Q4W and two patients (6.5%, event rate 4.02 per 100-PYs) receiving benralizumab Q8W experienced an AE leading to discontinuation during the on-treatment period (). There were no deaths reported for the FAS, which comprised patients who received benralizumab in ZONDA and entered BORA. However, two deaths were reported in the ZONDA trial (Citation9), and one death was reported for a patient who received placebo in ZONDA and benralizumab Q8W in BORA. Of ZONDA patients (APS, all patients who received benralizumab), one patient experienced a mild hypersensitivity reaction related to benralizumab (Q8W), followed by allergic dermatitis after a subsequent injection resulting in discontinuation. One patient in the benralizumab Q4W group (APS) experienced hypersensitivity as an SAE related to benralizumab. The allergic reaction event began on Day 112, the same day as the administration of the fifth dose of benralizumab, during ZONDA. The event was severe and resolved in one day, but it led to patient discontinuation and eventual study withdrawal.
The most common serious infections for those receiving benralizumab were lower respiratory tract infection, pneumonia, and bacterial urinary tract infection (n = 1 each [1.6%]; FAS). Similar rates of serious infections were observed at the end of the pivotal study and extension periods (n = 1 [1.6%], event rate 2.94 per 100-PYs vs. n = 2 [3.2%], event rate 3.2 per 100-PYs; FAS). No helminth infections, immune complex disease (type III hypersensitivity), or malignancies were reported in predecessor ZONDA patients.
In the Q8W group of predecessor ZONDA patients, 5 of 31 (16.1%) tested positive for anti-drug antibodies (ADA) at any time point (Table S3, Supplementary material). Few patients were persistently (4 months) positive (n = 3; 9.7%) or prolonged (12 months) persistently positive (n = 1; 3.2%). Serum concentrations of benralizumab remained above 71 ng/mL, and median eosinophil counts ranged from 0–50 cells/μL for ADA-positive patients at all assessments (from ZONDA Week 12 to BORA Week 56). The ADA response for ZONDA patients in the Q4W group was, in general, similar to the Q8W group. Overall, the number of ADA-positive ZONDA patients in this study was too small to assess a possible effect of ADA on efficacy or safety.
Discussion
This report presents the integrated efficacy and safety analysis for patients treated with benralizumab who entered the BORA safety extension study from the pivotal ZONDA Phase III steroid sparing study (Citation9,Citation10). We evaluated maintenance of effect for patients who received benralizumab in ZONDA and 56 weeks of benralizumab treatment in the first extension phase of the BORA study. This is the first report on maintenance of OCS use reduction during 1.5 years of benralizumab treatment.
These integrated analyses of the ZONDA and BORA studies further support benralizumab as a safe and efficacious treatment for patients with severe, uncontrolled eosinophilic asthma, complementing the SIROCCO and CALIMA Phase III studies in which patients receiving benralizumab had reduced exacerbations and improved lung function compared with placebo (Citation7,Citation8). Of the 64 patients in the benralizumab Q8W group of the OAS, 70.3% and 42.2% were able to maintain their daily OCS dosage reductions by ≥50% and ≥90%, respectively. Exacerbation rates and improvements in lung function (changes in pre-BD FEV1 and ACQ-6 scores from baseline) were consistent across the integrated study period.
Estimated median cumulative OCS exposure reduction over 1.5 years for patients receiving benralizumab Q8W compared with patients if they had remained on their baseline OCS dosages was 2.76 g. However, interpretation of these data is limited by the lack of a placebo arm and the level of imputation, as well as the fact that OCS dosage was not further reduced during BORA, which decreased the potential for cumulative OCS dosage reduction during the integrated period. Such reductions in OCS exposure are important, as annual cumulative exposures of systemic corticosteroids ≥1.0 g have been demonstrated to increase risk of TEAEs such as weight gain, anxiety, glaucoma, cardiovascular disease, osteoporosis, impaired immunity, and diabetes (Citation2,Citation4). Furthermore, the reduction in exacerbations with benralizumab treatment also reduces the risk of acute AEs associated with short-term OCS escalation during an exacerbation, such as sepsis, venous thromboembolism, and fracture (Citation11). These data put into context the improvements observed for patients with baseline OCS dosages ≤12.5 mg/day whose median cumulative OCS exposure associated with benralizumab over 1.5 years was <1.0 g (0.87 g). Future real-world analysis will further elucidate the potential of add-on benralizumab for reduction of cumulative OCS dosage by including OCS bursts administered to treat exacerbations, which have not been accounted for here.
TEAE rate reported during the BORA extension was numerically smaller than that reported during ZONDA (73.07 vs. 141.71 events per 100-PYs). Overall incidence of SAEs during the integrated on-treatment period was 15.4% for the benralizumab Q8W group and was stable across ZONDA and BORA study periods. Safety results from this study are consistent with published data (e.g., SIROCCO and CALIMA, 71–75% [benralizumab only]; BORA, 65–71%), and no unexpected AEs were reported with extended benralizumab use during approximately 1.5 years (Citation7,Citation8,Citation10). Adrenal insufficiency, associated with long-term OCS use, was not evaluated in this study but will be investigated in the PONENTE (NCT03557307) Phase IIIb trial designed to evaluate the efficacy and safety of tapering OCS use after initiation of benralizumab treatment for adult patients with severe, uncontrolled asthma with eosinophilic inflammation. There were no reports of eosinophilic granulomatosis with polyangiitis for predecessor ZONDA patients during OCS tapering.
This study was limited by the restricted number of patients followed for the full 1-year extension because of the rollover of patients into MELTEMI. Analysis of long-term efficacy for ZONDA patients was conducted on the FAS, which did not include patients who entered MELTEMI, limiting the interpretation of the long-term efficacy outcomes from the integrated analysis. There is also potential for selection bias, as patients entered BORA only if they completed their respective treatments in the pivotal studies, and patients who discontinued early may have had different outcomes. Nevertheless, the efficacy values and safety profile presented for the extension period of this study are consistent with what was reported in ZONDA (Citation9). The prevalence of ADA development remained stable throughout the treatment period. Overall, the number of ADA-positive predecessor ZONDA patients in this study was too small to assess a possible effect of ADA on safety or efficacy.
Conclusions
In summary, prolonged treatment with benralizumab exhibits a maintained efficacy and safety profile that enables patients with severe asthma to reduce long-term OCS exposure while maintaining disease control. The estimated cumulative OCS exposure reduction achieved with benralizumab is likely to reduce risk of adverse outcomes for patients, which further supports the safe and effective use of benralizumab for patients with severe, uncontrolled eosinophilic asthma.
Data-sharing statement
Data underlying the findings described in this manuscript may be requested in accordance with AstraZeneca’s data-sharing policy described at https://astrazenecagroup-dt.pharmacm.com/DT/Home.
Supplemental Material
Download MS Word (514 KB)Acknowledgements
Writing and editing support, including preparation of the draft manuscript under the direction and guidance of the authors, incorporating author feedback, and manuscript submission, was provided by Hayley Ellis, PhD, of JK Associates, Inc., and Michael A. Nissen, ELS, of AstraZeneca. This support was funded by AstraZeneca.
Declaration of interest
A. Bourdin has received personal fees from Actelion, AstraZeneca, Boehringer Ingelheim, Chiesi Pharmaceuticals, GSK, Novartis, Regeneron, Roche, and Teva; received grants from Boehringer Ingelheim and GSK; received non-financial support from Actelion, AstraZeneca, Biogen, Boehringer Ingelheim, Chiesi Pharmaceuticals, Galapagos, Novartis, Roche, and Vertex; and has been an advisory board member for Actelion, AstraZeneca, Boehringer Ingelheim, Chiesi Pharmaceuticals, Gilead, GSK, Novartis, Regeneron, and Teva. D. Shaw was an advisory board member for AstraZeneca, Boehringer Ingelheim, and GlaxoSmithKline and has received travel fees from Teva and Novartis. A. Menzies-Gow reports consultancy agreements with AstraZeneca, Sanofi, and Vectura; was an advisory board member for AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Sanofi, and Teva; received speaker fees from AstraZeneca, Boehringer Ingelheim, Novartis, Teva, and Vectura; has received clinical funding from AstraZeneca; has participated in research that his institution has been remunerated from Boehringer Ingelheim, GlaxoSmithKline, AstraZeneca, and Hoffman La Roche; and has attended international conferences sponsored by Teva and Boehringer Ingelheim.
J.M. FitzGerald is an advisory board member for AstraZeneca, Boehringer Ingelheim, Novartis, Sanofi-Regeneron, and Teva, and has received honoraria for lectures from AstraZeneca, Boehringer Ingelheim, GSK, and Novartis. E.R. Bleecker has performed clinical trials through his former employer, the Wake Forest School of Medicine, and has served as a paid consultant for AstraZeneca, Boehringer Ingelheim, GSK, MedImmune, Novartis, Regeneron, and Sanofi-Aventis. W.W. Busse reports personal fees from AstraZeneca, Boston Scientific, Genentech, GSK, Novartis, Sanofi, and Teva. G.T. Ferguson reports grants and personal fees from AstraZeneca, Novartis, Pearl Therapeutics, and Sunovian, as well as grants from Forest and personal fees from GSK. L. Brooks, P. Barker, E. Garcia Gil, and U.J. Martin are employees of AstraZeneca.
Additional information
Funding
References
- Chen S, Golam S, Myers J, Bly C, Smolen H, Xu X. Systematic literature review of the clinical, humanistic, and economic burden associated with asthma uncontrolled by GINA Steps 4 or 5 treatment. Curr Med Res Opin. 2018;34(12):2075–2088. doi:10.1080/03007995.2018.1505352.
- Menzies-Gow A, Canonica GW, Winders TA, Correia de Sousa J, Upham JW, Fink-Wagner AH. A charter to improve patient care in severe asthma. Adv Ther. 2018;35(10):1485–1496. doi:10.1007/s12325-018-0777-y.
- Ramsahai JM, Wark PA. Appropriate use of oral corticosteroids for severe asthma. Med J Aust. 2018;209(S2):S18–S21.
- Price DB, Trudo F, Voorham J, Xu X, Kerkhof M, Ling Zhi Jie J, Tran TN. Adverse outcomes from initiation of systemic corticosteroids for asthma: long-term observational study. J Asthma Allergy. 2018;Volume 11:193–204. doi:10.2147/JAA.S176026.
- Kolbeck R, Kozhich A, Koike M, Peng L, Andersson CK, Damschroder MM, Reed JL, Woods R, Dall'acqua WW, Stephens GL, et al. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol. 2010;125(6):1344–1353. doi:10.1016/j.jaci.2010.04.004.
- Pham TH, Damera G, Newbold P, Ranade K. Reductions in eosinophil biomarkers by benralizumab in patients with asthma. Respir Med. 2016;111:21–29. doi:10.1016/j.rmed.2016.01.003.
- Bleecker ER, FitzGerald JM, Chanez P, Papi A, Weinstein SF, Barker P, Sproule S, Gilmartin G, Aurivillius M, Werkström V, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2115–2127. doi:10.1016/S0140-6736(16)31324-1.
- FitzGerald JM, Bleecker ER, Nair P, Korn S, Ohta K, Lommatzsch M, Ferguson GT, Busse WW, Barker P, Sproule S, et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2128–2141. doi:10.1016/S0140-6736(16)31322-8.
- Nair P, Wenzel S, Rabe KF, Bourdin A, Lugogo NL, Kuna P, Barker P, Sproule S, Ponnarambil S, Goldman M. ZONDA trial investigators. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N Engl J Med. 2017;376(25):2448–2458. doi:10.1056/NEJMoa1703501.
- Busse WW, Bleecker ER, FitzGerald JM, Ferguson GT, Barker P, Sproule S, Olsson RF, Martin UJ, Goldman M, et al. BORA study investigators. Long-term safety and efficacy of benralizumab in patients with severe, uncontrolled asthma: 1-year results from the BORA phase 3 extension trial. Lancet Respir Med. 2019;7(1):46–59., doi:10.1016/S2213-2600(18)30406-5.
- Waljee AK, Rogers MA, Lin P, Singal AG, Stein JD, Marks RM, Ayanian JZ, Nallamothu BK. Short term use of oral corticosteroids and related harms among adults in the United States: population based cohort study. BMJ. 2017;357:j1415. doi:10.1136/bmj.j1415.